ABSTRACT
Heterologous display of enzymes on microbial cell surfaces is an extremely desirable approach, since it enables the engineered microbe to interact directly with the plant wall extracellular polysaccharide matrix. In recent years, attempts have been made to endow noncellulolytic microbes with genetically engineered cellulolytic capabilities for improved hydrolysis of lignocellulosic biomass and for advanced probiotics. Thus far, however, owing to the hurdles encountered in secreting and assembling large, intricate complexes on the bacterial cell wall, only free cellulases or relatively simple cellulosome assemblies have been introduced into live bacteria. Here, we employed the “adaptor scaffoldin” strategy to compensate for the low levels of protein displayed on the bacterial cell surface. That strategy mimics natural elaborated cellulosome architectures, thus exploiting the exponential features of their Lego-like combinatorics. Using this approach, we produced several bacterial consortia of Lactobacillus plantarum, a potent gut microbe which provides a very robust genetic framework for lignocellulosic degradation. We successfully engineered surface display of large, fully active self-assembling cellulosomal complexes containing an unprecedented number of catalytic subunits all produced in vivo by the cell consortia. Our results demonstrate that the enzyme stability and performance of the cellulosomal machinery, which are superior to those seen with the equivalent secreted free enzyme system, and the high cellulase-to-xylanase ratios proved beneficial for efficient degradation of wheat straw.
IMPORTANCE The multiple benefits of lactic acid bacteria are well established in health and industry. Here we present an approach designed to extensively increase the cell surface display of proteins via successive assembly of interactive components. Our findings present a stepping stone toward proficient engineering of Lactobacillus plantarum, a widespread, environmentally important bacterium and potent microbiome member, for improved degradation of lignocellulosic biomass and advanced probiotics.
KEYWORDS: cellulase, cellulosomes, enzymatic complex, lactic acid bacteria, self-assembly, xylanase
INTRODUCTION
The plant cell wall is a tough and rigid layer that surrounds the cell to enable it to withstand internal osmotic pressure resulting from the difference in solute concentrations between the cell interior and external water (1). It is composed of various polysaccharides (mostly cellulose and hemicellulose) and the cross-linked, phenolic polymer lignin. Degradation of the plant cell wall is performed in nature by various microbial systems that have evolved in order to utilize its sugars as a main carbon source. The cellulosome (2), first characterized in the thermophilic anaerobe Clostridium thermocellum (3), is a large, highly cellulolytic multienzymatic complex that can be either anchored to the bacterial cell surface (4, 5) or secreted to the extracellular medium. Cellulosomal complex formation is based on a unique type of intermodular interaction between its components, i.e., the enzymes and the scaffoldins. Multiple cohesin modules on the scaffoldin and individual dockerin modules on the enzyme subunits interact in a noncovalent manner with very high affinity that approaches and surpasses that of antigen-antibody binding (6). The close proximity between the multiple enzymes serves to enhance synergistic activity (7), and the carbohydrate-binding module (CBM), usually contained in the scaffoldin subunit, targets the entire complex to the substrate. When anchored to the bacterial surface, the cellulosome also contributes to minimal diffusion loss of enzymes and degradation products.
In the past, several studies have reported the fabrication of artificial, chimeric, cellulosomal structures, engineered for display on the surfaces of various microbial strains, notably, Saccharomyces cerevisiae (8–11), Bacillus subtilis (12, 13), Clostridium acetobutylicum (14), and Lactococcus lactis (15). For this purpose, designer cellulosome technology has been employed to mimic the architecture of cellulosome complexes and/or to specifically control their enzyme composition (16–19). One of the major issues of cell surface attachment of chimeric scaffoldins is the low level of surface display, which leads to slow catalysis and low fermentation efficiency (10, 20). The feasibility of transferring cellulosomal technologies to a bacterium with potential industrial and clinical applications has been demonstrated recently in Lactobacillus plantarum (21–23). Although this bacterium lacks the native capacity both to degrade cellulosic substrates and to produce biofuels such as ethanol, it is highly tolerant of low levels of pH and ethanol (up to 13% [vol/vol]) (24) and has been identified as a main contaminant in biofuel refineries (25). Therefore, it could also represent an attractive candidate vehicle for consolidated bioprocessing (CBP) (21, 22). L. plantarum is also a member of the human gut microbiome (26) and of additional gut ecosystems (27) and affects host attributes such as mate selection and growth (28, 29). Moreover, strains belonging to this species were recently shown to promote juvenile growth and buffer the effect of chronic undernutrition in germ-free mice (30).
Previously, the lignocellulolytic capabilities of engineered L. plantarum strains with respect to simple polysaccharides and wheat straw were demonstrated by introducing two key enzymes, a cellulase and a xylanase, from the thermophilic bacterium Thermobifida fusca, using previously developed pSIP vectors (31) for efficient secretion of heterologous proteins (21, 22). The two enzymes were also shown to be displayed directly on the cell surface of cellulosomes, and the assembly of the enzymes onto a chimeric scaffoldin was controlled by specific cohesin-dockerin interactions. The secreted enzymes represented the most active of the three strategies at early times of degradation; but, as component parts of the surface-attached designer cellulosomes, the enzymes were more stable over time and achieved levels of degradation similar to those of the secreted enzymes during later times of degradation. In the latter studies, we devised a novel cell consortium approach in which each engineered L. plantarum strain expressed and secreted different components of the complex to be assembled on the cell wall of a scaffoldin-expressing strain (21, 22). The labor of producing and secreting the cellulosomal components was therefore divided among the members of the bacterial community.
Nevertheless, due to the hurdles encountered in attempts at anchoring large scaffoldins on the L. plantarum cell wall, we were limited to assembling only small numbers of enzymes in the cellulosomal complex, thus restricting the fiber degradation capabilities of the engineered cell consortium. In order to reach superior levels of degradation of the recalcitrant fiber, and to exploit the potential of the cellulosomal complex, more enzymatic functions have to be incorporated into the cellulosomal machinery. In order to overcome these issues, in the current work, we mimicked naturally existing molecular tactics to amplify the inherent enzyme combinatorics and stoichiometric plasticity used by several cellulosome-producing bacteria (32, 33). This approach allows the expression of large, stable, and active self-assembling protein complexes on the bacterial cells and may provide an effective strategy to achieve enhanced cell surface display of the engineered enzymes, thereby expanding the lignocellulolytic potential in L. plantarum.
RESULTS
Engineering of fully active mesophilic enzymes for assembly of cellulosomal structures on the L. plantarum cell wall.
Since the host “vehicle” for our study, L. plantarum, is a mesophile, we searched for appropriate enzymes derived from mesophilic bacteria to be used as designer cellulosome components for surface display. Our recent involvement in genomic sequencing of the mesophilic cellulolytic bacterium Clostridium papyrosolvens (34) provided a wealth of potentially compatible enzymes for our study.
The C. papyrosolvens enzymes, selected for heterologous secretion in L. plantarum and destined for self-assembly into active designer cellulosomes on the L. plantarum cell surface, are shown schematically in Fig. 1A. C. papyrosolvens exhibits strong genome homology with the closely related mesophile Clostridium cellulolyticum, which was demonstrated previously to possess highly efficient polysaccharide-degrading enzymes, both in the context of in vitro designer cellulosomes (17, 35) and for yeast (Saccharomyces cerevisiae)- or bacterium-based CBP (14, 36). Two putative C. papyrosolvens cellulases, GH5 and GH9, were selected on the basis of their homology with the two known synergistic cellulases from C. cellulolyticum, i.e., the Cel9G processive endoglucanase and the Cel5A endoglucanase (17). Two putative xylanases were also selected: one from the GH11 family that is homologous to C. cellulolyticum Xyn11A and another from the GH10 family that is homologous to C. cellulolyticum Xyn10A. Both of the C. cellulolyticum enzymes were characterized as efficient xylanases (37, 38).
FIG 1.
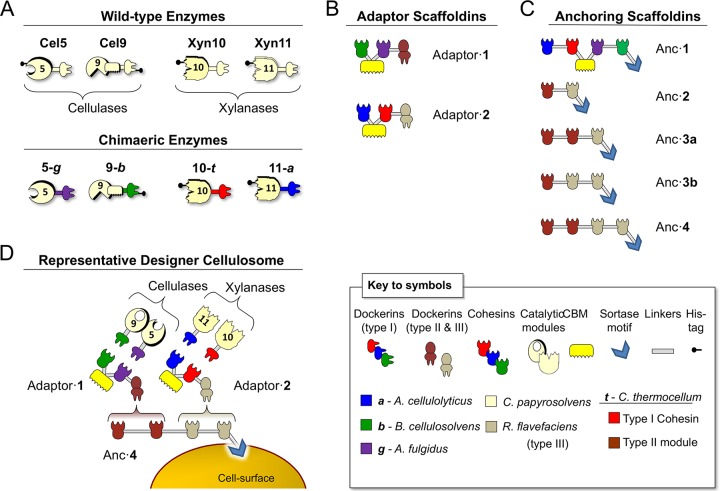
Schematic representation of the wild-type and chimeric proteins used in this study. The bacterial species from which the representative modules are derived are shown in color-coded form in the pictograms. (A) Wild-type and chimeric C. papyrosolvens enzymes. In the shorthand notation for the recombinant enzymes, the numbers 5, 9, 10, and 11 correspond to members of the GH family of the respective catalytic modules; the origin of a given dockerin module is also indicated by lowercase italic characters as described in the key to the symbols. (B) Modular architectures of the two different adaptor scaffoldins designed for this work. Each example of adaptor scaffoldin contains two divergent cohesins for selective integration of different dockerin-containing enzymes and a type II or type III dockerin for attachment to the appropriate cohesin-containing anchoring scaffoldin. (C) Modular architectures of the various types of anchoring scaffoldins designed in this study. Each contains a C-terminal sortase signal motif for covalent attachment to the cell surface. Anc·1 contains 4 divergent cohesins for selective integration of 4 different dockerin-bearing enzymes. Anc·2 through Anc·4 are anchoring scaffoldins differing in numbers (2 to 4) or positions (3a or 3b) of cohesins that integrate the two adaptor scaffoldins and their resident enzymes. (D) Example of designer cellulosome assembly, resulting from a consortium of different strains of transformed L. plantarum.
The cohesin-dockerin assembly is species specific (39, 40). Therefore, in order to control the composition and architecture of the desired designer cellulosomes, each enzyme was designed to contain a dockerin derived from a distinct bacterial species that matches a specific cohesin on the chimeric scaffoldin (16). The chimeric enzymes were thus modified by replacing the original C. papyrosolvens dockerin module (sharing the same binding specificity) with dockerins from other bacterial species, resulting in enzymes with different cohesin-binding specificities.
The hydrolytic activity of each of the five purified recombinant chimeric enzymes from C. papyrosolvens was compared to that of the corresponding recombinant wild-type enzyme, all produced in Escherichia coli (see Fig. S1 in the supplemental material). The wild-type enzymes and their respective recombinant chimeras were fully active on all cellulosic substrates or on xylan.
Newly designed recombinant synthetic scaffoldins.
In order to increase the combinatorics of the synthetic cellulosomal machinery, we have mimicked the existing natural microbial “adaptor-scaffoldin” approach with respect to heterogeneous bacterial cells (36, 41, 42). In this approach, several scaffoldins are assembled together through mediation via an adaptor scaffoldin(s), thereby increasing the number of enzymatic components in the cellulosomal complex (Fig. 1D). We designed two types of adaptor scaffoldins for enzyme integration (Fig. 1B). One type, adaptor·1, contains the two cohesin modules that bind the two dockerin-containing cellulases, and the second type, adaptor·2, contains two cohesin modules that incorporate the two dockerin-containing xylanases. In addition to the 2 enzyme-integrating cohesins, each adaptor scaffoldin contains a substrate-targeting CBM and a type II or type III dockerin for interaction with the cell surface-anchoring scaffoldin (42).
As mentioned above, one of the advantages of using the adaptor-scaffoldin approach is that it amplifies the combinatoric and stoichiometric possibilities for enzyme integration. In order to explore the combinatoric possibilities and to increase methodically the number of enzymes integrated into the complex, we created 5 different types of anchoring scaffoldins, as represented in Fig. 1C, that enable the insertion of up to 8 enzymes into the displayed designer cellulosomes. All of the anchoring scaffoldins contain a sortase signal motif for covalent attachment to the cell surface via a resident L. plantarum sortase (43). While anchor 1 (Anc·1) is composed of four different type I cohesin modules that directly interact with the four dockerin-bearing enzymes (the two cellulases and the two xylanases), Anc·2, Anc·3, and Anc·4 possess several copies of type II and III cohesins with different specificities. This setup of divergent specificities also allows us to analyze the influence of stoichiometry of the xylanases versus the cellulases on plant fiber degradation by enabling the attachment of either one or two copies of the cellulase-bearing adaptor scaffoldin (adaptor·1) or one or two copies of the xylanase-bearing adaptor scaffoldin (adaptor·2). An example of the various cell surface-displayed cellulosome assemblies produced by the different cell consortia is shown in Fig. 1D.
In order to examine the binding abilities of our engineered complexes, the two adaptor scaffoldins and five anchoring scaffoldins were initially purified recombinantly in E. coli. The respective binding specificities of the cohesin and dockerin modules of both the purified adaptor scaffoldins and anchoring scaffoldins were examined by performing native gel electrophoresis, and each recombinant protein was shown to interact selectively with its expected partner (see example in Fig. S2 in the supplemental material).
Secretion of active recombinant C. papyrosolvens mesophilic enzymes by L. plantarum.
The secretion and functionality of enzymes by L. plantarum were analyzed by comparing the enzymatic activity of concentrated culture supernatant fluids from transformed lactobacilli with that of the pure recombinant proteins from E. coli (Fig. S3). The two xylanases actively degraded xylan, and their concentrations were thus estimated (Fig. S3A and B) (Table 1). The two cellulases were not properly secreted using leader peptide 3050 (Lp3050) (data not shown). We therefore selected an alternative leader peptide (Lp2588), which was also previously reported to be an efficient candidate for secretion of foreign proteins in L. plantarum (31). The observed cellulase activities (data shown in Fig. S3C and D) served to allow estimation of the concentrations of the respective proteins (Table 1) (21). In parallel, we verified the presence of full-length recombinant enzymes and their ability to properly bind their respective cohesin modules by Far-Western blot analysis (Fig. S4).
TABLE 1.
Quantities of secreted and anchored proteins from transformed L. plantarum, determined using standard curves of purified proteins by ELISA-based binding assay
| Protein | Quantity in nM (± SD)a |
|---|---|
| Chimeric scaffoldins | |
| Adaptor·1 | 1.1 (0.01) |
| Adaptor·2 | 2.2 (0.02) |
| Anc·2 | 0.6 (0.11) |
| Anc·3a | 0.5 (0.20) |
| Anc·3b | 0.2 (0.07) |
| C. papyrosolvens enzymes | |
| Xylanase 10-t | 2.7 (0.07) |
| Xylanase 11-a | 0.12 (0.02) |
| Cellulase 5-g | 59.1 (0.9) |
| Cellulase 9-b | 41.4 (1.4) |
Data represent concentrations (in nanomoles) of individual transformed L. plantarum cells (OD600 = 1).
L. plantarum secretes and anchors active chimeric scaffoldins.
After examining the proper functionality and integrity of the enzymes with respect to function within the cellulosomal complex, we examined the expression of anchoring and adaptor scaffoldins in L. plantarum, which integrates the enzymes to form the desired elaborate cellulosomal structures.
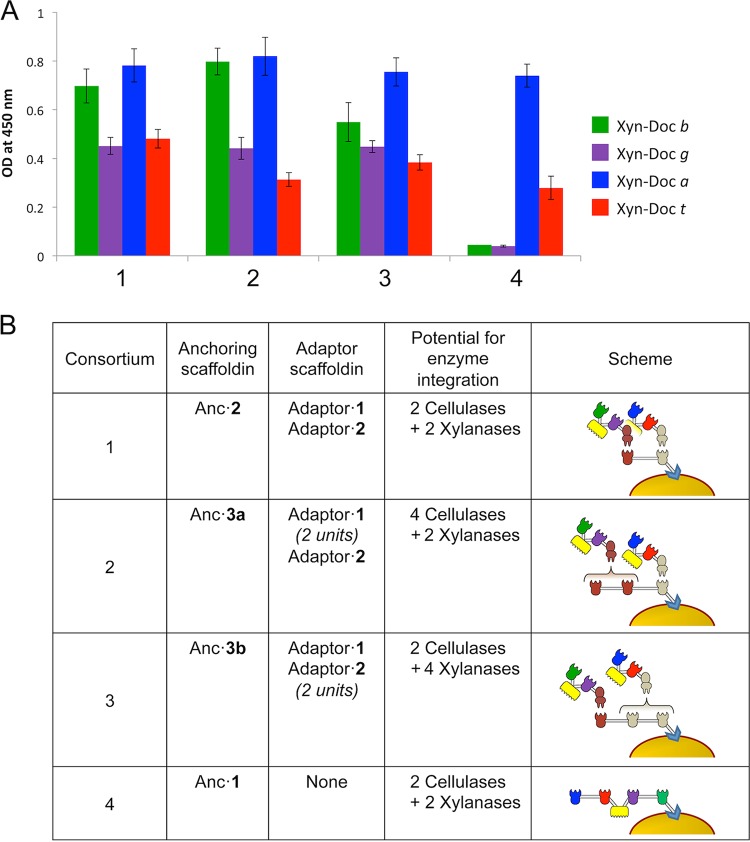
The secretion of the adaptor scaffoldins and their functionality were analyzed using an enzyme-linked immunosorbent assay (ELISA)-based binding assay by comparing the binding properties of the pure recombinant proteins (produced in E. coli) to those of culture supernatants from transformed lactobacilli. We found that the adaptor scaffoldins were properly secreted into the extracellular medium and exhibited the expected cohesin-dockerin binding capacities (Fig. S5). In addition, Fig. 2 shows that the binding properties of the adaptor scaffodins attached to the anchoring scaffoldins are functional. The presence of full-length adaptor scaffoldins was also verified by Western blotting (Fig. S4). The anchoring and functionality of the chimeric scaffoldins were also analyzed by ELISA-based binding assay by comparing the binding properties of pure proteins (xylanase tag fused to the different dockerins) to those from washed whole bacterial cells from transformed lactobacilli (Fig. S6). We observed that anchoring scaffoldins composed of two or three cohesins (Anc·2, Anc·3a, and Anc·3b) were attached and fully functional on the L. plantarum cell surface. The two cohesin/dockerin pairs appeared to enable comparably high levels of interaction events (Fig. S6). On the other hand, the anchoring scaffoldins composed of 4 cohesins (Anc·1 and Anc·4) did not exhibit full binding abilities, as they showed insufficient binding for two of their cohesins (Fig. 2; see also Fig. S6). In both cases, the cohesins adjacent to the anchoring signal motif were not functional. This result emphasizes the importance of using the adaptor scaffoldin strategy in this system, since incorporation of four different dockerin-bearing enzymes extends the number of catalytic subunits, and this would not be achieved by using a single scaffoldin directly anchored onto the L. plantarum cell surface.
FIG 2.
ELISA-based binding assay demonstrating the presence of active cohesin modules on the L. plantarum cell surface. (A) The three different consortia of individually transformed L. plantarum cells (see panel B legend for description) and the individual L. plantarum strain transformed with the gene for anchoring scaffoldin Anc·1 were examined for their capacity to interact with specific dockerin-bearing fusion proteins. Microtiter plates were coated with 1 μg/ml of the specified dockerins fused to the carrier protein (xylanase T6 from G. stearothermophilus). Washed whole bacterial cells from transformed lactobacilli of the different consortia and the Anc·1-bearing strain were then allowed to interact. The primary antibody used was prepared against the CBM of the scaffoldins. Washed bacterial cells (wild-type L. plantarum) served as a control. (B) Description of the incorporated chimeric scaffoldins for the indicated cellulosome complex. The different cell consortia comprised the following: consortium 1, consortium of anchoring scaffoldin Anc·2 with adaptor·1 and adaptor·2 (1 copy each); consortium 2, consortium of anchoring scaffoldin Anc·3a with 2 copies of adaptor·1 and 1 copy of adaptor·2; consortium 3, consortium of anchoring scaffoldin Anc·3b with 1 copy of adaptor·1 and 2 copies of adaptor·2; consortium 4, anchoring scaffoldin Anc·1.
ELISA-based binding assays both for adaptor and anchoring scaffoldins was also used to evaluate the quantity of secreted or anchored proteins of the different transformed strains of L. plantarum by using standard curves of known concentrations of pure proteins (Table 1).
L. plantarum cells displaying synthetic elaborate cellulosomal machinery show performance superior to that seen with secreted enzymes and simple synthetic cellulosome strategies.
As we reported previously (22), the cell consortium approach is a highly efficient method for assembling large complexes on bacterial cell walls. In this approach, the effort of producing and secreting the cellulosomal complex is divided among several strains—each secreting a different cellulosomal component of the cellulosome—thereby enabling assembly in combined form on the cell wall of the anchored scaffoldin-containing strain. In addition, since we obtained relatively large differences in the quantities of secreted/anchored proteins, the flexibility provided by the cell consortium approach is essential for control of the production of cellulosomal complexes with stoichiometric amounts of the relevant components.
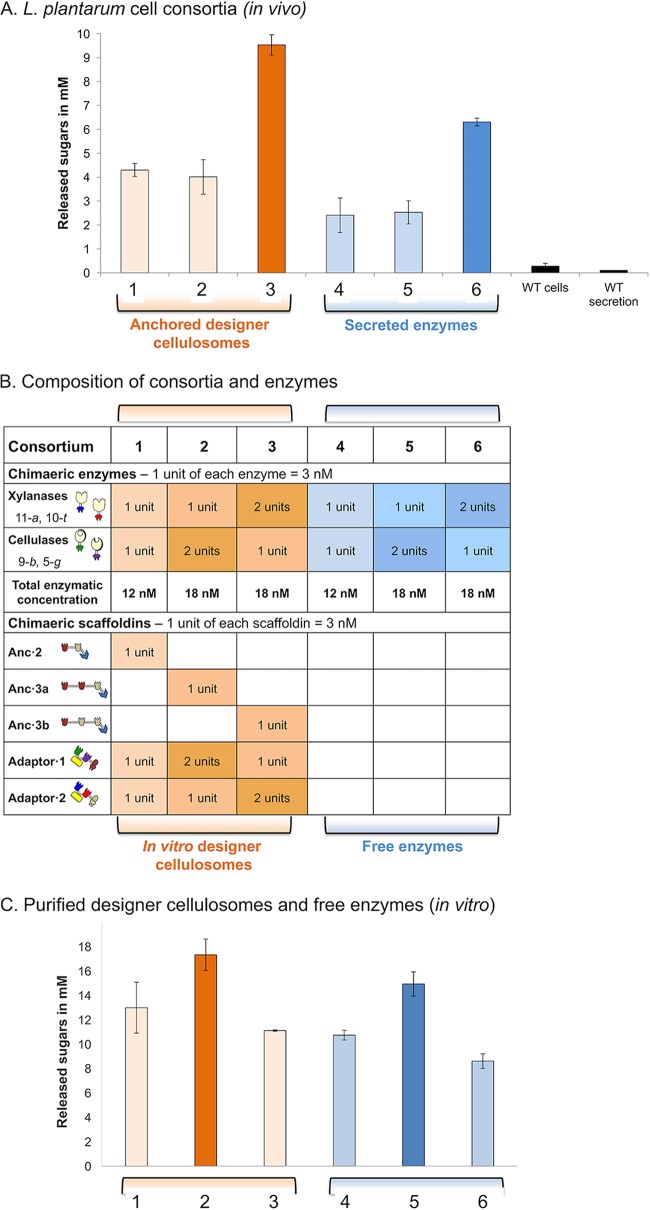
Here, we examined the ability of the elaborate cellulosomal machineries to degrade natural plant fiber material (pretreated wheat straw) and compared their activity to that resulting from the free secreted enzyme approach. To this end, six different types of microbial consortia were examined as detailed in Fig. 3B. Using these consortia, we experimented with different stoichiometries of the cellulosomal and secreted enzyme components (Fig. 3A).
FIG 3.
Comparative analysis of hydrolysis of hypochlorite-pretreated wheat straw by free enzymes versus cell-associated and cell-free designer cellullosomes. (A) Soluble sugars produced in the extracellular medium by different transformed L. plantarum consortia versus the wild-type (WT) strain. Reaction mixtures were incubated for 96 h at 37°C. For consortia 1, 2, and 3 and for WT cells, washed cells were used in the enzymatic reaction, whereas for consortia 4, 5, and 6 and for WT secretion cells, concentrated supernatant fluids were used. Hypochlorite-pretreated wheat straw was used at a concentration of 40 g/liter, and enzymatic activities are represented by the concentrations of total reducing sugars (expressed in millimoles). Experiments were conducted three times with triplicate samples, and standard deviations are indicated. (B) The recombinant enzymes and chimeric scaffoldins that were introduced in the different L. plantarum consortia are indicated and correspond to the respective bars in the chart. The molar ratios between the proteins, the numbers of units, and total enzyme concentrations are stipulated. (C) Soluble sugars produced by recombinant cell-free designer cellulosome assemblies and free enzyme mixtures parallel to the ones used as described for panel A and assembled from purified proteins produced by E. coli. The cellulosomal components were assembled stoichiometrically, where the concentration of the anchoring scaffoldin was set at 12 nM. The designer cellulosomes were allowed to assemble for 3 h at room temperature with all components of the enzymatic assay except the wheat straw substrate. The enzymatic reaction mixture was incubated for 96 h at 37°C under shaking conditions. Experiments were conducted three times with triplicate samples, and standard deviations are indicated.
The microbial consortia examined in this work produce either the free enzymes or the surface-displayed designer cellulosomes, and the bacterial cells would directly consume the sugars produced by their different enzymatic arrangements. Hence, in order to elucidate the portion of sugars that is consumed by our microbial consortia, we performed in vitro enzymatic hydrolysis with enzymes or assembled designer cellulosomes produced and purified from E. coli (Fig. 3C) in order to determine the level of soluble sugar production in the absence of L. plantarum cells. Indeed, as shown in Fig. 3C, we observed that the amounts of sugars produced by the enzymatic mixtures were larger than those produced by the residual sugars measured after incubation with the microbial consortia (Fig. 3A). In addition, we could see from the results represented in both panel A and panel C of Fig. 3 that the designer cellulosomes consistently outperformed their respective free enzyme counterparts (compare bars 1, 2, and 3 to bars 4, 5, and 6).
As shown in Fig. 3C, the comparative levels of degradation of pretreated wheat straw by the in vitro-applied enzymes and designer cellulosomes revealed that the enzymatic complex consisting of designer cellulosomes comprising two copies of the cellulases (bar 2) was the best-performing enzymatic complex. By comparison, as shown in Fig. 3A, the soluble (residual) sugar measurements reflected the amounts of sugars that were not consumed by the L. plantarum consortia. We then further evaluated the kinetics of the reaction by the use of microbial consortia, which revealed that the activity of the anchored designer cellulosomes continued until 96 h, whereas the free enzymes failed to produce additional soluble sugars after 48 h (Fig. S7). Further analysis of unconsumed released sugars from pretreated wheat straw degradation using a high-performance anionic exchange chromatography (HPAEC) system (see Table S1 in the supplemental material) revealed large amounts of xylan degradation products (mainly xylobiose and xylotriose), suggesting that L. plantarum could not assimilate these carbon sources.
DISCUSSION
In this study, we used the adaptor scaffoldin strategy for assembly of elaborate cellulosomal structures (36, 41, 42) on the cell surface of L. plantarum for both augmenting cell surface display and improving its fiber-degrading potential. Potent enzymes originating from a mesophilic cellulosome-producing species, Clostridium papyrosolvens, are well suited to expression in the gut ecosystem (a common L. plantarum habitat) for this purpose. Here, all the cellulosomal components were produced in vivo by the cell consortia and were not supplemented ex vivo as previously reported (36, 41).
Cellulosomal complexes have attracted increased interest in recent years, since lignocellulosic biomass represents a particularly abundant resource for conversion into fermentable sugars, suitable for production of biofuels (44). We recently reported the successful incorporation of simple divalent designer cellulosome components into the cell wall of Lactobacillus plantarum (22), an attractive candidate for consolidated bioprocessing (22, 45, 46). Here, the adaptor scaffoldin strategy was demonstrated to be an effective approach (i) for increasing the number of catalytic units in the cellulosome complex displayed on the cell surface, thereby bypassing the relatively low level of cell surface display of scaffoldins, and (ii) for achieving high capacities of binding of the bacterial cell to the substrate.
In this work, we produced elaborate cellulosomal complexes by employing a cell consortium approach where each recombinant strain of L. plantarum expresses an individual cellulosomal component (secreted to the extracellular medium or anchored to the bacterial cell surface). A total of four chimeric cellulosomal enzymes (cellulases and xylanases derived from C. papyrosolvens) and two adaptor scaffoldins were functionally secreted into the extracellular media. In addition, five different types of anchoring scaffoldins were tested for their ability to properly interact subsequently with the secreted cellulosomal elements. By composing various cocultures of recombinant bacteria expressing the heterologous proteins separately, we were able to attach up to three adaptor scaffoldins to the anchoring scaffoldins for potential display of up to six catalytic subunits on the cell surface. Using cocultures offers the advantage that the composition of the surface-anchored designer cellulosome, produced by an appropriate cell consortium, can be easily controlled by adjusting the ratios of the levels of the cell types during inoculation. It was also demonstrated that cocultures of recombinant bacteria expressing heterologous proteins did not affect the initial ratio of the strains and therefore did not affect the ratio of proteins expressed (21). The cell consortium approach decreases considerably the burden of the cellular machinery of each strain, thereby maximizing the ability of the cells to grow and to express the various cellulosomal components. In nature, this type of spatial differentiation strategy is commonly employed by prokaryotic species in a given ecosystem and results in a collaboration among the different cell types to achieve a unique objective from which they will all benefit (47).
The variety of available anchoring and adaptor scaffoldins allowed us to examine the importance of the ratios among the different enzymatic components in order to obtain efficient substrate degradation. The highest levels of degradation in the present studies were obtained when a trivalent anchoring scaffoldin enabled attachment to the cell surface of three adaptor scaffoldins incorporating a total of four cellulases (2 copies of adaptor·1) and two xylanases (a single copy of adaptor·2) (see Fig. 3C, bar 2). This enzymatic combination was also optimal among the purified free enzymes (Fig. 3C, bar 5). Since the kinetics of xylan removal by the employed xylanases is much higher than that represented by the slower cellulose degradation by cellulases used in this study (see Fig. S1 in the supplemental material), it would be therefore logical that a higher cellulase/xylanase ratio would be required for optimized wheat straw deconstruction.
We observed here that the cellulosome paradigm was more efficient than the secreted free enzyme approach and that elaborate cellulosome structures (consortia 2 and 3) conferred high stability to the catalytic subunits (Table 2) and high cellulose-binding abilities to the bacterial cells (Table 3). The stability of the enzymes seems to be a key parameter in terms of enzymatic efficiency. At later cultivation times (beyond 48 h), the anchoring paradigm appears more advantageous than the secreted free enzyme paradigm for the same enzymatic composition (Fig. S7). This corresponds to the decrease seen in the stability of the secreted cellulases (Table 2), whereas the anchored enzymes remained fully active. While the cellulosomal machinery is considered to induce synergistic activity among the catalytic modules as a consequence of their close proximity within the complex (5, 7), data from the present study as well as from previous studies (48, 49) strongly support the idea of the importance of the stability conferred to the enzymes by the scaffoldin subunit.
TABLE 2.
Stability in activity of the different consortia at 37°C for a 48-h period
| Degradation category | Activity stability (% ± SD) of: |
|||||
|---|---|---|---|---|---|---|
| Anchoring A | Anchoring B | Anchoring C | Secreting D | Secreting E | Secreting F | |
| CMC | 100 ± 0 | 94 ± 6 | 100 ± 0 | 17 ± 5 | 6 ± 1 | 1 ± 0 |
| Xylan | 100 ± 0 | 100 ± 1 | 100 ± 2 | 51 ± 7 | 41 ± 3 | 38 ± 1 |
TABLE 3.
Adherence analysis: variations in turbidity of different L. plantarum cultures grown to an OD of approximately 1 and incubated with 3% Avicel solution for 1 h at 4°C
| L. plantarum washed cell culture | Change in turbidity in ΔOD (± SD) |
|---|---|
| Consortium A | 0.29 (0.02) |
| Consortium B | 0.41 (0.01) |
| Consortium C | 0.43 (0.04) |
| Wild type | 0.09 (0.006) |
The ability of the bacteria to utilize the wheat straw substrate as the sole carbon source was assessed on a chemically defined medium (CDM). However, the use of wheat straw failed to sustain the growth of either the wild-type bacteria or those of consortium 2, which produced the largest amount of sugars (data not shown). We further examined the minimal amount of sugar required to sustain growth by growing wild-type L. plantarum cells on glucose, cellobiose, xylose, or xylobiose. No growth was observed on pure xylose and xylobiose, while the minimal concentration that sustained growth was 0.2% glucose (∼11 mM) or 0.2% cellobiose (∼5.5 mM) on CDM (Fig. S8). The concentrations of the sugars produced by the best-performing cellulosomal machinery (Fig. 3C, bar 2) were a concentration of about 17 mM of a mixture of soluble sugar products, some of which are not utilizable, and a concentration of about 4 mM of unconsumed sugars (Fig. 3A, bar 2). The lack of growth suggests that the amounts and types of sugars produced by the consortia are the limiting factors for growth sustainability. To bridge this gap in future studies, we will screen for additional highly expressed cellulases in order to generate higher production of sugars that can be assimilated. In addition, it is important to fine-tune the amounts and/or types of xylanases, which, on the one hand, serve to remove the embedded xylan that prevents physical access of the cellulases to the cellulose but, on the other, produce sugars that are not consumed by the L. plantarum cells. Data presented in Table S2 in the supplemental material suggest that xylose may be assimilated by the cells, since low concentrations of xylose were detected in the presence of L. plantarum. The complete genome sequence of L. plantarum WCFS1 indeed suggests the presence of genes involved in xylose transport, but genes for d-xylose isomerase and d-xylose kinase were not detected (50), indicating that xylose cannot be fermented. Indeed, effective xylose transport was also demonstrated for strain 3NSH, but xylose was not metabolized in those studies (51). Therefore, further research should consider the implementation of xylose assimilation genes in strain WCSF1 as previously used for strain NCIMB 8826 (52). Alternative explanations for the lack of bacterial growth by the cell consortia, such as the potential release of cellulase inhibitors by xylanase action (53), should be sought in future studies.
Within the context of gut microbiome ecosystems, the xylan degradation products produced by the cell consortia developed in this work could also benefit the overall microbial community. Given that it is a potent gut microbe, the engineering of L. plantarum for fiber degradation could be highly beneficial for clinical applications, such as in the form of probiotics (26). Indeed, by enabling this bacterium to degrade plant fiber, we potentially increase its fitness in the gut by extending its status in the ecosystem, which will allow it to better persist as a probiotic organism. Furthermore, the augmented cell surface display conferred by the adaptor scaffoldin strategy could serve to promote higher efficiency of mucosal vaccines, based on the use of bacteria as delivery vehicles (54).
MATERIALS AND METHODS
All the experiments were replicated three times in triplicate, and the data served to perform the statistical calculations in the relevant figures and tables.
Cloning.
All recombinant proteins employed in this study (see representation in Fig. 1) were first cloned into pET28a plasmids and designed to contain a His tag for subsequent purification by standard restriction-based cloning procedures (55). The recombinant enzymes from C. papyrosolvens were produced by replacing their native dockerins with dockerins of different specificities using respective genomic DNA as follows: 5-g was obtained by fusing the catalytic module of C. papyrosolvens GH5 (GenBank accession no. EPR12097.1) to a dockerin from Archaeoglobus fulgidus (Orf2375), 9-b was obtained by fusing the catalytic module of C. papyrosolvens GH9 (GenBank accession no. EPR13542.1) to a dockerin of Bacteroides cellulosolvens (ScaA), 10-t was obtained by fusing the catalytic module of C. papyrosolvens GH10 (GenBank accession no. EPR14039.1) to a dockerin of C. thermocellum (Cel48S), and 11-a was obtained by fusing the catalytic module of C. papyrosolvens GH11 (GenBank accession no. EPR13563.1) to a dockerin of Acetivibrio cellulolyticus (ScaB).
The adaptor scaffoldins adaptor·1 and adaptor·2 were obtained by fusing previously employed bivalent scaffoldins (19) to a type II dockerin from C. thermocellum (CipA) for adaptor·1 and to a type III dockerin from Ruminococcus flavefaciens 17 (ScaB) for adaptor·2.
The anchoring scaffoldins Anc·2, Anc·3a, Anc·3b, and Anc·4 were obtained by fusing one or two type II cohesins from C. thermocellum (OlpB) to one or two type III cohesins from R. flavefaciens 17 (ScaE), as shown in Fig. 1.
The enzymes and the genes coding for the two adaptor scaffoldins were introduced into L. plantarum using the previously employed pSIP vectors for efficient secretion/attachment of heterologous proteins (21, 22) and leader peptide 3050 (Lp3050) via pLp_3050sAmy, produced by replacing the amylase gene in these plasmids with an appropriately amplified gene fragment (31). As the five different anchoring scaffoldins (Anc·1, Anc·2, Anc·3a, Anc·3b, and Anc·4) were to be integrated into the bacterial cell wall, we fused them into potent cell wall anchor (cwa) anchoring signal cwa2 (22) via the use of modular pLp_0373sOFA-anchoring plasmids (56).
To amplify DNA fragments by PCR for cloning, a T-Gradient device (Biometra, Germany) was used. The PCR was performed in 50-μl reaction mixtures.
For short (up to 500-bp) PCR products, PCR ready mix (Abgen, Epsom, Surrey, United Kingdom) was employed. For longer PCR fragments, PCRs were performed using Phusion high-fidelity DNA polymerase F530-S (New England BioLabs, Inc.). Primers were added to reach a final concentration of 0.5 μM. PCR was programmed according to each manufacturer's instructions. Primers are listed in Table S2 in the supplemental material.
Protein expression and purification from Escherichia coli.
Recombinant proteins were expressed in E. coli BL21(DE3) and purified as described earlier (18, 42).
Protein expression in L. plantarum.
The methodology previously described by Moraïs et al. (22) was followed. For the consortium experiments, strains producing cellulase or xylanase or adaptor or anchoring scaffoldins were mixed at stoichiometric molar ratios for the enzyme/scaffoldin interactions and the scaffoldin/scaffoldin interactions and then grown on De Man, Rogosa, and Sharpe (MRS) broth (as prepared by BD Difco without protease peptone) supplemented with 40 mM CaCl2.
Western and Far-Western blotting of L. plantarum secreted proteins.
The methodology previously described by Moraïs et al. (22) was followed for the Western blot experiments (see Fig. S4E and F in the supplemental material). In the Far-Western blot experiments (Fig. S4A, B, C, and D), an interaction step was included after blocking. Binding interactions with the blotted proteins were assayed with tagged fusions of specific cohesins fused to the CBM3a module (from the C. thermocellum scaffoldin) (CBM-Coh) (57, 58). Specific rabbit antibody against the fused tag (CBM) diluted at 1:3,000 (58, 59) was used as the primary antibody. The Far-Western blot experiments served here to verify both the presence of the full-length dockerin-bearing enzymes and their ability to bind their respective cohesins.
ELISA binding assay.
The methodology previously described by Barak et al. (57) was followed with the following modification. MaxiSorp ELISA plates (Nunc A/S, Roskilde, Denmark) were coated overnight at 4°C either with 1 μg/ml of the specific dockerin fused to xylanase T6 from Geobacillus stearothermophilus (Xyn-Doc) or with CBM-Coh (100 μl/well) in 0.1 M sodium carbonate (pH 9). After the blocking step, incremental dilutions of washed L. plantarum whole cells or concentrated supernatant fluids (optical density at 600 nm [OD600] of 1) or 100 ng/ml of purified recombinant proteins in blocking buffer was added. Specific rabbit antibody, raised against either the CBM diluted at 1:3,000 in blocking buffer or the type II cohesin from C. thermocellum (1:10,000 dilution), was used as the primary antibody.
Enzymatic activity in pretreated wheat straw degradation.
Prior to enzymatic assay, culture supernatant fluids (for secreted proteins) were concentrated using Amicon centrifugal filters with a 30-kDa cutoff (Millipore, Molsheim, France) and washed with Tris-buffered saline (TBS [×10]: 80 g NaCl, 2 g KCl, and 30 g Tris, with double-distilled water [ddH2O] added to reach a volume of 1 liter and with pH adjusted to 7.4 with 32% HCl) containing 40 mM CaCl2; cells (for anchored designer cellulosomes) were washed with TBS containing 1% Triton X-100 by centrifugation and resuspension to eliminate the sugars present in the MRS medium. Hatched wheat straw, pretreated with 12% sodium hypochlorite, was prepared as described before (18). This type of pretreatment selectively removes lignin from the biomass, leaving the hemicellulose fraction largely intact. A typical assay mixture consisted of either washed whole cells or concentrated supernatant fluids from L. plantarum at specified concentrations applied to a suspension of 40 g/liter pretreated wheat straw in the relevant specified reaction volume (50 mM citrate buffer [pH 6.0], 12 mM CaCl2, 2 mM EDTA). Reaction mixtures were incubated at 37°C under shaking conditions. The total amount of sugars released was determined using the dinitrosalicylic acid (DNS) assay as described previously (42, 60).
When pure proteins or designer cellulosomes were employed (Fig. 3C), similar conditions were used with a stoichiometric concentration of enzymes (and scaffoldins), whereby the anchoring scaffoldin concentration was set at 12 nM. The designer cellulosomes were allowed to assemble for 3 h at room temperature with all the enzymatic assay components except the wheat straw substrate. Upon addition of the wheat straw, the enzymatic reaction mixture was incubated for 96 h at 37°C under shaking conditions.
Sugar analysis.
Sugar content was analyzed using a high-performance anion exchange chromatography (HPAEC) system equipped with a PA1 column (Dionex, Sunnyvale, CA). Supernatants of the reaction mixtures obtained after centrifugation were loaded onto the PA1 column and eluted with 200 mM NaOH (flow rate, 1 ml/min). At first, standards consisting of pure arabinose, xylose, xylobiose, xylotriose, glucose, cellobiose, and cellotriose were loaded separately to determine elution times and peak areas as a function of the sugar concentration. Sugars present under blank conditions were deducted in all the samples.
Stability assay.
The stability of the enzymatic combination at 37°C was determined by incubating the described consortia (at a 3 nM concentration for each enzyme) without the substrate over a 48-h period at 37°C. The residual enzymatic activity was calculated as the relative activity of the consortium incubated at 37°C compared to that of the anchored consortium (washed whole cells) or secreted consortium (concentrated supernatant fluids) that was directly introduced into the substrate (with no incubation period) on carboxymethyl cellulose (CMC) or beechwood xylan for a period of 2 h at 37°C.
Adherence/turbidity assay.
Wild-type L. plantarum bacteria or consortia of transformed strains were grown with inducer until an OD600 of 1 was reached. A 1-ml volume of the cultures was then subjected to an interaction with 30 mg of Avicel for 1 h at 4°C in TBS supplemented with 40 mM CaCl2. Gentle centrifugation (about 1 min at 1,000 rpm) was then performed to separate the Avicel substrate. The absorbance at OD600 was then verified using MRS medium supplemented with Avicel under the same conditions as those used for a blank solution without bacterial cells. The difference in the levels of absorbance at OD600 reflects the adhesion of the cells to cellulose.
Bacterial growth.
A chemically defined medium (CDM) previously developed by Wegkamp et al. (61) was prepared with 20 g/liter pretreated wheat straw. In parallel, consortium 2 bacteria or the wild-type bacteria were cultured in MRS without protease peptone as described above. Cells were harvested and washed twice in 10 ml 0.85% NaCl–10 mM CaCl2, and the washed cells served as an inoculum of the CDM that contained wheat straw as a carbon source. The medium was supplemented with pSIP inducer, 10 mM CaCl2, and erythromycin in the case of consortium 2. Growth at 37°C under conditions of agitation (200 rpm) was followed for a week by measuring the OD at 600 nm of the supernatant culture after the wheat straw precipitated (5 min). Growth of the wild-type bacteria was assessed on CDM supplemented with 0% to 1% cellobiose, glucose, xylose, or xylobiose.
Supplementary Material
ACKNOWLEDGMENTS
The research was supported by grants from the Israel Science Foundation (no. 1313/13 to I.M. and 1349/13 to E.A.B.), by the Israeli Chief Scientist Ministry of Agriculture and Rural Development Fund (no. 362-0426) to E.A.B. and I.M., by the Israeli Chief Scientist Ministry of Science Foundation (no. 3-10880) to E.A.B. and I.M., by the European Research Council (No. 640384) to I.M., and by the United States-Israel Binational Science Foundation (BSF). We appreciate the support of the European Union, CellulosomePlus (no. 604530), and European Union Horizon 2020: Waste2Fuels. E.A.B. is the incumbent of The Maynard I. and Elaine Wishner Chair of Bio-organic Chemistry.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00282-18.
REFERENCES
- 1.Doblin MS, Pettolino F, Bacic A. 2010. Plant cell walls: the skeleton of the plant world. Funct Plant Biol 37:357–381. doi: 10.1071/FP09279. [DOI] [Google Scholar]
- 2.Artzi L, Bayer EA, Moraïs S. 12 December 2016. Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides. Nat Rev Microbiol doi: 10.1038/nrmicro.2016.164. [DOI] [PubMed] [Google Scholar]
- 3.Lamed R, Setter E, Bayer EA. 1983. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol 156:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoham Y, Lamed R, Bayer EA. 1999. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol 7:275–281. doi: 10.1016/S0966-842X(99)01533-4. [DOI] [PubMed] [Google Scholar]
- 5.Bayer EA, Belaich J-P, Shoham Y, Lamed R. 2004. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol 58:521–554. doi: 10.1146/annurev.micro.57.030502.091022. [DOI] [PubMed] [Google Scholar]
- 6.Adams JJ, Pal G, Jia Z, Smith SP. 2006. Mechanism of bacterial cell-surface attachment revealed by the structure of cellulosomal type II cohesin-dockerin complex. Proc Natl Acad Sci U S A 103:305–310. doi: 10.1073/pnas.0507109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayer EA, Setter E, Lamed R. 1985. Organization and distribution of the cellulosome in Clostridium thermocellum. J Bacteriol 163:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai S-L, Oh J, Singh S, Chen R, Chen W. 2009. Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production. Appl Environ Microbiol 75:6087–6093. doi: 10.1128/AEM.01538-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai S-L, Goyal G, Chen W. 2010. Surface display of a functional minicellulosome by intracellular complementation using a synthetic yeast consortium and its application to cellulose hydrolysis and ethanol production. Appl Environ Microbiol 76:7514–7520. doi: 10.1128/AEM.01777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen F, Sun J, Zhao H. 2010. Yeast surface display of trifunctional minicellulosomes for simultaneous saccharification and fermentation of cellulose to ethanol. Appl Environ Microbiol 76:1251–1260. doi: 10.1128/AEM.01687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilly M, Fierobe H-P, van Zyl WH, Volschenk H. 2009. Heterologous expression of a Clostridium minicellulosome in Saccharomyces cerevisiae. FEMS Yeast Res 9:1236–1249. doi: 10.1111/j.1567-1364.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 12.You C, Zhang X-Z, Sathitsuksanoh N, Lynd LR, Zhang Y-HP. 2012. Enhanced microbial utilization of recalcitrant cellulose by an ex vivo cellulosome-microbe complex. Appl Environ Microbiol 78:1437–1444. doi: 10.1128/AEM.07138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson TD, Robson SA, Jiang XW, Malmirchegini GR, Fierobe HP, Lazazzera BA, Clubb RT. 2011. Assembly of minicellulosomes on the surface of Bacillus subtilis. Appl Environ Microbiol 77:4849–4858. doi: 10.1128/AEM.02599-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Willson BJ, Kovács K, Wilding-Steele T, Markus R, Winzer K, Minton NP. 2016. Production of a functional cell wall-anchored minicellulosome by recombinant Clostridium acetobutylicum ATCC 824. Biotechnol Biofuels 9:109. doi: 10.1186/s13068-016-0526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieczorek AS, Martin VJJ. 2010. Engineering the cell surface display of cohesins for assembly of cellulosome-inspired enzyme complexes on Lactococcus lactis. Microb Cell Fact 9:69. doi: 10.1186/1475-2859-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayer EA, Morag E, Lamed R. 1994. The cellulosome — a treasure-trove for biotechnology. Trends Biotechnol 12:379–386. doi: 10.1016/0167-7799(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 17.Fierobe H, Mingardon F, Mechaly A, Be A, Rincon MT, Lamed R, Tardif C, Be J, Bayer EA. 2005. Action of designer cellulosomes on homogeneous versus complex substrates. J Biol Chem 280:16325–16334. doi: 10.1074/jbc.M414449200. [DOI] [PubMed] [Google Scholar]
- 18.Moraïs S, Morag E, Barak Y, Goldman D, Hadar Y, Lamed R, Shoham Y, Wilson DB, Bayer EA. 2012. Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes. 3:e00508-12. doi: 10.1128/mBio.00508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moraïs S, Barak Y, Caspi J, Hadar Y, Lamed R, Shoham Y, Wilson DB, Bayer EA. 2010. Cellulase-xylanase synergy in designer cellulosomes for enhanced degradation of a complex cellulosic substrate. mBio 1:e00285-10. doi: 10.1128/mBio.00285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang GL, Anderson TD, Clubb RT. 18 December 2013. Engineering microbial surfaces to degrade lignocellulosic biomass. Bioengineered doi: 10.4161/bioe.27461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moraïs S, Shterzer N, Grinberg IR, Mathiesen G, Eijsink VGH, Axelsson L, Lamed R, Bayer EA, Mizrahi I. 2013. Establishment of a simple Lactobacillus plantarum cell consortium for cellulase-xylanase synergistic interactions. Appl Environ Microbiol 79:5242–5249. doi: 10.1128/AEM.01211-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moraïs S, Shterzer N, Lamed R, Bayer EA, Mizrahi I. 2014. A combined cell-consortium approach for lignocellulose degradation by specialized Lactobacillus plantarum cells. Biotechnol Biofuels 7:112. doi: 10.1186/1754-6834-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzoli R, Bosco F, Mizrahi I, Bayer EA, Pessione E. 2014. Towards lactic acid bacteria-based biorefineries. Biotechnol Adv 32:1216–1236. doi: 10.1016/j.biotechadv.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 24.G-Alegría E, López I, Ruiz JI, Sáenz J, Fernández E, Zarazaga M, Dizy M, Torres C, Ruiz-Larrea F. 2004. High tolerance of wild Lactobacillus plantarum and Oenococcus oeni strains to lyophilisation and stress environmental conditions of acid pH and ethanol. FEMS Microbiol Lett 230:53–61. doi: 10.1016/S0378-1097(03)00854-1. [DOI] [PubMed] [Google Scholar]
- 25.Lucena BT, dos Santos BM, Moreira JL, Moreira APB, Nunes AC, Azevedo V, Miyoshi A, Thompson FL, de Morais M. 2010. Diversity of lactic acid bacteria of the bioethanol process. BMC Microbiol 10:298. doi: 10.1186/1471-2180-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter J. 2008. Ecological role of Lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol 74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martino ME, Bayjanov JR, Caffrey BE, Wels M, Joncour P, Hughes S, Gillet B, Kleerebezem M, Lyon CB. 2016. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ Microbiol 18:4974–4989. doi: 10.1111/1462-2920.13455. [DOI] [PubMed] [Google Scholar]
- 28.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. 2011. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. 2010. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A 107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. 2016. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 31.Mathiesen G, Sveen A, Brurberg MB, Fredriksen L, Axelsson L, Eijsink VG. 2009. Genome-wide analysis of signal peptide functionality in Lactobacillus plantarum WCFS1. BMC Genomics 10:425. doi: 10.1186/1471-2164-10-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q, Gao W, Ding S, Kenig R, Shoham Y, Bayer EA, Lamed R. 2003. The cellulosome system of Acetivibrio cellulolyticus includes a novel type of adaptor protein and a cell surface anchoring protein. J Bacteriol 185:4548–4557. doi: 10.1128/JB.185.15.4548-4557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artzi L, Dassa B, Borovok I, Shamshoum M, Lamed R, Bayer EA. 2014. Cellulosomics of the cellulolytic thermophile Clostridium clariflavum. Biotechnol Biofuels 7:100. doi: 10.1186/1754-6834-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zepeda V, Dassa B, Borovok I, Lamed R, Bayer EA, Cate JHD. 12 September 2013. Draft genome sequence of the cellulolytic bacterium Clostridium papyrosolvens C7 (ATCC 700395). Genome Announc doi: 10.1128/genomeA.00698-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingardon F, Chantal A, López-Contreras AM, Dray C, Bayer EA, Fierobe H-P. 2007. Incorporation of fungal cellulases in bacterial minicellulosomes yields viable, synergistically acting cellulolytic complexes. Appl Environ Microbiol 73:3822–3832. doi: 10.1128/AEM.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan L-H, Zhang Z-J, Yu X-Y, Xue Y-X, Tan T-W. 2012. Self-surface assembly of cellulosomes with two miniscaffoldins on Saccharomyces cerevisiae for cellulosic ethanol production. Proc Natl Acad Sci U S A 109:13260–13265. doi: 10.1073/pnas.1209856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blouzard JC, Bourgeois C, De Philip P, Valette O, Bélaïch A, Tardif C, Bélaïch JP, Pagès S. 2007. Enzyme diversity of the cellulolytic system produced by Clostridium cellulolyticum explored by two-dimensional analysis: Identification of seven genes encoding new dockerin-containing proteins. J Bacteriol 189:2300–2309. doi: 10.1128/JB.00917-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohand-Oussaid O, Payot S, Guedon E, Gelhaye E, Youyou A, Petitdemange H. 1999. The extracellular xylan degradative system in Clostridium cellulolyticum cultivated on xylan: evidence for cell-free cellulosome production. J Bacteriol 181:4035–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haimovitz R, Barak Y, Morag E, Voronov-Goldman M, Lamed R, Bayer EA. 2008. Cohesin-dockerin microarray: diverse specificities between two complementary families of interacting protein modules. Proteomics 8:968–979. doi: 10.1002/pmic.200700486. [DOI] [PubMed] [Google Scholar]
- 40.Pagès S, Belaich A, Belaich J-P, Morag E, Lamed R, Shoham Y, Bayer EA. 1997. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: prediction of specificity determinants of the dockerin domain. Proteins 29:517–527. doi:. [DOI] [PubMed] [Google Scholar]
- 41.Tsai SL, DaSilva NA, Chen W. 2013. Functional display of complex cellulosomes on the yeast surface via adaptive assembly. ACS Synth Biol 2:14–21. doi: 10.1021/sb300047u. [DOI] [PubMed] [Google Scholar]
- 42.Stern J, Moraïs S, Lamed R, Bayer EA. 2016. Adaptor scaffoldins: an original strategy for extended designer cellulosomes, inspired from nature. mBio 7:e00083-16. doi: 10.1128/mBio.00083-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remus DM, Bongers RS, Meijerink M, Fusetti F, Poolman B, de Vos P, Wells JM, Kleerebezem M, Bron PA. 2013. Impact of Lactobacillus plantarum sortase on target protein sorting, gastrointestinal persistence, and host immune response modulation. J Bacteriol 195:502–509. doi: 10.1128/JB.01321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayer EA, Lamed R, Himmel ME. 2007. The potential of cellulases and cellulosomes for cellulosic waste management. Curr Opin Biotechnol 18:237–245. doi: 10.1016/j.copbio.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Bosma EF, Forster J, Nielsen AT. 2017. Lactobacilli and Pediococci as versatile cell factories – evaluation of strain properties and genetic tools. Biotechnol Adv 35:419–442. doi: 10.1016/j.biotechadv.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Lynd L, Zyl W, McBride J, Laser M. 2005. Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16:577–583. doi: 10.1016/j.copbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Agapakis CM, Boyle PM, Silver PA. 2012. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat Chem Biol 8:527–535. doi: 10.1038/nchembio.975. [DOI] [PubMed] [Google Scholar]
- 48.Krauss J, Zverlov VV, Schwarz WH. 2012. In vitro reconstitution of the complete Clostridium thermocellum cellulosome and synergistic activity on crystalline cellulose. Appl Environ Microbiol 78:4301–4307. doi: 10.1128/AEM.07959-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C, Qin Y, Li Y, Ji Y, Huang J, Song H, Xu J. 2010. Factors influencing cellulosome activity in consolidated bioprocessing of cellulosic ethanol. Bioresour Technol 101:9560–9569. doi: 10.1016/j.biortech.2010.07.065. [DOI] [PubMed] [Google Scholar]
- 50.Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RM, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A 100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heiss S, Hörmann A, Tauer C, Sonnleitner M, Egger E, Grabherr R, Heinl S. 2016. Evaluation of novel inducible promoter/repressor systems for recombinant protein expression in Lactobacillus plantarum. Microb Cell Fact 15:50. doi: 10.1186/s12934-016-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okano K, Yoshida S, Yamada R, Tanaka T, Ogino C, Fukuda H, Kondo A. 2009. Improved production of homo-d-lactic acid via xylose fermentation by introduction of xylose assimilation genes and redirection of the phosphoketolase pathway to the pentose phosphate pathway in l-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75:7858–7861. doi: 10.1128/AEM.01692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Tang M, Viikari L. 2012. Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour Technol 121:8–12. doi: 10.1016/j.biortech.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Fredriksen L, Kleiveland CR, Hult LTO, Lea T, Nygaard CS, Eijsink VGH, Mathiesen G. 2012. Surface display of N-terminally anchored invasin by Lactobacillus plantarum activates NF-kB in monocytes. Appl Environ Microbiol 78:5864–5871. doi: 10.1128/AEM.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peleg Y, Unger T. 2014. DNA cloning and assembly methods, p 73–87. In Valla S, Lale R (ed), Methods in molecular biology. Springer Science+Business Media, New York, NY. [Google Scholar]
- 56.Fredriksen L, Mathiesen G, Sioud M, Eijsink VGH. 2010. Cell wall anchoring of the 37-kilodalton oncofetal antigen by Lactobacillus plantarum for mucosal cancer vaccine delivery. Appl Environ Microbiol 76:7359–7362. doi: 10.1128/AEM.01031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barak Y, Handelsman T, Nakar D, Mechaly A, Lamed R, Shoham Y, Bayer EA. 2005. Matching fusion-protein systems for affinity analysis of two interacting families of proteins: The cohesin-dockerin interaction. J Mol Recognit 18:491–501. doi: 10.1002/jmr.749. [DOI] [PubMed] [Google Scholar]
- 58.Lapidot A, Mechaly A, Shoham Y. 1996. Overexpression and single-step purification of a thermostable xylanase from Bacillus stearothermophilus T-6. J Biotechnol 51:259–264. doi: 10.1016/S0168-1656(96)01604-5. [DOI] [PubMed] [Google Scholar]
- 59.Morag E, Lapidot A, Govorko D, Lamed R, Wilchek M, Bayer EA, Shoham Y. 1995. Expression, purification, and characterization of the cellulose-binding domain of the scaffoldin subunit from the cellulosome of Clostridium thermocellum. Appl Environ Microbiol 61:1980–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem 31:426–428. [Google Scholar]
- 61.Wegkamp A, Teusink B, de Vos WM, Smid EJ. 2010. Development of a minimal growth medium for Lactobacillus plantarum. Lett Appl Microbiol 50:57–64. doi: 10.1111/j.1472-765X.2009.02752.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.