Abstract
Summary
We conducted the first comparison of dual-energy X-ray absorptiometry (DXA) and peripheral quantitative computed tomography (pQCT) outcomes in adolescent girls with anorexia nervosa. We observed deficits in bone density by both tools. pQCT assessments were associated with many of the same clinical parameters as have been previously established for DXA.
Introduction
Adolescents with anorexia nervosa (AN) commonly exhibit bone loss, but effects on bone geometry are less clear. We compared measures obtained by DXA and pQCT in girls with AN.
Methods
Seventy females (age 15.5 ± 1.9 years ) with AN and 132 normal-weighted controls underwent tibial measures by pQCT including trabecular volumetric bone mineral density (vBMD) at the 3 % site, cortical vBMD and dimensions at the 38 % site, and muscle cross-sectional area (CSA) at the 66 % site. Participants with AN also underwent standard DXA measures. Independent t tests compared the pQCT results, while Pearson coefficient assessed correlations among DXA and pQCT measures.
Results
Trabecular vBMD Z-scores were lower in AN compared to controls (AN −0.31 ± 1.42 vs +0.11 ± 1.01, p = 0.01) and cortical vBMD Z-scores were higher (AN +0.18 ± 0.92 vs −0.50 ± 0.88, p < 0.001). Trabecular vBMD and cortical CSA Z-scores positively correlated with DXA BMD Z-scores (r range 0.57–0.82, p < 0.001). Markers of nutritional status positively correlated with Z-scores for trabecular vBMD, cortical CSA, section modulus, and muscle CSA (p < 0.04 for all).
Conclusions
This study is the first to compare DXA and pQCT measurements in adolescent girls with AN. We observed deficits in BMD by both DXA and pQCT. pQCT assessments correlated well with DXA bone and body composition measures and were associated with many of the same clinical parameters and disease severity markers as have been previously established for DXA. The differences in cortical vBMD merit further study.
Keywords: Anorexia nervosa, DXA, Malnutrition, Peripheral quantitative computed tomography
Introduction
Anorexia nervosa (AN), a disorder characterized by energy restriction, intense fear of weight gain, and disturbance of body weight perception, is becoming increasingly prevalent [1]. Disease onset often occurs during adolescence, when bone mineral accretion rates should be at their highest. Early bone loss of the axial skeleton is a frequent complication of AN, occurring in over half of patients [2]. The pathogenesis of bone loss in adolescents with AN is characterized by impaired bone formation and accelerated bone resorption [3–5] resulting from a combination of hypogonadism, hypercortisolism, low body mass, poor nutrition, and changes in bone marrow composition. Previous studies using dual-energy X-ray absorptiometry (DXA) have shown a significant reduction in bone density compared with age-matched controls, with the lumbar spine (a site consisting primarily of trabecular bone) appearing to be particularly vulnerable [6, 7]. Bone mineral density (BMD) in adolescents with AN is correlated with body mass index (BMI), age at onset of illness and of menarche, lean body mass, and duration of illness [3, 7–11].
The implications for life-long skeletal health are substantial; patients with AN have a sevenfold increased incidence of fractures, which may occur at multiple sites [12]. DXA has been the most widely used tool in clinical practice for the assessment of bone mass. DXA measures bone in two dimensions and allows for calculation of areal BMD (g/cm2). However, bone strength depends on skeletal properties such as geometry, bone material properties, and internal architecture which are not reflected directly in DXA measurements. Additionally, cortical and trabecular bone mineral content (BMC) are superimposed with DXA, which may conceal distinct disease and treatment effects on the separate components of BMC.
Peripheral QCT (pQCT) allows for direct selective measurement of trabecular and cortical volumetric BMD (vBMD) and additional cross-sectional geometry measures that are highly correlated with fracture load [13]. We previously reported deficits in estimates of cortical bone geometry and strength measured at the hip in adolescents with AN [14, 15]. As measured by high-resolution pQCT, cortical area and thickness at the distal radius were lower in girls with AN than control subjects [16]. In children with nephrotic syndrome, use of pQCT highlights the potential discrepancy between DXA and pQCT measures [17]. By DXA, areal BMD was lower than the reference group. However, these children had significantly lower mean pQCT trabecular vBMD Z-scores and significantly greater cortical vBMD Z-scores than the reference population [17]. Similar discrepancies between DXA and pQCT have been seen in studies of pediatric patients with Crohn disease and cystic fibrosis [18, 19].
To our knowledge, no studies have concurrently compared measures by DXA and pQCT in adolescents with AN at a weight-bearing site. The current study thus aimed to compare measurements from DXA with tibial measures by pQCT in a cohort of adolescent girls with AN. We also examined the clinical correlates with skeletal measures obtained by each technique and the associations of pQCT with muscle density. We enrolled patients over a broader degree of illness presentation than has occurred in some earlier reports, with the aim being to illustrate the spectrum of disease seen by clinicians and examine the skeletal implications of this range.
Materials and methods
Participant selection
Between January 2012 and January 2015, female adolescents (ages 11–20 years) were recruited from two urban hospital-based eating disorders programs for participation in a longitudinal clinical trial (Clinicaltrials.gov, NCT01343771). Eligible patients (n = 70) were postmenarchal or had a bone age ≥13 years and met DSM-V diagnostic criteria for AN including persistent restriction of energy intake leading to significantly low body weight, disturbance in the way one’s body weight/shape is experienced, and intense fear of weight gain. Subjects were excluded if they had other medical conditions known to affect bone health (such as celiac disease or cystic fibrosis) or if they were receiving medications known to affect the skeleton (such as oral contraceptives). All presented data were obtained at the baseline visit of the randomized trial.
Control subjects were recruited from general adolescent medicine clinics at the same two hospital sites: Boston (n = 74) and Providence (n = 58). All control subjects were normal-weighted, free of concomitant chronic diseases which affect bone health, and did not regularly use medications (with the exception of vitamins or supplements) over the year prior to study enrollment. We compared data from participants with AN to these “local” controls to avoid any confounding effects related to the regional differences between our local population and the reference population.
The local institutional review boards approved the study protocol. Written informed consent was obtained from study patients or their parent/guardian. Minor subjects also provided assent for participation.
Skeletal assessments
In subjects with AN only, DXA (Discovery A, Hologic, Inc., Bedford, MA) measurements were obtained for the total body excluding the head (TBLH), posteroanterior lumbar spine (L1–L4), and total hip on the same model scanner in Boston and Providence. Skeletal outcomes included BMC (g) and areal BMD (g/cm2). Scans of the phantom were performed daily. The CV was <1.0 %. Image analysis was performed using QDR for Windows XP 12.4 software (Hologic, Inc.).
In both participants with AN and healthy controls, measurements through serial cross sections of the left tibia were obtained by pQCT using a Stratec XCT 3000 pQCT device (Orthometrix, White Plains, NY) with a 12-detector unit, voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 20 mm/s. Scans were obtained using established measures. A scout view was first obtained to place a reference line at the proximal border of the fused distal tibia growth plate. The location of measurements was determined using percentages of the tibia length proximal to this reference line to avoid issues related to height. At the 3 % metaphyseal site, scans were analyzed for trabecular vBMD (g/cm3). At the 38 % meta-diaphysis site, scans were analyzed for cortical vBMD, cortical BMC (g), cortical cross-sectional area (CSA, mm2), periosteal circumference (mm), endosteal circumference (mm), and polar section modulus (Zp, mm3). Calf muscle and fat CSA (mm2) were assessed at the 66 % diaphyseal site. As previously described [20, 21], pQCT measure of muscle density (g/cm3) was also obtained. Scans were analyzed using Stratec software, version 6.20. The manufacturer’s phantom was scanned daily for quality assurance. Average coefficient of variation (CV) ranged from 0.2 to 0.6 %.
Biochemical assessments
Serum 25-hydroxyvitamin D [25(OH)D] concentration was measured by liquid chromatography tandem mass spectrometry [22] with local interassay precision of 7.3 %. Biomarkers of bone formation [serum levels of osteocalcin (OC) and bone-specific alkaline phosphatase (BSAP)] and bone resorption [serum C-telopeptides (CTx)] were also measured. OC was measured by ELISA (ALPCO Diagnostics, Salem, NH; %CV 5.0–6.5 %); CTx was measured by immunoradiometric assay (Immunodiagnostic Systems, Tyne and Wear, UK; %CV 5.2–6.8 %); and BSAP was measured by access chemiluminescent immunoassay (Beckman Coulter, Brea, CA; %CV 1.5–2.6 %). Samples were batched to minimize chance of batch effects.
Additional study measurements
Anthropometrics were obtained in all participants. Height (cm) was measured using standardized procedure with a wall-mounted stadiometer. Weight (kg) was measured in the morning, postvoiding, with subjects wearing a hospital gown. BMI was calculated using the following formula: BMI = weight (kg) / [height (m)]2. The Hamwi method for women was used to calculated percentage ideal body weight (% IBW) = 100 lbs. for 60 in. tall, and 5 lbs./in. for each inch over 5 ft [23, 24]. Body composition measures [total body lean mass (kg), total body fat mass (kg), percentage body fat] were obtained by DXA in all participants with AN. All participants responded to a structured interview to obtain demographic information and health history, which included information about menstrual history, smoking status, personal history of fracture, and family history of osteoporosis.
Statistical analysis
DXA and pQCT results were converted to gender- and race-specific (black vs nonblack) Z-scores relative to age using the LMS method (LMS Chartmaker version 2.3) [25]. The LMS method accounts for the nonlinearity, heteroscedasticity, and skew of bone data with age. The DXA BMD data in the participants with AN were converted to Z-scores using the LMS curves generated by the multicenter Bone Mineral Density in Childhood Study (BMDCS) using Hologic scanners [26]. Results were adjusted for height Z-scores to account for the correlations between height and bone measures. The pQCT bone, muscle, and fat results were converted to Z-scores using LMS curves generated in over 650 healthy children, adolescents, and young adults at a single center, as previously described [27, 28].
We used standard descriptive statistics to characterize the distribution of bone measures and clinical variables in AN and control participants. We used the independent t test to compare the AN and control distribution of pQCT measures, which were obtained in both groups. The Pearson coefficient was used to assess correlation between DXA and pQCT measures in the AN participants, in whom both scans were performed, and to assess the association of clinical markers with both DXA and pQCT measures. To compare two Pearson correlations involving a common variable, we used the method of Zou [29].
We employed complete-case analysis, as under 0.3 % of pQCT and DXA values were missing or discarded because of motion artifact. Correlations that were significantly different from zero with p < 0.05 were taken as statistically significant. No adjustment of type I error rate was made for multiple comparisons, as the Bonferroni correction is unduly conservative since it assumes independence of measures, and our measurements are highly correlated. SAS software (version 9.4, Cary, NC) was used for all computations.
Results
The adolescents with AN (n = 70) and healthy control subjects (n = 132) were similar in terms of age and height (Table 1). As expected due to the nature of their illness, adolescents with AN had a lower BMI and percentage ideal body weight (Table 1). The distribution of weight in the control subjects was normal. Race was self-reported by all subjects (Table 2). The racial and ethnic distribution of the control subjects matched across recruitment sites. Given differences in race and ethnicity between subjects with AN and the 132 healthy control subjects, we created a group of n = 40 adolescents matched for race and ethnicity (Tables 1 and 2). Approximately 1/3 of the girls with AN reported a history of fracture, while 24 % had a known family history of osteoporosis. Among controls, the prevalence of fractures was similar; 38 (29 %) reported a history of fracture. A family history of osteoporosis was significantly lower among controls, reported in 13 (10 %) (p = 0.01).
Table 1.
Measured characteristics of 70 adolescent girls with anorexia nervosa, compared with 132 female control subjects and a subsample of 40 control subjects matched for race and ethnicity
| Measure | Group | Mean ± SD | Median (Q1, Q3) | Minimum, maximum | p valuea |
|---|---|---|---|---|---|
| Age (years) | AN | 15.5 ± 1.9 | 15.6 (14.1, 16.9) | 11.5, 18.9 | – |
| Control | 16.9 ± 2.3 | 17.1 (15.1, 18.9) | 11.0, 20.8 | <0.0001 | |
| Matched control | 17.2 ± 2.4 | 17.9 (15.5, 19.3) | 11.0, 20.8 | 0.0002 | |
| Height (cm) | AN | 160.5 ± 7.9 | 159.2 (154.9, 166.8) | 142.0, 178.0 | – |
| Control | 161.9 ± 7.3 | 162.6 (156.5, 166.4) | 134.7, 178.4 | 0.28 | |
| Matched control | 164.7 ± 8.6 | 166.0 (161.6, 168.9) | 134.7, 178.4 | 0.009 | |
| Weight (kg) | AN | 48.4 ± 6.5 | 49.0 (45.3, 52.8) | 25.9, 59.4 | – |
| Control | 59.4 ± 9.9 | 58.2 (52.5, 67.5) | 30.8, 81.4 | <0.0001 | |
| Matched control | 58.6 ± 9.8 | 58.1 (50.3, 66.7) | 31.8, 76.6 | <0.0001 | |
| BMI (kg/m2) | AN | 18.7 ± 1.7 | 18.9 (17.9, 19.8) | 12.8, 22.4 | – |
| Control | 22.6 ± 3.1 | 22.2 (20.3, 24.8) | 15.6, 29.7 | <0.0001 | |
| Matched control | 21.5 ± 2.4 | 21.4 (19.5, 23.9) | 17.1, 24.9 | <0.0001 | |
| BMI Z-score | AN | −0.60 ± 0.79 | −0.52 (−0.93, −0.09) | −3.13, 0.82 | – |
| Control | 0.41 ± 0.74 | 0.49 (−0.10, 0.99) | −1.52, 2.01 | <0.0001 | |
| Matched control | 0.11 ± 0.69 | 0.12 (−0.47, 0.68) | −1.52, 1.13 | <0.0001 | |
| % IBW | AN | 91.0 ± 9.4 | 91.4 (85.1, 97.4) | 56.9, 111.0 | – |
| Control | 109.6 ± 15.4 | 107.8 (98.9, 119.8) | 67.7, 146.2 | <0.0001 | |
| Matched control | 102.8 ± 12.4 | 103.7 (94.3, 113.7) | 69.9, 120.8 | <0.0001 |
AN anorexia nervosa
Compared with AN by Wilcoxon rank-sum test, to allow for skewed distributions
Table 2.
Characteristics of 70 adolescent girls with anorexia nervosa, compared with 132 female control subjects and a subsample of 40 control subjects matched for race and ethnicity
| Characteristic | Group | Category | n (%) | p valuea |
|---|---|---|---|---|
| Race | AN | White | 61 (87) | – |
| Asian | 2 (3) | |||
| Other | 7 (10) | |||
| Control | White | 48 (36) | <0.0001 | |
| Black | 31 (23) | |||
| Asian | 22 (17) | |||
| Native American | 3 (2) | |||
| Other | 28 (21) | |||
| Matched control | White | 36 (90) | 0.89 | |
| Asian | 1 (3) | |||
| Other | 3 (8) | |||
| Hispanic ethnicity | AN | 4 (6) | – | |
| Control | 32 (24) | 0.008 | ||
| Matched control | 2 (5) | 1 | ||
| History of fracture | AN | 22 (31) | – | |
| Control | 38 (29) | 0.75 | ||
| Matched control | 12 (30) | 1 | ||
| Family history of osteoporosis | AN | Yes | 17 (24) | – |
| No | 45 (64) | |||
| Unknown | 8 (11) | |||
| Control | Yes | 13 (10) | 0.003 | |
| No | 113 (86) | |||
| Unknown | 6 (5) | |||
| Matched control | Yes | 7 (18) | 0.03 | |
| No | 33 (83) | |||
| Unknown | 0 (0) |
AN anorexia nervosa
Compared with AN by Fisher exact test
All control subjects reported regular menstruation. Girls with AN reported amenorrhea for a median duration of 4 months (range 1–18 months). Of the subjects with AN, seven were premenarchal and three had primary amenorrhea. These ten subjects were not included in the calculation of duration of amenorrhea. Those subjects noted to have 1 month of amenorrhea had been receiving oral contraceptive pills, which they stopped for 1 month prior to study enrollment. Almost half of the girls with AN were vitamin D insufficient; 10 % were vitamin D deficient (Table 3).
Table 3.
Clinical and biochemical characteristics of 70 girls with anorexia nervosa
| Mean ± SD | Median (Q1, Q3) | Minimum, maximum | |
|---|---|---|---|
| Duration of AN (months) | 8.1 ± 10.2 | 4.5 (2, 10) | 1, 60 |
| Duration of amenorrhea (months)a | 5.1 ± 4.2 | 4 (2, 7) | 1, 18 |
| BSAP (ng/mL) | 29 ± 22 | 23 (16, 34) | 0.4, 146 |
| Osteocalcin (ng/mL) | 35 ± 30 | 28 (16,43) | 2.7, 197 |
| CTx (ng/mL) | 0.77 ± 0.47 | 0.64 (0.40, 1.01) | 0.13, 1.96 |
| 25(OH)D (ng/mL) | 32 ± 10 | 30 (25, 39) | 12.1, 73 |
| N (%) | |||
| 25(OH)D status | |||
| Vitamin D insufficient (<30 ng/mL) | 34 (49) | ||
| Vitamin D deficient (<20 ng/mL) | 7 (10) | ||
AN anorexia nervosa, 25(OH)D 25-hydroxy-vitamin D, BSAP bone-specific alkaline phosphatase, CTx C-telopeptides
Rounded down; premenarchal participants exluded
The DXA BMD and BMC results for the participants with AN are summarized in Table 4. A substantial number of girls with AN had a BMD less than expected for age. Deficits at the lumbar spine were most common; 31 % had a BMD Z-score < −1 SD, and 11 % ≤ −2 SD. The BMD Z-score mean was significantly below zero for both the hip and spine (p = 0.04 and p = 0.002, respectively). Body composition was notable for the fairly low mean body fat percentage (24.4 %).
Table 4.
Dual-energy X-ray absorptiometry measures in 70 adolescent girls with anorexia nervosa
| Measure | Mean ± SD or n (%) | Minimum | Maximum |
|---|---|---|---|
| Bone mineral density Z-score | |||
| Total body less head | −0.25 ± 1.23 | −4.60 | +2.20 |
| Total hip | −0.31 ± 1.23 | −3.50 | +2.00 |
| Lumbar spine | −0.53 ± 1.35 | −3.90 | +2.90 |
| Bone mineral density Z-score < −1 | |||
| Total body less head | 14 (20) | – | – |
| Total hip | 16 (23) | – | – |
| Lumbar spine | 22 (31) | – | – |
| Bone mineral density Z-score ≤ −2 | |||
| Total body less head | 4 (6) | – | – |
| Total hip | 7 (10) | – | – |
| Lumbar spine | 8 (11) | – | – |
| Bone mineral content (g) | |||
| Total body less head | 1412 ± 295 | 634 | 2105 |
| Total hip | 29 ± 6 | 12 | 43 |
| Lumbar spine | 50 ± 11 | 19 | 76 |
| Bone mineral content Z-score | |||
| Total body less head | −0.29 ± 0.99 | −4.00 | +1.60 |
| Total hip | +0.15 ± 0.98 | −2.50 | +2.20 |
| Lumbar spine | −0.11 ± 1.14 | −3.60 | +2.00 |
| Body composition | |||
| Fat mass (kg) | 11.9 ± 3.3 | 3.6 | 18.6 |
| Lean mass (kg) | 34.5 ± 4.9 | 18.9 | 50.0 |
| Body fat (%) | 24.4 ± 5.2 | 11.0 | 33.5 |
Trabecular vBMD Z-scores were significantly lower in subjects with AN compared to the larger control subject sample (p = 0.01), but not compared to the matched control subjects (p = 0.45; Table 5). The mean cortical vBMD Z-score of girls with AN was higher than that of the control subjects (p < 0.0001), whether matched or not. Periosteal circumference and section modulus were both lower in subjects with AN compared to matched control subjects (Table 5). Tibial pQCT BMC, CSA, and cortical thickness Z-scores were not different between groups (Table 5).
Table 5.
Tibial pQCT Z-scores and muscle density in 70 adolescent girls with anorexia nervosa and 132 female control subjects of comparable age
| Site | Measure | Group | Mean ± SD | Minimum | Maximum | p valuea |
|---|---|---|---|---|---|---|
| 3 % | Trabecular vBMD | AN | −0.31 ± 1.42 | −4.34 | +2.58 | – |
| Control | +0.11 ± 1.01 | −2.47 | +3.07 | 0.01 | ||
| Matched control | −0.11 ± 1.23 | −2.47 | +3.07 | 0.45 | ||
| Total BMC | AN | −0.09 ± 1.29 | −3.29 | +2.11 | – | |
| Control | +0.10 ± 0.98 | −1.91 | +4.04 | 0.25 | ||
| Matched control | +0.11 ± 1.25 | −1.81 | +4.04 | 0.42 | ||
| 38 % | Cortical vBMD | AN | +0.18 ± 0.92 | −2.06 | +2.31 | – |
| Control | −0.50 ± 0.88 | −2.42 | +1.68 | <0.0001 | ||
| Matched control | −0.77 ± 0.73 | −2.42 | +0.47 | <0.0001 | ||
| Cortical BMC | AN | −0.22 ± 1.14 | −3.19 | +2.08 | – | |
| Control | −0.28 ± 1.05 | −4.03 | +2.97 | 0.75 | ||
| Matched control | +0.05 ± 1.08 | −2.66 | +2.97 | 0.23 | ||
| Cortical CSA | AN | −0.30 ± 1.17 | −3.21 | +1.90 | – | |
| Control | −0.21 ± 1.09 | −3.70 | +3.23 | 0.60 | ||
| Matched control | +0.14 ± 1.15 | −2.81 | +3.23 | 0.06 | ||
| Periosteal circumference | AN | −0.32 ± 1.06 | −2.54 | +1.84 | – | |
| Control | −0.25 ± 1.08 | −2.68 | +2.51 | 0.66 | ||
| Matched control | +0.13 ± 1.12 | −2.19 | +2.41 | 0.04 | ||
| Endosteal circumference | AN | −0.23 ± 1.15 | −2.24 | +2.05 | – | |
| Control | −0.21 ± 1.07 | −3.43 | +2.06 | 0.89 | ||
| Matched control | +0.01 ± 1.13 | −2.51 | +2.03 | 0.28 | ||
| Cortical thickness | AN | −0.17 ± 1.20 | −3.39 | +2.84 | – | |
| Control | −0.07 ± 0.96 | −2.73 | +3.65 | 0.49 | ||
| Matched control | +0.13 ± 1.14 | −2.49 | +3.65 | 0.20 | ||
| Polar section modulus | AN | −0.32 ± 1.09 | −2.78 | +1.94 | – | |
| Control | −0.23 ± 1.11 | −3.39 | +2.68 | 0.61 | ||
| Matched control | +0.17 ± 1.15 | −2.46 | +2.68 | 0.03 | ||
| 66 % | Maximal muscle CSA | AN | −0.42 ± 0.98 | −2.98 | +1.72 | – |
| Control | +0.20 ± 0.96 | −1.83 | +2.66 | <0.0001 | ||
| Matched control | +0.31 ± 0.97 | −1.78 | +2.33 | 0.0003 | ||
| Fat CSA | AN | −0.69 ± 0.99 | −3.62 | +1.11 | – | |
| Control | +0.14 ± 0.93 | −2.12 | +2.15 | <0.0001 | ||
| Matched control | −0.05 ± 0.88 | −2.03 | +2.07 | 0.0009 | ||
| Muscle density, g/cm2 | AN | 79.1 ± 1.5 | 74.2 | 82.9 | – | |
| Control | 79.7 ± 1.8 | 73.5 | 85.4 | 0.03 | ||
| Matched control | 79.9 ± 1.6 | 75.5 | 82.5 | 0.02 |
pQCT peripheral quantitative computed tomography, AN anorexia nervosa, vBMD volumetric bone mineral density, BMC bone mineral content, CSA cross-sectional area
Compared with AN, by independent-sample t test
Body composition measured by pQCT also differed. The calf muscle and fat CSA Z-scores were much lower in girls with AN than in control subjects (Table 5; p < 0.0001 for both). Muscle density was also lower in the AN group (p = 0.03). Body composition measurement Z-scores by pQCT correlated consistently with similar measures by DXA. Muscle CSA Z-score was positively associated with whole body lean mass on DXA (Pearson r = 0.67, p < 0.0001) and not associated with fat mass (p = 0.52). Similarly, fat CSA Z-score was highly correlated with body fat mass by DXA (Pearson r = 0.62, p < 0.001) and not with lean mass by DXA (p = 0.60).
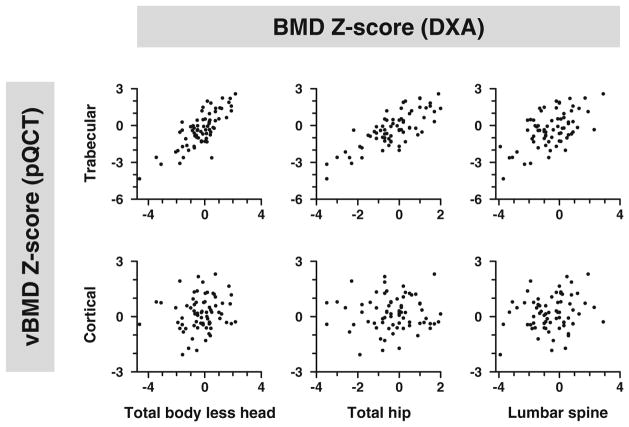
In girls with AN, tibia trabecular vBMD Z-scores correlated with DXATBLH and hip BMD Z-scores (Table 6; Pearson r = 0.79 and 0.78, respectively, p < 0.001 for both). The association between trabecular vBMD Z-score and lumbar spine BMD Z-score was still significant (Pearson r = 0.57, p < 0.001), but not as strong as for TBLH or hip (p = 0.001 for the differences in Pearson r). Trabecular vBMD Z-scores also correlated with BMC Z-scores at all three measured sites (Pearson r = 0.54 to 0.79, p < 0.001 for all), again less strongly for the spine than for TBLH or hip (p < 0.01 for differences). In contrast, no significant associations were found between cortical vBMD Z-scores and BMD Z-scores. Only lumbar spine BMC Z-scores correlated with cortical vBMD Z-scores (Table 6, rFig. 1). Relationships between other pQCT measures and DXA were significant. Cortical bone mineral content, cortical CSA, and cortical thickness Z-scores were all associated with TBLH and hip BMD and BMC Z-scores (Pearson = 0.59 to 0.83, p < 0.001 for all). Relationships between pQCT Z-scores and lumbar spine BMD and BMC Z-scores were still significant, but were less robust than those seen at the other two DXA sites (Pearson r = 0.39 to 0.69).
Table 6.
Correlation between tibial pQCT Z-scores and DXA measures in 70 adolescent girls with anorexia nervosa
| Site | pQCT Z-score | Correlation with BMD Z-score
|
Correlation with BMC Z-score
|
||||
|---|---|---|---|---|---|---|---|
| Total body less head | Total hip | Lumbar spine | Total body less head | Total hip | Lumbar spine | ||
| 3 % | Trabecular vBMD | 0.79 | 0.78 | 0.57 | 0.79 | 0.73 | 0.54 |
| 38 % | Cortical vBMD | 0.15** | 0.02** | 0.19** | 0.18** | −0.03** | 0.24* |
| Cortical BMC | 0.62 | 0.83 | 0.69 | 0.62 | 0.62 | 0.42 | |
| Cortical CSA | 0.59 | 0.82 | 0.66 | 0.59 | 0.61 | 0.39 | |
| Cortical thickness | 0.69 | 0.81 | 0.59 | 0.65 | 0.65 | 0.40 | |
| Polar section modulus | 0.41 | 0.70 | 0.61 | 0.44 | 0.48 | 0.31* | |
| Correlation with fat mass | Correlation with lean mass | Correlation with percentage body fat | |||||
| 66 % | Maximum muscle CSA | −0.08** | 0.67 | −0.40 | |||
| Fat CSA | 0.62 | −0.06** | 0.67 | ||||
All correlations are significantly different from zero, p < 0.001, except as indicated
DXA dual-energy X-ray absorptiometry, pQCT peripheral quantitative computed tomography, BMD bone mineral density, BMC bone mineral content, CSA cross-sectional area
Significantly different from zero, p < 0.05;
not significantly different from zero, p > 0.10
Fig. 1.
DXA BMD Z-scores (BMD-Z) at the hip, spine, and total body less head (horizontal axis) are plotted against Z-scores for trabecular and cortical volumetric BMD (vBMD, vertical axis) among 70 adolescent girls with anorexia nervosa. A positive significant correlation was seen between DXA measures and trabecular vBMD (upper row), but not with cortical BMD (lower row)
DXA measurements correlated with many clinical parameters and markers of AN. Age was negatively associated with lumbar spine BMD Z-score (Pearson r = −0.40, p = 0.0005). BMI Z-score was positively associated with every measure of BMD and BMC by DXA (r range 0.44 to 0.57, p < 0.001 for all). The time since diagnosis of AN showed a mild inverse correlation with BMD Z-scores at the spine (Pearson r = −0.32, p = 0.006), but not at the hip or TBLH. The duration of amenorrhea was negatively associated with TBLH (Pearson r = −0.26, p = 0.04) and spine BMD Z-scores (Pearson r = −0.27, p = 0.04). Similar results were seen for TBLH (Pearson r = −0.28, p = 0.03) and spine (Pearson r = −0.38, p = 0.003) BMC Z-scores.
To examine clinical features of AN that were associated with peripheral skeletal deficits, we explored relationships between markers of disease severity and pQCT measurements. Age was negatively associated with cortical vBMD Z-scores (Pearson r = −0.30, p = 0.01). Markers of malnutrition (BMI Z-score and percentage of ideal body weight) were positively correlated with almost all pQCT measurement Z-scores. For BMI Z-score, correlations with trabecular vBMD, cortical BMC, cortical CSA, section modulus, and muscle CSA Z-scores ranged from Pearson r 0.35 to 0.50, p < 0.005 for all). Correlations with the percentage of ideal body weight were similar (Pearson r ranging 0.22 to 0.42, p < 0.07 for all). The exception was cortical vBMD Z-scores, which were not significantly associated with either nutritional measure. Neither the duration of disease nor duration of amenorrhea was associated with the pQCT measures.
We also examined whether there were associations between bone turnover markers and other skeletal outcomes, to evaluate for potential mechanisms to explain the observed results. Bone-specific alkaline phosphatase, a marker of bone formation, was negatively correlated with DXA aBMD Z-scores of the total body less head and hip. We found no significant correlations between BSAP and any pQCT variables, or between CTx or osteocalcin and the current DXA or pQCT variables. Similarly, there were no significant correlations between vitamin D concentrations and bone measures.
Discussion
While DXA is a widely utilized clinical tool, this methodology possesses inherent limitations that impact its ability to characterize fully and accurately skeletal wellness. In the current study, we revealed deficits in bone density by both DXA and pQCT in adolescent girls with AN. Assessments by pQCT correlated well with bone and body composition measures by DXA. The strong correlations noted between findings from pQCT and DXA are noteworthy as questions have arisen as to whether these technologies capture the same skeletal properties. pQCT measurements were associated with many of the same clinical parameters and markers of disease severity as have been previously established for DXA.
In our cohort, TBLH BMC Z-score and TBLH BMD Z-score both appear to capture cortical bone size as measured by pQCT cortical CSA Z-score (Pearson r = 0.62, p < 0.001 for both). It has previously been demonstrated that this relationship occurs because cortical BMD varies in such a narrow range that BMC is almost entirely a function of bone size [18, 30]. Our data in AN are consistent with these previous reports. Cortical measures by DXA correlated with cortical measures by pQCT; this same association was seen with the trabecular measures. Lumbar spine BMD Z-scores by DXA are correlated with trabecular vBMD Z-scores.
Conversely, we did not find significant associations between cortical vBMD and DXA measurements in our sample. As the majority of skeletal mass is composed of cortical bone, measures such as DXA TBLH BMC Z-scores would be expected to be highly correlated with cortical vBMD. We hypothesize that the adverse effects of this eating disorder on bone remodeling contribute to the observed greater cortical vBMD Z-scores in our patients. In AN, both the low body mass and multiple hormonal aberrations associated with the disease lead to alterations from normal in both bone resorption and bone formation. We have previously demonstrated suppressed bone formation and resorption in hospitalized adolescents with AN [31]. This low bone turnover state translates to less new bone being formed. Newly formed bone is less mineralized than mature bone. If AN induces suppression of cortical modeling, older bone with greater material density will accumulate, leading to greater cortical vBMD. This effect has been previously described in cohorts of children and adolescents receiving chronic glucocorticoid therapy for renal disease [17, 32]. Long-term glucocorticoid therapy led to reduced bone formation markers, decreased height Z-scores, and elevated cortical vBMD Z-scores [32]. This finding is of concern, since too much bone mineralization can lead to brittle bones [33] and thus to increased fracture risk [34, 35].
Poor bone formation prevents improvements in cortical dimensions. In a study of chronically ill adult women with AN, Milos et al. assessed the ultradistal radius with three-dimensional pQCT [36] and reported reduced cortical thickness in subjects with AN. Similar deficits in cortical thickness at the radius have also been demonstrated in other skeletal “risk groups,” including children with chronic kidney disease [37]. In our sample, we did not find substantial deficits in cortical thickness at the tibial midshaft. Similarly, we did not observe impairments in growth, based upon the normal height Z-scores in our sample. These findings may reflect the younger age (mean 15.5 years) of our cohort compared to the Milos investigation (mean age 23.4 years), as well as shorter disease duration in the current study (4.5 months versus 5.8 years). In addition, many of our subjects were weight-restored at an average 91 % ideal body weight. Weight restoration, early detection of illness, and early intervention may have ameliorated deficits in cortical thickness in this sample.
Patients with onset of AN prior to puberty have bone deficits at both axial and appendicular sites, while those with onset of disease during adulthood are more likely to have axial disease [38]. The majority of our subjects with AN had onset of disease following menarche, after a period of rapid bone growth. Given this timing, our findings suggest that malnutrition may have less severely affected the appendicular skeleton (as detected by pQCT) and more substantially impacted trabecular-rich areas (as assessed by DXA). This hypothesis is further supported by the inverse relationship seen between age and spine BMD Z-score in our subjects with AN. Age may be a proxy measure for duration of disease. As patients grow older and the duration of disease lengthened, age-expected trabecular bone accretion may not have occurred at the lumbar spine. Metabolically active trabecular bone is also predisposed to reflect acute changes in nutritional status or other clinical parameters compared to cortical bone. In contrast, we see the expected positive correlation of age with TBLH BMC and hip BMC Z-scores.
Previously demonstrated relationships between body mass [3, 39, 40] and BMD Z-scores were confirmed. Similar positive correlations were seen between BMI Z-score and bone mineral content at all sites, lean mass and fat variables. These results support our long-time premise that weight gain and normalization of body composition parameters are good for bone health in patients with AN. Weight gain should be encouraged as a baseline intervention regardless of what new skeletal agent is being tested.
Study limitations should be acknowledged. The sample size was relatively small, and the study design cross-sectional, with its inherent limitations. The current sample included many patients with relatively mild illness severity. Our recruitment strategy aimed to examine bone health across a broader spectrum of disease than has been captured in earlier reports. However, considering this study design, the lack of striking skeletal abnormalities between subjects with AN and controls may not be generalizable to those patients with long-standing and severe disease. Study subjects were only required to complete a 1-month washout of OCPs prior to participation. Some of the findings noted were unexpected, such as the higher cortical vBMD in participants with AN compared to control subjects. This finding may represent the underlying compromised bone formation among anorexic subjects or compensatory response to alterations in bone turnover and endocrine parameters. pQCT cannot assess bone microarchitecture. There are also known inherent differences in any densitometric technique that could be at play and merit additional study. From the cross-calibration data presented, we show substantial difference between scanners with respect to cortical density, significantly impacting Z-scores. It is noteworthy that differences between machines may have explained the higher cortical vBMD observed in the current participants with AN. Biochemical and nutritional data from the control group are lacking.
In conclusion, we found skeletal deficits at the appendicular skeleton by pQCT in a sample of adolescent girls with AN. While pQCT measures can capture changes in cortical structure and trabecular vBMD Z-scores, no DXA measures are able to detect alterations in cortical vBMD. Many of the same clinical markers of disease known to impact the axial skeleton also effect measures of bone health in the periphery. To our knowledge, this is one of the first studies to compare DXA measures of bone health to pQCT results in the same cohort of girls with AN. Our results indicate that malnutrition and the accompanying systemic alterations may impact both the peripheral and axial skeleton of young women with AN, although the timing of the impact and severity may differ. While DXA may be insufficient to measure true structural bone properties that determine fracture risk, it may provide a useful screening tool to identify at-risk patients, particularly given its wide availability and good safety profile. Our findings offer a provocative new look at an old problem and should drive larger longitudinal trials exploring true fracture risk in adolescents with AN.
Acknowledgments
The authors thank Alicia McAllister, RT, CBDT, Valerie Marsocci, RT, and Nicole DaSilva, RT, CNMT for technical assistance; the excellent care of the Boston Children’s Hospital Clinical and Translational Study Unit (CTSU) nurses; and our patients and their families, who made this research possible. Our study was supported by NIH R01 AR060829, NICHD K23 HD060066, NIH UL1 RR-025758 (Harvard Clinical and Translational Science Center), the Boston Children’s Hospital Department of Medicine and CTSU, and the Brown Alpert Medical School Department of Orthopaedics.
Footnotes
Compliance with ethical standards The local institutional review boards approved the study protocol. Written informed consent was obtained from study patients or their parent/guardian. Minor subjects also provided assent for participation.
Conflicts of interest None.
References
- 1.Smink FRE, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14(4):406–414. doi: 10.1007/s11920-012-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson A, Gordon CM. Skeletal consequences of eating disorders. Metabolism. 2015 In press. [Google Scholar]
- 3.Gordon CM, Goodman E, Emans SJ, et al. Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr. 2002;141(1):64–70. doi: 10.1067/mpd.2002.125003. [DOI] [PubMed] [Google Scholar]
- 4.Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87(9):4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 5.Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80(3):898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- 6.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84(12):4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 7.Misra M, Aggarwal A, Miller KK, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114(6):1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 8.Jagielska G, Wolanczyk T, Komender J, Tomaszewicz-Libudzic C, Przedlacki J, Ostrowski K. Bone mineral density in adolescent girls with anorexia nervosa–a cross-sectional study. Eur Child Adolesc Psychiatry. 2002;11(2):57–62. doi: 10.1007/s007870200011. [DOI] [PubMed] [Google Scholar]
- 9.Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68(3):548–554. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 10.Audi L, Vargas DM, Gussinye M, Yeste D, Marti G, Carrascosa A. Clinical and biochemical determinants of bone metabolism and bone mass in adolescent female patients with anorexia nervosa. Pediatr Res. 2002;51(4):497–504. doi: 10.1203/00006450-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Turner JM, Bulsara MK, McDermott BM, Byrne GC, Prince RL, Forbes DA. Predictors of low bone density in young adolescent females with anorexia nervosa and other dieting disorders. Int J Eat Disord. 2001;30(3):245–251. doi: 10.1002/eat.1081. [DOI] [PubMed] [Google Scholar]
- 12.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA. 1991;265(9):1133–1138. [PubMed] [Google Scholar]
- 13.Liu D, Manske SL, Kontulainen SA, et al. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int. 2007;18(7):991–997. doi: 10.1007/s00198-007-0325-0. [DOI] [PubMed] [Google Scholar]
- 14.DiVasta AD, Beck TJ, Petit MA, Feldman HA, Leboff MS, Gordon CM. Bone strength in adolescents with anorexia nervosa: a hip structural analysis study. Osteoporos Int. 2006 doi: 10.1007/s00198-006-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiVasta AD, Feldman HA, Beck TJ, LeBoff MS, Gordon CM. Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J Bone Miner Res. 2014;29(1):151–157. doi: 10.1002/jbmr.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faje AT, Karim L, Taylor A, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. 2013;98(5):1923–1929. doi: 10.1210/jc.2012-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetzsteon RJ, Shults J, Zemel BS, et al. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24(3):503–513. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsampalieros A, Griffin L, Terpstra AM, et al. Changes in DXA and quantitative CT measures of musculoskeletal outcomes following pediatric renal transplantation. Am J Transplant. 2014;14(1):124–132. doi: 10.1111/ajt.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis O, Clerinx P, Gies I, De Wachter E, De Schepper J. Well-nourished cystic fibrosis patients have normal mineral density, but reduced cortical thickness at the forearm. Osteoporos Int. 2009;20(2):309–314. doi: 10.1007/s00198-008-0646-7. [DOI] [PubMed] [Google Scholar]
- 20.Mostoufi-Moab S, Magland J, Isaacoff EJ, et al. Adverse fat depots and marrow adiposity are associated with skeletal deficits and insulin resistance in long-term survivors of pediatric hematopoietic stem cell transplantation. J Bone Miner Res. 2015;30(9):1657–1666. doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker JF, Von Feldt J, Mostoufi-Moab S, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66(11):1612–1618. doi: 10.1002/acr.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. 25-OH-vitamin D assays. J Clin Endocrinol Metab. 2005;90(5):3128–3129. doi: 10.1210/jc.2005-0162. [DOI] [PubMed] [Google Scholar]
- 23.Hamwi GJ. Therapy: changing dietary concepts. In: Danowski TS, editor. Diabetes mellitus: diagnosis and treatment. American Diabetes Association; New York: 1964. pp. 73–78. [Google Scholar]
- 24.Organization WH. Report of a WHO Expert Committee. Geneva: 1995. Physical status: the use and interpretation of anthropometry. [PubMed] [Google Scholar]
- 25.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45–60. [PubMed] [Google Scholar]
- 26.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95(4):1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin LM, Thayu M, Baldassano RN, et al. Improvements in bone density and structure during anti-TNF-α therapy in pediatric Crohn’s disease. J Clin Endocrinol Metab. 2015;100(7):2630–2639. doi: 10.1210/jc.2014-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou GY. Toward using confidence intervals to compare correlations. Psychol Methods. 2007;12(4):399–413. doi: 10.1037/1082-989X.12.4.399. [DOI] [PubMed] [Google Scholar]
- 30.Griffin LM, Kalkwarf HJ, Zemel BS, et al. Assessment of dual-energy X-ray absorptiometry measures of bone health in pediatric chronic kidney disease. Pediatr Nephrol. 2012;27(7):1139–1148. doi: 10.1007/s00467-012-2116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiVasta AD, Feldman HA, Quach AE, Balestrino M, Gordon CM. The effect of bed rest on bone turnover in young women hospitalized for anorexia nervosa: a pilot study. J Clin Endocrinol Metab. 2009;94(5):1650–1655. doi: 10.1210/jc.2008-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsampalieros A, Gupta P, Denburg MR, et al. Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res. 2013;28(3):480–488. doi: 10.1002/jbmr.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R. Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int. 2005;16(12):2031–2038. doi: 10.1007/s00198-005-1992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davison KS, Siminoski K, Adachi JD, et al. Bone strength: the whole is greater than the sum of its parts. Semin Arthritis Rheum. 2006;36(1):22–31. doi: 10.1016/j.semarthrit.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res. 2005;20(8):1372–1380. doi: 10.1359/JBMR.050326. [DOI] [PubMed] [Google Scholar]
- 36.Milos G, Spindler A, Ruegsegger P, et al. Cortical and trabecular bone density and structure in anorexia nervosa. Osteoporos Int. 2005;16(7):783–790. doi: 10.1007/s00198-004-1759-2. [DOI] [PubMed] [Google Scholar]
- 37.Leonard MB. A structural approach to the assessment of fracture risk in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2007;22(11):1815–1824. doi: 10.1007/s00467-007-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeman E, Karlsson MK, Duan Y. On exposure to anorexia nervosa, the temporal variation in axial and appendicular skeletal development predisposes to site-specific deficits in bone size and density: a cross-sectional study. J Bone Miner Res. 2000;15(11):2259–2265. doi: 10.1359/jbmr.2000.15.11.2259. [DOI] [PubMed] [Google Scholar]
- 39.Gordon CM, Grace E, Emans SJ, et al. Effects of oral de-hydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87(11):4935–4941. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- 40.Divasta AD, HAF, Giancaterino C, CJR, MSL, CMG The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism. 2012;61(7):1010–1020. doi: 10.1016/j.metabol.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]