Abstract
Objective:
To detect the expression of brain-derived neurotrophic factor (BDNF) in dorsal root ganglion (DRG) of rats with bone cancer pain, and to explore the effects of BDNF and anti-BDNF on pain behavior.
Methods:
40 SD rats were randomly divided into Sham group and Cancer group (n=20). Rats in Sham were injected with PBS into the tibia, while rats in Cancer group were injected with Walker 256 cells to construct rat tibial bone cancer pain model. Rats in Cancer group were further divided into physiological saline (NS) group and anti-BDNF group (n=10) to observe the effects of anti-BDNF on pain behavior in rats with bone cancer pain.
Results:
Expression level of BDNF and its receptor in DRG of Cancer group was significantly higher than that of Sham group at 3 weeks after inoculation (p<0.05). The expression level of BDNF mRNA in the Cancer group was significantly higher than that in the Sham group at 5d, 10d and 20d (p<0.05). Hindpaw withdrawal latency was significantly shorter in Cancer group than in Sham group (p<0.05). Hindpaw withdrawal mechanical threshold was significantly lower in Cancer group than in Sham group (p<0.05). Hindpaw withdrawal latency was significantly longer and hindpaw withdrawal mechanical threshold was significantly higher in anti-BDNF group than in NS group after the use of anti-BDNF (p<0.05).
Conclusion:
BDNF can aggravate bone pain in rats, and anti-BDNF has obvious antinociceptive function in bone cancer pain.
Keywords: Bone Cancer Pain, BDNF, anti-BDNF, Pain Behavior, DRG
Introduction
Cancer patients usually experience pain. Cancer pain can affect not only the patient’s mood and quality of life, but also prevent patients from effective treatment[1]. Bone cancer pain is a major type of cancer pain. Our study aimed to study the occurrence and development of bone cancer pain to reveal its mechanism.
Brain-derived neurotrophic factor (BDNF) is a nutritious protein[2], which is widely distributed in the human body, especially in the central nervous system, peripheral nervous system, endocrine system, bone and cartilage tissue[3,4]. Recent studies have found that BDNF is an important signal molecule between neurons and glial cells[5,6]. It is speculated that the release of BDNF is likely to be involved in the development of bone pain. Without appropriate models, cancer pain still hasn’t been well studied. Bone cancer pain models established by injecting tumor cells into the bone marrow cavity have been reported recently. Those models provide similar pain behavior to clinical symptoms, which in turn accelerate the study of the mechanism of cancer pain and provide effective strategies for clinical treatment.
Materials and methods
Experimental animals
Rat tibial bone cancer pain model was used in this study. A total of 40 female SD rats were purchased from Experimental Animal Center of Jilin University (certificate number: SCXK (Ji) 2008-0004). Rats were fed with LAD0011 feed (Trophic Animal Feed High-Tech Co., Ltd, Nantong, China). The average age of SD rats was 8 weeks, and the body weight was 210~230 g, indoor temperature was kept at 20°C±1°C, and humidity was kept at 45~55%. Rats were allowed to access food and water freely. All experimental animal procedures were carried out in accordance with the European Directive 2010/63/EU.
Instruments and reagents
Roche 480 fluorescence quantitative PCR instrument (Roche, America), E-Gel Imager (ThermoFisher, America), MP-510 electrophoresis (Major Science, America), PBS buffer (Shanghai Zerun Biotechnology Co., Ltd., Shanghai, China), rabbit anti-BDNF antibody, tissue protein extraction and quantification kit (Kaiji Bio-Technology Co. Ltd., Nanjing, China), Walker256 cells (Bioleaf, Shanghai, China).
Experimental methods
Experiment 1: A total of 40 rats were divided into Sham group and Cancer group (n=20). Rats in Sham group were injected with PBS into the tibia, while rats in Cancer group were injected with Walker256 cells. Expression levels of BDNF and its receptors were detected by western blot and real-time quantitative PCR.
Experiment 2: Intrathecal intubation was performed for rats in Cancer group at 12 days after model construction. Rats were randomly divided into NS group and anti-BDNF group (n=10). Intrathecal injection of physiological saline and anti-BDNF (10 µl per rat, 3 times per day) were performed at 15 days after model construction. Pain behavioral changes in rats were observed for 5 consecutive days.
Pain behavioral observation
After operation, rats in two groups were placed in a transparent plexiglass box to avoid mutual bite the situation. Walking posture and autophagy were observed. Walking posture and spontaneous nociception in 2 min were recorded. Measurement was performed 3 times with light radiation focused on the middle of the foot, and the measurement interval was 10 min. The average value of three measurements was used as thermal pain threshold. Mechanical pain threshold was measured using von Frey filaments. Positive response was the paw withdrawal during test or during the removement of filament. Three paw withdrawals in 5 test (10 s interval) was set as threshold.
Determination of BDNF protein expression
Three weeks after model construction, 0.3% pentobarbital sodium was used for anesthesia, and the rats were sacrificed by decapitation. DRG was separated along the L4 and L5 spinal nerves. Total protein was extracted and quantified using kits according to the instructions. Protein samples (40 ul) were subjected to electrophoresis and transmembrane. After washing with TBS for 10 min, membranes were blocked with 5% skimmed milk for 1h. After washing, membranes were incubated with rabbit anti-BDNF antibody (1: 1000) at room temperature for 2h. After washing, membranes were further incubated with goat anti-rabbit IgG (1:2000) at room temperature for 2h. Staining and color development were performed, and PVDF membranes were dried and scanned.
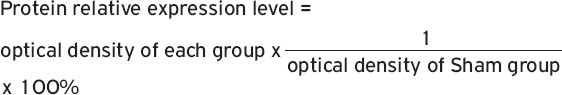
Determination of BDNF mRNA expression
Total RNA was extracted using Trizol reagent (ThermoFisher, USA) according to the instructions. UV spectrophotometer was used to measure RNA concentration. All RNA samples were stored at -80°C before use. Roche 480 quantitative PCR instrument was used for PCR reactions. Reaction conditions for reverse transcription were: 37°C for 15 min and 95°C for 5s. Experiments were performed for 3 times to calculate the average value. Primers were designed and synthesized by invitrogen (Table I). Ct values of the target gene were determined for each sample. Ct total=Ct target gene -Ct endogenous control.
Table I.
Primers used in PCR reaction.
| mRNA | RT-PCR | |
|---|---|---|
| BDNF | Forward | 5‘-AGCCTCCTCTGCTCTTTCTGC-3’ |
| Reverse | 5‘-TCACCCACTCGCTAATACTGTCAC-3’ | |
| β-actin | Forward | 5‘-GGGACCTGACTGACTACCTC-3’ |
| Reverse | 5‘-TCATACTCCTGCTTGCTGAT-3’ |
Statistical analysis
SPSS 23.0 software was used for statistical analysis. Measurement data were expressed as x±s processed by nonparametric Kolmogorov–Smirnov test. p<0.05 was considered to be statistically significant.
Results
General information
Αll rats were healthy with mild temperament and were fed with LAD0011 feed. No significant differences in body weight and length were between two groups (p<0.05). No rats died during model construction (Table II).
Table II.
Comparison of basic data of two groups of rats.
| Basic data | Sham group | Cancer group | p value |
|---|---|---|---|
| Weight (g) | 219.8±10.2 | 222.4±11.3 | 0.785 |
| Length (cm) | 18.3±1.2 | 19.5±1.1 | 0.693 |
| Age (weeks) | 8.1±0.4 | 8.5±0.2 | 0.899 |
| Glucose (mmol / L) | 82.3±11.2 | 79.7±12.3 | 0.685 |
| Mechanical injury sensing threshold | 27.9±1.9 | 26.8±1.3 | 0.763 |
| Hot pain field | 7.6±1.8 | 7.2±1.2 | 0.8424 |
| Red blood cell count | 6.8±0.3 | 6.5±0.3 | 0.745 |
| White blood cell count | 9.5±3.3 | 9.1±2.9 | 0.776 |
| Percentage of lymphocytes (%) | 88.4±0.4 | 84.2±0.6 | 0.757 |
| Ca | 10.6±0.5 | 11.2±0.7 | 0.782 |
| ALP | 665.3±104.6 | 642.8±107.9 | 0.793 |
| ALB | 3.2±0.1 | 3.5±0.2 | 0.747 |
Comparison of body weight between two groups of SD rats
Hair of rats in Cancer group became dim after the inoculation of walker256 tumor cells. Unbalanced walking posture was observed at 12 days, and rats showed fear of touching the ground. Development was temporarily suspended at 15 to 20 days after inoculation. No significant differences in body weight were found between two groups at 15 days (250±8.6 vs 231±4.2) and 20 days (292±9.1 vs 239±8.0) after inoculation (p>0.05) (Table III).
Table III.
Comparison of body weight between two groups of SD rats.
| Weight (g) | Sham group | Cancer group | p value |
|---|---|---|---|
| 15 days | 250±8.6 | 231±4.2 | 0.356 |
| 20 days | 292±9.1 | 239±8.0 | 0.052 |
Behavioral changes in rats
The frequency of spontaneous nociception was higher in Cancer group than in Sham group at 1 week after inoculation (p<0.05). Hindpaw withdrawal latency was shorter in Cancer group than in Sham group (p<0.05). Hindpaw withdrawal mechanical threshold was lower in Cancer group than in Sham group (p<0.05). Hindpaw withdrawal latency was longer and hindpaw withdrawal mechanical threshold was higher in anti-BDNF group than in NS group (p<0.05) (Figures 1-5).
Figure 1.
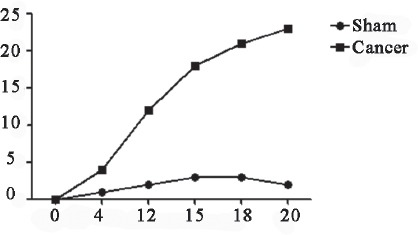
Spontaneous nociception (2 min). The frequency of spontaneous nociception was higher in Cancer group than in Sham group, significant differences were observed in 7d.
Figure 2.
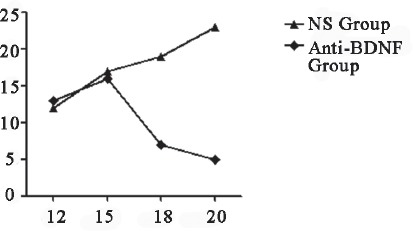
Numbers of spontaneous nociception after intrathecal injection of anti-BDNF (2 min). Number of spontaneous nociception was significantly lower in anti-BDNF group than in NS group.
Figure 3.
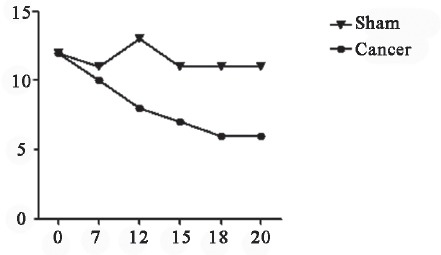
Hindpaw withdrawal latency revealed by heat radiation. Difference between two groups appeared at 7d, the peak difference observed.
Figure 4.
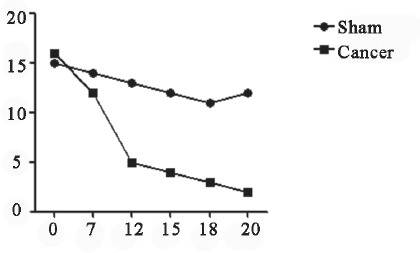
Determination of mechanical pain threshold. Mechanical pain threshold was lower in Cancer group than in Sham group at 5d, but decrease was slowed down at 12d, followed by increase at 19d.
Figure 5.
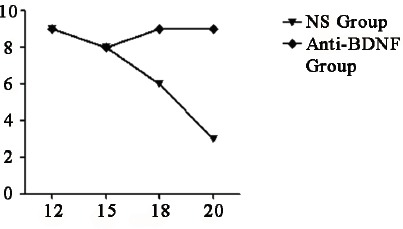
Hindpaw withdrawal latency after intrathecal injection of anti-BDNF. No significant differences were found between two groups before 15d, the value was lower in NS group than in anti-BDNF group from 16d, and gap was gradually widened after that.
Expression of BDNF protein in SD rats
Relative expression levels of BDNF and its receptors in DRG of Cancer group were significantly higher than those in Sham rats at 20 days after inoculation (p<0.05), indicating that bone cancer pain can upregulate the expression of BDNF (Figure 6).
Figure 6.
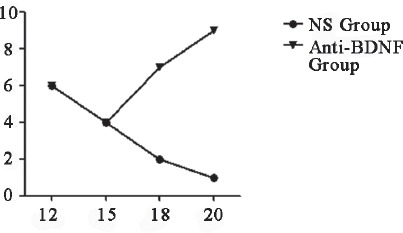
Determination of mechanical pain threshold after intrathecal injection of anti-BDNF. Difference was observed on day 15, and the value of anti-BDNF group was higher than NS group, the value of NS group reached 0 on day 20.
Expression of BDNF mRNA in rats
Expression level of BDNF mRNA was detected by qRT-PCR at 1d, 5d, 10d and 20d after inoculation. The expression level of BDNF mRNA was significantly increased in DRG of Cancer group at 5d, 10d and 20d after inoculation (p<0.05) (Table IV).
Table IV.
Expression level of BDNF mRNA in rats.
| Time points | Sham group | Cancer group | p value |
|---|---|---|---|
| 1d | 0.00 | 0.01 | 0.683 |
| 5d | 0.12 | 0.63 | 0.012 |
| 10d | 0.35 | 1.34 | 0.008 |
| 20d | 1.00±0.12 | 2.10±0.26 | 0.002 |
Discussion
As a type of chronic pain with extremely complex mechanism, bone cancer pain is not only caused by tumor invasion itself, but also by peripheral nerve sensitization, cytokines, tumor cell compression, ischemia and other factors[7-9]. The complex mechanism of bone cancer pain makes it difficult to be controlled[10-12]. With appropriate animal models, studies of bone cancer pain have been greatly accelerated.
Results showed that expression level of BDNF was significantly increased in Cancer group (p<0.05), antinociceptive ability was significantly increased in anti-BDNF group after the use of anti-BDNF. Those results suggest that BDNF expression may be related to the occurrence and development of bone cancer pain. Ugolini G[13] reported that the number of white blood cells would be increased due to tumor cell proliferation and invasion, which in turn lead to the excessive secretion of growth factors. Arco MD[14] showed that tumor cells, necrotic tumor cells and tissues invaded by tumor cells could secrete BDNF. Paw withdrawal was more obvious in Cancer group than in Sham group, hyperalgesia was also observed in Cancer group. Yuan J[15] has shown that changes in level of BDNF in some tissues can induce hyperalgesia. Electrophysiological studies carried out by Merighi A[16] and Rose CR[17] showed that the excitability of nociceptors was closely related to the content of BDNF. In this study, expression levels of both BDNF mRNA and protein were increased in rats with bone cancer pain compared with control group, indicating that BDNF plays an important role in sensitization process.
This study found that treatment with anti-BDNF significantly alleviated hyperalgesia caused by bone cancer pain. The frequency of spontaneous nociception was reduced, the hindpaw withdrawal latency was elongated, and hindpaw withdrawal mechanical threshold was increased, indicating that anti-BDNF can block the BDNF pathway and improve the rat’s anti-injury sensing ability. Kynast KL[18] and Bali KK[19] found that the blocking of BDNF pathway can significantly reduce hyperalgesia, but the effects on mechanical pain were not significant. This can be explained by the changes in levels of neurotransmitter precursor material and excitatory ion channels caused by a single administration. In this study, anti-BDNF was used for 5 continuous days to induce sensitization of peripheral nerves, indicating that BDNF expression is involved in the regulation of rat bone cancer pain. BDNF and other growth factors are closely correlated with the development of bone cancer pain[20,21]. Blocking of BDNF pathway and regulation of BDNF expression may effectively relieve bone cancer pain, but further studies are needed to confirm the conclusions.
In our study, SD rats were used to construct model to detect the expression of BDNF in DRG, and to explore its effects on pain behavior. Clinical data are needed to confirm the conclusions.
In conclusion, BDNF was widely expressed in DRG of rats with bone cancer pain to aggravate cancer pain and affect pain behavior, which in turn shortened hindpaw withdrawal latency and reduced mechanical pain threshold. In addition, anti-BDNF has a significant anti-injury sensing effect in bone cancer pain.
Footnotes
The author has no conflict of interest.
Edited by: G. Lyritis
References
- 1.Rush AM, Waxman SG. PEG2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004;1023(2):264–71. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 2.Fjell J, Cummins TR, Fried K, Black JA, and Waxman SG. In vivo NG F deprivation reduces SNS expression and TTX-R sodium currents in IB4-negative DRG neurons. J Neurophysiol. 1999;81(2):803–10. doi: 10.1152/jn.1999.81.2.803. [DOI] [PubMed] [Google Scholar]
- 3.Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108(3):237–47. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Zahn PK, Subieta A, Park SS, Brennan TJ. Effect of blockade of never growth factor and tumor necrosis factor on pain behaviors after plantar incision. J Pain. 2004;5(3):157–63. doi: 10.1016/j.jpain.2004.02.538. [DOI] [PubMed] [Google Scholar]
- 5.Qiu F, Jiang Y, Zhang H, Liu Y, and Mi W. Increased expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 with dorsal root ganglia in a rat model of bone cancer pain. Neurosci Lett. 2012;512(2):61–6. doi: 10.1016/j.neulet.2012.01.069. [DOI] [PubMed] [Google Scholar]
- 6.Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, and Eglen RM. A comparison of the potential role of the tetrodotoxin-insensitive sodium channels,PN3/SNS and NaN/SNS2,in rat models of chronic pain. Proc Natl Acad Sci U S A. 1999;96(14):7640–4. doi: 10.1073/pnas.96.14.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal an ion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 8.Slack SE, Thompson SW. Brain-derived neurotrophic factor induces NMDA receptor 1 phosphorylation in rat spinal cord. Neuroreport. 2002;13(15):1967–70. doi: 10.1097/00001756-200210280-00027. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Zhang Y, Dai J, Yang J, and Gang S. Electroacupuncture(EA) modulates the expression of NMDA receptors in primary sensory neurons in relation to hyperalgesia in rats. Brain Res. 2006;1120(1):46–53. doi: 10.1016/j.brainres.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 11.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O'Reilly T, et al. A rat model of bone cancer pain. Pain. 2002;96(1-2):129–40. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 12.Boudes M, and Menigoz A. Non-neuronal BDNF, a key player in development of central sensitization and neuropathic pain. J Physiol. 2009;587(Pt 10):2111–2. doi: 10.1113/jphysiol.2009.172130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ugolini G, Marinelli S, Covaceuszach S, Cattaneo A, and Pavone F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc Natl Acad Sci U S A. 2007;104(8):2985–90. doi: 10.1073/pnas.0611253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arco MD, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, Fabbretti E. Neutralization of Nerve Growth Factor Induces Plasticity of ATP-Sensitive P2X3 Receptors of Nociceptive Trigeminal Ganglion Neurons. Journal of Neuroscience. 2007;27(31):8190–8201. doi: 10.1523/JNEUROSCI.0713-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan J, Zhang Y, Wang X, and Ma H. Exogenous Brain-Derived Neurotrophic Factor at a 50 ng/mL Concentration has a Significant Protective Effect on Bilirubin-Induced Cerebral Cortex Neuronal Injury. Clin Lab. 2017;63(9):1421–1429. doi: 10.7754/Clin.Lab.2017.170303. [DOI] [PubMed] [Google Scholar]
- 16.Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, and Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85(3):297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator;rapid effects of BDNF on ion channels. Bioessays. 2004;26(11):1185–94. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- 18.Kynast KL, Russe OQ, Geisslinger G, Niederberger E. Novel findings in pain processing pathways;implications for miRNAs as future therapeutic targets. Expert Rev Neurother. 2013;13(5):515–25. doi: 10.1586/ern.13.34. [DOI] [PubMed] [Google Scholar]
- 19.Bali KK, Kuner R. Noncoding RNAs:key molecules in understanding and treating pain. Trends Mol Med. 2014;20(8):437–48. doi: 10.1016/j.molmed.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou LJ, Zhong Y, Ren WJ, Li YY, Zhang T, Liu XG. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp Neurol. 2008;212(2):507–14. doi: 10.1016/j.expneurol.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16(11):1258–66. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]


