Abstract
Objective:
Motor-related cortical potentials (MRCP) often compared separated muscle activations; however, MRCP preceding combined contraction onsets and relaxation offsets of one consecutive motor task sequence remain to be elucidated.
Methods:
Twelve healthy males (27.92±4.33 years, 181.83±7.15 cm, 84.58±7.15 kg) performed 40 submaximal isometric right-limb wrist flexions (i.e. motor task sequences). Each motor task sequence combined timed contractions to and relaxations from distinct torque levels, i.e. 20% and 40% of maximum voluntary contractions (MVC). Synchronized continuous EEG (32 Ag/AgCl-electrodes mounted over motor-related areas) and EMG (i.e. flexor carpi radialis, FCR) recordings served to detect torque level-on/offsets for MRCP analyses.
Results:
Motor task sequences were accurately maintained with participants’ mean values of FCR muscle activity revealing no signs of fatigue (p>0.05). Main findings (i.e. readiness potential) were larger amplitudes over frontal electrode sites (p<0.05) preceding contractions compared to relaxations, whereas amplitudes were larger (i.e. peak) over centro-parietal electrode sites (p<0.05) preceding 40% compared to 20% MVC.
Conclusion:
When performed in one consecutive motor task sequence, controlling the production as well as the releasing of force may require similar proprioceptive and visuo-motor processing preceding the same force level (i.e. 20% or 40%); however, this is irrespective of the muscle activation type (i.e. contraction or relaxation).
Keywords: MRCP, Readiness Potential, Force, EEG, EMG
1. Introduction
Encoding motor behaviour results, at least in part, from modulations in activities of central neuronal motor areas. Motor-related cortical potentials (MRCP) describe EEG-derived potentials preceding voluntary movements. A most prominent MRCP component is the readiness potential (RP) setting in as a negative going slope, observed 2 to 1 seconds prior to a voluntary movement onset. Previous RP research on the consecutive sequences of motor behaviour and its mechanisms investigated the preparation[1,2], initiation[3,4], execution[5,6] and control[7] of a voluntary movement performance and, referred to separated contractions or, less often, relaxations with respect to an EMG-derived onset or offset.
With muscle contraction serving as the onset of a voluntary movement performance, changes in RP have been reported for different force levels. Larger RP amplitudes have been associated with greater joint force[8-12]. Force estimation and force production are of critical importance to control daily life movement performances and sports-specific movements as well[13]. Kern and colleagues[14] report that daily life movement performances usually require 20% of a maximum voluntary contraction (MVC). Oda and colleagues[15] compared RP preceding 10% or 50% MVC during controlled repetitive isometric right-elbow flexions and suggested a greater activation over premotor and primary motor areas for the preparation of greater force production. Following a more comprehensive approach, increasing MRCP amplitudes with 10%, 35%, 60% and 85% MVC preceding isometric elbow-flexion contractions have been reported to show larger amplitudes in sensorimotor areas compared to supplementary motor areas[16]. Siemionow and colleagues[16] further concluded that, if MRCP amplitudes depend on the force level, the amplitude would be larger preceding 20% to 40% MVC compared to 0% to 20% MVC, despite the same difference in force production. In addition to distinct force levels, MRCP have also been investigated preceding distinct contraction rates (i.e. slow, intermediate, and fast[16]). There were strong correlations between increasing MRCP amplitudes and increasing force levels as well as increasing contraction rates, representing central neuronal motor commands that scale the level of a muscle activation. To the contrary, Wasaka and colleagues[17] reported no significant differences in RP amplitudes between 20% and 50% MVC of self-initiated plantar flexions.
The recording of MRCP preceding muscle relaxations have enabled a comparison to muscle contractions, further elucidating central neuronal mechanisms[18-20]. Similar to a muscle contraction, the muscle relaxation has been suggested to also involve activity modulations in cerebral neuronal motor areas, at least in supplementary motor areas[18,21], premotor areas[21], and primary motor areas[22]. With the muscle relaxation onset serving as an active part of a voluntary movement performance, the RP occurring over primary motor areas when preceding isotonic muscle relaxation performances (i.e. passive flexions) has been reported to be remarkably similar to the RP associated with muscle contractions[18,20]. On the contrary, Rothwell and colleagues[19] suggested smaller RP amplitudes occurring in primary motor areas preceding muscle relaxations of non-movement performances (i.e. isometric). Thus, Pope and colleagues[23] directly compared muscle contractions to muscle relaxations in both, isotonic and isometric tasks and found larger RP amplitudes preceding muscle contractions compared to muscle relaxations in isometric tasks; however, RP amplitudes preceding isotonic tasks were shown similar between contraction and relaxation. It is therefore suggested that isotonic compared to isometric performances require active processes for muscle relaxation in motor-related areas[19,23]. Additionally, smaller MRCP amplitudes preceding muscle relaxations compared to muscle contractions of self-initiated voluntary movements[19] are considered to reflect movement preparation processing[3]. According to healthy participants, participants with neurological movement disorders (i.e. patients with dystonia) show even smaller MRCP preceding muscle relaxations compared to muscle contractions indicating functional degenerations of voluntary movement performances, particularly in inhibitory circuits[20]. Further, larger RP preceding isotonic muscle relaxation have been related to proprioceptive feedback[23]. Therefore, the neural mechanisms of muscle relaxations are dependent upon isotonic or isometric performances. However, it seems important to note that these mechanisms are based upon research comparing muscle contractions to muscle relaxations in two separated tasks. With each relaxation having to follow a contraction, even in separated tasks, there is a necessity to conduct a contraction-relaxation sequence in one consecutive task.
Daily life movement performances as well as sports-specific movement techniques, however, require combined and more complex voluntary movements other than separated muscle contractions or relaxations. With this, it has been suggested to detect RP during sports-specific movements[24,25] that combine contractions and relaxations in one consecutive motor task sequence. Vogt and colleagues[2] have shown that technological developments allow the recording of RP during sports-specific movement techniques and, thus, rather complex motor task sequences. However, it is further suggested to investigate RP according to its characteristics that control complex motor task sequences, e.g. its central neuronal motor behavioural processes of consecutive contracting and relaxing muscle activation onsets[2]. This in mind, well discussed physiological and behavioural factors that influence RP preceding separated voluntary movement performances were discussed[4,5,26]. However, these factors may be complemented when combined in one consecutive motor task sequence (i.e. more complex movement[7]) taking predefined motor-related areas into account[2,3]: frontal electrode sites over premotor areas (e.g. plan, prepare), central electrode sites over primary motor areas (e.g. initiate, execute), centro-parietal electrode sites over somatosensory areas (e.g. control, proprioception), and posterior-parietal electrode sites (e.g. visuo-motor processing).
Based on the afore introduced reports on MRCP preceding either a contraction or a relaxation obtained from two separated motor tasks, the aim of this study was to investigate MRCP preceding a combined contraction and relaxation within one consecutive motor task sequence. Therefore, this study investigated RP at electrode sites over motor- and sensorimotor-related areas preceding contraction or relaxation onsets of isometric dominant-hand (i.e. right) wrist flexions performed in one consecutive motor task sequence at distinct torques (i.e. 20%, 40% MVC). Supporting previous results from separated contracting or relaxing isometric motor tasks, it is hypothesized that (1) RP amplitudes are larger when preceding contractions compared to relaxations, particularly over frontal electrode sites. Further, (2) larger RP amplitudes are hypothesized when preceding higher compared to lower force levels, particularly over centro-parietal electrode sites.
2. Materials and methods
2.1. Participants
Twelve healthy, right-handed males (27.92±4.33 years, 181.83±7.15 cm, 84.58±7.15 kg) volunteered to participate in this study. Participants were injury-free with no known history of neuromuscular or skeletal disorders and considered themselves as recreationally active. Each participant gave written informed consent. Experimental procedures were approved by the German Sport University Human Research Ethics Committee and were conducted in accordance with the Declaration of Helsinki.
2.2. Experimental procedure
A familiarization was completed during which participants rehearsed performing controlled submaximal isometric motor task sequences. Each motor task sequence combined consecutive wrist-flexion contractions to as well as relaxations from a distinct torque level. Motor task sequences were predefined with torque levels from 20%in (a rapid contraction from full relaxation to 20% of maximal voluntary torque, MVT) up to 40%in (a rapid contraction from 20% to 40% MVT), down to 40%out (a rapid relaxation from 40% to 20% MVT), and down to 20%out (a rapid relaxation from 20% MVT to full relaxation. To be able to re-reference (see 2.3.2) the baseline within each consecutively performed motor task sequence, each torque level was hold for approximately 3 seconds before continuing with a contraction/relaxation to the next torque level and subsequently finishing one motor task sequence (Figures 1 and 2).
Figure 1.
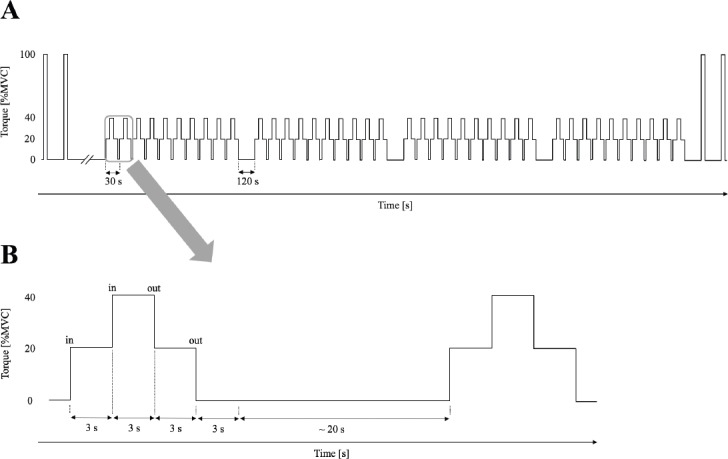
Schematic view of the experimental protocol including two initial and tow closing maximum voluntary contractions (MVC) as well as 40 motor task sequences (A). With approximately 20 seconds rest between motor task sequences, a motor task sequence contained contractions (20%in, 40%in) and relaxations (40%out, 20%out) at distinct torques, hold for 3 seconds each (B).
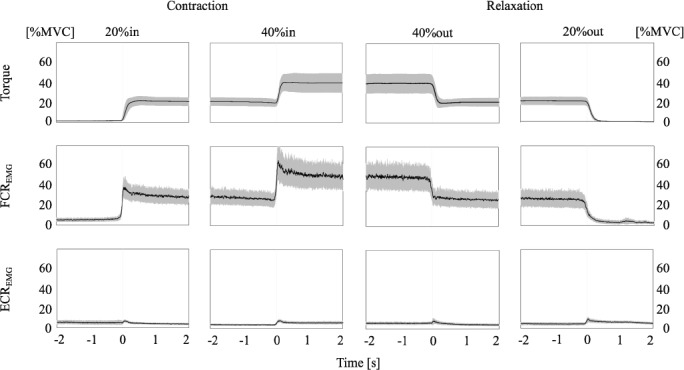
Figure 2.
Displayed are mean performances (black line) ± standard deviation (grey) from -2 to 2 second (s) of the prescribed motor task sequences based upon torque onsets and offsets (each at 0 seconds) and including flexor carpi radialis (FCR) and extensor carpi radialis (ECR) EMG with the respective muscle contracting (20%in, 40%in) or relaxing (40%out, 20%out) activation.
Attending one experimental trial, participants were seated and strapped into a dynamometer to determine MVT (see 2.3.3). Subsequently, participants remained seated to perform 4 sets of 10 motor task sequences (total 40) with approximately 20 seconds rest between each motor task sequence[15,16]. Electromyography (EMG), EEG and torque were synchronised and measured continuously during each motor task sequence. Real-time visual feedback of torque was presented on a screen at good sight approximately 1 meter in front of the participant. Screens were scaled to serve as an orientation to approximately meet the instructed 3-second timings at each torque level; participants were asked to avoid ‘silent counting’. All motor task sequences were performed with the participant’s dominant right hand.
2.3. Data collection
2.3.1. EEG recordings
A portable actiCAP system and Brain Vision Recorder 1.20 (Brain Products GmbH, Germany) recorded EEG at a sampling rate of 1000 Hz. To improve signal transduction, 32 actiCAP-attached Ag/AgCl-electrodes were filled with SuperVisc™ electrode gel (EasyCap GmbH, Herrsching, Germany), head-mounted according to the international 10/20 system[27]: Fp1, Fp2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CPz, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, PO9, O1, Oz and O2. Ground (FCz) and reference (AFz) electrodes were added, whereas PO10 served as right horizontal electrooculorgram (EOG, mounted next to the participants’ right eye) to detect lateral eye movements[2]. The actiCAP adapted to individual head size and was permeable to air to avoid heating; although not measured, perspiration of participants could not be observed. In addition, electrodes were distant 25 mm to avoid salt bridge-induced cross-talk.
Analogue EEG recordings were amplified and converted to digital signals for analyses. Synchronised torque recordings served to detect motor task sequence-onsets (refer to 2.3.3).
2.3.2. RP analysis
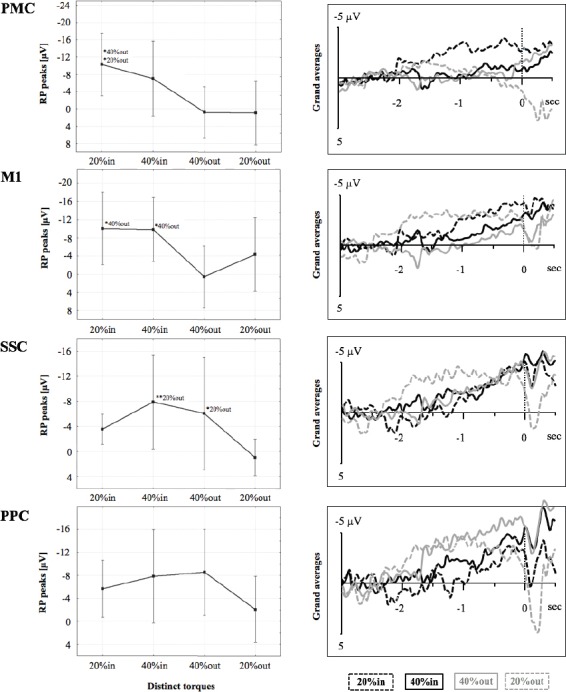
Converted EEG data were analysed using Brain Vision Analyser 2.1 (Brain Products GmbH, Germany). Following initial visual inspections for manual artifact rejections, electrode sites exceeding 10kØ were topographically interpolated to counteract extensive data exclusion[28,29]. Low- and high-cut off filtering remained a frequency range from 0.03 to 10.0 Hz (time constant 5.305165 s) for MRCP analyses[2]. Standard ocular corrections with reference to EOG recordings were applied[30]. According to movement onsets at distinct torques (refer to 2.3.4), each motor task sequence was segmented from -3000 to 500 ms, baseline corrected from -3000 to -2000 ms in each torque level respectively (i.e. re-referenced) and averaged (mean minimum trials for 20%in n=22, 40%in n=23, 40%out n=23, 20%out n=23). Grand averages were performed to identify MRCP (Figure 3). Subsequently, RP peaks of pooled electrode sites according to relevant predefined motor and sensorimotor areas[3] were exported for statistical computations[2]: frontal electrode sites over the premotor cortex (PMC: F3, Fz, F4, FC1, FC2), central electrode sites over the primary motor cortex (M1: C3, Cz, C4), centro-parietal electrodes sites over the somatosensory cortex (SSC: CP1, CP2) and posterior-parietal electrodes sites over the posterior-parietal cortex (PPC: P3, Pz, P4).
Figure 3.
Displayed are mean readiness potential (RP) peaks (µV) with confidence intervals at distinct torques, i.e. 20% (dashed lines in grand averages) and 40% (continuous lines in grand averages), preceding contraction (in; black in grand averages) or relaxation onsets (out; grey in grand averages) of one continuous motor task sequence over PMC (premotor cortex electrode sites), M1 (primary motor cortex electrode sites), SSC (somatosensory cortex electrode sites), and PPC (posterior-parietal cortex electrode sites). The levels of significance are marked by asterisks (* p<.05, ** p<.01), each referenced with annotations respectively.
2.3.3. EMG and torque recordings
Following warm-up procedures of submaximal continuous isometric contractions and relaxations of the flexor carpi radialis muscle (FCR), participants performed two MVC with 20 seconds rest in between prior to the motor task sequences and immediately afterwards to confirm that there were no possible fatiguing effects. Participants were instructed to exert maximum effort of wrist-flexion torque continuously for approximately 3 seconds with visual feedback and verbal encouragement. MVT was determined as mean of the two MVC peaks. Subsequently, participants rested for approximately 15 minutes allowing to mount the EEG cap and verify EEG signals before performing 40 consecutive submaximal isometric motor task sequences (see 2.2; Figure 1). All motor task sequences and MVC were performed on a dynamometer (IsoMed 2000, D&R GmbH, Germany).
Surface EMG was recorded at 1000 Hz from right FCR (i.e. agonist) and right extensor carpi radialis muscle (ECR; i.e. antagonist) using an A/D converter system (Brain Products GmbH, Germany[2]). Following shaving, gentle abrasion and cleansing with ethanol of the respective skin areas, Ag/AgCl electrodes (1 cm diameter) were disposed over the respective superficial muscle bellies with an interelectrode distance of approximately 2 cm. After mounting, interelectrode impedances and EMG signals were visually verified.
2.3.4. EMG and torque analyses
To determine torque onsets of each contraction and relaxation performance (20%in, 40%in, 40%out and 20%out), experimenters visually identified triggered changes in torque referring to the synchronised recordings. Then, for each trial, EMG activity of each muscle (i.e. FCR, ECR) was rectified and calculated over a period from -1 to 1 second based upon torque onset and offset. Mean EMG values were normalized according to MVC and subsequently, EMG values were averaged over all participants (Figure 2).
2.4. Statistical analyses
Statistical analyses were performed using Statistica 7.1 (StatSoft Inc., Tulsa, USA).
Repeated measures analysis of variance (ANOVA) were computed to display motor task sequence-induced changes (i.e. at torques 20%in, 40%in, 40%out, 20%out) for RP peak over distinct motor areas (i.e. PMC, M1, SSC, and PPC).
Data from one participant included a high-amplitude noise component in the recorded EEG signals during almost all performed motor task sequences and had to be excluded, remaining a sample size of n=11 for analyses (i.e. EEG mappings, EMG).
To confirm absence of fatiguing, a paired t-test was performed comparing mean values of the initial and closing MVC.
Data in the figures are presented as mean ± confidence intervals (CI; 95%), in the text and tables as mean ± standard deviation (SD). The level of significance was set at p<.05.
3. Results
3.1. EMG and torque activity
Participants were able to accurately maintain the prescribed motor task sequences, according to torque outputs and the steadiness of muscle contraction and relaxation of FCR, referenced to ECR (Fig. 2).
Mean values of initially performed MVC revealed no differences compared to closing MVC (p=.22; pre 22.23 ± 4.52 Nm vs. post 21.24 ± 4.68 Nm).
3.2. RP peak
RP peak showed significant interactions for PMC (F(3, 24)= 3.57, p<.05), M1 (F(3, 24)=3.07, p<.05), and SSC (F(3, 24)=3.52, p<.05); however, there was no interaction for PPC (F(3, 24)=2.49, p=.08, Figure 3).
For PMC, post-hoc tests revealed larger RP peaks (i.e. increased negativity) from 20%in to 40%out and to 20%out. For M1, post-hoc tests revealed larger RP peaks (i.e. increased negativity) from 20%in to 40%out as well as from 40%in to 40%out. For SSC, post-hoc tests revealed larger RP peaks (increased negativity) from 40%in to 20%out as well as from 40%out to 20%out.
4. Discussion
This study aimed to investigate RP over task-relevant motor areas preceding contraction and relaxation onsets that are performed in one consecutive motor task sequence using controlled right-handed isometric wrist flexions at distinct torques (i.e. 20%, 40% MVC). Main findings were: 1) larger RP amplitudes over motor-related areas preceding contractions compared to relaxations and 2) larger peaking RP amplitudes over sensorimotor-related areas preceding higher (40%in, 40%out) compared to lower (20%in, 20%out) force levels.
According to the present study’s first hypothesis, larger RP amplitudes preceded contractions compared to relaxations. This was clearly shown at electrode sites over PMC and M1 and supports Pope and colleagues[23] to reflect a greater muscle activation in preparation of a contracting compared to a relaxing isometric force production. Additionally, these findings are in line with Rothwell and colleagues[19] reporting smaller RP amplitudes to precede isometric muscle relaxations, most pronounced over primary motor areas. However, increasing the force level either during contraction to produce greater force (i.e. from 20%in to 40%in) or during relaxation to actively release from a greater force (i.e. from 40%out to 20%out) did not influence RP amplitudes in the present study. This, at least in part, contradicts Siemionow and colleagues[16] who found larger RP amplitudes over sensorimotor areas relative to greater force levels; however, they compared separated motor tasks following a more comprehensive approach including an extensive range of force production (i.e. up to 85% MVC) at different contraction rates. Performing similar force levels (i.e. 20% and 50% MVC) in, more importantly, motor task sequential activities, Wasaka and colleagues[17] reported no differences in RP amplitudes preceding contracting muscle activations according to force levels. Following this line of thought, central neuronal motor behavioural processes preceding a muscle activation require greater cortical excitability for isometric contractions not only in separated motor tasks[3,4,26] but also when combined with relaxations performed in one consecutive motor task sequence. It seems reasonable that this results in similar premotor and primary motor central neuronal patterns that may reflect a greater relevance for the type of the muscle activation (i.e. contraction or relaxation) other than its relation to the produced and estimated force level (i.e. 20% or 40% MVC). This would be, at least, related to central neuronal motor behavioural processes of planning, preparing, initiating and executing a contraction. However, in one consecutive motor task sequence these central neuronal motor behavioural patterns seem to change given its functional focus, i.e. with respect to controlling, proprioception and visuo-motor processing.
According to the present study’s second hypothesis, larger RP amplitudes preceded higher (i.e. 40% MVC) compared to lower (i.e. 20% MVC) force levels for both contractions and relaxations. In contrast to electrode sites over motor-related areas, this was clearly shown at electrode sites over somatosensory areas, reflecting performance control, proprioception and visuo-motor processing. Previous research suggested remarkably similar RP amplitudes over central neuronal motor areas for both contractions and relaxations[18,20,31]. Additionally, Pope and colleagues[23] related larger RP amplitudes preceding relaxation to proprioceptive feedback; however, again comparing separated motor tasks. This and the functional focus of central motor behavioural processes relevant for the respective motor-task during one motor task sequence in mind, it seems reasonable that controlling the production of force from one to another distinct torque (e.g. contractions from 20%in to 40%in) as well as the releasing of force from one to another distinct torque (e.g. relaxation from 40%out to 20%out) requires similar proprioceptive as well as visuo-motor processing that result in similar RP amplitudes preceding the same force level but irrespective of different types of muscle activity (i.e. contraction or relaxation).
4.1. Limitations
We are well aware that there are limitations in controlling the performance of a motor task sequence: despite instructing all participants to self-initiate and time-control each motor task sequence (i.e. approximately 3 seconds of 20%in, 40%in, 40%out, and 20%out), neither acoustic nor active visual Go-signals to help initiating a subsequent muscle activation (i.e. contraction or relaxation) and, thus, adequately meeting the respective torque could be instructed to avoid interference with the voluntary performance itself or with additional cognitive processing (e.g. time-counting strategies). However, in particular additional cognitive strategies to meet the instructions may not be excluded, although passive visual guidance was provided (i.e. monitoring of the visual torque-feedback was set to fit the duration of one motor task sequence before resetting the screen for the next motor task sequence). Additionally, choosing the correct force level (i.e. 20% and 40% MVC) may be discussed: this is with respect to meeting the suggested repetitions of motor task sequences when aiming to record RP on the one hand, and, on the other hand, providing detectable differences between force levels as well as avoiding fatigue when performing several consecutive and continuous motor task sequences with, from a practicable point of view, rather enduring chair time for each participant. According to this, the number of performed motor task sequences may be considered as limiting.
4.2. Conclusion
In conclusion, the present study aimed to investigate central neuronal motor behaviour preceding contractions and relaxations performed in one consecutive motor task sequence at distinct torques.
Larger RP amplitudes at electrode sites over motor areas preceding contracting compared to relaxing muscle activation indicate greater relevance for the type of muscle activity (i.e. contraction or relaxation) rather than its relation to the produced and estimated force level with respect to processes of planning, preparing, initiating and executing a motor task sequence. This is reversed with respect to controlling a motor task sequence, its proprioception and visuo-motor processing, particularly for more demanding force production, which is reflected by larger RP amplitudes at electrode sites over sensorimotor areas preceding higher compared to lower force levels; however, more intense force levels (e.g. 60% or 80% of MVC) need further investigation.
Acknowledgements
The authors would like to extend their sincere gratitude to all those who spend their valuable time participating in this study, to Stefan Schneider for providing research equipment during data collection and to two unknown reviewers for their valuable contributions during the reviewing process.
The study was funded by an international grant of the German Academic Exchange Service (DAAD project no. 57320531) awarded to the corresponding author.
Footnotes
The authors have no conflict of interest.
Edited by: A. Ireland
References
- 1.Barrett G, Shibasaki H, Neshige R. Cortical potentials preceding voluntary movement:evidence for three periods of preparation in man. Electroencephalogr Clin Neurophysiol. 1986;63:327–39. doi: 10.1016/0013-4694(86)90017-9. [DOI] [PubMed] [Google Scholar]
- 2.Vogt T, Kato K, Schneider S, Türk S, Kanosue K. Central neuronal motor behaviour in skilled and less skilled novices - Approaching sports-specific movement techniques. Hum Mov Sci. 2017;52:151–9. doi: 10.1016/j.humov.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Shibasaki H, Hallet M. What is the Bereitschafspotential? Clin Neurophysiol. 2006;117(11):2341–56. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Slobonouv S, Hallett M, Newell KM. Perceived effort in force production as reflected in motor-related cortical potentials. Clin Neurophysiol. 2004;115:2391–402. doi: 10.1016/j.clinph.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Masaki H, Takasawa N, Yamazaki K. Enhanced negative slope of the readiness potential preceeding a target force production task. Electroencephal Clin Neurophysiol. 1998;108:390–7. doi: 10.1016/s0168-5597(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 6.Tarkka IM, Hallett M. Topography of scalp-recorded motor potentials in human finger movements. J Clin Neurophysiol. 1991;8:331–41. doi: 10.1097/00004691-199107010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Benecke R, Dick JPR, Rothwell JC, Day BL, Marsden CD. Increase of the Bereitschaftspotential in simultaneous and sequential movement. Neurosci Lett. 1985;62:347–52. doi: 10.1016/0304-3940(85)90573-7. [DOI] [PubMed] [Google Scholar]
- 8.Becker W, Kristeva R. Cerebral potentials prior to various force developments. Prog Brain Res. 1980;54:189–95. doi: 10.1016/S0079-6123(08)61624-6. [DOI] [PubMed] [Google Scholar]
- 9.Hink RF, Deecke L, Kornhuber HH. Force uncertainty of voluntary and human movement-related potentials. Biol Psychol. 1983;16:197–210. doi: 10.1016/0301-0511(83)90024-8. [DOI] [PubMed] [Google Scholar]
- 10.Kutas M, Donchin E. Studies of squeezing:handness, responding hand, response force, and asymmetry of readiness potential. Science. 1974;186:545–8. doi: 10.1126/science.186.4163.545. [DOI] [PubMed] [Google Scholar]
- 11.Nishihira Y, Araki H, Ishihara A. Cerebral motor potential preceding grip strength movement. J Sports Med Phys Fitness. 1989;29:297–303. [PubMed] [Google Scholar]
- 12.Shibata M, Moritani T, Kubota K. The relationships between movement-related cortical potentials and the force level in man. Med Sci Sports Exerc. 1993;25:197. [Google Scholar]
- 13.Stevens JC, Mack JD. Scales of apparent force. J Exp Psychol. 1959;58:405–13. doi: 10.1037/h0046906. [DOI] [PubMed] [Google Scholar]
- 14.Kern DS, Semmler JG, Enoka RM. Long-term activity in upper- and lower-limb muscles of humans. J Appl Physiol. 2001;91(5):2224–32. doi: 10.1152/jappl.2001.91.5.2224. [DOI] [PubMed] [Google Scholar]
- 15.Oda S, Shibata M, Moritani T. Force-dependet changes in movement-related cortical potentials. J Electromyogr Kinesiol. 1996;6(4):247–52. doi: 10.1016/s1050-6411(96)00010-7. [DOI] [PubMed] [Google Scholar]
- 16.Siemionow V, Yue GH, Ranganathan VK, Liu JZ, Sahgal V. Relationship between motor activity-related cortical potential and voluntary muscle activation. Exp Brain Res. 2000;133:303–11. doi: 10.1007/s002210000382. [DOI] [PubMed] [Google Scholar]
- 17.Wasaka T, Nakata H, Kida T, Kakigi R. Changes in the centrifugal gating effect on somatosensory evoked potentials depending on the level of contractile force. Exp Brain Res. 2005;166(1):118–25. doi: 10.1007/s00221-005-2333-7. [DOI] [PubMed] [Google Scholar]
- 18.Terada K, Ikeda A, Nagamine T, Shibasaki H. Movement-related cortical potentials associated with voluntary muscle relaxation. Electroencephal Clin Neurophysiol. 1995;95:335–45. doi: 10.1016/0013-4694(95)00098-j. [DOI] [PubMed] [Google Scholar]
- 19.Rothwell JC, Higuchi K, Obeso JA. The offset cortical potential:an electrical correlate of movement inhibition in man. Mov Dis. 1998;13:330–5. doi: 10.1002/mds.870130221. [DOI] [PubMed] [Google Scholar]
- 20.Yazawa S, Ikeda A, Kaji R, Terada K, nagamine T, Toma K, Kubori T, Kimura J, Shibasaki H. Abnormal cortical processing of voluntary muscle relaxation in patients with focal hand dystonia studied by movement-related potentials. Brain. 1999;122:1357–66. doi: 10.1093/brain/122.7.1357. [DOI] [PubMed] [Google Scholar]
- 21.Yazawa S, Ikeda A, Kunieda T, Mima T, Nagamine T, Ohara S, Terada K, Taki W, Kimura J, Shibasaki H. Human supplementary motor area is active in preparation for both voluntary muscle relaxation and contraction:subdural recording of Bereitschaftspotential. Neurosci Lett. 1998;244(3):145–8. doi: 10.1016/s0304-3940(98)00149-9. [DOI] [PubMed] [Google Scholar]
- 22.Terada K, Ikeda A, Yazawa S, Nagmine T, Shibasaki H. Movement-related cortical potentials associated with voluntary relaxation of foot muscles. Clin Neurophysiol. 1999;110(3):397–403. doi: 10.1016/s1388-2457(98)00017-0. [DOI] [PubMed] [Google Scholar]
- 23.Pope PA, Holton A, Hassan S, Kourtis D, Praamstra P. Cortical control of muscle relaxation:a lateralized readiness potential (LRP) investigation. Clin Neurophysiol. 2007;118(5):1044–52. doi: 10.1016/j.clinph.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallett M. Synchronization of parietal and premotor areas during preparation and execution of praxis hand movements. Clin Neurophysiol. 2005;116:1382–90. doi: 10.1016/j.clinph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallett M. Temporal activation pattern of parietal and premotor areas related to praxis movements. Clin Neurophysiol. 2005;116:1201–12. doi: 10.1016/j.clinph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Lang W. Surface recordings of the Bereitschaftspotential in normal. In: Jahanshanhi Hallett M, editor. The Bereitschaftspotential. Movement-related cortical potentials. New York: Kluver Academic/Plenum Publishers; 2003. pp. 19–34. [Google Scholar]
- 27.Jasper HH. The ten-twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology Supplements. 1958;35:371–75. [PubMed] [Google Scholar]
- 28.Vogt T, Herpers R, Scherfgen D, Strüder HK, Schneider S. Neuroelectric adaptations to cognitive processing in virtual environments:an exercise-related approach. Exp Brain Res. 2015;233(4):1321–9. doi: 10.1007/s00221-015-4208-x. [DOI] [PubMed] [Google Scholar]
- 29.Vogt T, Schneider S, Anneken V, Strüder HK. Moderate cycling exercise enhances neurocognitive processing in adolescents with intellectual and developmental disabilities. Res Develop Dis. 2013;34(9):2708–16. doi: 10.1016/j.ridd.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 30.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephal Clin Neurophysiol. 1983;5:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 31.Toma K, Honda M, Hanakawa T, Okada T, Fukuyama H, Ikeda A, Nishizawa S, Konishi J, Shibasaki H. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation:an event-related fMRI study. J Neurosci. 1999;19(9):3527–34. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


