Abstract
Background
2–4% of newly diagnosed cases of malignant disease involve cancer of unknown primary (CUP). This mixed entity is one of the 6 most common types of malignant disease in Germany. Highly refined treatment strategies can now be offered to patients with CUP.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed with an emphasis on articles from the past decade. The current guidelines and recommendations of specialty societies were also considered in the evaluation.
Results
CUP most commonly manifests itself as metastases to the lymph nodes, lungs, liver, or bones. With the aid of imaging studies, including functional hybrid imaging and further medical examination, a primary tumor can be discovered in up to 40% of patients initially diagnosed with CUP. Immunohistochemistry guided by histomorphology often enables precise characterization of the lesion and can be supplemented, in selected cases, by molecular-genetic diagnostic evaluation. The most commonly detected types of primary tumor are cancers of the lung, pancreas, liver, and biliary system. For patients with local metastases, surgical resection or radiotherapy with curative intent is usually indicated, sometimes in the framework of a multimodal treatment concept. The median 2-year survival of patients with disseminated CUP is only 20%. For such patients, specific types of systemic therapy are recommended on the basis of the diagnostic characterization of the disease. Immune-modulatory antibodies can be effective, particularly in the treatment of CUP that has been characterized with biomarkers, but should still be considered experimental at present.
Conclusion
A combination of conventional and innovative diagnostic methods enables the provision of highly refined therapeutic strategies to patients with CUP who are undergoing treatment in interdisciplinary cancer centers.
With an incidence of 6–12 cases per 100 000 inhabitants per year, metastatic cancers of unknown primary origin („CUP“) account for approximately 2–4% of all new cancer cases in Germany (1, 2). The cumulative incidence of CUP is thereby almost equal to that of common malignant tumors, such as gastric and pancreatic carcinomas, and is even higher than the single incidences of malignant lymphomas or leukemia.
Despite this quantitative significance, medical progress on CUP syndrome has not experienced the same dynamics as seen for many cancers defined by homogeneous histological criteria. However, with the clinical introduction of high-resolution imaging as well as molecular pathological and molecular genetic diagnostic procedures, the apparent homogeneity of histomorphologically well-defined tumors is now under question. In light of this, a reassessment of the CUP syndrome is also indicated. The aim of this work is to provide a current review of clinically relevant diagnostic algorithms and criteria as well as the resulting therapeutic concepts.
Methods
Based on the clinical and scientific experience of the authors, a selective literature search was performed in PubMed that included reviews, controlled studies, registry studies, and prospective case series, using especially those published in the past ten years. In addition, current guidelines and recommendations of scientific societies were taken into account.
Clinical presentation
The most common manifestations of CUP syndromes are metastases in the lymph nodes, lung, liver, or bone (3). Disseminated metastases are seen in most cases (75–85%). Solitary metastases or metastasis limited to lymph nodes are only observed in 15–25% of cases (3).
Symptoms of CUP syndrome are determined in particular by the respective organ involvement (table 1) and by the extent of metastasis. In addition, diagnosis can be made as a secondary or incidental finding of radiology imaging in largely asymptomatic patients.
Table 1. Affected organs in CUP (%).
| Affected organ | % |
| Lymph nodes | 40–45 |
| Liver | 30–40 |
| Skeleton | 25–35 |
| Lung | 30–40 |
| Pleura | 5–15 |
| Peritoneum | 5–10 |
| Central nervous system | 5–10 |
| Adrenal glands | ˜ 6 |
| Skin | ˜ 4 |
CUP, „cancer of unknown primary“ (modified according to [2] and [3])
For CUP, a comprehensive medical history, an in-depth physical examination, imaging and possibly endoscopic procedures, and the selection of the tumor manifestation suitable for biopsy and histopathological and molecular pathological characterization are critical. As CUP is ultimately an exclusion diagnosis, it is necessary to avoid both too many and too few diagnostics. Evidence-based guidelines from national and international specialist societies provide important support for this (2, 4, 5).
Frequently, the diagnostic algorithm is based on the clinical presentation of metastasis as well as on the histomorphological findings. For example, a patient with axillary lymph node filaments should undergo not only basic imaging diagnostics but also senological diagnostics, comprising ultrasound, mammography, and magnetic resonance imaging (MRI) of the breast (6).
Colonoscopy is recommended for liver metastases, and an ear, nose, and throat medical examination (if necessary, using endoscopy), for cervical lymph node metastases. For younger men, findings of mediastinal and retroperitoneal metastases along the midline or lung metastasis should always be followed by a urological examination of the testes as well as determination of the tumor cell markers typical of germ cell tumors, beta human chorionic gonadotropin (ß-HCG), and alpha-1-fetoprotein (AFP). Prostate cancer should be considered if osseous metastases are determined in older men; a prostate-specific antigen (PSA) diagnosis is justified.
Further targeted examinations may be useful depending on the specific history and histology (3). If this procedure leads to no plausible detection of a primary tumor, further diagnosis should be made with the working hypothesis of the presence of CUP syndrome.
Imaging diagnostics
The basic diagnostic of CUP usually includes contrast-enhanced computed tomography (CT) of the thorax, abdomen, and pelvis and, depending on the clinical manifestation, other affected body regions (7). The introduction of positron emission tomography (PET) using the tracer 2-fluoro-2-deoxy-D-glucose (FDG) determines not only the metabolic activity of lesions but also detects and characterizes lesions that are difficult to define anatomically. Thanks to the availability of hybrid PET/CT devices, a contrast-enhanced CT scan of the entire body with PET can be performed in a single examination and evaluated together.
Meta-analyses show that combined FDG-PET/CT detects a primary tumor in approximately 40% of cases classified clinically as CUP (figure 1), even when evidence for it is lacking based on previously used diagnostic methods (8, 9). However, the cited meta-analyses show high degrees of heterogeneity.
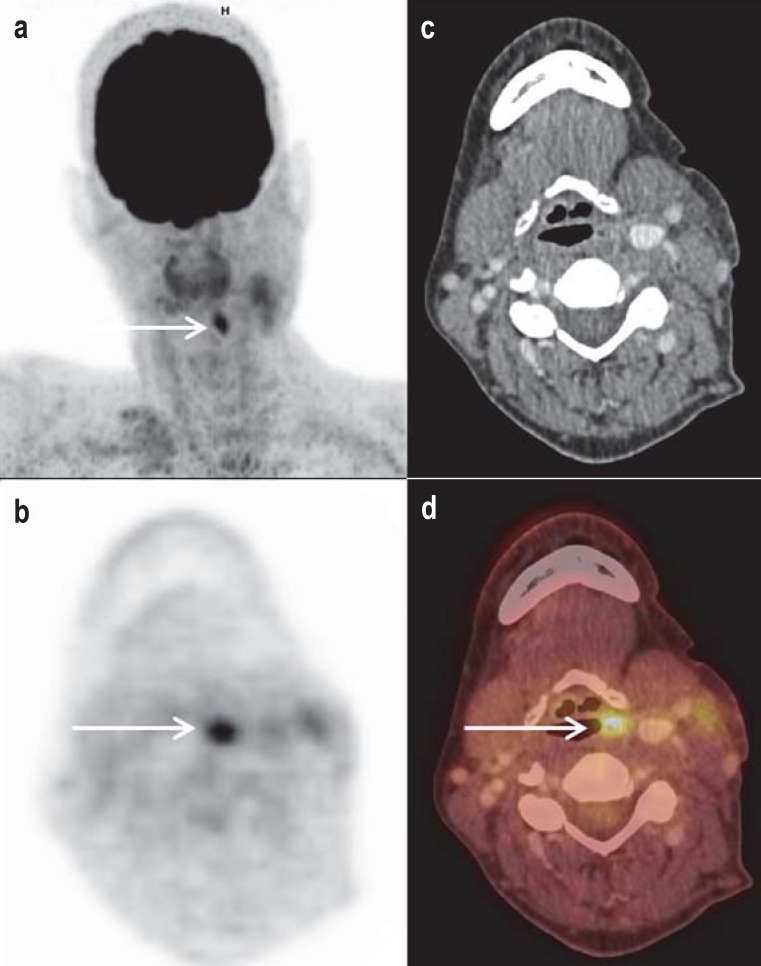
Figure 1.
Detection of cervical lymph node filaments on the left side, and unobtrusive endoscopic and conventional morphological imaging in a 49-year-old female patient. a) The „maximum intensity projection“ (MIP) shows focal FDG uptake in the associated axial sections (b, PET; c, contrast medium CT; d, fused PET/CT) that was assigned to soft tissue asymmetry to the left above the hyoid bone. The primary tumor was then histopathologically confirmed and completely resected.
CT, computed tomography; FDG, 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography
The introduction of hybrid PET and MRI devices may further improve the detection rate. Nonetheless, despite promising initial results, this modality is currently reserved for scientific applications (10).
Based on published studies, evidence-based recommendations for the use of FDG-PET/CT in the cervical region are now available. In March 2017, the German Federal Joint Committee (Gemeinsame Bundesausschuss) determined that using FDG-PET/CT for head and neck tumors and „for CUP syndrome in the head-neck area“ is a reimbursable procedure (11). For CUP syndrome outside the head area, there are currently no evidence-based positive recommendations.
Histology and molecular pathology
Histological and/or cytological examinations are key for further diagnostics and therapy. Additionally, interdisciplinary cooperation and an effective exchange of information about patient history and imaging procedures are also decisive.
In our clinical experience, adenocarcinomas make up the main clinical diagnosis of CUP syndrome (with 40–60%). This also corresponds to data of the guideline of the German Society for Hematology and Oncology (Deutsche Gesellschaft für Hämatologie und Onkologie, DGHO) (2). Of the remaining, undifferentiated carcinomas account for about 15–30%, and squamous cell carcinomas, for about 15–20%. Differentiated neuroendocrine carcinomas, including small cell carcinomas, are relatively rare (around 5%) but are increasing in frequency (2). An indication of differentiated neuroendocrine or small cell carcinoma usually comes from detection of neuroendocrine markers in the serum. Sarcoma CUPs are very rare; in this case, diagnosis relies on molecular markers (translocations).
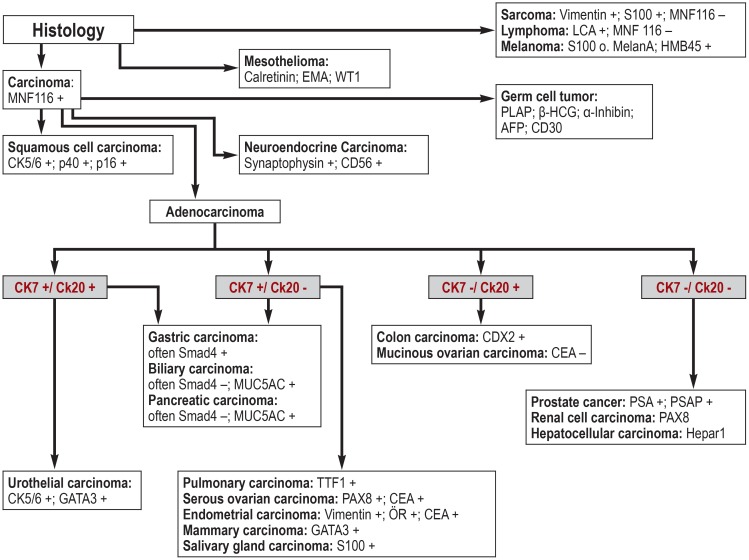
For the mostly moderately to poorly differentiated metastases, immunohistology and/or cytology are very important. In particular, immunohistological examinations enable the presence of malignant lymphomas and neuroendocrine tumors to be detected or excluded. Important antibodies and their algorithmic uses are summarized in Figure 2. The p16 protein can be used as an immunohistochemical marker for squamous cell carcinoma to identify primary tumors in the head/neck region (12).
Figure 2.
Important diagnostic antibodies and their algorithmic use (modified from [13])
(S100, MNF116, HMB45, etc. = antibody names)
If the primary tumor cannot be localized using clinical, radiological, or histological methods, a complex molecular pathology can be used to partially identify the primary tumor. Some publications report a success rate of 98% (14– 17). Molecular tumor profiling (MTP) kits for this are commercially available that use RNA or DNA similarity analyses to identify the primary tumor (15). Additionally, analyses using multiple epigenetic alterations (e.g., the methylation profile of metastases) to identify the primary tumor have recently been published (16, 17).
However, these studies need to be critically analyzed due to a very high clinical pretest probability, as the conventional histological or immunohistological analyses were very indicative in most of the published cases we examined. For instance, the immunohistochemical marker combination of cytokeratins (CK5/6, CK7, and GATA3) can predict the likelihood of having a urothelial carcinoma, which can then be confirmed by gene expression profiles. If so-called CUP chip tests (gene chip analysis) are carried out commercially, the examiner must first receive all clinical information.
General therapy recommendations
If the diagnostic algorithm mentioned above does not allow a tumor to be definitively classified, the CUP diagnosis is maintained. In this case, the following recommendations for action apply: If only a solitary metastasis or the incidence of a single lymph node region is detected, a local radical surgical (18) or radiotherapy can be carried out with curative intent (19). However, more than 75% of cases involve multilocular metastases, which is associated with a less favorable prognosis (3).
In principle, radiotherapy for CUP should be considered for adjuvant, definitive, and palliative therapy intents. Together with surgical and medical approaches, radiotherapy can contribute to organ preservation or curative procedures (19). Radiotherapy can also be an option for palliative care, especially in the following situations:
in metastases in technically inoperable areas, for instance when surgery threatens loss of organ function;
to optimize analgesia, such as in osseous metastasis (19).
For both radiotherapy and surgical treatment options, patients should be divided into prognostic subgroups. Table 2 shows prognostically favorable subgroups for the potentially life-prolonging surgery in CUP manifestations. In addition to only resection, multimodal therapy based on the suspected primary tumor should also be considered in these cases (18, 20).
Table 2. Prognostically favorable subgroups for surgical ?intervention and their survival.
| Subgroups | Survival |
| Peritoneal carcinomatosis of a papillary adenocarcinoma in women |
Median 15–42 months |
| Axillary lymph node metastasis of adenocarcinoma in women |
5 YS 72% |
| Cervical lymph node metastases of squamous cell |
5 YS 40–60% |
| Inguinal lymph node metastases | 5 YS 37.5% |
| Mediastinal / retroperitoneal metastasis along the midline |
Insufficient data |
| Localized, resectable metastases | Insufficient data |
(modified according to [18, 20]); 5 YS: 5-year survival rate
Systemic therapy based on the finding of extensive pathological characterization of the metastases is recommended for widely disseminated CUP syndromes. Larger studies on systemic chemotherapy report a median survival of 8–11 months and a 2-year survival of 20% for disseminated CUP syndrome (21).
Internationally accepted standards of treatment for adenocarcinomas with no indication of enteral origin, and for undifferentiated carcinomas, are combination chemotherapy of a platinum substance (cisplatin, carboplatin) and a taxane, gemcitabine, or irinotecan, or (in the case of contraindication to platinum) platinum-free combination therapies or monotherapies (21). In the case of clinical presentation of adenocarcinoma compatible with a colon tumor, therapy should be analogous to that of metastatic colon carcinoma. Thus, a recommendation grade B is available for fluoropyrimidine-based combination therapy (for example, as part of the FOLFOX or FOLFIRI chemotherapy regimen) (2, 20, 22).
If the histopathological characterization points to a germ cell tumor, a platinum- and etoposide-based therapy with curative intent is indicated (23). Furthermore, neuroendocrine (2–4%) and squamous (5–8%) differentiated CUP syndromes can be distinguished as special histological categories (24, 25).
Neuroendocrine CUP syndromes are treated according to the degree of differentiation. Platinum-based chemotherapy is useful for undifferentiated neuroendocrine CUP syndromes. Differentiated neuroendocrine tumors can express somatostatin receptors. Highly selective somatostatin receptor-specific PET tracers, such as DOTA-TOC and DOTA-TATE, are used in PET/CT centers for tumor detection and primary staging. DOTA-TOC and DOTA-TATE can also be coupled with therapeutic radionuclides and thus represent a promising treatment option in well-differentiated neuroendocrine CUP tumors as peptide receptor radionuclide therapy (PRRT). However, PRRT is only recommended if all detectable tumor manifestations express the somatostatin receptor to a sufficient extent (26).
For differentiated squamous cell CUP syndrome, combination therapy with 5-fluorouracil and cisplatin is the standard, which can be supplemented with radiotherapy in the case of regional spread (24, 25).
Modern therapy concepts and outlook
To increase the probability of primary tumor detection, options are currently being researched that can optimize diagnostics via DNA methylation profiling or molecular tumor profiling (27). This can be followed by a tumor-specific therapy to improve prognosis of patients with clinically-defined CUP syndrome (16, 27). Also, the demonstration of potentially targeted, oncogenic driver mutations offers a possible treatment approach that is independent of the primary tumor (28, 29). However, these strategies are still in the scientific testing stage and are currently characterized by a very heterogeneous response across different histomorphologically defined entities. A German research group is evaluating whether the results of standard therapy with carboplatin/paclitaxel can be improved by supplementing the anti-EGFR antibody cetuximab in a multicenter, randomized phase III study (30). The final study results are pending.
The clinical introduction of monoclonal antibodies directed against immunoregulatory receptors or ligands, such as the so-called checkpoint inhibitors of PD-1, PD-L1 and CTLA-4, for broad-spectrum, drug-based therapy also holds the potential to improve treatment options for patients with CUP syndrome. For some histologically defined entities, immunohistochemical detection of PD-L1 expression on tumor cells or stromal cells may detect patient groups with higher probability to react to therapeutic antibodies directed against PD-1 or PD-L1 (31).
To date, no systematic studies on CUP syndrome have been carried out for this hypothesis. However, an isolated case has been reported in which a refractory, disseminated CUP syndrome with high PD-L1 expression was effectively treated with an immunocheckpoint inhibitor (32).
In this context, the current approval in the USA of the antibody pembrolizumab for the entity-independent treatment of tumor diseases with molecular pathology-assured microsatellite instability (MSI) should also be considered. In a multi-center study, 86 patients with various MSI-positive tumors, including CUP, were treated with the anti-PD-1 antibody. Promising initial results were reported: 53% patients showed objective tumor responses, with complete recovery in 21% of patients (33). Further studies are required for conformation before this therapy, which is solely biomarker-based, can be broadly applied.
Key Messages.
About 2% to 4% of all newly diagnosed cancers are cancers with unknown primary origin („CUP“).
The most common manifestations of CUP syndromes are metastases in the lymph nodes, lung, liver, or bone.
Diagnosis is based on evidence-based guidelines from national and international medical societies and follows rational algorithms. Elaborate methods, such as immunohistochemistry and molecular pathology, are used selectively.
Hybrid imaging techniques (and especially FDG-PET/CT) can locate a primary tumor in a high proportion of clinical CUPs (FDG, 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography; CT, computed tomography).
With close cooperation between clinical practice and modern diagnostics, patients with CUP can be offered differentiated treatment options at interdisciplinary cancer centers, preferably within the framework of scientifically controlled clinical studies.
Acknowledgments
Translated from the original German by Veronica A. Raker.
Footnotes
Conflict of interest statement
Prof. Schuler has received consultant honoraria from Bristol-Meyers Squibb, MSD, AstraZeneca, and Roche, travel expenses and conference fee reimbursement from Bristol-Meyers Squibb, MSD, and AstraZenca, speaking honoraria from Bristol-Meyers Squibb und MSD, and study support (third-party funds) from Bristol-Meyers Squibb.
The remaining authors declare that no conflict of interest exists.
References
- 1.Hübner G, Link H, Kohne CH, et al. Paclitaxel and carboplatin vs gemcitabine and vinorelbine in patients with adeno- or undifferentiated carcinoma of unknown primary: a randomised prospective phase II trial. Br J Cancer. 2009;100:44–49. doi: 10.1038/sj.bjc.6604818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie (DGHO) (eds.) CUP Syndrom - Krebserkrankungen mit unbekanntem Primärtumor. Leitlinie: Stand 2014. www.onkopedia.com/de/onkopedia/guidelines/cup-syndrom-krebserkrankungen-mit-unbekanntem-primaertumor/@@view/html/index.html#ID0EVKAE (last accessed on 26 January 2018) [Google Scholar]
- 3.Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428–1435. doi: 10.1016/S0140-6736(11)61178-1. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN) NCCN Guidelines for occult primary. www.nccn.org/professionals/physician_gls/f_guidelines. asp#occult(last accessed on 30 June 2017) [Google Scholar]
- 5.European Society for Medical Oncology (ESMO) Cancers of unknown primary site: ESMO clinical practice guidelines. www.esmo.org/Guidelines/Cancers-of-Unknown-Primary-Site/Cancers-of-Unknown-Primary-Site (last accessed on 26 January 2018 [Google Scholar]
- 6.Pentheroudakis G, Lazaridis G, Pavlidis N. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): a systematic review of published evidence. Breast Cancer Res Treat. 2010;119:1–11. doi: 10.1007/s10549-009-0554-3. [DOI] [PubMed] [Google Scholar]
- 7.Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371:757–765. doi: 10.1056/NEJMra1303917. [DOI] [PubMed] [Google Scholar]
- 8.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol. 2009;19:731–744. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burglin SA, Hess S, Høilund-Carlsen PF, Gerke O. 18F-FDG PET/CT for detection of the primary tumor in adults with extracervical metastases from cancer of unknown primary: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000006713. e6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhlmann V, Ruhlmann M, Bellendorf A, et al. Hybrid imaging for detection of carcinoma of unknown primary: a preliminary comparison trial of whole-body PET/MRI versus PET/CT. Eur J Radiol. 2016;85:1941–1947. doi: 10.1016/j.ejrad.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Gemeinsamer Bundesausschuss PET/CT bei Kopf-Hals-Tumoren. Einsatz zur Vermeidung invasiver Eingriffe wird Kassenleistung. Pressemitteilung Berlin, 16. März 2017. www.g-ba.de/institution/presse/pressemitteilungen/672/ (last accessed on 26 January 2018) [Google Scholar]
- 12.Vent J, Haidle B, Wedemeyer I, et al. p16 expression in carcinoma of unknown primary: diagnostic indicator and prognostic marker. Head Neck. 2013;35:1521–1526. doi: 10.1002/hed.23190. [DOI] [PubMed] [Google Scholar]
- 13.Munding J, Tannapfel A. Pathologie des CUP-Syndroms. Onkologe. 2013,;19:15–21. [Google Scholar]
- 14.Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14:634–642. doi: 10.1007/s11864-013-0257-1. [DOI] [PubMed] [Google Scholar]
- 15.Greco FA, Lennington WJ, Spigel DR, Hainsworth JD. Molecular profiling diagnosis in unknown primary cancer: accuracy and ability to complement standard pathology. J Natl Cancer Inst. 2013;105:782–790. doi: 10.1093/jnci/djt099. [DOI] [PubMed] [Google Scholar]
- 16.Moran S, Martínez-Cardús A, Sayols S, et al. Epigenetic profiling to classify cancer of unknown primary: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:1386–1395. doi: 10.1016/S1470-2045(16)30297-2. [DOI] [PubMed] [Google Scholar]
- 17.Moran S, Martinez-Cardús A, Boussios S, Esteller M. Precision medicine based on epigenomics: the paradigm of carcinoma of unknown primary. Nat Rev Clin Oncol. 2017;14:682–694. doi: 10.1038/nrclinonc.2017.97. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt T, Ulrich A. [Surgical options in cancer of unknown primary (CUP)] Radiologe. 2014;54:140–144. doi: 10.1007/s00117-013-2549-7. [DOI] [PubMed] [Google Scholar]
- 19.Krug D, Debus J, Sterzing F. [Radiotherapeutic concepts in cancer of unknown primary site] Radiologe. 2014;54:145–151. doi: 10.1007/s00117-013-2550-1. [DOI] [PubMed] [Google Scholar]
- 20.Krämer A, Löffler H. Cancer of unknown primary Cham, Schweiz. Springer International Publishing. 2016:47–48. [Google Scholar]
- 21.Hainsworth JD, Spigel DR, Clark BL, et al. Paclitaxel/carboplatin/etoposide versus gemcitabine/irinotecan in the first-line treatment of patients with carcinoma of unknown primary site: a randomized, phase III Sarah Cannon Oncology Research Consortium Trial. Cancer J. 2010;16:70–75. doi: 10.1097/PPO.0b013e3181c6aa89. [DOI] [PubMed] [Google Scholar]
- 22.Greco FA, Lennington WJ, Spigel DR, Varadhachary GR, Hainsworth JD. Carcinoma of unknown primary site (CUP): outcomes in patients with a colorectal molecular profile treated with site-specific chemotherapy. J Clin Oncol. 2011;29 Abstract 3563. [Google Scholar]
- 23.Richardson RL, Schoumacher RA, Fer MF, et al. The unrecognized extragonadal germ cell cancer syndrome. Ann Intern Med. 1981;94:181–186. doi: 10.7326/0003-4819-94-2-181. [DOI] [PubMed] [Google Scholar]
- 24.Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary—a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 2014;111:481–487. doi: 10.3238/arztebl.2014.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neben K, Hübner G, Folprecht G, Jäger D, Krämer A. Metastases in the absence of a primary tumor: advances in the diagnosis and treatment of CUP syndrome. Dtsch Arztebl Int. 2008;105:733–740. doi: 10.3238/arztebl.2008.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poeppel TD, Boy C, Bockisch A, et al. Peptide receptor radionuclide therapy for patients with somatostatin receptor expressing tumours German Guideline (S1) Nuklearmedizin. 2015;54:1–11. [PubMed] [Google Scholar]
- 27.Hainsworth JD, Rubin MS, Spigel DR, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon Research Institute. J Clin Oncol. 2013;31:217–223. doi: 10.1200/JCO.2012.43.3755. [DOI] [PubMed] [Google Scholar]
- 28.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massard C, Michiels S, Ferté C. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 2017;7:586–595. doi: 10.1158/2159-8290.CD-16-1396. [DOI] [PubMed] [Google Scholar]
- 30.Krämer A, Hübner G, Schneeweiss A, Folprecht G, Neben K. Carcinoma of unknown primary—an orphan disease? Breast Care. 2008;3:164–170. doi: 10.1159/000136001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 32.Gröschel S, Bommer M, Hutter B, et al. Integration of genomics and histology revises diagnosis and enables effective therapy of refractory cancer of unknown primary with PDL1 amplification. Cold Spring Harb Mol Case Stud. 2016;2 doi: 10.1101/mcs.a001180. a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le DT, Durham JN, Smith KN, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]