ABSTRACT
Members of the genus Pseudovibrio have been isolated worldwide from a great variety of marine sources as both free-living and host-associated bacteria. So far, the available data depict a group of alphaproteobacteria characterized by a versatile metabolism, which allows them to use a variety of substrates to meet their carbon, nitrogen, sulfur, and phosphorous requirements. Additionally, Pseudovibrio-related bacteria have been shown to proliferate under extreme oligotrophic conditions, tolerate high heavy-metal concentrations, and metabolize potentially toxic compounds. Considering this versatility, it is not surprising that they have been detected from temperate to tropical regions and are often the most abundant isolates obtained from marine invertebrates. Such an association is particularly recurrent with marine sponges and corals, animals that play a key role in benthic marine systems. The data so far available indicate that these bacteria are mainly beneficial to the host, and besides being involved in major nutrient cycles, they could provide the host with both vitamins/cofactors and protection from potential pathogens via the synthesis of antimicrobial secondary metabolites. In fact, the biosynthetic abilities of Pseudovibrio spp. have been emerging in recent years, and both genomic and analytic studies have underlined how these organisms promise novel natural products of biotechnological value.
KEYWORDS: Pseudovibrio, symbiosis, holobiont, microbiome, marine bacteria, antibiotics, biotechnology, biosynthetic gene clusters
INTRODUCTION
The genus Pseudovibrio belongs to the class Alphaproteobacteria, as a member of the family Rhodobacteraceae that includes the highly diverse and environmentally abundant bacteria of the Roseobacter clade. This genus was established 14 years ago with the description of the first species Pseudovibrio denitrificans. The type strain DN34 was isolated from a seawater sample collected in the coastal region of Taiwan. DN34 was described as a Gram-negative motile rod-shaped bacterium capable of growing anaerobically by performing either denitrification or fermentation (1). A few years later, strain WSF2T was isolated from surface seawater off the coastline of Japan, and it was described as belonging to the new species Pseudovibrio japonicus (2). To date, these two are the only type strains isolated from coastal seawater, whereas the remaining four strains were all obtained from marine invertebrates. Pseudovibrio ascidiaceicola strain F423T was isolated in Japan from the ascidian Polycitor proliferus (3); Pseudovibrio axinellae strain AD2T was obtained from the marine sponge Axinella dissimilis collected off the coast of Ireland (4); and finally, Pseudovibrio stylochi strain UST20140214-052T and Pseudovibrio hongkongensis strain UST20140214-015BT were both isolated from marine polyclad flatworms collected in Hong Kong (5, 6).
Although to date only six type strains have been described, in the last 15 years, Pseudovibrio-like bacteria have been detected via cultivation-dependent and -independent methods worldwide, mostly associated with marine invertebrates. Among these, sponges and corals represent the major source of isolation. Due to the recurrent association with marine invertebrates and to their ability to inhibit various medically relevant microbial pathogens, in the last decade, increasing interest has mounted around this genus. However, information on the distribution and physiology of these bacteria is fragmented, as systematic efforts to better understand their biology have been missing so far. Moreover, several findings date back to earlier years when the genus was not yet established, keeping some important features of these bacteria undisclosed. The aim of this review is to provide an overview of the distribution, abundance, and metabolic characteristics of bacteria belonging to the Pseudovibrio genus. Attention will be given to their biosynthetic abilities, which have led to the discovery of new natural products of medical and biotechnological value. The intention is to stimulate research on this genus, as it might be an ideal candidate for both the understanding of some of the mechanisms defining the marine invertebrate holobionts and the discovery of novel bioactive compounds.
The holobiont is the unit formed by the host and its associated microbes (7). Both sponges and corals, the two most common hosts of Pseudovibrio spp., harbor a big diversity of microorganisms and play pivotal roles in the ecology of the seas. Reef-forming corals are crucial for the formation of the physical substrates where coral reefs develop and are important primary producers thanks to their association with photosynthetic microorganisms (8). Coral reefs are fundamental ecosystems for marine life, as their primary production has been estimated to support ∼25% of all marine species (8). Both corals and sponges play essential roles in the biogeochemistry of coral reefs (8, 9). In benthic environments, sponges can occupy >80% of available surfaces, representing the major organisms in term of biomass (7). Found from temperate to polar regions and from shallow to deep waters, sponges are important in maintaining ocean health, as they function as natural “bioreactors” affecting a variety of biogeochemical cycles (9, 10). They link the benthic and pelagic zones by pumping through their bodies thousands of liters of seawater per day, respiring organic matter, and facilitating the recirculation of nutrients. Sponge-associated microorganisms can represent more than a third of the animal body weight, and within them, bacteria can reach the considerable cell density of 109 cells/cm3 of tissue, which is around three orders of magnitude higher than what is observed in surface seawaters (7).
The pivotal role that both sponges and corals play in maintaining ocean health underlines the importance of understanding their holobiont in order to protect the biodiversity and the function of these key ecological players. Several researchers have pointed out that in the near future, the primary emerging frontiers for the marine host-microbiome research will be the understanding of the physiological role fulfilled by bacterial symbionts, the mechanisms used by these bacteria to interact with their hosts, the extent of the coevolution within the holobiont, and the influences of microbiomes on host development and adaptation to a changing environment (7, 11). Therefore, combined efforts embracing both the study of the biology of single associated bacterial groups and the study of the holobiont ecology will be needed. The present review has to be considered an integral component of this framework, as it aims to provide insights into the biology of a bacterial genus recurrently identified within the microbiomes of marine invertebrates.
DISTRIBUTION OF BACTERIA BELONGING TO THE PSEUDOVIBRIO GENUS
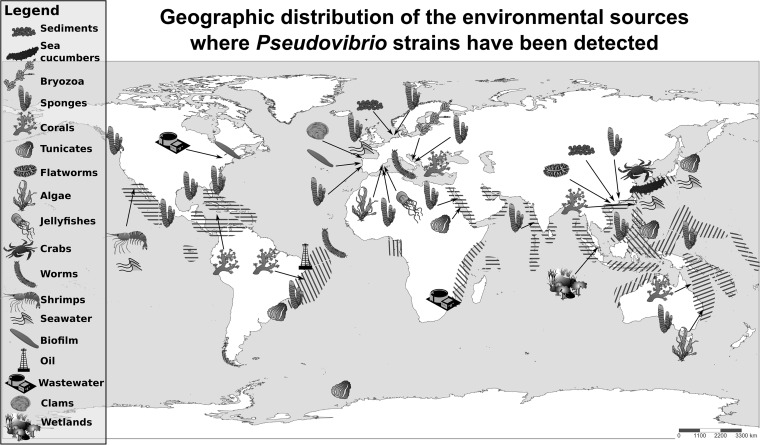
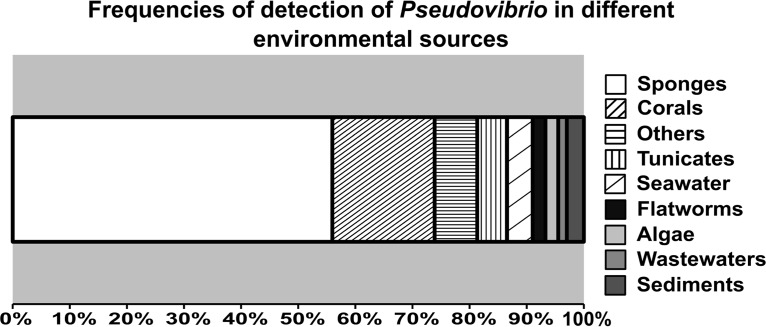
Besides being found recurrently associated with various marine invertebrates, Pseudovibrio strains have been also detected in marine and estuarine sediments, seawater, oil samples, wastewater treatment systems, and wetland mesocosms (Fig. 1 and 2) (1, 2, 12–18). An overview of the sources where Pseudovibrio strains have been detected is shown in Fig. 2. Remarkably, these bacteria have been described to be associated with Rhodophyta and Embryophyta algae (19, 20) and with nine animal phyla, including more than 15 different sponge genera. In fact, in more than half of the 134 studies here considered, Pseudovibrio spp. were described as being associated with sponges (phylum Porifera) (Fig. 2). The phyla Cnidaria, with corals and jellyfish (21–24), and Chordata, with tunicates (25), represent additional recurrent sources of detection (Fig. 2). The remaining six animal phyla are Annelida, such as fire worms (26); Bryozoa (27); Echinoderma, such as sea cucumbers (28); Arthropoda, such as crabs and shrimp (29, 30); and Platyhelminthes and Mollusca, such as flatworms and clams (5, 6, 31). Geographically, these bacteria have a wide distribution ranging from temperate to polar regions, with the majority of the strains obtained from Ireland, Brazil, and Southeast Asia (Fig. 1).
FIG 1.
Schematic representation depicting the marine sources and their respective geographic locations in which Pseudovibrio strains have been identified. The positions of the symbols do not necessarily respect the precise geographic coordinates of the collection sites. Symbols are courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/). Striped areas indicate the approximate locations of coral reefs, added following the data reported by the National Oceanic and Atmospheric Administration (NOAA).
FIG 2.
Overview of the most common sources of detection of Pseudovibrio-like bacteria. One hundred thirty-four studies that employed either cultivation-dependent or -independent methods were reviewed. The category “Others” includes jellyfishes, Bryozoa, shrimp, crabs, clams, sea cucumbers, polychaetes, biofilms, oil wells, and wetlands.
To date, the data about the abundance of these bacteria in the host microbiomes are contradictory. In general, Pseudovibrio strains are either the most abundant or among the most abundant isolates obtained from a great variety of sponges (22, 23). However, some studies have shown that although Pseudovibrio spp. were recurrently isolated from various marine sponges, they were never detected in sequencing data obtained from the host's bacterial communities (32, 33). In contrast, additional culture-independent approaches found Pseudovibrio-like bacteria within the host's microbiomes in various abundances (34–37). Interestingly, in the demosponge Aplysilla rosea, pyrosequencing detected Pseudovibrio as the dominant genus among the host-associated microbes, representing 8% of the total bacterial community (38). Fluorescence in situ hybridization (FISH) data confirmed the presence of Pseudovibrio cells within the demosponges Spongia officinalis, Rhopaloeides odorabile, and Mycale laxissima larvae (39–41). Moreover, studies conducted on the sponge R. odorabile revealed a permanent association in space and time between Pseudovibrio and the host, and the inability to detect these strains in the seawater surrounding the animals suggested that such bacteria might have been a sponge-specific lineage (41).
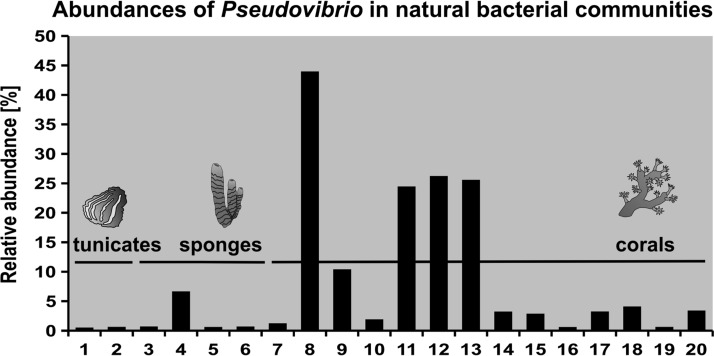
In order to gain an overview of the environmental distribution and abundance of Pseudovibrio spp., here, all available 16S rRNA amplicon data sets from the Sequence Read Archive (SRA; as of 31 December 2017) were interrogated for the presence of sequences assigned to this genus. Forty-nine publicly available Pseudovibrio genome assemblies were annotated as previously described, and 16S rRNA genes were collected and clustered using VSEARCH version 1.1.1 with a 99% identity threshold (42, 43). The 14 representative sequences (centroids) were then submitted to the Integrated Microbial Next Generation Sequencing platform (IMNGS), using a genus threshold of 95% (44). IMNGS systematically screens the SRA for prokaryotic 16S rRNA gene amplicon data sets, processes them, and builds sample-specific sequence databases. After retaining only sequences classified as belonging to the Pseudovibrio genus, 498 data sets containing Pseudovibrio-related sequences were obtained, which were reduced to 318 data sets after removing singletons. Pseudovibrio spp. reached an abundance of ≥0.1% in 48 samples, mainly derived from marine corals, sponges, and ascidians (see Table S1 in the supplemental material). Astonishingly, in four samples, the abundances of Pseudovibrio spp. ranged from 43 to 89% (Table S1). However, according to the SRA descriptions, these data sets did not derive from environmental samples but from enrichment cultures obtained from Mediterranean sponges. Considering only the data obtained from natural bacterial communities, Fig. 3 reports an overview of the data sets where the relative abundance of Pseudovibrio spp. was ≥0.5%. Remarkably, Pseudovibrio was a dominant genus in the coral Stylophora pistillata, reaching an impressive abundance of 44% (SRA accession number SRR1992887) (Fig. 3). The above-mentioned data indicate that the Pseudovibrio genus harbors broad-host-range bacteria, which reach various abundances in the natural bacterial community of marine invertebrates, especially sponges and corals, and in some cases, they represent significant fractions of the host's microbiomes (Fig. 3) (38). Sponge microbiomes are astonishingly diverse, covering more than 50 microbial phyla and thousands of operational taxonomic units (7, 45). On average, the relative abundance of the whole Alphaproteobacteria class varies between 1 and 10% (45). Considering such diversity, finding Pseudovibrio-related sequences within these microbiomes is noteworthy. Likely, the discrepancy between the cultivation-dependent and -independent data derives from the high metabolic versatility, fast growth, and antagonist features of Pseudovibrio strains, which allow them to outcompete other cells during cultivation.
FIG 3.
Abundances of Pseudovibrio-related sequences in all available 16S rRNA amplicon data sets from the Sequence Read Archive (SRA; as of 31 December 2017), interrogated using the Integrated Microbial Next Generation Sequencing (IMNGS) web platform (44). Only samples retrieved from natural bacterial communities in which Pseudovibrio-related sequences reached an abundance of ≥0.5% are shown. The SRA accession numbers of the data sets are as follows: 1, SRR1392309; 2, SRR1393908; 3, SRR651959; 4, SRR768455; 5, SRR2154157; 6, SRR2154188; 7, SRR1992874; 8, SRR1992887; 9, SRR1992892; 10, SRR1992897; 11, SRR1992900; 12, SRR1992926; 13, SRR1992927; 14, SRR2134226; 15, SRR2134228; 16, SRR2134230; 17, SRR2167813; 18, SRR2167815; 19, SRR2167816; and 20, SRR2167822. Symbols are courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/).
BACTERIA BELONGING TO THE PSEUDOVIBRIO GENUS ARE METABOLICALLY VERSATILE AND ABLE TO GROW UNDER VARIOUS NUTRIENT REGIMES
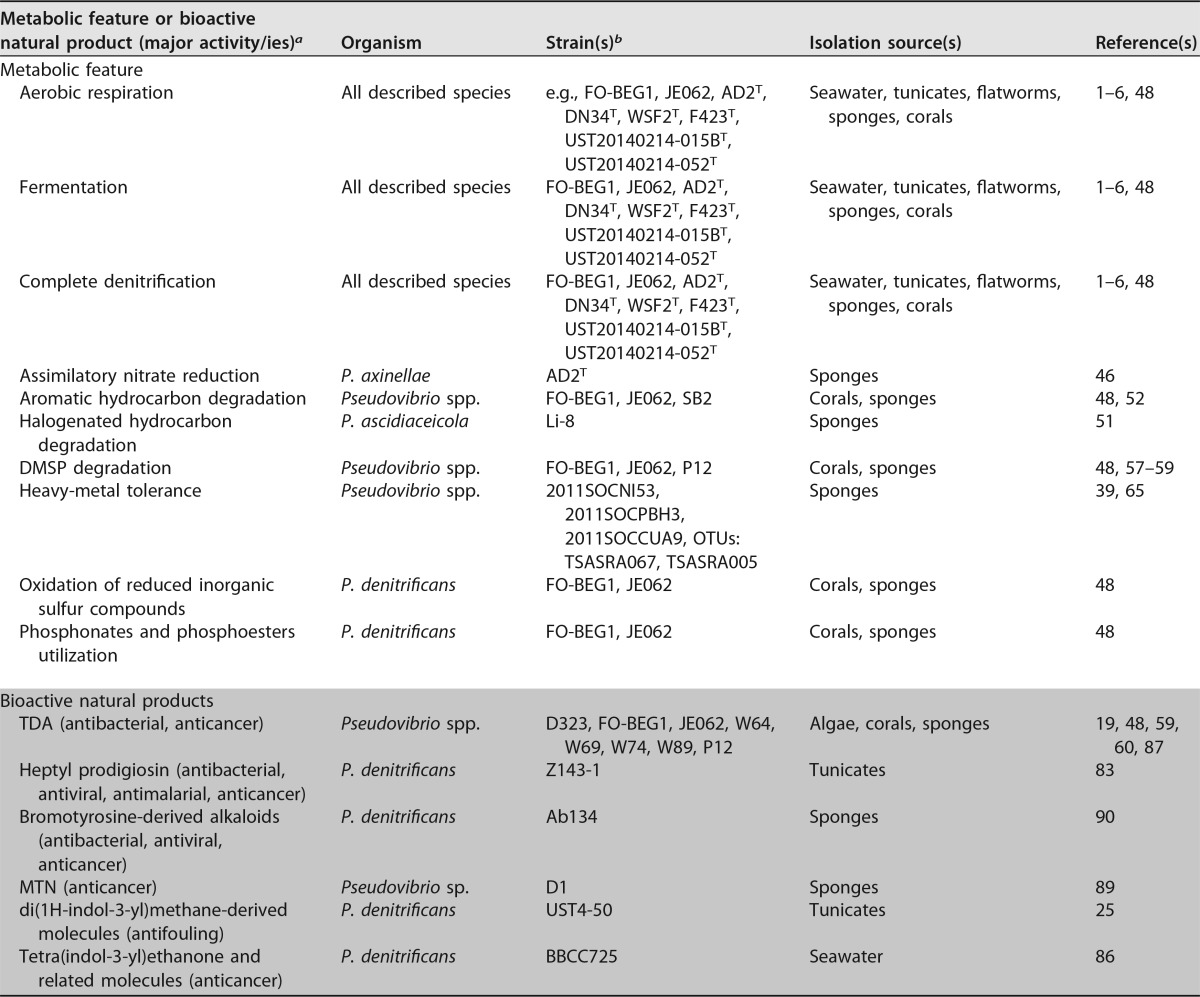
To date, 49 genomes belonging to 44 different strains of Pseudovibrio are publicly available (Table S2A). The most comprehensive comparative genomic analyses performed on subsets of these genomes revealed a high metabolic versatility shared among various Pseudovibrio strains (46, 47). So far, the biggest direct body of evidence concerning the physiology of these bacteria comes from the work of Bondarev and colleagues (48) and from the type strain descriptions. Additionally, various environmental studies indirectly supported some of these findings. These microbes are described as mesophilic, strictly marine (therefore requiring NaCl for growth), and oxidase and catalase positive. The main feature shared among the described strains is their involvement in the nitrogen cycle, as the ability to perform a complete denitrification has been recurrently observed (Table 1). Interestingly, assimilatory nitrate reduction, which allows bacteria to use nitrate as the sole nitrogen source, has been so far demonstrated only for P. axinellae strain AD2T (Table 1) (46). Considering that this bacterium was isolated from the same sponge and location as many other Pseudovibrio spp., it is likely that a certain degree of metabolic differentiation allows multiple strains to occupy different niches and therefore coexist within the host's microbiome.
TABLE 1.
Overview of the metabolic features and bioactive compounds described in the Pseudovibrio genus
The major bioactivities of the compounds isolated from Pseudovibrio cultures are reported, and they refer to the general class of compounds to which the characterized metabolites belong. TDA, tropodithietic acid; MTN, 2-methylthio-1,4-naphthoquinone.
Strain FO-BEG1 was isolated from a Beggiatoa sp. enrichment culture, which originally derived from a black band-diseased scleractinian coral (48). For clarity, this strain will be considered to be of coral origin.
The presence of Pseudovibrio-like bacteria in denitrifying consortia was shown via cultivation-independent methods in both engineered and natural systems (13, 17). A recent metagenomic study that followed the dynamic of denitrifying communities in a chemostat inoculated with coastal sediments showed that Pseudovibrio-like bacteria were among the dominant microbes involved in the nitrogen cycle (13). The analysis of the genomic bins assigned to Pseudovibrio revealed the unusual presence of both NirS- and NirK-encoding genes. These are structural dissimilar nitrite reductases that seldom cooccur in the same organism. It has been suggested that bacteria encoding these enzymes respond differently to environmental conditions and occupy different niches, a scenario hypothesized also for denitrifying bacteria within sponge microbiomes (7, 49). Therefore, finding both genes within the same genus is noteworthy, as it suggests a high degree of specialization of Pseudovibrio subpopulations, which can allow their coexistence during the denitrifying process (13).
The array of organic compounds used as energy and carbon sources by Pseudovibrio spp. is large, including several sugars, amino acids, and short-chain fatty acids, with differences observed among the type strains (1, 3–6, 48). In recent years, additional unexpected physiological traits have been described, with some features being of potential biotechnological interest. For example, a cultivation-independent study detected Pseudovibrio to be among the most abundant groups in marine and estuarine sediments contaminated with organochlorine pesticides and triazole herbicides (12). Similarly, the presence of Pseudovibrio spp. was detected in the cavity fluid of sponges exposed to hexabromobenzene (50). Consistent with these results, a Pseudovibrio strain isolated from the marine demosponge Hymeniacidon perlevis showed a remarkable capacity to use as sole carbon source the organic pollutant lindane (hexachlorocyclohexane; Table 1). This is an organochlorine pesticide of great environmental concern because of its toxicity, persistence, and tendency to bioaccumulate in the ecosystems (51). Information on the pathways used to metabolize this compound is not yet available for Pseudovibrio. However, in several strains of different phylogenetic lineages, genes encoding dehalogenases, which are enzymes involved in the cleavage of the C-halogen bond, are present (e.g., P. denitrificans, NCBI accession no. WP_041768374, in strain FO-BEG1; P. ascidiaceicola, NCBI accession no. KZL18572, in strain WM33; P. axinellae, NCBI accession no. WP_068002989, in strain AD2T; and Pseudovibrio sp., NCBI accession no. WP_093179375 in strain Tun.PSC04-5.I4).
Underlining the metabolic versatility of these bacteria, Bondarev et al. showed that strains FO-BEG1 and JE062, both related to P. denitrificans, were able to aerobically use 4-hydroxybenzoate as the only source of carbon and energy (Table 1) (48). These data are consistent with an early study showing the ability of the sponge surface-associated bacterium SB2, which we now know is related to P. denitrificans, to use the dihydroxylated aromatic compound protocatechuate as the sole source of energy and carbon (Table 1) (52). Evidence suggests that sponges might produce hydroxylated and dihydroxylated aromatic compounds, which could represent ideal growth substrates for Pseudovibrio species (52). Besides aromatic compounds, these microbes are likely able to metabolize various other hydrocarbons. In fact, Pseudovibrio-related strains were identified in an oil sample from a Brazilian onshore basin and were enriched in media containing hexadecane and other hydrocarbon mixes (15, 26, 53). Moreover, polychaete-associated Pseudovibrio spp. produced biosurfactant and were able to grow in media containing oil mixtures, showing an emulsifying capacity (26, 54). Consistent with these ecological and physiological data, the genomes of many strains of different phylogenetic lineages encode enzymes belonging to the alkane/xylene monooxygenase family (IPR033885; e.g., P. denitrificans, NCBI accession no. AEV37994.1, in strain FO-BEG1; P. ascidiaceicola, NCBI accession no. SFK72151.1, in strain DSM 16392T; and P. axinellae, NCBI accession no. WP_068000935, in strain AD2T), which includes enzymes involved in the oxidation and degradation of various hydrocarbons. A better characterization of these pathways and of the degradation efficiency of such compounds, and their halogenated derivative, will help to understand whether these physiological features of Pseudovibrio can be of interest for bioremediation processes.
Additional substrates can be used as sources of energy and carbon by Pseudovibrio species. Bondarev et al. (48) found sox genes in the genomes of both strain FO-BEG1 and JE062. Such findings not only suggest that these strains are able to use inorganic sources as alternative electron donors but also that they are involved in the marine sulfur cycle. The sox genes encode proteins for the oxidation of reduced inorganic sulfur compounds, and physiological experiments showed that both strains could, in fact, oxidize thiosulfate directly to sulfate, boosting aerobic growth (48). The involvement of Pseudovibrio spp. in the marine sulfur cycle is strengthened by their ability to degrade the organic sulfur compound dimethylsulfoniopropionate (DMSP). DMSP is synthesized in great quantity by algae and phytoplankton, with phytoplankton producing more than a billion tons of DMSP a year. This makes DMSP the most abundant reduced sulfur compound in the sea and thus a key molecule in the marine sulfur cycle (55). This compound is found also in corals and coral mucus, where it can reach a concentration orders of magnitude higher than in the surrounding water, indicating the pivotal role of coral reefs in the oceanic DMSP cycle (56). DMSP has been shown to be used by multiple Pseudovibrio strains as a source of energy and carbon (Table 1) (48, 57), and recently, the ability to both assimilate it with other sulfur-containing metabolites produced by the zooxantella Symbiodinium, and use it as sulfur source were shown as well (58, 59).
In addition to the above-mentioned features, recent genomic, proteomic, and physiological data showed the involvement of Pseudovibrio spp. in the marine phosphorus cycle. Genes encoding proteins involved in the metabolism of phosphoesters, molecules containing C-O-P bonds, phosphonates, molecules containing the stable C-P bond, and phosphate are recurrent in the Pseudovibrio genomes and are highly expressed under phosphate limitation (46, 48, 60). Additionally, physiological data showed that Pseudovibrio spp. can use phosphonates as the sole phosphorus source (Table 1) (48). Accessing the dissolved organic phosphorus pool, which is considered one of the biggest phosphorous reservoirs in the ocean (61), would allow Pseudovibrio spp. to survive under phosphate-limiting conditions, which are common in many marine environments. Interestingly, phosphonates can be produced by both phytoplankton and benthic marine invertebrates (61, 62), from which associated Pseudovibrio spp. might recover such compounds.
The metabolic versatility of Pseudovibrio spp. is not only explained by the diversity of substrates used to meet their growth needs, but also by their ability to proliferate under suboptimal growth conditions. In fact, strain FO-BEG1 was able to proliferate in ultraoligotrophic artificial seawater, having a dissolved organic carbon concentration almost an order of magnitude lower than that of natural oligotrophic seawater (63). Ultrahigh-resolution mass spectrometry suggested that bacterial proliferation was likely supported by the consumption of a variety of substrates having chemical features consistent with condensed hydrocarbons, aromatic compounds, aliphatic amines/amides, proteins, and amino sugars/carbohydrates. Most likely, this capability is associated with the high number of encoded ATP-independent periplasmic (TRAP) transporters and ATP-binding cassette (ABC) transporters (46, 48), which allow cells to take up limiting substrates with high affinity. Additionally, strain FO-BEG1 was able to proliferate under unbalanced growth conditions, characterized by a limited amount of phosphorous. Overexpression of genes involved in recovering phosphorous from organic molecules, synthesis of phosphorous-free membrane lipids, and degradation of previously accumulated polyphosphate were all physiological strategies adopted by strain FO-BEG1 to grow under such nutrient restrictions (60). These data indicate that Pseudovibrio strains are well equipped to survive in environments characterized by fluctuating nutrient regimes, such as surface seawater, where, in fact, they have often been detected (1, 2, 14) (Fig. 1 and 2).
Another interesting physiological feature of these microbes is their tolerance to toxic concentrations of heavy metals (Table 1). Among the isolates from the demosponge S. officinalis, three Pseudovibrio spp. were the most resistant strains to copper, lead, and nickel, tolerating a metal concentration up to 25 mM (39). This value is remarkable, as in Escherichia coli, the MIC for copper and nickel is 1 mM, and the MIC for lead is 5 mM (64). Another recent study revealed tolerance to 5 mM arsenic (As) in Pseudovibrio isolates associated with the sponge Theonella swinhoei (65). Arsenic is a highly toxic element, which for this reason has no function as trace metal. In nature, arsenic is mainly found as arsenate, which due to its chemical similarity to phosphate is taken up by bacteria via the phosphate transport systems (64). The tolerance to arsenate is consistent with the presence in several Pseudovibrio genomes of genes encoding the detoxifying enzyme arsenate reductases (e.g., P. denitrificans, NCBI accession no. WP_014286665, in strain FO-BEG1; P. ascidiaceicola, NCBI accession no. WP_063312480, in strain AD26; and Pseudovibrio sp., NCBI accession no. WP_093187096, in strain Tun.PSC04-5.I4). Such tolerances are ideal adaptations for sponge-associated bacteria, since the filtration activity of these animals can lead them to accumulate elements in concentrations potentially toxic for many microorganisms.
The metabolism of metals in Pseudovibrio spp. appears to be intriguing and for certain aspects puzzling. In fact, strain FO-BEG1 drastically influenced the concentrations of cobalt and iron in phosphate-limited media (66). Under such conditions, this strain dissolved the precipitated iron by secreting so-far-uncharacterized chelators. Moreover, phosphate limitation likely induced a significant increase in the cellular uptake of cobalt and iron, a phenomenon not observed in strains belonging to the closely related Roseobacter clade cultivated under the same conditions (66).
Overall, these data underline the metabolic versatility of the Pseudovibrio genus. The ability to live under nutrient restrictions, the high number of encoded high-affinity transporters, and the plethora of substrates used to meet their nutritional demand make these bacteria well adapted to proliferate as free-living organisms in surface seawater and in other environments characterized by fluctuating nutrient regimes. Moreover, their ability to use host metabolites allows them to take advantage of the host-associated lifestyle. The main hosts of Pseudovibrio spp., corals and sponges, are highly abundant in coral reefs (Fig. 1), which are typically confined to oligotrophic waters. The productivity of such ecosystems is mostly dependent on the cycling of nutrients performed by the bacterial communities associated with the dominant invertebrates (7–9). Therefore, the metabolic versatility of this genus, together with the high abundance it can reach in some hosts, underlines its ecological implication in several biogeochemical cycles in these environments, including the carbon, nitrogen, and both the organic and inorganic phosphorus and sulfur cycles.
ASSOCIATION WITH EUKARYOTES AND MECHANISMS USED TO INTERACT WITH THE HOSTS AND THEIR MICROBIOMES
The interaction between Pseudovibrio spp. and their hosts is likely beneficial, or at least neutral, as these bacteria are commonly associated with healthy animals. Only recently, Pseudovibrio strains were isolated from bleached scleractinian corals (67), and it was shown that their abundance increased in corals kept in aquaria and affected by brown jelly syndrome (68). However, from such studies, a relationship of causality between the presence of Pseudovibrio spp. and diseases cannot be inferred. It is important to consider that the quantity and quality of coral mucus change between healthy and diseased or stressed individuals (57, 69). For example, temperature-stressed corals produce a mucus much richer in DMSP (57), a substrate used by Pseudovibrio-related bacteria as energy, carbon, and sulfur sources. Therefore, the association between diseased corals and Pseudovibrio spp. can also be interpreted as an opportunistic strategy, as the metabolic versatility of these bacteria would allow them to take advantage of a changed bacterial community by occupying newly available niches.
In contrast, in the sponge Callyspongia, during the study of the microbiome composition throughout the development of the sponge necrosis syndrome, pyrosequencing revealed that a ribotype affiliated with Pseudovibrio was dominant in healthy tissues, reduced in abundance in moderately healthy tissues, and absent in the diseased parts of the sponge (70). Similarly, pyrosequencing detected Pseudovibrio spp. in two coral species irrespective of animal age and health status (24, 71). Interestingly, several Pseudovibrio isolates strongly inhibited potential sponge pathogens of the genus Bacillus and the coral pathogens Vibrio coralliilyticus and Vibrio owensii (59, 72, 73). These data suggest that Pseudovibrio spp. are not only present in healthy animals, but they are also able to protect the host from potential pathogens via shaping the composition of the host microbiome. Considering the metabolic versatility of Pseudovibrio spp., it is possible that these bacteria are simply commensalistic symbionts of marine invertebrates. However, the facts that Pseudovibrio spp. inhibit coral and sponge pathogens, that they have never been associated with diseased sponges, and that they decrease in abundance in diseased tissues suggests that, as hypothesized by Webster and Hill, Pseudovibrio-like bacteria do not harm their hosts and might even be beneficial to their survival and health (41).
Another two open questions are the location of Pseudovibrio strains within their hosts and their transmission mode through host generations. The majority of bacteria associated with sponges reside in an extracellular matrix known as mesohyl, which forms most of the sponge body, even though intracellular bacteria have been described (7). Similarly, in two different sponge species, Pseudovibrio cells were detected via FISH in the mesohyl of the animals (39, 41). However, in the sponge R. odorabile, experiments directed to separate sponge components from bacteria failed, as Pseudovibrio spp. were always found in fractions containing sponge material. This led to the hypothesis that these strains can reside inside or tightly attached to sponge cells (41). The transmission mode of sponge symbionts is still unclear, and the same is true for Pseudovibrio species. Enticknap and colleagues (40) showed that Pseudovibrio-like bacteria were particularly enriched in the larvae of the sponge M. laxissima, suggesting a direct transmission from the parental line to the progeny (vertical transmission) of these microbes within their hosts (40). This sponge species is viviparous; thus, it cannot be excluded that Pseudovibrio-like bacteria were acquired from the environment (horizontal transmission). In fact, as pointed out by Enticknap et al., considering the impressive filtration rates of sponges, even bacteria present in the seawater at very low abundances could be acquired by the adults and in turn colonize the larvae early in their development (40). An examination of gametes and larvae at different developmental stages would be needed to elucidate the modality of transmission.
One puzzling and fascinating thematic in sponge microbiology is the ability of these animals to discriminate food from symbionts. Feeding experiments performed with the sponge Aplysina aerophoba revealed that food bacteria were taken up to a higher rate than sponge-associated strains (74). In that study, the authors also analyzed the uptake of strain SB89, which was detected to be specifically associated with sponges and which we today know is affiliated with Pseudovibrio species. This strain was retained by the sponge at a rate similar to the rates at which the other food bacteria were retained, leading to the speculation that SB89 might be able to resist phagocytosis and subsequently establish itself within the host (74). Recent data suggest that symbionts may adopt specific mechanisms to manipulate host behavior and avoid digestion (7). In line with this view, all Pseudovibrio genomes analyzed so far encode a great diversity of systems potentially used to interact with the sponge cells, evade defense systems and digestion, and colonize both the host cells and the extracellular space (46–48, 75). From these genomic analyses, three types of systems detected in the majority of Pseudovibrio genomes stood out for their role in the interaction with the hosts: type III and type VI secretion systems (T3SS and T6SS, respectively), toxin-like proteins, and proteins containing eukaryotic-like domains (ELD). T3SS and T6SS are syringe-like structures assembled across the bacterial membranes, and via injecting specific proteins (effectors) into target cells, they induce modifications in either prokaryote or eukaryote behaviors (76). Their effectors have been described in many pathogenic bacteria to interfere with both eukaryotic cellular phagocytosis and immune response, allowing bacteria to evade the defense mechanisms of the host and in turn colonize its cells and tissues (76). The majority of Pseudovibrio genomes encode a large diversity of effector proteins that can potentially target both the host and its microbiome, allowing them to elude the host defenses and outcompete neighboring bacterial cells (46). Similarly, toxin-like proteins have been described to be involved in adhesion and penetration into host cells and fulfill an antagonist role against both prokaryotes and eukaryotes. For example, Rhs- and Rtx-containing proteins together with serralysin peptidases have been shown to be used by symbionts to protect aphids from pathogens, to suppress in vitro phagocytosis, and to allow bacteria to adhere to and cleave extracellular matrix components (77, 78). All these types of toxins are encoded in Pseudovibrio genomes, and proteomic data indicate that some of them are actively synthesized during growth (46, 60). Finally, it has been shown that symbiont proteins containing ELD are enriched in sponge microbiomes and can protect bacteria from phagocytosis (7). Consistent with these data, in all Pseudovibrio genomes, multiple proteins containing ELD are encoded (e.g., P. denitrificans, NCBI accession numbers WP_014285680, AEV37624, and WP_014283455, in strain FO-BEG1; P. ascidiaceicola, NCBI accession numbers KZL16458, KZL16380, and KZL03167, in strain AD26) (47). These data indicate that Pseudovibrio has a big arsenal to successfully interact and colonize its hosts and outcompete bacterial competitors within the host's microbiome.
The benefits Pseudovibrio spp. receive from these associations can be easily envisioned, as they will be exposed to a continuous flux of seawater and nutrients and be able to feed on host metabolites. On the other hand, the benefits the hosts might receive from this association need to be better framed. As will be described below, many Pseudovibrio isolates have strong antimicrobial properties against a great variety Gram-positive and -negative bacteria. The production of antimicrobial compounds greatly improves the antagonistic ability of Pseudovibrio spp., representing a competitive advantage within densely populated communities as those within sponges and corals. On the other hand, the hosts will greatly benefit from such productions, as they will be protected from potential pathogens. The host would also benefit from other biosynthetic abilities of Pseudovibrio spp., as Bondarev et al. showed that strains FO-BEG1 and JE062 encode many vitamin biosynthetic pathways, which allowed them to proliferation in vitamin-free media (48). This leads to the speculation that these strains might provide the hosts with an external supply of those vitamins. Considering these features and the metabolic versatility underlined above, it is likely that more than one type of interaction exists between Pseudovibrio spp. and their hosts. These might range from a permanent symbiosis characterized by vertical transmission to a facultative interaction that relies on the horizontal acquisition of these strains by the hosts. Moreover, the data available so far indicate that such association is neutral or beneficial for the hosts, as to date, there is no direct evidence indicating that these bacteria have a pathogenic lifestyle.
PSEUDOVIBRIO SPP. ARE A PROMISING SOURCE OF NOVEL BIOACTIVE COMPOUNDS
One of the most appealing features of bacteria belonging to the Pseudovibrio genus is their antagonistic properties against a wide variety of microorganisms, including E. coli, Bacillus subtilis, Kluyveromyces marxianus, Salmonella enterica serotype Typhimurium, methicillin-resistant Staphylococcus aureus (MRSA), and Clostridium difficile (22, 23, 79). These properties have been described for Pseudovibrio strains isolated around the world, from different types of sponges, and from different marine invertebrates (19, 72, 80–83). Additional bioactivities have been assigned to these bacteria in the last number of years. For example, crude extracts obtained from cultures of strains SB1 and SB2, isolated from the sponge Suberites domuncula, showed antibacterial, angiogenic, hemolytic, and cytotoxic activities against eukaryotic cell lines (84). Also, isolates from the sponge Aplysina gerardogreeni showed antifouling activity against both bacteria and microalgae (85). Accordingly, eight compounds isolated from an ascidian-associated Pseudovibrio sp. inhibited larval settlement of the barnacle Balanus amphitrite. These molecules showed antifouling performances comparable to those of a commercial biocide but maintained a lower degree of toxicity (25).
So far, the recurrent bioactivity is unmatched by the number of characterized compounds (Table 1). The above-mentioned antifouling molecules derived from di(1H-indol-3-yl)methane (25) and their discovery in Pseudovibrio spp. were noteworthy, as they have never, to our knowledge, been detected in an alphaproteobacterium before. Recently, five additional indole alkaloids were isolated from the seawater strain P. denitrificans BBCC725 (86). Among these, the novel molecule tetra(indol-3-yl)ethanone was described, and it showed high cytotoxicity against mouse fibroblasts and a human adenocarcinoma cell line (86). The antibacterial activity associated with Pseudovibrio strains has often been ascribed to the sulfur-containing metabolite tropodithietic acid (TDA; Table 1) (19, 87). This is a tropolone derivate, which is produced by several bacteria, including members of the Roseobacter clade. There is a strong interest in this compound, because to date, only a few TDA-resistant strains have been found, and it also showed strong anticancer properties (88). Another sulfur-containing secondary metabolite, 2-methylthio-1,4-naphthoquinone (MTN), was isolated from the sponge strain D1 (89). MTN is medically interesting because it caused strong inhibition of both angiogenesis and vertebrate tumor cell proliferation (89).
One of the first compounds isolated from a strain affiliated with P. denitrificans was the red pigment heptyl prodigiosin (Table 1), which showed anti-S. aureus activity (83). Prodigiosin-related compounds are of great interest because they have antibacterial, antifungal, anticancer, antimalarial, and antiviral properties. This discovery was notable because these molecules have never been described in the class Alphaproteobacteria. Surely, one of the most striking findings was the recent identification of multiple bromotyrosine-derived alkaloids in a culture of strain P. denitrificans Ab134, originally isolated from the demosponge Arenosclera brasiliensis (Table 1). These types of compounds showed antibacterial, antiviral, and anticancer properties and were previously isolated only from sponges of the order Verongida and used as phylogenetic markers (90). This discovery is remarkable for two reasons; first, it proved that some of the compounds long considered to be markers for Verongida sponges are instead of bacterial origin; second, it proved for the first time, to our knowledge, that bacteria can produce such a class of compounds, underlining the unrecognized biosynthetic capabilities of Pseudovibrio species. This is particularly relevant because novel antimicrobials are needed more than ever, as the frequency of antimicrobial resistance is steadily increasing and the emergence of “superbugs” resistant to last-resort antibiotics is becoming recurrent.
Further data indirectly underlined the biosynthetic abilities of Pseudovibrio species. For example, Riesenfeld et al. showed that the bacterial community associated with the tunicate Synoicum adareanum, which is the source of the potent antitumoral macrolide palmerolide A, was enriched in Microbulbifer- and Pseudovibrio-related bacteria (91). Since it has been hypothesized that palmerolide A is of bacterial origin, it is likely that the abundant microbes associated with the tunicate could produce such compound. Also, the sponge-derived strain Ac17 showed strong quorum-quenching (QQ) properties (92). QQ is an alternative therapeutic approach to fight multidrug-resistant pathogens, as it disrupts the signal communication that coordinates pathogenic behaviors, thus limiting infections. Finally, a molecular study revealed the presence of the gene phzE in strain AB108, which was closely related to P. ascidiaceicola and isolated from Mediterranean sponges (93). This gene is involved in the production of phenazines, a class of compounds having multiple bioactive properties.
Supporting the widespread experimental bioactivity, recent genomic data showed the diversity of secondary metabolite biosynthetic gene clusters (BGCs) in Pseudovibrio genomes (42, 47, 48, 75). Among others, BGCs for terpene, polyketide synthase (PKS), nonribosomal peptide synthetase (NRPS), bacteriocin, and homoserine lactone were identified. Based on the data of Naughton et al. (42) and on the information found in the Integrated Microbial Genomes portal (94), here it was calculated that in Pseudovibrio spp., 3.2% ± 1.3% of the whole genome and 3.2% ± 1.9% of all genes are dedicated to secondary metabolite synthesis, with some strains devoting ∼5 to 6% of their genomes to such a purpose (Table S2A). These values remain below the biosynthetic ability of the most gifted actinomycetes but are comparable with those of other relevant bioactive molecule producers, such as bacteria belonging to the Bacillaceae, Oxalobacteraceae, and Pseudoalteromonadaceae families (Table S2B) (95–97). Interestingly, even closely related and coisolated Pseudovibrio strains showed variation in their BGCs, indicating both different mechanisms potentially used for the interaction with the host and its microbiome and the diversity of natural products potentially produced (42, 47). Chemical characterization of secondary metabolites is a slow process, but it is important to keep in mind that often the standard procedures used for the cultivation of these strains might limit the expression of their BGCs (98). So far, Pseudovibrio strains have been recalcitrant to genetic manipulation. However, application of the “One strain many compounds” (OSMAC) principle, which is based on the use of multiple growth conditions to induce various secondary metabolite production, could lead to the activation of such BGCs (99). In fact, the proliferation of Pseudovibrio spp. under unbalanced growth conditions has already revealed the impact that nutrient limitations have on antibiotic production and the overall pattern of secreted metabolites (60, 66, 100).
CONCLUDING REMARKS
The picture depicted above indicates that the Pseudovibrio genus harbors bacteria well adapted to a free-living and a host-associated lifestyle. The metabolic versatility and the predisposition for cultivation, the encoded mechanisms potentially used to interact with the hosts and its microbiome, and the worldwide distribution and recurrent association with marine invertebrates are all aspects suggesting that these strains are ideal candidates to deepen our understating of the mechanisms underpinning the interactions within the marine invertebrate holobionts. Overall, the available data support the view of these bacteria as beneficial sponge symbionts, which represent important players in shaping the structure of their host's microbiomes. Pure cultures of such bacteria open the door for the experimental elucidation of those systems that sponges or corals and bacteria use to sense each other. This would help understand the molecular and regulatory processes that the hosts use to discriminate beneficial symbionts from pathogens/food and that the bacteria use to interact with the hosts. Bioactive secondary metabolites are surely one of the mechanisms used by Pseudovibrio spp. to interact with their hosts and their microbiomes, potentially defending the hosts from pathogens. Such features are also extremely appealing from a biotechnological point of view, as Pseudovibrio spp. might produce novel bioactive molecules of medical relevance. The recurrent inhibitory properties showed by these bacteria, together with the biosynthetic capabilities observed at the genomic level, call for a systematic effort directed to elucidate this unexploited biosynthetic potential. Such studies will not only discover novel and valuable bioactive natural products but would also provide insights into the intricate interactions characterizing the chemical ecology of the sponge and coral holobionts.
Supplementary Material
ACKNOWLEDGMENTS
I sincerely acknowledge R. Ansorge for providing feedback on the review. I am grateful to three anonymous reviewers for their constructive comments on the manuscript.
I declare no conflicts of interest.
ADDENDUM IN PROOF
While this paper was in press, Fróes et al. (Front Mar Sci, 2018, https://doi.org/10.3389/fmars.2018.00081) described the genomic features of strain Ab134, which was previously shown to produce bromotyrosine-derived alkaloids. The authors propose the new species Pseudovibrio brasiliensis, and in consistency with previous genomic analyses conducted on other strains, genes encoding T3SS, T6SS, and toxin-like proteins were identified in the Ab134 genome.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02516-17.
REFERENCES
- 1.Shieh WY, Lin YT, Jean WD. 2004. Pseudovibrio denitrificans gen. nov., sp. nov., a marine, facultatively anaerobic, fermentative bacterium capable of denitrification. Int J Syst Evol Microbiol 54:2307–2312. doi: 10.1099/ijs.0.63107-0. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya S, Yokota A. 2007. Pseudovibrio japonicus sp. nov., isolated from coastal seawater in Japan. Int J Syst Evol Microbiol 57:1952–1955. doi: 10.1099/ijs.0.64922-0. [DOI] [PubMed] [Google Scholar]
- 3.Fukunaga Y, Kurahashi M, Tanaka K, Yanagi K, Yokota A, Harayama S. 2006. Pseudovibrio ascidiaceicola sp. nov., isolated from ascidians (sea squirts). Int J Syst Evol Microbiol 56:343–347. doi: 10.1099/ijs.0.63879-0. [DOI] [PubMed] [Google Scholar]
- 4.O'Halloran JA, Barbosa TM, Morrissey JP, Kennedy J, Dobson ADW, O'Gara F. 2013. Pseudovibrio axinellae sp. nov., isolated from an Irish marine sponge. Int J Syst Evol Microbiol 63:141–145. doi: 10.1099/ijs.0.040196-0. [DOI] [PubMed] [Google Scholar]
- 5.Lai Q, Xu Y, Zhang Y, Li Q, Tian R. 2016. Pseudovibrio stylochi sp. nov., isolated from a marine flatworm. Int J Syst Evol Microbiol 66:2025–2029. doi: 10.1099/ijsem.0.000984. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Li Q, Tian R, Lai Q, Zhang Y. 2015. Pseudovibrio hongkongensis sp. nov., isolated from a marine flatworm. Antonie Van Leeuwenhoek 108:127–132. doi: 10.1007/s10482-015-0470-y. [DOI] [PubMed] [Google Scholar]
- 7.Webster NS, Thomas T. 2016. The sponge hologenome. mBio 7:e00135-16. doi: 10.1128/mBio.00135-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowlton N, Jackson JB. 2001. The ecology of coral reefs, p 395–422. In Bertness MD, Gaines SD, Hay ME (ed), Marine community ecology. Sinauer Associates, Sunderland, MA. [Google Scholar]
- 9.de Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM, Admiraal W. 2013. Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110. doi: 10.1126/science.1241981. [DOI] [PubMed] [Google Scholar]
- 10.Bell JJ. 2008. The functional roles of marine sponges. Estuar Coast Shelf Sci 79:341–353. doi: 10.1016/j.ecss.2008.05.002. [DOI] [Google Scholar]
- 11.Thompson JR, Rivera HE, Closek CJ, Medina M. 2015. Microbes in the coral holobiont: partners through evolution, development, and ecological interactions. Front Cell Infect Microbiol 4:176. doi: 10.3389/fcimb.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang H, Cai L, Yang Y, Ju F, Li X, Yu Y, Zhang T. 2014. Metagenomic analysis reveals potential biodegradation pathways of persistent pesticides in freshwater and marine sediments. Sci Total Environ 470-471:983–992. doi: 10.1016/j.scitotenv.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 13.Kraft B, Tegetmeyer HE, Meier D, Geelhoed JS, Strous M. 2014. Rapid succession of uncultured marine bacterial and archaeal populations in a denitrifying continuous culture: rapid succession in a denitrifying chemostat. Environ Microbiol 16:3275–3286. doi: 10.1111/1462-2920.12552. [DOI] [PubMed] [Google Scholar]
- 14.Lim YW, Cuevas DA, Silva GGZ, Aguinaldo K, Dinsdale EA, Haas AF, Hatay M, Sanchez SE, Wegley-Kelly L, Dutilh BE, Harkins TT, Lee CC, Tom W, Sandin SA, Smith JE, Zgliczynski B, Vermeij MJA, Rohwer F, Edwards RA. 2014. Sequencing at sea: challenges and experiences in Ion Torrent PGM sequencing during the 2013 Southern Line Islands Research Expedition. PeerJ 2:e520. doi: 10.7717/peerj.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva TR, Verde LCL, Santos Neto EV, Oliveira VM. 2013. Diversity analyses of microbial communities in petroleum samples from Brazilian oil fields. Int Biodeterior Biodegrad 81:57–70. doi: 10.1016/j.ibiod.2012.05.005. [DOI] [Google Scholar]
- 16.Meli K, Kamika I, Keshri J, Momba MNB. 2016. The impact of zinc oxide nanoparticles on the bacterial microbiome of activated sludge systems. Sci Rep 6:39176. doi: 10.1038/srep39176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auclair J, Parent S, Villemur R. 2012. Functional diversity in the denitrifying biofilm of the methanol-fed marine denitrification system at the Montreal Biodome. Microb Ecol 63:726–735. doi: 10.1007/s00248-011-9960-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Luo J, Lee ZMP, Gersberg RM, Liu Y, Tan SK, Ng WJ. 2016. Characterization of microbial communities in wetland mesocosms receiving caffeine-enriched wastewater. Environ Sci Pollut Res 23:14526–14539. doi: 10.1007/s11356-016-6586-4. [DOI] [PubMed] [Google Scholar]
- 19.Penesyan A, Tebben J, Lee M, Thomas T, Kjelleberg S, Harder T, Egan S. 2011. Identification of the antibacterial compound produced by the marine epiphytic bacterium Pseudovibrio sp. D323 and related sponge-associated bacteria. Mar Drugs 9:1391–1402. doi: 10.3390/md9081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchet E, Prado S, Stien D, Oliveira da Silva J, Ferandin Y, Batailler N, Intertaglia L, Escargueil A, Lami R. 2017. Quorum sensing and quorum quenching in the Mediterranean seagrass Posidonia oceanica microbiota. Front Mar Sci 4:218. doi: 10.3389/fmars.2017.00218. [DOI] [Google Scholar]
- 21.Cortés-Lara S, Urdiain M, Mora-Ruiz M, Prieto L, Rosselló-Móra R. 2015. Prokaryotic microbiota in the digestive cavity of the jellyfish Cotylorhiza tuberculata. Syst Appl Microbiol 38:494–500. doi: 10.1016/j.syapm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Flemer B, Kennedy J, Margassery LM, Morrissey JP, O'Gara F, Dobson ADW. 2012. Diversity and antimicrobial activities of microbes from two Irish marine sponges, Suberites carnosus and Leucosolenia sp: antimicrobial activities of sponge microbes J Appl Microbiol 112:289–301. doi: 10.1111/j.1365-2672.2011.05211.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Halloran JA, Barbosa TM, Morrissey JP, Kennedy J, O' Gara F, Dobson ADW. 2011. Diversity and antimicrobial activity of Pseudovibrio spp. from Irish marine sponges: Pseudovibrio spp. with antimicrobial activity. J Appl Microbiol 110:1495–1508. doi: 10.1111/j.1365-2672.2011.05008.x. [DOI] [PubMed] [Google Scholar]
- 24.Williams AD, Brown BE, Putchim L, Sweet MJ. 2015. Age-related shifts in bacterial diversity in a reef coral. PLoS One 10:e0144902. doi: 10.1371/journal.pone.0144902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K-L, Xu Y, Lu L, Li Y, Han Z, Zhang J, Shao C-L, Wang C-Y, Qian P-Y. 2015. Low-toxicity diindol-3-ylmethanes as potent antifouling compounds. Mar Biotechnol 17:624–632. doi: 10.1007/s10126-015-9656-6. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo C, Michaud L, Hörmann B, Gerçe B, Syldatk C, Hausmann R, De Domenico E, Lo Giudice A. 2013. Bacteria associated with sabellids (Polychaeta: Annelida) as a novel source of surface active compounds. Mar Pollut Bull 70:125–133. doi: 10.1016/j.marpolbul.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Heindl H, Wiese J, Thiel V, Imhoff JF. 2010. Phylogenetic diversity and antimicrobial activities of bryozoan-associated bacteria isolated from Mediterranean and Baltic Sea habitats. Syst Appl Microbiol 33:94–104. doi: 10.1016/j.syapm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Nakahara T, Murase S, Nakata H, Inoue T, Kudo T. 2013. Physiological characterization of aerobic culturable bacteria in the intestine of the sea cucumber Apostichopus japonicus. J Gen Appl Microbiol 59:1–10. doi: 10.2323/jgam.59.1. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Di P, Wang H, Li B, Pan Y, Yan S, Wang Y. 2015. Bacterial community associated with the intestinal tract of Chinese mitten crab (Eriocheir sinensis) farmed in Lake Tai, China. PLoS One 10:e0123990. doi: 10.1371/journal.pone.0123990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas-Albores F, Porchas-Cornejo MA, Martínez-Porchas M, Villalpando-Canchola E, Gollas-Galván T, Martínez-Córdova LR. 2017. Bacterial biota of shrimp intestine is significantly modified by the use of a probiotic mixture: a high throughput sequencing approach. Helgol Mar Res 71:5. doi: 10.1186/s10152-017-0485-z. [DOI] [Google Scholar]
- 31.Dubert J, da Costa F, Aranda-Burgos JA, Martínez-Patiño D, Prado S, Barja JL. 2016. Beneficial effects of carpet shell clam (Ruditapes decussatus) depuration during short periods of conditioning in shellfish hatchery: role of the temperature and phytoplankton on reduction and diversity of vibrios. Aquaculture 459:65–72. doi: 10.1016/j.aquaculture.2016.03.030. [DOI] [Google Scholar]
- 32.Montalvo NF, Davis J, Vicente J, Pittiglio R, Ravel J, Hill RT. 2014. Integration of culture-based and molecular analysis of a complex sponge-associated bacterial community. PLoS One 9:e90517. doi: 10.1371/journal.pone.0090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Versluis D, McPherson K, van Passel MWJ, Smidt H, Sipkema D. 2017. Recovery of previously uncultured bacterial genera from three Mediterranean sponges. Mar Biotechnol 19:454–468. doi: 10.1007/s10126-017-9766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuc NTK, Dat TTH, Hong TT, Cuong PV. 2017. Phylogenetic diversity of microorganisms associated with three marine sponges from Mien Trung sea of Vietnam. J Sci Technol 55:168. [Google Scholar]
- 35.Esteves AIS, Amer N, Nguyen M, Thomas T. 2016. Sample processing impacts the viability and cultivability of the sponge microbiome. Front Microbiol 7:499. doi: 10.3389/fmicb.2016.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lema KA, Willis BL, Bourne DG. 2014. Amplicon pyrosequencing reveals spatial and temporal consistency in diazotroph assemblages of the A. cropora millepora microbiome: diazotroph communities associated with the coral Acropora millepora. Environ Microbiol 16:3345–3359. doi: 10.1111/1462-2920.12366. [DOI] [PubMed] [Google Scholar]
- 37.Rubio-Portillo E, Santos F, Martínez-García M, de los Ríos A, Ascaso C, Souza-Egipsy V, Ramos-Esplá AA, Anton J. 2016. Structure and temporal dynamics of the bacterial communities associated to microhabitats of the coral Oculina patagonica: role of pathogens in the development of coral diseases. Environ Microbiol 18:4564–4578. doi: 10.1111/1462-2920.13548. [DOI] [PubMed] [Google Scholar]
- 38.Mehbub MF, Tanner JE, Barnett SJ, Franco CMM, Zhang W. 2016. The role of sponge-bacteria interactions: the sponge Aplysilla rosea challenged by its associated bacterium Streptomyces ACT-52A in a controlled aquarium system. Appl Microbiol Biotechnol 100:10609–10626. doi: 10.1007/s00253-016-7878-9. [DOI] [PubMed] [Google Scholar]
- 39.Bauvais C, Zirah S, Piette L, Chaspoul F, Domart-Coulon I, Chapon V, Gallice P, Rebuffat S, Pérez T, Bourguet-Kondracki M-L. 2015. Sponging up metals: bacteria associated with the marine sponge Spongia officinalis. Mar Environ Res 104:20–30. doi: 10.1016/j.marenvres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Enticknap JJ, Kelly M, Peraud O, Hill RT. 2006. Characterization of a culturable alphaproteobacterial symbiont common to many marine sponges and evidence for vertical transmission via sponge larvae. Appl Environ Microbiol 72:3724–3732. doi: 10.1128/AEM.72.5.3724-3732.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webster NS, Hill RT. 2001. The culturable microbial community of the Great Barrier Reef sponge Rhopaloeides odorabile is dominated by an α-proteobacterium. Mar Biol 138:843–851. doi: 10.1007/s002270000503. [DOI] [Google Scholar]
- 42.Naughton LM, Romano S, O'Gara F, Dobson ADW. 2017. Identification of secondary metabolite gene clusters in the Pseudovibrio genus reveals encouraging biosynthetic potential toward the production of novel bioactive compounds. Front Microbiol 8:1494. doi: 10.3389/fmicb.2017.01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagkouvardos I, Joseph D, Kapfhammer M, Giritli S, Horn M, Haller D, Clavel T. 2016. IMNGS: a comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci Rep 6:33721. doi: 10.1038/srep33721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas T, Moitinho-Silva L, Lurgi M, Björk JR, Easson C, Astudillo-García C, Olson JB, Erwin PM, López-Legentil S, Luter H, Chaves-Fonnegra A, Costa R, Schupp PJ, Steindler L, Erpenbeck D, Gilbert J, Knight R, Ackermann G, Victor Lopez J, Taylor MW, Thacker RW, Montoya JM, Hentschel U, Webster NS. 2016. Diversity, structure and convergent evolution of the global sponge microbiome. Nat Commun 7:11870. doi: 10.1038/ncomms11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romano S, Fernàndez-Guerra A, Reen FJ, Glöckner FO, Crowley SP, O'Sullivan O, Cotter PD, Adams C, Dobson ADW, O'Gara F. 2016. Comparative genomic analysis reveals a diverse repertoire of genes involved in prokaryote-eukaryote interactions within the Pseudovibrio genus. Front Microbiol 7:387. doi: 10.3389/fmicb.2016.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Versluis D, Nijsse B, Naim MA, Koehorst JJ, Wiese J, Imhoff JF, Schaap PJ, Passel V, J MW, Smidt H, Sipkema D. 2018. Comparative genomics highlights symbiotic capacities and high metabolic flexibility of the marine genus Pseudovibrio. Genome Biol Evol 10:125–142. doi: 10.1093/gbe/evx271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bondarev V, Richter M, Romano S, Piel J, Schwedt A, Schulz-Vogt HN. 2013. The genus Pseudovibrio contains metabolically versatile bacteria adapted for symbiosis: the genus Pseudovibrio. Environ Microbiol 15:2095–2113. doi: 10.1111/1462-2920.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones CM, Hallin S. 2010. Ecological and evolutionary factors underlying global and local assembly of denitrifier communities. ISME J 4:633–641. doi: 10.1038/ismej.2009.152. [DOI] [PubMed] [Google Scholar]
- 50.Seo H-S, Yang S-H, Bae SS, Lee J-H, Kwon KK. 2014. Change of sponge (Axinella sp.)-associated bacterial community during the cultivation with hexabromobenzene. J Mar Biosci Biotechnol 6:76–83. doi: 10.15433/ksmb.2014.6.2.076. [DOI] [Google Scholar]
- 51.Loredana S, Graziano P, Antonio M, Carlotta N, Caterina L, Maria A, Carlo Z, Giuseppe C, Pietro A. 2017. Lindane bioremediation capability of bacteria associated with the demosponge Hymeniacidon perlevis. Mar Drugs 15:108. doi: 10.3390/md15040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller WEG, Grebenjuk VA, Thakur NL, Thakur AN, Batel R, Krasko A, Muller IM, Breter HJ. 2004. Oxygen-controlled bacterial growth in the sponge Suberites domuncula: toward a molecular understanding of the symbiotic relationships between sponge and bacteria. Appl Environ Microbiol 70:2332–2341. doi: 10.1128/AEM.70.4.2332-2341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasumoto-Hirose M, Nishijima M, Ngirchechol MK, Kanoh K, Shizuri Y, Miki W. 2006. Isolation of marine bacteria by in situ culture on media-supplemented polyurethane foam. Mar Biotechnol 8:227–237. doi: 10.1007/s10126-005-5015-3. [DOI] [PubMed] [Google Scholar]
- 54.Rizzo C, Michaud L, Syldatk C, Hausmann R, De Domenico E, Lo Giudice A. 2014. Influence of salinity and temperature on the activity of biosurfactants by polychaete-associated isolates. Environ Sci Pollut Res 21:2988–3004. doi: 10.1007/s11356-013-2259-8. [DOI] [PubMed] [Google Scholar]
- 55.Simó R. 2001. Production of atmospheric sulfur by oceanic plankton: biogeochemical, ecological and evolutionary links. Trends Ecol Evol 16:287–294. doi: 10.1016/S0169-5347(01)02152-8. [DOI] [PubMed] [Google Scholar]
- 56.Van Alstyne KL, Schupp P, Slattery M. 2006. The distribution of dimethylsulfoniopropionate in tropical Pacific coral reef invertebrates. Coral Reefs 25:321–327. doi: 10.1007/s00338-006-0114-9. [DOI] [Google Scholar]
- 57.Garren M, Son K, Raina J-B, Rusconi R, Menolascina F, Shapiro OH, Tout J, Bourne DG, Seymour JR, Stocker R. 2014. A bacterial pathogen uses dimethylsulfoniopropionate as a cue to target heat-stressed corals. ISME J 8:999–1007. doi: 10.1038/ismej.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raina J-B, Clode PL, Cheong S, Bougoure J, Kilburn MR, Reeder A, Forêt S, Stat M, Beltran V, Thomas-Hall P, Tapiolas D, Motti CM, Gong B, Pernice M, Marjo CE, Seymour JR, Willis BL, Bourne DG. 2017. Subcellular tracking reveals the location of dimethylsulfoniopropionate in microalgae and visualises its uptake by marine bacteria. Elife 6:e23008. doi: 10.7554/eLife.23008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raina J-B, Tapiolas D, Motti CA, Foret S, Seemann T, Tebben J, Willis BL, Bourne DG. 2016. Isolation of an antimicrobial compound produced by bacteria associated with reef-building corals. PeerJ 4:e2275. doi: 10.7717/peerj.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romano S, Schulz-Vogt HN, González JM, Bondarev V. 2015. Phosphate limitation induces drastic physiological changes, virulence-related gene expression, and secondary metabolite production in Pseudovibrio sp. strain FO-BEG1. Appl Environ Microbiol 81:3518–3528. doi: 10.1128/AEM.04167-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dyhrman ST, Benitez-Nelson CR, Orchard ED, Haley ST, Pellechia PJ. 2009. A microbial source of phosphonates in oligotrophic marine systems. Nat Geosci 2:696–699. doi: 10.1038/ngeo639. [DOI] [Google Scholar]
- 62.Quin LD. 1965. The presence of compounds with a carbon-phosphorus bond in some marine invertebrates. Biochemistry 4:324–330. doi: 10.1021/bi00878a022. [DOI] [Google Scholar]
- 63.Schwedt A, Seidel M, Dittmar T, Simon M, Bondarev V, Romano S, Lavik G, Schulz-Vogt HN. 2015. Substrate use of Pseudovibrio sp. growing in ultra-oligotrophic seawater. PLoS One 10:e0121675. doi: 10.1371/journal.pone.0121675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nies DH. 1999. Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 65.Keren R, Lavy A, Ilan M. 2016. Increasing the richness of culturable arsenic-tolerant bacteria from Theonella swinhoei by addition of sponge skeleton to the growth medium. Microb Ecol 71:873–886. doi: 10.1007/s00248-015-0726-0. [DOI] [PubMed] [Google Scholar]
- 66.Romano S, Bondarev V, Kölling M, Dittmar T, Schulz-Vogt HN. 2017. Phosphate limitation triggers the dissolution of precipitated iron by the marine bacterium Pseudovibrio sp. FO-BEG1. Front Microbiol 8:364. doi: 10.3389/fmicb.2017.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreira APB, Chimetto Tonon LA, do Valle Pereira PC, Alves N, Amado-Filho GM, Francini-Filho RB, Paranhos R, Thompson FL. 2014. Culturable heterotrophic bacteria associated with healthy and bleached scleractinian Madracis decactis and the fireworm Hermodice carunculata from the remote St. Peter and St. Paul Archipelago, Brazil. Curr Microbiol 68:38–46. doi: 10.1007/s00284-013-0435-1. [DOI] [PubMed] [Google Scholar]
- 68.Sweet MJ, Craggs J, Robson J, Bythell JC. 2013. Assessment of the microbial communities associated with white syndrome and brown jelly syndrome in aquarium corals. J Zoo Aquar Res 1:20–27. [Google Scholar]
- 69.Harvell D, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis B. 2007. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 20:172–195. doi: 10.5670/oceanog.2007.91. [DOI] [Google Scholar]
- 70.Sweet M, Bulling M, Cerrano C. 2015. A novel sponge disease caused by a consortium of micro-organisms. Coral Reefs 34:871–883. doi: 10.1007/s00338-015-1284-0. [DOI] [Google Scholar]
- 71.Chiou S-F, Kuo J, Wong T-Y, Fan T-Y, Tew KS, Liu J-K. 2010. Analysis of the coral associated bacterial community structures in healthy and diseased corals from off-shore of southern Taiwan. J Environ Sci Health Part B 45:408–415. doi: 10.1080/03601231003800032. [DOI] [PubMed] [Google Scholar]
- 72.Esteves AIS, Cullen A, Thomas T. 2017. Competitive interactions between sponge-associated bacteria. FEMS Microbiol Ecol 93:fix008. doi: 10.1093/femsec/fix008. [DOI] [PubMed] [Google Scholar]
- 73.Vizcaino MI, Johnson WR, Kimes NE, Williams K, Torralba M, Nelson KE, Smith GW, Weil E, Moeller PDR, Morris PJ. 2010. Antimicrobial resistance of the coral pathogen Vibrio coralliilyticus and Caribbean sister phylotypes isolated from a diseased octocoral. Microb Ecol 59:646–657. doi: 10.1007/s00248-010-9644-3. [DOI] [PubMed] [Google Scholar]
- 74.Wehrl M, Steinert M, Hentschel U. 2007. Bacterial uptake by the marine sponge Aplysina aerophoba. Microb Ecol 53:355–365. doi: 10.1007/s00248-006-9090-4. [DOI] [PubMed] [Google Scholar]
- 75.Alex A, Antunes A. 2015. Whole genome sequencing of the symbiont Pseudovibrio sp. from the intertidal marine sponge Polymastia penicillus revealed a gene repertoire for host-switching permissive lifestyle. Genome Biol Evol 7:3022–3032. doi: 10.1093/gbe/evv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa TRD, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M, Waksman G. 2015. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol 13:343–359. doi: 10.1038/nrmicro3456. [DOI] [PubMed] [Google Scholar]
- 77.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 78.Linhartova I, Osicka R, Bumba L, Masin J, Sebo P. 2015. RTX toxins: a review, p 1–29. In Gopalakrishnakone P, Stiles B, Alape-Giŕon A, Dubreuil JD, Mandal M (ed), Microbial toxins. Springer Netherlands, Dordrecht, The Netherlands. [Google Scholar]
- 79.Margassery LM, Kennedy J, O'Gara F, Dobson AD, Morrissey JP. 2012. Diversity and antibacterial activity of bacteria isolated from the coastal marine sponges Amphilectus fucorum and Eurypon major: diversity and antibacterial activity of bacteria. Lett Appl Microbiol 55:2–8. doi: 10.1111/j.1472-765X.2012.03256.x. [DOI] [PubMed] [Google Scholar]
- 80.Muscholl-Silberhorn A, Thiel V, Imhoff JF. 2008. Abundance and bioactivity of cultured sponge-associated bacteria from the Mediterranean Sea. Microb Ecol 55:94–106. doi: 10.1007/s00248-007-9255-9. [DOI] [PubMed] [Google Scholar]
- 81.Rypien KL, Ward JR, Azam F. 2010. Antagonistic interactions among coral-associated bacteria. Environ Microbiol 12:28–39. doi: 10.1111/j.1462-2920.2009.02027.x. [DOI] [PubMed] [Google Scholar]
- 82.Santos OCS, Pontes PVML, Santos JFM, Muricy G, Giambiagi-deMarval M, Laport MS. 2010. Isolation, characterization and phylogeny of sponge-associated bacteria with antimicrobial activities from Brazil. Res Microbiol 161:604–612. doi: 10.1016/j.resmic.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 83.Sertan-de Guzman AA, Predicala RZ, Bernardo EB, Neilan BA, Elardo SP, Mangalindan GC, Tasdemir D, Ireland CM, Barraquio WL, Concepcion GP. 2007. Pseudovibrio denitrificans strain Z143-1, a heptylprodigiosin-producing bacterium isolated from a Philippine tunicate. FEMS Microbiol Lett 277:188–196. doi: 10.1111/j.1574-6968.2007.00950.x. [DOI] [PubMed] [Google Scholar]
- 84.Thakur AN, Thakur NL, Indap MM, Pandit RA, Datar VV, Müller WEG. 2005. Antiangiogenic, antimicrobial, and cytotoxic potential of sponge-associated bacteria. Mar Biotechnol 7:245–252. doi: 10.1007/s10126-004-4085-y. [DOI] [PubMed] [Google Scholar]
- 85.Aguila-Ramírez RN, Hernández-Guerrero CJ, González-Acosta B, Id-Daoud G, Hewitt S, Pope J, Hellio C. 2014. Antifouling activity of symbiotic bacteria from sponge Aplysina gerardogreeni. Int Biodeterior Biodegrad 90:64–70. doi: 10.1016/j.ibiod.2014.02.003. [DOI] [Google Scholar]
- 86.Rodrigues AMS, Rohée C, Fabre T, Batailler N, Sautel F, Carletti I, Nogues S, Suzuki MT, Stien D. 2017. Cytotoxic indole alkaloids from Pseudovibrio denitrificans BBCC725. Tetrahedron Lett 58:3172–3173. doi: 10.1016/j.tetlet.2017.07.005. [DOI] [Google Scholar]
- 87.Harrington C, Reen F, Mooij M, Stewart F, Chabot J-B, Guerra A, Glöckner F, Nielsen K, Gram L, Dobson A, Adams C, O'Gara F. 2014. Characterisation of non-autoinducing tropodithietic acid (TDA) production from marine sponge Pseudovibrio species. Mar Drugs 12:5960–5978. doi: 10.3390/md12125960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson MZ, Wang R, Gitai Z, Seyedsayamdost MR. 2016. Mode of action and resistance studies unveil new roles for tropodithietic acid as an anticancer agent and the γ-glutamyl cycle as a proton sink. Proc Natl Acad Sci U S A 113:1630–1635. doi: 10.1073/pnas.1518034113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müller WE, Thakur NL, Ushijima H, Thakur AN, Krasko A, Le Pennec G, Indap MM, Perović-Ottstadt S, Schröder HC, Lang G, Bringmann G. 2004. Matrix-mediated canal formation in primmorphs from the sponge Suberites domuncula involves the expression of a CD36 receptor-ligand system. J Cell Sci 117:2579–2590. doi: 10.1242/jcs.01083. [DOI] [PubMed] [Google Scholar]
- 90.Nicacio KJ, Ióca LP, Fróes AM, Leomil L, Appolinario LR, Thompson CC, Thompson FL, Ferreira AG, Williams DE, Andersen RJ, Eustaquio AS, Berlinck RGS. 2017. Cultures of the marine bacterium Pseudovibrio denitrificans Ab134 produce bromotyrosine-derived alkaloids previously only isolated from marine sponges. J Nat Prod 80:235–240. doi: 10.1021/acs.jnatprod.6b00838. [DOI] [PubMed] [Google Scholar]
- 91.Riesenfeld CS, Murray AE, Baker BJ. 2008. Characterization of the microbial community and polyketide biosynthetic potential in the palmerolide-producing tunicate Synoicum adareanum. J Nat Prod 71:1812–1818. doi: 10.1021/np800287n. [DOI] [PubMed] [Google Scholar]
- 92.Saurav K, Bar-Shalom R, Haber M, Burgsdorf I, Oliviero G, Costantino V, Morgenstern D, Steindler L. 2016. In search of alternative antibiotic drugs: quorum-quenching activity in sponges and their bacterial isolates. Front Microbiol 7:416. doi: 10.3389/fmicb.2016.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneemann I, Wiese J, Kunz AL, Imhoff JF. 2011. Genetic approach for the fast discovery of phenazine producing bacteria. Mar Drugs 9:772–789. doi: 10.3390/md9050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Pillay M, Ratner A, Huang J, Woyke T, Huntemann M, Anderson I, Billis K, Varghese N, Mavromatis K, Pati A, Ivanova NN, Kyrpides NC. 2014. IMG 4 version of the integrated microbial genomes comparative analysis system. Nucleic Acids Res 42:D560–D567. doi: 10.1093/nar/gkt963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chowdhury SP, Hartmann A, Gao X, Borriss R. 2015. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42–a review. Front Microbiol 6:780. doi: 10.3389/fmicb.2015.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harunari E, Komaki H, Ichikawa N, Hosoyama A, Kimura A, Hamada M, Igarashi Y. 2018. Draft genome sequence of Streptomyces hyaluromycini MB-PO13T, a hyaluromycin producer. Stand Genomic Sci 13:2. doi: 10.1186/s40793-017-0286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olsson BE, Korsakova ES, Anan'ina LN, Pyankova AA, Mavrodi OV, Plotnikova EG, Mavrodi DV. 2017. Draft genome sequences of strains Salinicola socius SMB35T, Salinicola sp. MH3R3-1 and Chromohalobacter sp. SMB17 from the Verkhnekamsk potash mining region of Russia. Stand Genomic Sci 12:39. doi: 10.1186/s40793-017-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reen F, Romano S, Dobson A, O'Gara F. 2015. The sound of silence: activating silent biosynthetic gene clusters in marine microorganisms. Mar Drugs 13:4754–4783. doi: 10.3390/md13084754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bode HB, Bethe B, Höfs R, Zeeck A. 2002. Big effects from small changes: possible ways to explore nature's chemical diversity. Chembiochem 3:619. doi:. [DOI] [PubMed] [Google Scholar]
- 100.Romano S, Dittmar T, Bondarev V, Weber RJM, Viant MR, Schulz-Vogt HN. 2014. Exo-metabolome of Pseudovibrio sp. FO-BEG1 analyzed by ultra-high resolution mass spectrometry and the effect of phosphate limitation. PLoS One 9:e96038. doi: 10.1371/journal.pone.0096038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.