ABSTRACT
Phytoplasmas are uncultivated plant pathogens and cell wall-less bacteria and are transmitted from plant to plant by hemipteran insects. The phytoplasma's circulative propagative cycle in insects requires the crossing of the midgut and salivary glands, and primary adhesion to cells is an initial step toward the invasion process. The flavescence dorée (FD) phytoplasma possesses a set of variable membrane proteins (Vmps) exposed on its surface, and this pathogen is suspected to interact with insect cells. The results showed that VmpA is expressed by the flavescence dorée phytoplasma present in the midgut and salivary glands. Phytoplasmas cannot be cultivated at present, and no mutant can be produced to investigate the putative role of Vmps in the adhesion of phytoplasma to insect cells. To overcome this difficulty, we engineered the Spiroplasma citri mutant G/6, which lacks the ScARP adhesins, for VmpA expression and used VmpA-coated fluorescent beads to determine if VmpA acts as an adhesin in ex vivo adhesion assays and in vivo ingestion assays. VmpA specifically interacted with Euscelidius variegatus insect cells in culture and promoted the retention of VmpA-coated beads to the midgut of E. variegatus. In this latest case, VmpA-coated fluorescent beads were localized and embedded in the perimicrovillar membrane of the insect midgut. Thus, VmpA functions as an adhesin that could be essential in the colonization of the insect by the FD phytoplasmas.
IMPORTANCE Phytoplasmas infect a wide variety of plants, ranging from wild plants to cultivated species, and are transmitted by different leafhoppers, planthoppers, and psyllids. The specificity of the phytoplasma-insect vector interaction has a major impact on the phytoplasma plant host range. As entry into insect cells is an obligate process for phytoplasma transmission, the bacterial adhesion to insect cells is a key step. Thus, studying surface-exposed proteins of phytoplasma will help to identify the adhesins implicated in the specific recognition of insect vectors. In this study, it is shown that the membrane protein VmpA of the flavescence dorée (FD) phytoplasma acts as an adhesin that is able to interact with cells of Euscelidius variegatus, the experimental vector of the FD phytoplasma.
KEYWORDS: phytoplasma, spiroplasma, bacterial adhesion to cell, insect vector, transmission
INTRODUCTION
Phytoplasmas are bacteria responsible for diverse epidemic diseases in various cultivated and ornamental plants (1, 2). Characterized by the absence of a cell wall and no peculiar morphology, they belong to the class Mollicutes in the order Acholeplasmatales, “Candidatus Phytoplasma.” These bacteria are exclusively located in the sieve elements of plant hosts and are propagated by numerous insect vectors from the order Hemiptera (3). In hosts, namely, plants and insects, phytoplasmas are found intracellularly. In insects, these bacteria colonize different organs, such as the intestinal tract, muscles, and salivary glands (4). Phytoplasma members of the 16SrV-C and V-D taxonomic subgroups cause a severe epidemic disease of grapevines called flavescence dorée (FD) and have therefore been classified as quarantine pests. These phytoplasmas are propagated within and from vineyard to vineyard by the Deltocephalinae leafhopper Scaphoideus titanus Ball (5), which was introduced in France well before 1950 (6). These grapevine-specialized insects, from the first nymphal to imago stages (7), acquire phytoplasmas while feeding on infected grapevines and subsequently become infectious after a latency period. The use of insecticide treatments against the vector is one of three main ways to control flavescence dorée, with the other two being planting phytoplasma-free material for planting and removing infected grapes. However, chemical treatments cause unwanted economic, social, and environmental impacts and must be reduced. To strengthen such an improvement in FD management, a better understanding of the mechanisms leading to phytoplasma transmission, especially the acquisition phase, is necessary.
In the insect vector, the cycle is persistent and multiplicative (8). This property implies the crossing of the two barriers represented by the intestine epithelium and the salivary gland cells but also the multiplication of bacteria into insects. Phytoplasmas have the capacity to multiply in a wide variety of cellular types, such as the intestine, particularly the muscle layer of the midgut, and salivary glands (9, 10), but FD phytoplasmas have not been detected in sexual organs (11). Their passing through intestinal and salivary gland cells is clearly mediated by endocytosis; next, there is movement into the cytoplasm and then exocytosis, as is the case for Spiroplasma citri, another plant pathogen of the class Mollicutes transmitted by leafhoppers (12, 13). These steps imply the direct interactions between the phytoplasma and eukaryotic cell proteins to promote endocytosis by cells that are not specialized in phagocytosis. Lacking specialized organelles for mobility or kinetic cytoskeletons, phytoplasmas have to move from apical to basal membranes and leave the host cell by exocytosis after multiplication. All of these steps must be achieved without altering tissue integrity to avoid toxicity to their vector. Several genomes of “Ca. Phytoplasma” species are available from which proteins that are predicted to be secreted or surface exposed are tentatively selected for functional studies. However, notably few protein function studies have been described in relation to insect transmission. Among the three types of immunodominant membrane proteins (IDPs) that are the major membrane proteins of phytoplasmas (14), Amp of “Ca. Phytoplasma asteris” was observed to interact with the three main proteins of the microfilament complex, i.e., the actin and myosin light and heavy chains of the intestinal smooth muscle, as well as with the ATP synthase of the leafhopper vector (15, 16). Although Amp is essential for transmission by insect vectors (17), Amp interacts with insect proteins after phytoplasmas have become intracellular and could be implicated in the movement of phytoplasmas across these cells. However, phytoplasma adhesins, which are necessary for the promotion of the adhesion of phytoplasma to cells in the digestive tract and salivary glands, have not been identified to date. Several immunogenic membrane proteins that are present at the surfaces of the phytoplasmas, such as the variable membrane protein Vmp1 of “Ca. Phytoplasma solani,” are targets of strong selective pressures (18). This finding suggests the proteins are implicated in interactions with host molecules. VmpA, similar to Vmp1, is a variable membrane protein predicted to be destined to the FD phytoplasma (FD-P) surface by the Sec-dependent pathway to be finally anchored to the membrane by a C-terminal transmembrane segment (19). Several other genes found in the genome of the FD-P encode variable membrane proteins, including VmpB, which share the same structure (20). The structure of FD-P VmpA also contains a hydrophilic central region that possesses 3 complete repeats of 78 amino acids exposed to the phytoplasma surface. This finding is consistent with the possible role of VmpA in the FD-P adhesion to insect cells, as repeated domains are commonly found in bacterial proteins involved in cell recognition (21). Such adhesins have been characterized in S. citri (22–24) and in Mycoplasma agalactiae (25, 26). Thus, the role of VmpA in the adhesion of the phytoplasma to insect cells was examined.
The in vitro culture of leafhopper vector cells provides an experimental tool to study the phytoplasma-insect interaction at the cellular level. For example, it was demonstrated that the ability of S. citri to invade insect cells ex vivo is correlated to its ability to be transmitted by the leafhopper vector Circulifer haematoceps (27). Additionally, a useful experimental cycle was used to transmit FD-P to the broad bean Vicia faba using the leafhopper Euscelidius variegatus (28), which, similar to S. titanus, belongs to the Deltocephalinae subfamily. This prompted us to use cultured cells of E. variegatus to explore the implication of the strain FD92 (FD92-P) VmpA in the adhesion process of FD-P to insect cells. In this study, antibodies were used to ascertain the VmpA expression by FD92-P in the insect E. variegatus, and we measured the adhesion to E. variegatus cells of recombinant spiroplasmas expressing VmpA and fluorescent latex beads coated with His6-tagged VmpA. The interaction of VmpA-His6-coated beads with the apical surface of midgut epithelial cells was assessed in in vivo ingestion assays.
RESULTS
VmpA protein is expressed by FD92 phytoplasmas in insects.
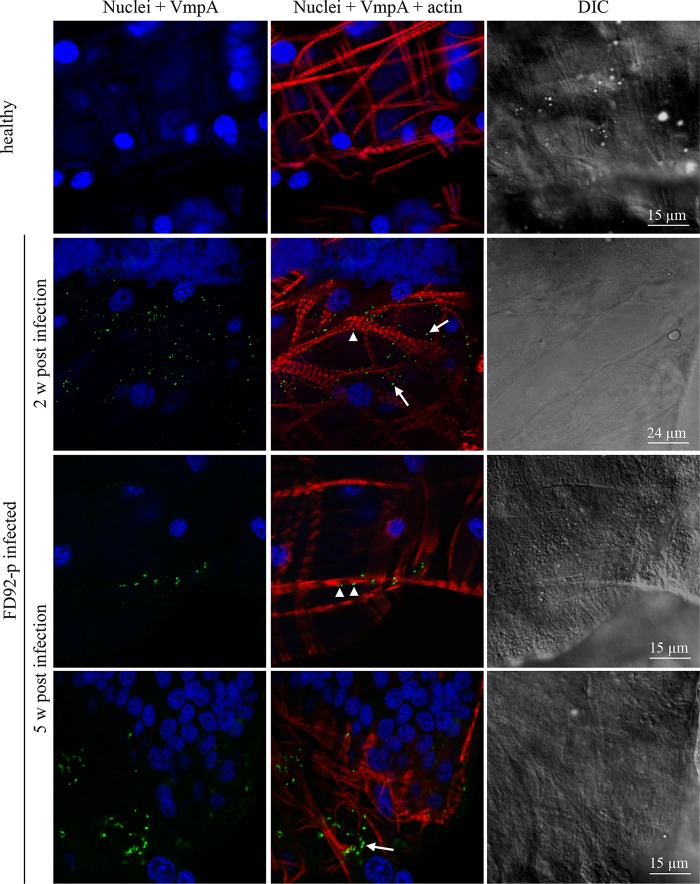
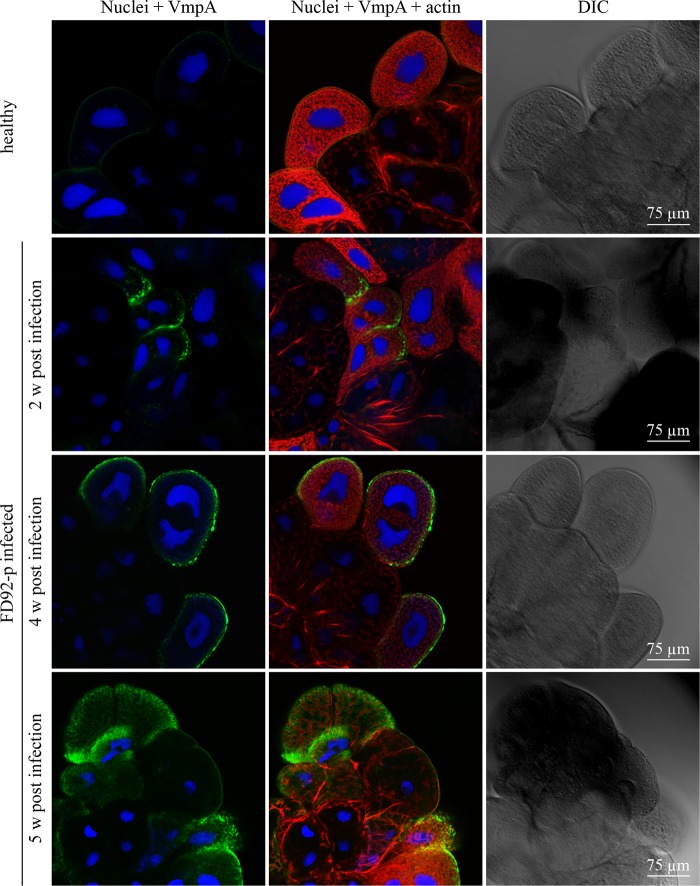
To assess VmpA expression by FD92-P in the intestinal tract and the salivary glands, indirect immunofluorescence labeling and confocal observations were used. VmpA proteins were visualized in the phytoplasmas in midguts 2 weeks after acquisition by feeding with infected broad beans, and they were still detected 5 weeks after infection (Fig. 1). Bacteria were located in intestine cells (Fig. 1, arrows), and a number were observed considerably closer to actin filaments of the muscle fibers covering the basal lamina (Fig. 1, arrowheads). VmpA was also already detected in the salivary glands of some insects 2 weeks after acquisition feeding (Fig. 2) and in the majority of insects after a longer latency period of 4 and 5 weeks (Fig. 2). No labeling was observed in the midgut and salivary glands of healthy insects. The detection of VmpA by immunolabeling showed that the FD92-P infecting E. variegatus produced VmpA both in the midgut and salivary glands.
FIG 1.
Confocal micrographs of Euscelidius variegatus-infected leafhoppers' midguts immunolabeled with anti-VmpA PAbs. The midguts of leafhoppers that were fed healthy broad beans or FD-P-infected broad beans were observed with fluorescent and differential interference contrast (DIC) microscopy. Actin was labeled with Alexa 568-phalloidin (red), nuclei were labeled with DAPI (blue), and FD-P was labeled with anti-His6-VmpA PAbs and secondary Alexa 488 antibodies (green). Arrows indicate internal phytoplasmas, and arrowheads show the phytoplasmas that were located close to actin filaments.
FIG 2.
Confocal micrographs of E. variegatus-infected leafhoppers' salivary glands immunolabeled with anti-VmpA PAbs. The salivary gland cells of leafhoppers fed healthy broad beans or FD-P-infected broad beans were observed with fluorescent and differential interference contrast (DIC) microscopy. Actin was labeled with Alexa 568-phalloidin (red), nuclei were stained with DAPI (blue), and FD-P was labeled with anti-His6-VmpA PAbs and secondary Alexa 488 antibodies (green).
Euva-1 cell line.
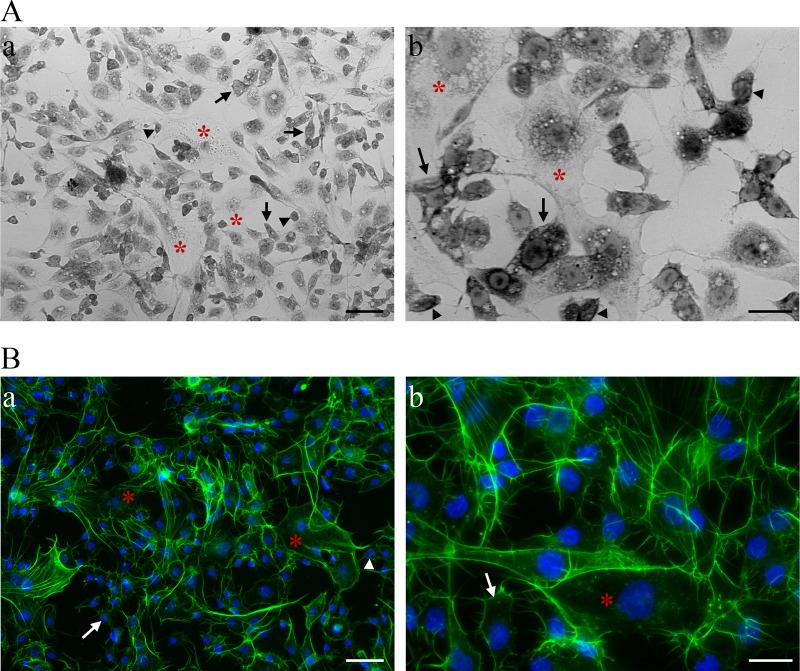
A cell line from E. variegatus was established to study the cellular and molecular interactions between phytoplasma proteins and insect cells. Ten months of continuous culturing of cells isolated from embryos of E. variegatus resulted in the Euva-1 cell line. The cell morphology was examined by light microscopy using methylene blue staining and the fluorescent labeling of actin filaments and nuclei. On the basis of the cellular morphology and colorations, the Euva-1 cell line possessed three main cellular types. The first cells were the largest ones and had only their nuclei stained with methylene blue (Fig. 3, asterisks). The cells in the second cell type had their nuclei and cytosol colored (Fig. 3, arrows), while the cells of the third type were the smallest in size and were colored darker by methylene blue (Fig. 3, arrowheads). Actin coloration showed that the type 2 and 3 cells had clear attachment fibers and filopodia, enabling them to adhere to the flask (Fig. 3B). The two first cell types resembled epithelial cells, whereas the nature of the third was unknown. The interaction experiments were performed with cells cultivated between passages 15 and 21.
FIG 3.
Observation of Euva-1 cells cultured from E. variegatus embryos by phase contrast (A) and epifluorescence (B) microscopy. (A) Low magnification (×20, a) and high magnification (×40, b) of the Euva-1 monolayer colored with methylene blue. Cells that have their nuclei colored by methylene blue are indicated by red asterisks. Cells with both the nucleus and cytosol colored by methylene blue are indicated by arrows for the larger ones or arrowheads for the smaller ones. (B) Cellular actin was labeled with Alexa 568-phalloidin (green), and nuclei were stained with DAPI (blue). Scale bars, 50 μm (subpanels a in A and B) and 20 μm (subpanels b in A and B).
The sequencing of the mitochondrial marker cytochrome oxidase subunit I (COI) was used to confirm the leafhopper origin of the Euva-1 cell line. The sequences from the mitochondrial marker amplified from Euva-1 cell DNA and from the E. variegatus insect DNA were found to be identical (data not shown). A BLAST search in the GenBank database revealed that this sequence shared 80% nucleotide identity with the COI gene of the Cicadellinae Acrogonia virescens, which may be its closest relative.
VmpA-coated beads adhere to E. variegatus leafhopper cells in culture.
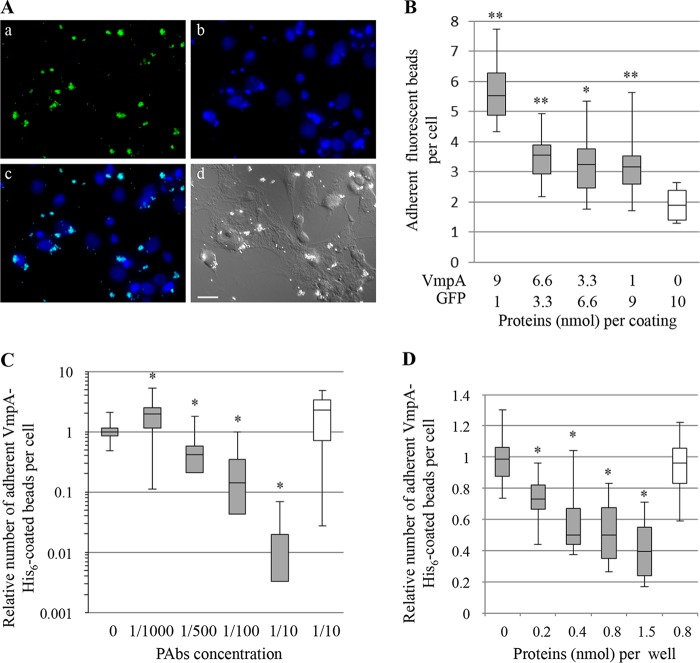
As a phytoplasma mutant cannot be engineered at present, we used recombinant VmpA proteins to test the interaction between VmpA and the insect cells ex vivo. For that purpose, we covalently linked VmpA-His6 recombinant protein and green fluorescent protein (GFP), which served as the negative control, to NH2 beads instead of COOH beads to better mimic the surface exposition of the VmpA N-terminal part. In these adherence assays, fluorescent beads were incubated for 1 h with Euva-1 cells and counted by epifluorescence observation (Fig. 4A). The adhesion of beads to Euva-1 cells was significantly augmented with increasing concentrations of VmpA, and the median number of adherent beads was 3-fold higher when the beads were linked with 9 nmol of VmpA than when the control beads were coated with GFP only (Fig. 4B).
FIG 4.
Adhesion of VmpA-His6-coated fluorescent beads to Euva-1 cells. (A) Observation of fluorescent VmpA-coated beads adherent to Euva-1 cells: a, fluorescent VmpA-coated beads (green); b, nuclei stained with DAPI (light blue); c, overlay; and d, same view by differential interference contrast microscopy. Scale bar, 20 μm. (B) Fluorescent beads were coated with different amounts of VmpA-His6 and GFP before coming in contact with insect cells in culture. **, P < 0.01; *, P < 0.05 compared to beads coated with 0 nmol of VmpA-His6 and 10 nmol of GFP by Student's tests. (C) The fluorescent beads coated with VmpA-His6 were preincubated with rabbit serum anti-His6-VmpA (gray bars) or anti-spiralin (control, white bar) at the indicated dilutions. *, P < 0.05 compared to nontreated beads (0) by Student's test. (D) Euva-1 cells were preincubated with the recombinant protein His6-VmpA (gray bars) or His6-VmpB (white bar) at the indicated concentrations. *, P < 0.05 compared to Euva-1 preincubated with medium alone (0) by Student's test.
To evaluate the specificity of VmpA adhesion to insect cells, we used competitive and inhibition adhesion assays. The adhesion of the fluorescent VmpA-His6-coated beads was strongly decreased in the presence of anti-His6-VmpA antibodies (PAb) in a dose-dependent manner (Fig. 4C), although a small but significant increase in bead adhesion was measured when few antibodies were used (1/1,000 dilution). When anti-spiraline PAbs were used as a negative baseline control, as expected, the adhesion rate was not significantly changed. No visible aggregation of the VmpA-His6-coated beads was observed in the presence of anti-His6-VmpA PAbs (data not shown). The results of competitive adhesion assays show that the presence of an increasing quantity of His6-VmpA overlaying the leafhopper cells decreased the adhesion of VmpA-His6-coated beads in a concentration-dependent manner (Fig. 4D). In parallel experiments, we used the other predicted surface-exposed protein, VmpB, which is also expressed in the insect E. variegatus (see Fig. S1 in the supplemental material). No reduction was observed when the cells were preincubated with the His6-VmpB recombinant protein. Altogether, these results strongly suggest that VmpA was able to specifically interact in vitro with the cells of the FD-P experimental insect vector E. variegatus, as an adhesin would do.
VmpA enables the adhesion of recombinant S. citri to leafhopper cells in culture.
To complete the functional studies on phytoplasma proteins, spiroplasmas that express VmpA at their surfaces were engineered to measure the impact of VmpA on bacterial adhesion to insect cells (19). We first verified that the recombinant S. citri G/6 strain still produced the protein VmpA after several passages before the adhesion assay. A comparison of VmpA expression in recombinant S. citri was conducted in the presence and absence of antibiotic selection pressures to promote the stability of pSTVA1. Two clones of S. citri G/6 carrying pSTVA1 (clones 5 and 6) were plated on SP4 agar, and different subclones were cultivated for 5 passages. The pSTVA1 plasmid was easily detected in the presence of tetracycline, while in the absence of the antibiotic, it was visualized in the subclone 6g only, but the restriction map was incorrect, suggesting a deletion (Fig. 5A). Thus, PCR amplifications and sequencing were performed to verify the presence of a correct vmpA sequence. In the case of subclone 6g, a deletion of approximately 700 bp was observed, corresponding to the size of the repeat domains that contain the VmpA protein, and confirmed the plasmid profile after HindIII restriction. In the case of the subclones 5e and 5h, amplicons were observed after electrophoresis, which suggests that pSTVA1 plasmids were present in these two subclones. No deletion or mutation in the vmpA gene was observed in clones propagated in the presence of the antibiotic and in the subclones 5e and 5h propagated without the antibiotic. When the expression of VmpA was monitored by Western blotting (Fig. 5A), differences in VmpA expression were observed between spiroplasmas cultivated in the presence and in the absence of tetracycline. When the antibiotic was omitted, the production of VmpA was abolished. In contrast, the culture of the subclones in the presence of tetracycline enabled the detection of a strong VmpA signal. Thus, a second culture passage of recombinant S. citri was performed only in the presence of tetracycline for adhesion assays, and the production of VmpA was verified by a colony blot.
FIG 5.

Expression of VmpA by Spiroplasma citri and the adhesion of VmpA-expressing S. citri to Euva-1 cells. (A) Plasmid extractions and restriction with HindIII enzyme (plasmid profile), PCR amplification of the vmpA gene, and Western immunoblotting of proteins from S. citri transformants. Subclones of the two clones 5 and 6 of VmpA expressing S. citri were cultivated without tetracycline (−) or in the presence of tetracycline (+) for 5 passages prior to extractions. The blot was probed with a 1:5,000 dilution of anti-His6-VmpA rabbit serum. Lane 1kb+, 1 kb Plus DNA ladder; lane +, plasmid pSTVA1; lane kDa, molecular mass (in kDa); lane rVmpA, recombinant protein VmpA lacking the C-terminal transmembrane segment. The images corresponding to PCR and Western immunoblotting were cut and assembled to be in the same order as the plasmid profile. (B) Adhesion of S. citri transformants to Euva-1 cells. The relative value of 100% corresponds to the condition of cell adhesion with the S. citri G/6 strain carrying the plasmid pSTP2. **, P < 0.001 compared to Euva-1 infected with S. citri G/6/pSTP2 devoid of VmpA by a Student's test.
Adhesion assays were performed by comparing the S. citri G/6 strain carrying the plasmid pSTP2 (vector without vmpA) to G/6 carrying pSTVA1. The colony blots showed that 50 to 100% of the spiroplasma colonies were expressing VmpA, depending on the replicates and as revealed by immunoblots. A significant increase in adhesion was observed when VmpA was expressed by the recombinant spiroplasmas (Fig. 5B). These results reinforce the previous results to show that VmpA acted as an adhesin binding to Euva-1 cells.
Interaction of VmpA with the midgut.
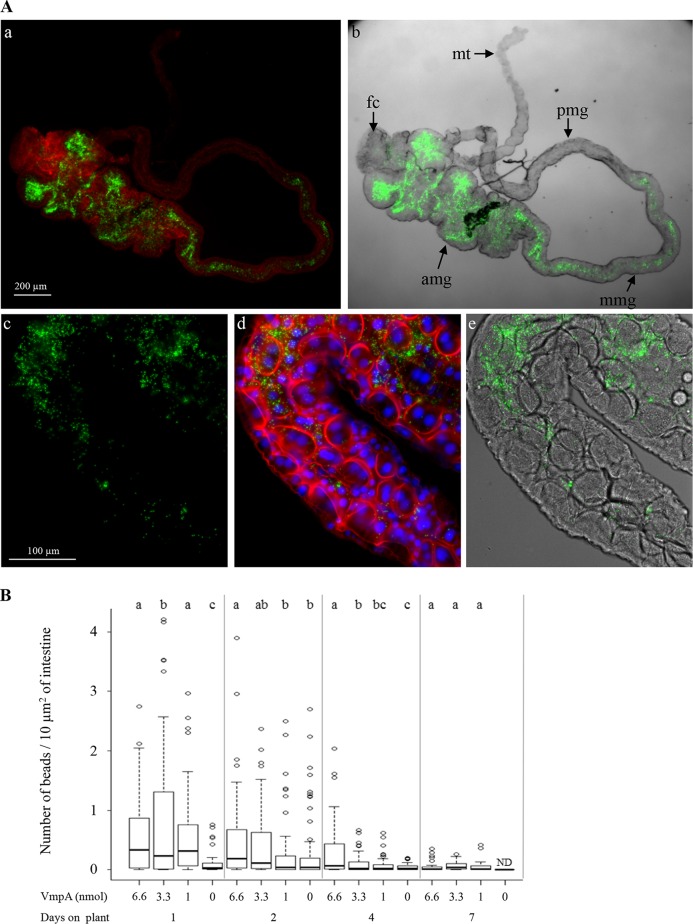
To assess the role of VmpA in the adhesion of phytoplasmas to intestinal epithelial cells, we used fluorescent beads to localize and count the VmpA-His6-coated beads in the midguts of E. variegatus in in vivo experiments. After ingestion by insects, the fluorescent latex beads were only observed in the midgut and occasionally in the filter chamber but were never detected in the Malpighian tubules (Fig. 6A). A large amount of variability in the numbers of retained beads could be observed between insects (Fig. 6B), but it was reproducible among 3 independent experiments. Regardless of the amount of VmpA-His6 bound to the fluorospheres, the number of beads per surface unit was observed to be higher in the anterior midgut compared to that in the middle midgut. Furthermore, the larger the quantity of VmpA-His6 coupled to the beads, the greater the number of beads attached to the midgut surface. When VmpA was in excess compared to bovine serum albumin (BSA), the beads attached to the anterior midgut were too numerous to be accurately quantified. For this reason, the counting was performed only at the middle midgut level. As shown in Fig. 6B, more VmpA-His6-coated fluorescent beads were retained in midguts than BSA-coated beads at 1, 2, and 4 days after acquisition feeding. Seven days postingestion, the number of VmpA-His6-coated beads fell and was similar to that of BSA-coated beads 4 days after ingestion. These results suggest that VmpA, unlike BSA, is more strongly retained in the luminal surface of midgut cells.
FIG 6.
Number of VmpA-His6-coated latex beads in E. variegatus midguts after ingestion and fluorescent observations. (A) Presence of fluorescent beads coated with VmpA-His6 in E. variegatus midgut after the ingestion of beads for 2 days. Low magnification (×4) by fluorescence microscopy showing the actin fluorescence (red, Alexa 568-phalloidin) of intestine cells and fluorescent beads (green) (a) and by phase-contrast microscopy overlaid with fluorescent image of beads (b). amg, anterior midgut; mmg, middle midgut; pmg, posterior midgut; fc, filter chamber; mt, Malpighian tubules. Higher magnification showing VmpA-His6-coated latex beads (green) (c), actin (red) and nuclei (blue, DAPI) (d), and the overlay of green VmpA-His6-coated latex beads and intestine tube viewed by phase-contrast microscopy (e). (B) Numbers of VmpA-His6-coated beads in the E. variegatus middle midguts. After feeding on HEPES-sucrose with BSA-coated beads or VmpA-His6-coated beads, E. variegatus insects were maintained one, two, four, or seven days on broad beans before their intestines were dissected. These assays were performed in three independent experiments, and 36 to 70 insects per group were examined. Different lowercase letters above sets indicate statistically significant differences calculated separately for each time on broad beans (Student's test, P < 0.05).
VmpA-His6-coated beads are localized and embedded in the perimicrovillar membrane of midguts.
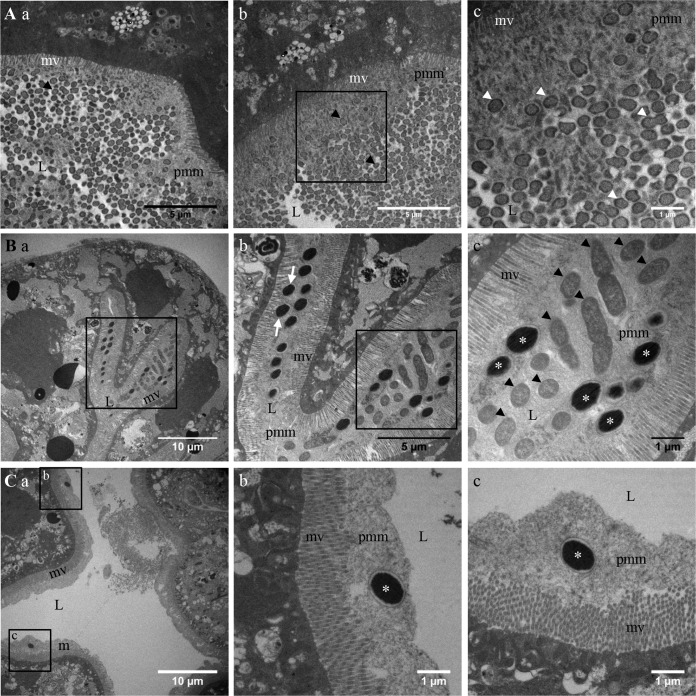
To more precisely localize the VmpA-His6-coated beads in the midgut at the cellular level, we used transmission electron microscopy (TEM). Midguts dissected from leafhoppers that had ingested VmpA-His6-coated beads in HEPES-sucrose for 2 days and then were fed healthy broad beans for 1 day were compared to those from leafhoppers that were only fed healthy broad beans (Fig. 7). Bacterium-like particles were visualized in the lumens of the midguts and in the anterior and middle midguts of insects, regardless of whether the leafhoppers were fed (Fig. 7, arrowheads). The particles were often associated with a structure that resembles the perimicrovillar membrane in the anterior and medium parts of the midgut. In the midguts of insects that had ingested beads coated with VmpA-His6, the beads were clearly visible in the lumen (asterisks in Fig. 7B and C). The beads were found alone or in groups embedded in the perimicrovillar membranes, and certain beads were clearly in contact with the microvilli of epithelial cells (Fig. 7C, arrow). In the anterior part of the midgut, the VmpA-His6-coated beads were present in a larger quantity than in the medium midgut, as previously observed by fluorescence microscopy. Beads were observed in the same gut lumen sections where bacterium-like particles were also visualized. No beads were seen inside cells, regardless of the region in which the observation was conducted. Other small, dense unidentified particles could also be seen within epithelial cells. As a control, microscopy observations did not show differences between leafhoppers fed HEPES-sucrose and those fed healthy broad beans (data not shown). Altogether, TEM observations suggest an affinity of VmpA for the perimicrovillar membranes that cover the apical surfaces of epithelial cells.
FIG 7.
Transmission electron microscopy images of the dissected midguts of E. variegatus insects that have ingested VmpA-His6-coated fluorescent beads. (A) The anterior (a) and middle (b and c) midgut of one healthy insect; subpanel c shows higher magnification of the boxed part of subpanel b. A section of the anterior (B) and middle (C) midgut of an insect that was fed VmpA-His6-coated beads in HEPES-sucrose for 2 days. In panel B, subpanel b shows higher magnification of the boxed part of subpanel a, and subpanel c shows higher magnification of the boxed part of subpanel b. In panel C, subpanels b and c represent higher magnifications of the boxed parts of subpanel a. Beads are shown with asterisks and bacteria with arrowheads. L, lumen; mv, microvillosity; pmm, perimicrovillar membrane.
DISCUSSION
Because phytoplasmas have not been cultivated in vitro to date, no defective mutants are available to study candidate genes putatively implicated in phytoplasma adhesion to insect cells. Fortunately, Spiroplasma citri, another plant pathogen also transmitted by Cicadellidae insects, is amenable to genetic manipulation. The S. citri GII3 mutant G/6, devoid of the ScARP adhesins (24, 29), was transformed to express the VmpA protein of the FD-P strain FD92 at the spiroplasmal surface (19). We used the mutant S. citri G/6, deficient for the adhesion to insect cells (24), to express VmpA with the aim of increasing the spiroplasmal adhesion to the E. variegatus cells. To screen the adhesion-like properties of phytoplasmal surface proteins, the recipient cell line Euva-1 was established from the experimental vector E. variegatus of the FD92 phytoplasmas. The percentages of Euva-1 cells with adherent G/6 spiroplasmas measured in this study were low and similar to those observed when this defective strain was incubated with Ciha-1 cells, a cell line derived from one of S. citri natural vectors, Circulifer haematoceps (24). The expression of VmpA at the surface of this G/6 mutant resulted in recombinant spiroplasmas that significantly increased their adhesion to Euva-1 cells. These results are reinforced by experiments that show an enhanced adhesion of VmpA-coated fluorescent beads to the same cells. Altogether, these data demonstrate that VmpA acts as an adhesin, regardless of the support used. Thus, S. citri was confirmed to be a good model to functionally characterize adhesins or other surface proteins of phytoplasmas. By extension, it should constitute a reliable experimental platform for the simultaneous expression of sets of phytoplasma proteins acting in concert in the adhesion and entry phenomena. However, the results also indicated that antibiotic pressure was necessary to maintain the VmpA expression encoded by the pSTVA1 plasmid. The pSTVA1 plasmid is a derivative of the plasmid pSci21NT, a modified pSci natural plasmid of S. citri GII3 (19), and it was stably expressed in the nontransmissible S. citri strain 44 (30). The pSci plasmids are present in 10 to 14 copies per cell (31), which might also be the case for pSci derivatives. The instability of pSTVA1 could therefore result from an increase in the metabolic energy necessary for plasmid maintenance and function or from the general deleterious effect on the bacterial growth rate (32, 33). There are many reports that show that overexpressed heterologous membrane proteins can affect the bacterial growth rate by imposing a metabolic burden, an overload of the membrane biogenesis machinery, a membrane stress, or local membrane disruptions in bacteria (33–35). Thus, one possible explanation for the lack of VmpA detection in the absence of tetracycline is that the expression of this protein decreases S. citri fitness and produces counter selection, leading to the loss of VmpA expression. Such instability of a viral-derived vector was previously observed for S. citri viral vectors engineered to express a fragment of a mycoplasma adhesin (35).
As shown in the present paper, phytoplasmas expressing VmpA were observed in the intestinal cells of E. variegatus, a necessary condition to envisage its role in adhesion to intestine cells. In addition, more of the ingested VmpA-His6-coated beads were retained in the midgut than those predominantly coated with BSA, especially when the beads were coated with a larger amount of VmpA. This validates the hypothesis that VmpA could play a major role in midgut colonization. Ingested coated beads have been observed to be embedded in a matrix associated with the apical surfaces of microvilli, occasionally with bacteria in the anterior midgut. One of these bacteria might be the congenitally transmitted enterobacterium previously observed by Cheung and Purcell (36). The bead location was similar to that of maize bushy stunt phytoplasma cells in the midgut lumen of Dalbulus maidis (37). Packed maize bushy stunt phytoplasmas were observed by the authors in the lumen near the microvilli and appeared to be surrounded by a slightly electron-dense structure resembling the structure in which the VmpA-coated beads were detected. The precise composition of the E. variegatus matrix observed in the lumen of E. variegatus in 1993 has not been deciphered to date (36). In our observations, the size of this structure is similar to that observed by these authors and is approximately 2 to 6 μm wide. This matrix had been called glycocalyx by Cheung and Purcell, but according to recent studies on hemipteran insects, we prefer calling it the perimicrovillar membrane (38). Unlike lepidopterans and coleopterans, euhemipterans lack a peritrophic membrane (PM). Their perimicrovillar membranes (PMMs) are partly composed of glycoproteins (38). In euhemipterans, this PMM seems to act as a protective barrier against invasive microbes and could have diverse functions in the digestion and absorption of nutrients. Microorganisms blocked by the PMM and that use adhesins to stick to the PMM and escape the feeding bowl flow must cross through to reach the apical surfaces of gut epithelial cells to finally undergo midgut colonization. One example is Trypanosoma cruzi, which is attached to the PMM of the Chagas disease vector bug Rhodnius prolixus. This attachment is mediated via lectin-like proteins of T. cruzi to glycoproteins of the midgut PMM (39). Similar to the surface lectin spiralin of S. citri (40, 43), VmpA enables S. citri and fluorescent beads to adhere to insect cells in culture and to the PMM, which is rich in glycoconjugates. VmpA has also been detected on phytoplasmas attached to the salivary glands, the surface of which is glycosylated similar to the different lobes of Circulifer haematoceps salivary glands (43). Because of these analogies between the two models, a lectin activity for VmpA could therefore be hypothesized and should be further investigated. In the case of another pathosystem, the Trichoplusia ni granulosis virus (TnGV) encodes the metalloprotease enhancin that alters the structural integrity and porosity of the lepidopteran PM and results in an increased movement of the virus (41). Regarding the structural and functional domains found in VmpA, a PepSY motif that is implicated in the regulation of peptidase activity (42) was found upon in silico analysis. In this regard, VmpA could promote the local degradation of the PMM protein component, enabling phytoplasmas to reach the apical membranes of the midgut epithelium. Nevertheless, these two VmpA activities remain to be investigated.
During their cycle within their vectors, phytoplasmas have to invade diverse types of cells or different vectoring insects. These successive steps must involve different phytoplasma membrane-associated proteins, as this has been deciphered in the Spiroplasma models (22, 24, 40, 43, 44). In the case of FD92-P, VmpB, which shows a similar structure, is a potential candidate to have a similar function. Competition adhesion assays showed that VmpB does not inhibit the adhesion of VmpA to insect cells, suggesting that if VmpB interacts with insect proteins, it is probably not targeting the same receptor(s). The recent deciphering of the FD92-P (20) chromosome will help to compile the list of the potential actors in phytoplasma-insect vector interactions. The use of the Euva-1 cells and recombinant spiroplasmas should be of great help in addressing this challenge.
MATERIALS AND METHODS
Insects, bacterial strains, and culture conditions.
Phytoplasma-free Euscelidius variegatus leafhoppers were reared in cages on broad beans (Vicia faba var. aquadulce) and oats (Avena sativa) at 25°C. The phytoplasma strain FD92 (FD92-P) was originally transmitted to broad bean (Vicia faba var. aquadulce) by infected Scaphoideus titanus sampled on FD-diseased vineyards in southwest France (45, 46) and was continuously maintained in broad beans by Euscelidius variegatus transmissions as described by Caudwell and colleagues (47).
The Spiroplasma citri strain GII3 was originally isolated from its leafhopper vector Circulifer haematoceps captured in Morocco (48). The low-passage-number wild-type strain GII3 contains seven plasmids, pSciA and pSci1 to pSci6 (31). The S. citri GII3 mutant G/6 was engineered via plasmid incompatibility curing and only contains the pSciA and pSci6 plasmids; therefore, it lacks ScARP genes (29). Spiroplasmas that expressed the FD92-P VmpA at their cell surfaces were obtained by electrotransformation of the S. citri GII3 mutant G/6 with 1 to 5 μg of purified plasmid pSTVA1, as described by Renaudin and colleagues (19). In summary, this plasmid carries the signal peptide-depleted vmpA coding sequence fused to the signal peptide sequence of the adhesin ScARP3d and is under the control of the S. citri tuf gene promoter and ribosome binding site (RBS). Spiroplasmas were cultivated at 32°C in SP4 medium from which the fresh yeast extract was omitted (49), and the medium was supplemented with 5 to 10 μg/ml tetracycline when necessary. The colonies were further propagated in SP4 broth medium containing 5 to 10 μg/ml tetracycline during 3 passages and submitted to a dot blot immunoassay and Western blotting (see below) to reveal the production of VmpA.
Establishment of the Euva-1 cell line derived from the leafhopper E. variegatus.
The cell line Euva-1 of the leafhopper E. variegatus was established according to a previously described protocol (27). Primary cell cultures, originally established from eggs with red eyespots, were maintained in monolayer culture at 25°C in culture medium made of 400 ml Schneider's Drosophila medium, 50 ml Grace's insect cell culture medium (Invitrogen), 50 ml heat-inactivated fetal bovine serum (Eurobio), 3 ml G-5 supplement (Invitrogen) supplemented with 1.25 μg · ml−1 amphotericin B (Fungizone; Invitrogen), and 50 μg · ml−1 penicillin-streptomycin (Invitrogen). After the cell line was established, leafhopper cells were passed every 10 days with a two-thirds dilution with an additional change of the medium during the week.
DNA isolation, restriction, and PCR.
To confirm the origin of the cells, the genomic DNA was extracted from a 20-ml culture of Euva-1 cells with the Wizard genomic DNA purification kit (Promega). A fragment of approximately 800 bp of the cytochrome oxidase subunits I and II (COI) mitochondrial gene was amplified using the primers C1-J-2195 (5′-TTGATTTTTTGGTCATCCAGAAGT-3′) and TL2-N-3014 (5′-TCCAATGCACTAATCTGCCATATTA-3′) (50). PCR amplifications were performed according to Bertin et al. (51) with 1 μl of DNA template in a final volume of 25 μl. The sequencing of the PCR products from both the 5′ and 3′ ends of purified PCR products was performed by Beckman Coulter Genomics (Takeley, UK).
Plasmid DNA was purified from 10-ml cultures of Spiroplasma citri with the Wizard SV Minipreps DNA purification kit (Promega). The plasmids were digested with HindIII, and the digested products were analyzed on 0.8% agarose gels. The amplification of the vmpA fragment was performed using the primers pSciF11 (5′-GTTATTGTGTGGGTCAGATG-3′) and VmpARev (5′-CCCTAGCTAACTGAATTCATGGATC-3′). The PCR conditions were 35 cycles of 30 s at 92°C, 30 s at 52°C, and 45 s at 68°C with the Taq polymerase (Biolab).
Expression and purification of recombinant Vmps and production of antibodies.
The primers Cl-VmpA-F1 (5′-ACAAACATATGAAAGCTATTACAGATTTGAGTGG-3′) and Cl-VmpA-R1 (5′-TCATTCTCGAGTTAACTTTTTTTCTTAACAGTAAAC-3′), as well as Cl-VmpB-92F (5′-ATCAACATATGGCAGATAAAGAAAAACCATTATC-3′) and Cl-VmpB-92R (5′-TAATTCTCGAGTTAGATTCTGTAACGGTTTCG-3′) were designed for the cloning of parts of the vmpA (accession number LN680870) and vmpB (accession number PRJEB22700) genes, respectively, without the transmembrane regions, as detailed in Fig. S2A and B in the supplemental material. The DNA fragments, 930 bp long for VmpA (amino acids [aa] 38 to 347) and 756 bp long for VmpB (aa 34 to 285), were PCR amplified with the Phusion high-fidelity DNA polymerase (Finnzymes) from the total DNA of V. faba infected by FD92-P. For the expression of the histidine-tagged proteins in Escherichia coli, the amplicons were cloned into the pET28 expression system (Novagen, Madison, WI). E. coli BL21 Star (DE3) cells (Invitrogen) were then transformed with pET28-His6-VmpA, pET-VmpA-His6, or with pET28-His6-VmpB, according to the manufacturer's protocol. Expression was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The tagged proteins were purified as described previously (24) on HIS-Select nickel affinity gel-packed columns (Sigma). For VmpA, the nickel column was conditioned with 0.05 M sodium phosphate buffer at pH 7.4 with 0.2% Triton X-100 and for VmpB, with 0.05 M sodium phosphate buffer at pH 7.4 with 0.025 M imidazole and 0.2% Triton X-100. Imidazole elution concentrations were 0.25 M for His6-VmpA and 0.5 M for His-VmpB. The purification of each protein was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting was applied with anti-FD monoclonal antibodies provided by the Sediag Company for His6-VmpA/VmpA-His6 and with the anti-histidine antibodies (Sigma) for His6-VmpB. Rabbit polyclonal antibodies (PAbs) raised against the His6-tagged recombinant VmpA (His6-VmpA) (19) and the His6-tagged recombinant VmpB (His6-VmpB) were produced by Covalab (Villeurbanne, France).
Western immunoblotting and dot blot immunoassay.
The methods for the immunoblotting analysis of spiroplasmal proteins were previously described (52). Briefly, spiroplasmas were pelleted from 20-ml cultures by centrifugation at 25,000 × g for 20 min and washed twice in HEPES-sucrose (HS) buffer (8 mM HEPES [pH 7.4] and 280 mM sucrose). Protein concentrations were determined using the DC protein assay kit (Bio-Rad, Hercules, CA, USA). Protein preparations were mixed with one volume of 2× Laemmli solubilization buffer and solubilized by heating at 80°C for 20 min. The preparation was stored at −20°C until use or directly separated by 10% SDS-PAGE, and then the proteins were electrotransferred to a nitrocellulose membrane. For dot blotting, spiroplasmas from 2-ml cultures were spotted onto nitrocellulose membranes after two washes in HEPES-sucrose (HS) buffer. The presence of VmpA was revealed using anti-His6-VmpA PAbs (1:5,000 dilution), goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate, and NBT-BCIP (Sigmafast; Sigma-Aldrich, St. Louis, MO, USA) as the substrate.
Coating of fluorescent beads.
The yellow-green fluorescent and amine-modified beads (4 × 109 beads at 1 μm) (Invitrogen) were covalently coated with 10 nmol of a mix of recombinant VmpA-His6 and GFP or BSA, according to the supplier's instructions. The relative quantities of VmpA-His6, GFP, and BSA varied according to the experiments and are indicated under the graphs. The coating of the beads was verified by immunofluorescence. VmpA-His6-coated beads were incubated with anti-VmpA PAbs diluted 1:500 in phosphate-buffered saline (PBS)-BSA solution (PBS containing 1% BSA) for 30 min; after 3 washes with PBS, the beads were incubated for 30 min with Alexa 633-conjugated goat anti-rabbit antibodies (Invitrogen) diluted at 1:200. The beads were included in the antifading ProLong Gold reagent (Invitrogen), mounted with coverslips, and imaged using a TCS SP2 upright Leica confocal laser scanning microscope (CLSM), with a 63× oil immersion objective lens with a pixel size of 70 nm. Fluorochromes were detected sequentially frame by frame. The coating of fluorescent beads was also verified for VmpA and BSA by measuring the remaining uncoated proteins using the Bradford procedure.
Coloration and microscopy of Euva-1 cells, salivary glands, and midguts of Euscelidius variegatus.
Euva-1 cells were grown on coverslips in 24-well plates for 1 day and then washed in PBS. The cells were fixed with 4% paraformaldehyde and incubated with methylene blue (0.1%) for 1 min after 3 washes in water or with Alexa 568-phalloidin (Thermo Fisher Scientific) and DAPI (4′,6-diamidino-2-phenylindole; Sigma). Samples were mounted with ProLong Gold antifade reagent (Thermo Fisher Scientific) and imaged using a Nikon Eclipse E800 microscope with 40× and 20× objective lenses.
To infect E. variegatus with FD-P, 5th instar nymphs were fed phytoplasma-infected broad beans for 1 week, corresponding to the acquisition period. The insects were subsequently caged on healthy broad beans for different latency periods in a greenhouse. The intestine and salivary glands were dissected from 10 insects. The organs were fixed with 4% paraformaldehyde, washed in PBS, incubated with a 1:3,000 dilution of anti-VmpA rabbit serum (PAbs) in PBS-BSA, washed, and then incubated with Alexa 488-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific) at a 1:200 dilution. F-actin and nuclei were stained using Alexa 568-phalloidin (Thermo Fisher Scientific) and DAPI (Sigma), respectively. Immunofluorescent samples were finally mounted with ProLong Gold antifade reagent (Thermo Fisher Scientific) and imaged using a TCS SP2 upright Leica confocal laser scanning microscope (CLSM) with 40× water immersion and 20× objective lenses.
For transmission electron microscopy (TEM), the insects were fed in microtubes as described above with caps filled with HEPES-sucrose with or without VmpA-His6-coated beads (6.6 nmol of recombinant VmpA-His6 with 3.3 nmol of BSA) for 2 days and caged on healthy plants for 1 day. The dissected midguts were fixed in glutaraldehyde, postfixed in osmium tetroxide, and dehydrated in ethanol, and inclusion was performed in Epon resin as described previously (24, 27). Micrographs were taken at 120 kV on an FEI Tecnai G2 Spirit equipped with an Eagle 4K digital camera (FEI France, Lyon).
Euva-1 adhesion assays.
Adhesion assays of yellow-green fluorescent and amine-modified beads were performed as previously described (43). Briefly, Euva-1 cells cultivated on coverslips in 24-well plates and were incubated with 2 × 106 coated latex beads in Schneider's Drosophila medium for 1 h at 25°C. After three washes, the cells were fixed with 4% paraformaldehyde, and the cell nuclei were stained with 1 μg · ml−1 DAPI for 5 min. The samples were mounted in the antifading ProLong Gold reagent (Thermo Fisher Scientific), and immunofluorescent samples were analyzed with a fluorescence microscope (Nikon Eclipse E800) at ×40 magnification. Each experiment was repeated three times independently in triplicates. For each experiment, 20 to 25 fields with approximately 30 cells per field were observed randomly. The counting of beads per cell was performed with the free software package ImageJ (http://imagej.nih.gov/ij/). For the relative number of adherent beads per cells, the average of the bead numbers under the control condition (0 in Fig. 4B and C) corresponded to a value of 1. The relative values of bead numbers obtained under the different conditions were then readjusted according to this endogenous standard. For the antibody inhibition assay, VmpA-His6-coated beads were preincubated in the presence of various concentrations of anti-His6-VmpA PAbs (1/10 to 1/1,000) or anti-spiralin (53) for 1 h at room temperature. For the competitive assay, Euva-1 cells were preincubated for 1 h at 25°C in the presence of various quantities of His6-VmpA (0.2 to 0.8 nmol) or His6-VmpB (0.8 nmol). After one wash in PBS, the fluorescent VmpA-His6-coated beads were added to the Euva-1 cells as above. Each experiment was repeated three times independently in triplicates.
The binding of spiroplasmas that express VmpA to Euva-1 cells was determined essentially as described previously in reference 27. In brief, approximately 105 Euva-1 cells per well were infected with S. citri at a multiplicity of infection between 20 and 50 and incubated at 30°C for 3 h. The insect cells were trypsinized with TrypLE (Thermo Fisher Scientific) for 5 min. Serial dilutions were plated onto SP4 containing 1% noble agar for CFU counting. To calculate the relative percentage of adherent spiroplasmas, the value 100% corresponded to the average of the adherent S. citri G/6/pSTP2 (control condition). Each experiment was performed in four distinct wells and was repeated three times.
Ingestion assays.
HEPES-sucrose solution (500 μl) containing 105 coated beads was introduced in the cap of 1.5-ml microtubes and closed with Parafilm. Three young adult E. variegatus insects were introduced by using a tube containing a narrow band of Whatman paper to enable the insects to move up to the cap, and a piece of gauze was placed just below the cap to permit the insect to hold on to it and eat. After 2 days at room temperature, to ensure insect adaptation to the artificial feeding system, the 25 to 30 surviving insects (approximately 80%) were transferred to a cage containing 2 broad beans. One, four, and seven days later, 20 insects were dissected. Other experiments with insects left 2 days on broad beans were independently conducted besides the first set of experiments but were shown on the same graph in Fig. 6. The midguts were fixed for 18 h with 4% paraformaldehyde in PBS containing 0.1% Triton X-100. Alexa 568-phalloidin (Invitrogen) was used to stain the actin filaments (diluted 1:200 in PBS-BSA for 1 h) and DAPI (Sigma) was used to stain nuclei (for 5 min in water). The organs were mounted in antifading ProLong Gold reagent (Invitrogen), and immunofluorescent samples were imaged using the fluorescence microscope (Nikon Eclipse E800). For each experiment, approximately 15 midguts were observed per condition, and the experiment was repeated three times. The counting of beads per midgut and the determination of the area of midguts were performed with the free software package ImageJ.
Statistical analyses.
The similarities of deviations between independent experiments were checked with the F test first. Then, for the purposes of statistical evaluation, a Student's t test was used for comparing two samples, and Student's z test was used for comparing four samples. The results of the statistical analyses were considered to be significant if their corresponding P values were less than 0.05 and 0.001.
Accession number(s).
The sequences for COI from Euva-1 cells were deposited in ENA (accession numbers LT960658 and LT960628).
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Brocard of the Bordeaux Imaging Center, a member of the France Bioimaging Infrastructure (ANR-10-INBS-04), for advice and experimental assistance in the preparation of samples for transmission electron microscopy. We also thank K. Guionneaud and D. Lacaze for rearing the insects and producing the plants.
This research was funded by the Conseil Interprofessionnel du Vin de Bordeaux, FranceAgriMer, the Regional Council of Aquitaine region, and INRA in the frame of the project “VMP Adapt” of the Plant Health and Environment.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02487-17.
REFERENCES
- 1.Lee I-M, Davis RE, Gundersen-Rindal DE. 2000. Phytoplasma: phytopathogenic mollicutes. Annu Rev Microbiol 54:221–255. doi: 10.1146/annurev.micro.54.1.221. [DOI] [PubMed] [Google Scholar]
- 2.Sugio A, MacLean AM, Kingdom HN, Grieve VM, Manimekalai R, Hogenhout SA. 2011. Diverse targets of phytoplasma effectors: from plant development to defense against insects. Annu Rev Phytopathol 49:175–195. doi: 10.1146/annurev-phyto-072910-095323. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub PG, Beanland L. 2006. Insect vectors of phytoplasmas. Annu Rev Entomol 51:91–111. doi: 10.1146/annurev.ento.51.110104.151039. [DOI] [PubMed] [Google Scholar]
- 4.Hogenhout SA, Oshima K, Ammar E-D, Kakizawa S, Kingdom HN, Namba S. 2008. Phytoplasmas: bacteria that manipulate plants and insects. Mol Plant Pathol 9:403–423. doi: 10.1111/j.1364-3703.2008.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schvester D, Carle P, Moutous G. 1961. Sur la transmission de la flavescence dorée des vignes par une cicadelle. C R Acad Sci 18:1021–1024. (In French.) [Google Scholar]
- 6.EFSA Panel on Plant Health (PLH), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen-Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Bosco D, Foissac X, Strauss G, Hollo G, Mosbach-Schulz O, Grégoire J-C. 2016. Risk to plant health of Flavescence dorée for the EU territory. EFSA J 14:e04603. doi: 10.2903/j.efsa.2016.4603. [DOI] [Google Scholar]
- 7.Boudon-Padieu E, Larrue J, Caudwell A. 1989. ELISA and dot-blot detection of flavescence dorée-MLO in individual leafhopper vectors during latency and inoculative state. Curr Microbiol 19:357–364. doi: 10.1007/BF01570882. [DOI] [Google Scholar]
- 8.Maillet PL, Gouranton J. 1971. Etude du cycle biologique du mycoplasme de la phyllodie du trefle dans l'insecte vecteur, Euscelis lineolatus Brulle (Homoptera, Jassidae). J Microsc (Paris) 11:143–162. (In French.) [Google Scholar]
- 9.Gouranton J, Maillet P. 1973. High-resolution autoradiography of mycoplasmalike organisms multiplying in some tissues of an insect vector for clover-phyllody. J Invertebr Pathol 21:158–163. doi: 10.1016/0022-2011(73)90196-1. [DOI] [Google Scholar]
- 10.Lefol C, Lherminier J, Boudonpadieu E, Larrue J, Louis C, Caudwell A. 1994. Propagation of flavescence Doree Mlo (mycoplasma-like organism) in the leafhopper vector Euscelidius variegatus Kbm. J Invertebr Pathol 63:285–293. doi: 10.1006/jipa.1994.1053. [DOI] [Google Scholar]
- 11.Bressan A, Girolami V, Boudon-Padieu E. 2005. Reduced fitness of the leafhopper vector Scaphoideus titanus exposed to flavescence doree phytoplasma. Entomol Exp Appl 115:283–290. doi: 10.1111/j.1570-7458.2005.00240.x. [DOI] [Google Scholar]
- 12.Fletcher J, Wayadande A, Melcher U, Ye F. 1998. The phytopathogenic mollicute-insect vector interface: a closer look. Phytopathology 88:1351–1358. doi: 10.1094/PHYTO.1998.88.12.1351. [DOI] [PubMed] [Google Scholar]
- 13.Liu H-Y. 1983. The relationship of Spiroplasma citri and Circulifer tenellus. Phytopathology 73:585–590. doi: 10.1094/Phyto-73-585. [DOI] [Google Scholar]
- 14.Kakizawa S, Oshima K, Nishigawa H, Jung H-Y, Wei W, Suzuki S, Tanaka M, Miyata S, Ugaki M, Namba S. 2004. Secretion of immunodominant membrane protein from onion yellows phytoplasma through the Sec protein-translocation system in Escherichia coli. Microbiology 150:135–142. doi: 10.1099/mic.0.26521-0. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Oshima K, Kakizawa S, Arashida R, Jung H-Y, Yamaji Y, Nishigawa H, Ugaki M, Namba S. 2006. Interaction between the membrane protein of a pathogen and insect microfilament complex determines insect-vector specificity. Proc Natl Acad Sci U S A 103:4252–4257. doi: 10.1073/pnas.0508668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galetto L, Bosco D, Balestrini R, Genre A, Fletcher J, Marzachì C. 2011. The major antigenic membrane protein of “Candidatus Phytoplasma asteris” selectively interacts with ATP synthase and actin of leafhopper vectors. PLoS One 6:e22571. doi: 10.1371/journal.pone.0022571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashidi M, Galetto L, Bosco D, Bulgarelli A, Vallino M, Veratti F, Marzachì C. 2015. Role of the major antigenic membrane protein in phytoplasma transmission by two insect vector species. BMC Microbiol 15:193. doi: 10.1186/s12866-015-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimerman A, Pacifico D, Salar P, Marzachì C, Foissac X. 2009. Striking diversity of vmp1, a variable gene encoding a putative membrane protein of the stolbur phytoplasma. Appl Environ Microbiol 75:2951–2957. doi: 10.1128/AEM.02613-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renaudin J, Béven L, Batailler B, Duret S, Desqué D, Arricau-Bouvery N, Malembic-Maher S, Foissac X. 2015. Heterologous expression and processing of the flavescence dorée phytoplasma variable membrane protein VmpA in Spiroplasma citri. BMC Microbiol 15:82. doi: 10.1186/s12866-015-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carle P, Malembic-Maher S, Arricau-Bouvery N, Desqué D, Eveillard S, Carrere S, Foissac X. 2011. Flavescence doree' phytoplasma genome: a metabolism oriented towards glycolysis and protein degradation. Bull Insectol 64:S13–S14. [Google Scholar]
- 21.Dramsi S, Dehoux P, Cossart P. 1993. Common features of Gram-positive bacterial proteins involved in cell recognition. Mol Microbiol 9:1119–1122. doi: 10.1111/j.1365-2958.1993.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Wayadande AC, Fletcher J. 2000. Spiroplasma citri surface protein P89 implicated in adhesion to cells of the vector Circulifer tenellus. Phytopathology 90:716–722. doi: 10.1094/PHYTO.2000.90.7.716. [DOI] [PubMed] [Google Scholar]
- 23.Berg M, Melcher U, Fletcher J. 2001. Characterization of Spiroplasma citri adhesion related protein SARP1, which contains a domain of a novel family designated sarpin. Gene 275:57–64. doi: 10.1016/S0378-1119(01)00655-2. [DOI] [PubMed] [Google Scholar]
- 24.Béven L, Duret S, Batailler B, Dubrana M-P, Saillard C, Renaudin J, Arricau-Bouvery N. 2012. The repetitive domain of ScARP3d triggers entry of Spiroplasma citri into cultured cells of the vector Circulifer haematoceps. PLoS One 7:e48606. doi: 10.1371/journal.pone.0048606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glew MD, Papazisi L, Poumarat F, Bergonier D, Rosengarten R, Citti C. 2000. Characterization of a multigene family undergoing high-frequency DNA rearrangements and coding for abundant variable surface proteins in Mycoplasma agalactiae. Infect Immun 68:4539–4548. doi: 10.1128/IAI.68.8.4539-4548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleury B, Bergonier D, Berthelot X, Peterhans E, Frey J, Vilei EM. 2002. Characterization of P40, a cytadhesin of Mycoplasma agalactiae. Infect Immun 70:5612–5621. doi: 10.1128/IAI.70.10.5612-5621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duret S, Batailler B, Danet J-L, Béven L, Renaudin J, Arricau-Bouvery N. 2010. Infection of the Circulifer haematoceps cell line Ciha-1 by Spiroplasma citri: the non-insect-transmissible strain 44 is impaired in invasion. Microbiology 156:1097–1107. doi: 10.1099/mic.0.035063-0. [DOI] [PubMed] [Google Scholar]
- 28.Caudwell A, Larrue J. 1977. La production de cicadelles saines et infectieuses pour les épreuves d'infectivité chez les jaunisses à mollicutes des végétaux. L'élevage de Euscelidius variegatus KBM et la ponte sur mousse de polyurethane. Ann Zool Ecol Anim 9:443–456. (In French.) [Google Scholar]
- 29.Breton M, Duret S, Danet J-L, Dubrana M-P, Renaudin J. 2010. Sequences essential for transmission of Spiroplasma citri by its leafhopper vector, Circulifer haematoceps, revealed by plasmid curing and replacement based on incompatibility. Appl Environ Microbiol 76:3198–3205. doi: 10.1128/AEM.00181-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breton M, Duret S, Arricau-Bouvery N, Béven L, Renaudin J. 2008. Characterizing the replication and stability regions of Spiroplasma citri plasmids identifies a novel replication protein and expands the genetic toolbox for plant-pathogenic spiroplasmas. Microbiology 154:3232–3244. doi: 10.1099/mic.0.2008/019562-0. [DOI] [PubMed] [Google Scholar]
- 31.Saillard C, Carle P, Duret-Nurbel S, Henri R, Killiny N, Carrère S, Gouzy J, Bové J-M, Renaudin J, Foissac X. 2008. The abundant extrachromosomal DNA content of the Spiroplasma citri GII3-3X genome. BMC Genomics 9:195. doi: 10.1186/1471-2164-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiba S, Tsunekawa H, Imanaka T. 1982. New approach to tryptophan production by Escherichia coli: genetic manipulation of composite plasmids in vitro. Appl Environ Microbiol 43:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rai M, Padh H. 2001. Expression systems for production of heterologous proteins. Curr Sci 80:1121–1128. [Google Scholar]
- 34.Steen A, Wiederhold E, Gandhi T, Breitling R, Slotboom DJ. 2011. Physiological adaptation of the bacterium Lactococcus lactis in response to the production of human CFTR. Mol Cell Proteomics 10:M000052MCP200. doi: 10.1074/mcp.M000052-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hattab G, Suisse AYT, Ilioaia O, Casiraghi M, Dezi M, Warnet XL, Warschawski DE, Moncoq K, Zoonens M, Miroux B. 2014. Membrane protein production in Escherichia coli: overview and protocols, p 87–106. In Mus-Veteau I. (ed), Membrane proteins production for structural analysis. Springer, New York, NY. [Google Scholar]
- 36.Cheung WWK, Purcell AH. 1993. Ultrastructure of the digestive system of the Le fhopper Euscelidius variegatus Kirshbaum (Homoptera: Cicadellidae), with and without congenital bacterial infections. Int J Insect Morphol Embryol 22:49–61. doi: 10.1016/0020-7322(93)90033-W. [DOI] [Google Scholar]
- 37.García González J, Ossamu Tanaka FA, Spotti Lopes JR. 2015. First findings in the route of the maize bushy stunt phytoplasma within its vector Dalbulus maidis (Hemiptera: Cicadellidae). J Econ Entomol 109:966–968. doi: 10.1093/jee/tov345. [DOI] [PubMed] [Google Scholar]
- 38.Gutiérrez-Cabrera AE, Córdoba-Aguilar A, Zenteno E, Lowenberger C, Espinoza B. 2016. Origin, evolution and function of the hemipteran perimicrovillar membrane with emphasis on Reduviidae that transmit Chagas disease. Bull Entomol Res 106:279–291. doi: 10.1017/S0007485315000929. [DOI] [PubMed] [Google Scholar]
- 39.Alves CR, Albuquerque-Cunha JM, Mello CB, Garcia ES, Nogueira NF, Bourguingnon SC, de Souza W, Azambuja P, Gonzalez MS. 2007. Trypanosoma cruzi: attachment to perimicrovillar membrane glycoproteins of Rhodnius prolixus. Exp Parasitol 116:44–52. doi: 10.1016/j.exppara.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Killiny N, Castroviejo M, Saillard C. 2005. Spiroplasma citri spiralin acts in vitro as a lectin binding to glycoproteins from its insect vector Circulifer haematoceps. Phytopathology 95:541–548. doi: 10.1094/PHYTO-95-0541. [DOI] [PubMed] [Google Scholar]
- 41.Peng J, Zhong J, Granados RR. 1999. A baculovirus enhancin alters the permeability of a mucosal midgut peritrophic matrix from lepidopteran larvae. J Insect Physiol 45:159–166. doi: 10.1016/S0022-1910(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 42.Yeats C, Rawlings ND, Bateman A. 2004. The PepSY domain: a regulator of peptidase activity in the microbial environment? Trends Biochem Sci 29:169–172. doi: 10.1016/j.tibs.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Duret S, Batailler B, Dubrana M-P, Saillard C, Renaudin J, Béven L, Arricau-Bouvery N. 2014. Invasion of insect cells by Spiroplasma citri involves spiralin relocalization and lectin/glycoconjugate-type interactions. Cell Microbiol 16:1119–1132. doi: 10.1111/cmi.12265. [DOI] [PubMed] [Google Scholar]
- 44.Killiny N, Batailler B, Foissac X, Saillard C. 2006. Identification of a Spiroplasma citri hydrophilic protein associated with insect transmissibility. Microbiology 152:1221–1230. doi: 10.1099/mic.0.28602-0. [DOI] [PubMed] [Google Scholar]
- 45.Caudwell A, Kuszala C, Bachelier JC, Larrue J. 1970. Transmission de la Flavescence doree de la vigne aux plantes herbacees par l'allongement du temps d'utilisation de la cicadelle Scaphoideus littoralis Ball et l'etude de sa survie sur en grand nombre d'especes vegetales. Ann Phytopathol 1572(Hors série):181–189. (In French.) [Google Scholar]
- 46.Angelini E, Clair D, Borgo M, Bertaccini A, Boudon-Padieu E. 2001. Flavescence doree in France and Italy - Occurrence of closely related phytoplasma isolates and their near relationships to Palatinate grapevine yellows and an alder yellows phytoplasma. Vitis 40:79–86. [Google Scholar]
- 47.Caudwell A, Kuszla C, Larrue J, Bachelier JC. 1972. Transmission de la flavescence doree de la feve a la feve par des cicadelles des genres Euscelis et Euscedlidius: Intervention possible de ces insectes dans l'epidemiologie du bois noir en Bourgogne. Ann Phytopathol 1972:181–189. (In French.) [Google Scholar]
- 48.Vignault JC, Bové JM, Saillard C, Vogel R, Faro A, Venegas L, Stemmer W, Aoki S, McCoy R, Al-Beldawi A, Larue M, Tuzcu O, Ozsan M, Nhami A, Abassi M, Bonfils J, Moutous G, Fos A, Poutiers F, Viennot-Bourgin G. 1980. Mise en culture de spiroplasmes à partir de matériel végétal et d'insectes provenant de pays circum méditerranéens et du Proche Orient. C R Acad Sci Hebd Seances Acad Sci D 290:775–780. (In French.) [Google Scholar]
- 49.Tully JG, Whitcomb RF, Clark HF, Williamson DL. 1977. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science 195:892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- 50.Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. 1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain-reaction primers. Ann Entomol Soc Am 87:651–701. doi: 10.1093/aesa/87.6.651. [DOI] [Google Scholar]
- 51.Bertin S, Picciau L, Ács Z, Alma A, Bosco D. 2010. Molecular identification of the Hyalesthes species (Hemiptera: Cixiidae) occurring in vineyard agroecosystems. Ann Appl Biol 157:435–445. doi: 10.1111/j.1744-7348.2010.00434.x. [DOI] [Google Scholar]
- 52.Duret S, Berho N, Danet J-L, Garnier M, Renaudin J. 2003. Spiralin is not essential for helicity, motility, or pathogenicity but is required for efficient transmission of Spiroplasma citri by its leafhopper vector Circulifer haematoceps. Appl Environ Microbiol 69:6225–6234. doi: 10.1128/AEM.69.10.6225-6234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wróblewski H, Johansson KE, Hjérten S. 1977. Purification and characterization of spiralin, the main protein of the Spiroplasma citri membrane. Biochim Biophys Acta 465:275–289. doi: 10.1016/0005-2736(77)90079-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.