Abstract
Children with osteogenesis imperfecta (OI) type VI often have high fracture rates despite the current standard treatment with bisphosphonates. Subcutaneous injections of denosumab have been proposed as an alternative treatment approach, but safety data on denosumab in children are limited. Here we describe fluctuations in bone and mineral metabolism during denosumab treatment in four children with OI type VI who started denosumab (basic protocol: 1 mg per kg body mass every 3 months) between 1.9 and 9.0 years of age, after having received intravenous bisphosphonates previously. All four children developed hypercalciuria during active denosumab therapy. In two children aged 3.9 and 4.6 years, episodes of hypercalcemia were observed between 7 and 12 weeks after the preceding denosumab injection. During times when the interval between denosumab injections was increased to 6 months for clinical reasons, lumbar spine bone mineral density z-scores decreased rapidly. It appears that the duration of action of denosumab is short and variable in children with OI type VI. These observations call into question the concept that denosumab can be used as a stand-alone alternative to bisphosphonates to treat children with OI.
Keywords: Children, Denosumab, Hypercalcemia, Hypercalciuria, Osteogenesis Imperfecta, Osteoporosis
Introduction
Osteogenesis imperfecta (OI) is a heritable condition that is usually caused by dominant mutations in one of the two collagen type I encoding genes, COL1A1 or COL1A2[1]. Rare mutations in more than 15 other genes can also give rise to an OI phenotype[2]. Among these is OI type VI (MIM 172860), a recessive disorder caused by biallelic mutations in SERPINF1[3,4]. Similar to other OI types, the most obvious clinical characteristic of OI type VI is increased bone fragility. The unique feature of OI type VI is a mineralization defect in bone tissue[5], which leads to an increased amount of unmineralized osteoid in trabecular and cortical bone (i.e., osteomalacia) despite normal calcium and phosphate serum levels and normal growth plate mineralization (i.e., absence of rickets). The mechanistic link between SERPINF1 mutations and the mineralization defect is not clear at present.
From a clinical perspective, children with OI type VI typically appear healthy at birth but start to have minimal-trauma fractures after the age of 6 months[5]. Treatment with intravenous bisphosphonates appears to have some beneficial effect, but fracture rates nevertheless tend to remain high, especially during the growing years[6,7]. One potential issue limiting the effectiveness of bisphosphonates in OI type VI is that these drugs need to be adsorbed to mineralized bone surfaces in order to exert their inhibitory effect on osteoclasts[8]. The mineralization defect of OI type VI may limit the access of bisphosphonates to mineralized bone surfaces and therefore interfere with the action of the drug.
In contrast to bisphosphonates, denosumab exerts its anti-osteoclast activity with a monoclonal antibody that inactivates RANKL, a protein that stimulates osteoclast formation[8]. The action of denosumab should therefore not be affected by the presence of a mineralization disorder. Indeed, subcutaneous injections of denosumab showed promising results in a group of four children with OI type VI who were followed for two years[9,10].
Nevertheless, data about the safety of denosumab in growing children is limited. One area of concern is the so-called ‘rebound hypercalcemia’[11]. A recent review on denosumab therapy for pediatric bone disorders found that the literature contained reports on 45 children who had received denosumab for a range of conditions[11]. ‘Severe hypercalcemia’ after denosumab discontinuation was reported in 5 (4 published, 1 unpublished) of these 45 children (11%) (Table 1). For most of the other denosumab-treated children, the cited reports did not contain information about post-treatment serum calcium levels, and therefore the currently reported rate of denosumab-associated hypercalcemia in children may be underestimated. The published reports indicate that the time between the last dose of denosumab and the detection of hypercalcemia ranged from 7 weeks to 5 months. As the proposed protocol for OI type VI consists of denosumab injections that are given every 10-12 weeks[9,10], it is therefore possible that disturbances in calcium metabolism develop not only after treatment discontinuation but also in the interval between two denosumab injections.
Table 1.
Published reports on denosumab-associated hypercalcemia in children.
| Reference | Patient | Diagnosis | Dmab Doses (N) | Dmab Dosing | Time after last Dmab when hypercalcemia was noted |
|---|---|---|---|---|---|
| Boyce 2012 [24] | Boy, 9 y | Fibrous dysplasia | 7 | 1-1.5 mg/kg once per month | 2 months |
| Grasemann 2013 [18] | Girl, 7 y | Juvenile Paget’s | 2 | 1 mg/kg and 0.5 mg/kg with 6-week interval | 7 weeks |
| Gossai 2015 [25] | Girl, 10 y | Giant cell tumor | 27 | 120 mg once per month | 5 months |
| Setsu 2016 [26] | Boy, 10 y | Giant cell tumor | 12 | 120 mg once per month | 4 months |
Abbreviations: Dmab, denosumab; y, years.
In the present report, we describe our observations on bone and mineral metabolism during ongoing denosumab treatment in four growing children with OI type VI, including hypercalcemia while actively on therapy.
Subjects and methods
The study population was comprised of 4 children with OI type VI, who were followed at the Shriners Hospital for Children in Montreal or at the Children’s Hospital of Eastern Ontario in Ottawa. Clinical data were obtained by retrospective chart review. Informed consent was obtained as required by the relevant Institutional Review Boards.
Patients received denosumab at a dose of 1 mg per kg of body mass by subcutaneous injection. The per-protocol treatment interval was 3 months, but varied according to clinical context, as indicated in the individual case descriptions. Blood samples were obtained after an overnight fast between 8:00 a.m. and 10:00 a.m. Serum levels of C-telopeptide of collagen type I (CTX) were measured by immunochemiluminescence assay on an automated analyzer (IDS-ISYS, Immunodiagnostic Systems Inc, Gaithersburg, MD, USA). Lumbar spine (L1-L4) areal bone mineral density (LS-aBMD) was determined in the anterior-posterior direction by dual-energy x-ray absorptiometry using a Hologic QDR Discovery (Hologic Inc., Waltham, MA, USA) or a Lunar Prodigy (General Electric; Madison, WI, USA) device. Results were transformed to age-specific z-scores using published reference data[12-15].
Results
Patient 1 was a boy with a homozygous stop mutation in SERPINF1 who had received intravenous pamidronate therapy every 4 months from 3.1 years to 8.7 years of age (Table 2). Despite regular denosumab injections, the bone resorption markers serum CTX (measured immediately prior to each denosumab injection) increased and hypercalciuria developed (Figure 1). However, total serum calcium levels were within the reference range (2.25 mmol/l to 2.63 mmol/l) at each clinic visit during denosumab therapy (data not shown).
Table 2.
Medical treatment history of the study group.
| Sex | Time of previous Bsp (y) | Age at first Dmab (y) | Time of FU with Dmab (y) | Height z-score at first Dmab | LS-aBMD z-score at first Dmab | LS-aBMD z-score at last Dmab | |
|---|---|---|---|---|---|---|---|
| Patient 1 | M | 5.6 | 9.0 | 3.5 | -1.8 | -0.9 | -1.8 |
| Patient 2 | M | 1.6 | 3.6 | 2.5 | -2.9 | -2.9 | -2.9 |
| Patient 3 | F | 0.7 | 2.7 | 1.3 | -2.2 | -3.5 | -0.6 |
| Patient 4 | M | 0.3 | 1.9 | 2.6 | -1.2 | -0.5 | -1.8 |
Abbreviations: Bsp, bisphosphonate; Dmab, denosumab; FU, follow-up; LS-aBMD, lumbar spine areal bone mineral density; y, years.
Figure 1.
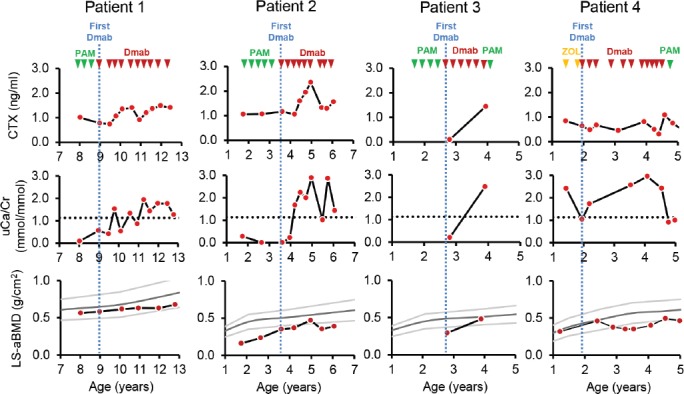
Treatments and outcome measures for each patient. The times of intravenous bisphosphonate infusions and of subcutaneous denosumab injections are indicated by triangles. The time of the first denosumab injection for each individual is highlighted by blue vertical lines. Abbreviations: CTX: C-telopeptide of collagen type I; Dmab: denosumab; LS-aBMD: lumbar spine areal bone mineral density; PAM: pamidronate; uCa/Cr: urinary calcium to creatinine ratio; ZOL: zoledronate.
Patient 2 was a boy with compound heterozygous SERPINF1 mutations that result in premature termination codons. He was treated with intravenous pamidronate between the ages of 1.8 years and 3.3 years. In the first 16 months following the start of denosumab, serum CTX and urinary calcium/creatinine ratio steadily increased, but LS-aBMD z-scores nevertheless rose into the reference range (Figure 1). Given the normalized LS-aBMD, we opted to decrease the exposure to denosumab by increasing the interval to the next denosumab injection to 6 months. Six months after the previous denosumab injection, the LS-aBMD z-score had dropped by 2.1, and serum CTX and the urinary calcium/creatinine ratio had decreased. Thereafter, denosumab injections were continued every 3 months and LS-aBMD started to increase again. Total serum calcium levels were within the reference range at each clinic visit during denosumab therapy (data not shown).
Patient 3 was a girl with a homozygous splice mutation in SERPINF1. She underwent four cycles of intravenous pamidronate starting at 1.7 years of age. The first denosumab injection was given at another institution. When she was first examined at our hospital five weeks later, serum CTX was at the lower limit of detection (Figure 1). She continued to receive denosumab every 3 months at another institution and was re-assessed at our hospital at the age of 3.9 years (12 weeks after the last denosumab injection). At that time, LS-aBMD z-score was at -0.6, serum CTX had increased about 15-fold compared to the first evaluation and there was marked hypercalciuria (Figure 1). The serum level of ionized calcium was at 1.54 mmol/l (norm: 1.19 mmol/l to 1.36 mmol/l), indicating hypercalcemia, and serum parathyroid hormone was suppressed to below the limit of detection. She received an injection of denosumab. Serum ionized calcium was within normal limits four weeks later, but mild hypercalcemia returned (ionized calcium of 1.38 mmol/l) seven weeks after the denosumab injection. Hypercalcemia was then successfully treated with a course of intravenous pamidronate.
Patient 4 was a boy with a homozygous splice mutation in SERPINF1. He received two infusions of zoledronate, starting at 1.4 years of age. Denosumab was first given every three months, but subsequently the interval between denosumab injections was up to six months because the boy underwent intramedullary rodding procedures and denosumab was delayed until there was sufficient healing of osteotomy sites (Figure 1). During this time, the LS-aBMD z-score decreased, to a nadir of -3.1. The interval between denosumab injections was subsequently reduced to 2 months and the LS-aBMD z-score increased. There was persistent hypercalciuria during denosumab therapy but serum calcium levels initially remained within the reference range at each treatment visit. However, at 4.6 years of age (9 weeks after the preceding denosumab injection), the boy developed hypercalcemia (serum ionized calcium at 1.62 mmol/l). After an intravenous infusion of pamidronate, serum calcium returned into the reference range. Kidney ultrasound was performed after the episode of hypercalcemia and showed evidence for nephrocalcinosis, whereas nephrocalcinosis had been absent prior to antiresorptive therapy.
Discussion
In this case series, we describe four children with OI type VI who were treated with subcutaneous denosumab injections, starting between 1.9 and 9.0 years of age. All four children developed hypercalciuria during ongoing denosumab therapy. In two children aged 3.9 and 4.6 years, episodes of hypercalcemia were observed between 7 and 12 weeks after the preceding denosumab injection, and one boy developed nephrocalcinosis.
The initial reports on denosumab treatment in children with OI type VI stated that hypercalcemia was not observed; data on urinary calcium excretion were not reported[9,10]. However, it was noted that the antiresorptive effect of a denosumab injection seemed to last only 6 to 8 weeks, which prompted the investigators to decrease the interval between denosumab injections from the original 12 weeks to a ‘minimum 10-week’ interval[10]. It is possible that this shortening of the treatment interval was sufficient to prevent hypercalcemia. However, one of the patients in the present series had hypercalcemia only 7 weeks after the preceding denosumab injection. Possibly, the propensity for developing hypercalcemia during denosumab treatment varies not only with the diagnosis, but individual characteristics, such as age, growth rate or activity of bone metabolism.
Denosumab is a monoclonal antibody that inactivates RANKL, a protein that stimulates osteoclast formation[8]. Very little is known about the pharmacokinetics and pharmacodynamics of denosumab in children, but in adults the clearance of denosumab seems to depend on the amount of available RANKL[16]. There is no information about the amount of RANKL produced by children at different ages and nothing is known about how RANKL production is influenced by the medical conditions that might be treated with denosumab in children at any age. In general terms, if the RANKL antibody concentration is low relative to the concentration of RANKL, the antibody will be eliminated rapidly[17]. It appears therefore that a denosumab dose of 1 mg per kg body mass is insufficient to reliably block the RANKL produced by children for a predictable period of time.
The short duration of denosumab action is often cited as an advantage over bisphosphonates, as bisphosphonates can suppress bone resorption for several years[9,10]. However, this perceived advantage of denosumab comes at a cost. From the perspective of the mechanostat model, bone tissue that has been added by the antiresorptive action of denosumab will be sensed as ‘unnecessary excess bone’ and will be removed as soon as the antiresorptive effect of the drug has run its course. In accordance with this view, two of our patients experienced a rapid decrease in bone density when the interval between denosumab injections was extended to 6 months for clinical reasons.
The speed and duration of the bone loss after the antiresorptive effect of a denosumab injection is over may depend on the underlying rate of RANKL production and the amount of ‘excess bone’ that has accumulated while the drug was effective. Denosumab-associated hypercalcemia can develop very quickly in children, as evidenced in an 8-year old denosumab-treated girl with juvenile Paget’s disease who had a very high serum calcium concentration only days after a blood test had shown normocalcemia[18]. How long this hypercalcemia persists if left untreated is unknown. We therefore can not exclude that some episodes of transient hypercalcemia were missed in our patients, as serum calcium levels were only determined when patients returned for denosumab injections.
These considerations raise the question whether the short duration of action of denosumab actually is an advantage for children who have persistent disorders such as OI. It is possible that the long-term antiresorptive action of a bisphosphonate is still needed to prevent intermittent hypercalciuria and hypercalcemia during denosumab treatment and to prevent rapid bone loss once denosumab is discontinued.
Hypercalcemia in the context of denosumab treatment has now been observed in children with a variety of conditions that are usually associated with high bone turnover (Table 1). Bone turnover is also increased in OI caused by mutations in collagen type I encoding genes[19]. Even though a study on children with OI did not report hypercalcemia, serum calcium levels increased and parathyroid hormone levels decreased throughout the 48 week observation interval[20]. It is however unclear whether children with chronic illnesses due to glucocorticoid exposure, systemic inflammation or mobility disorders that are usually associated with low bone turnover may be less susceptible to the effects of acute re-activation of osteoclast activity[21-23].
In summary, our observations suggest that the duration of action of denosumab (at a dose of 1 mg per kg body mass) is short and variable in children with OI type VI. This can lead to hypercalciuria, unpredictable episodes of hypercalcemia, nephrocalcinosis and rapid bone loss. These observations call into question the concept that denosumab can be used as a stand-alone alternative to bisphosphonates to treat children with OI type VI. Whether children with other low bone mass disorders are also susceptible to this rebound effect remains to be determined.
Acknowledgements
LMW is supported by the Research Chair program at the University of Ottawa and the Departments of Pediatrics and Surgery, Children’s Hospital of Eastern Ontario. This study was supported by the Shriners of North America.
Footnotes
The authors have no conflict of interest.
References
- 1.Trejo P, Rauch F. Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment. Osteoporos Int. 2016;27:3427–37. doi: 10.1007/s00198-016-3723-3. [DOI] [PubMed] [Google Scholar]
- 2.Bardai G, Moffatt P, Glorieux FH, Rauch F. DNA sequence analysis in 598 individuals with a clinical diagnosis of osteogenesis imperfecta:diagnostic yield and mutation spectrum. Osteoporos Int. 2016;27:3607–13. doi: 10.1007/s00198-016-3709-1. [DOI] [PubMed] [Google Scholar]
- 3.Homan EP, Rauch F, Grafe I, et al. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res. 2011;26:2798–803. doi: 10.1002/jbmr.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88:362–71. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R. Osteogenesis imperfecta type VI:a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002;17:30–8. doi: 10.1359/jbmr.2002.17.1.30. [DOI] [PubMed] [Google Scholar]
- 6.Land C, Rauch F, Travers R, Glorieux FH. Osteogenesis imperfecta type VI in childhood and adolescence:Effects of cyclical intravenous pamidronate treatment. Bone. 2007;40:638–44. doi: 10.1016/j.bone.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Trejo P, Palomo T, Montpetit K, Fassier F, Sato A, Glorieux FH, et al. Long-term follow-up in osteogenesis imperfecta type VI. Osteoporos Int. 2017 doi: 10.1007/s00198-017-4141-x. [DOI] [PubMed] [Google Scholar]
- 8.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates:different mechanisms of action and effects. Bone. 2011;48:677–92. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Semler O, Netzer C, Hoyer-Kuhn H, Becker J, Eysel P, Schoenau E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. 2012;12:183–8. [PubMed] [Google Scholar]
- 10.Hoyer-Kuhn H, Netzer C, Koerber F, Schoenau E, Semler O. Two years'experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet J Rare Dis. 2014;9:145. doi: 10.1186/s13023-014-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyce AM. Denosumab:an Emerging Therapy in Pediatric Bone Disorders. Curr Osteoporos Rep. 2017 doi: 10.1007/s11914-017-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salle BL, Braillon P, Glorieux FH, Brunet J, Cavero E, Meunier PJ. Lumbar bone mineral content measured by dual energy X-ray absorptiometry in newborns and infants. Acta Paediatr. 1992;81:953–8. doi: 10.1111/j.1651-2227.1992.tb12152.x. [DOI] [PubMed] [Google Scholar]
- 13.Southard RN, Morris JD, Mahan JD, Hayes JR, Torch MA, Sommer A, et al. Bone mass in healthy children:measurement with quantitative DXA. Radiology. 1991;179:735–8. doi: 10.1148/radiology.179.3.2027984. [DOI] [PubMed] [Google Scholar]
- 14.Glastre C, Braillon P, David L, Cochat P, Meunier PJ, Delmas PD. Measurement of bone mineral content of the lumbar spine by dual energy x-ray absorptiometry in normal children:correlations with growth parameters. J Clin Endocrinol Metab. 1990;70:1330–3. doi: 10.1210/jcem-70-5-1330. [DOI] [PubMed] [Google Scholar]
- 15.del Rio L, Carrascosa A, Pons F, Gusinyé M, Yeste D, Domenech FM. Bone mineral density of the lumbar spine in white Mediterranean Spanish children and adolescents:changes related to age, sex, and puberty. Pediatr Res. 1994;35:362–6. doi: 10.1203/00006450-199403000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Sutjandra L, Rodriguez RD, Doshi S, Ma M, Peterson MC, Jang GR, et al. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet. 2011;50:793–807. doi: 10.2165/11594240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Zheng S, Gaitonde P, Andrew MA, Gibbs MA, Lesko LJ, Schmidt S. Model-based assessment of dosing strategies in children for monoclonal antibodies exhibiting target-mediated drug disposition. CPT Pharmacometrics Syst Pharmacol. 2014;3:e138. doi: 10.1038/psp.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasemann C, Schundeln MM, Hovel M, Schweiger B, Bergmann C, Herrmann R, et al. Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget's disease. J Clin Endocrinol Metab. 2013;98:3121–6. doi: 10.1210/jc.2013-1143. [DOI] [PubMed] [Google Scholar]
- 19.Rauch F, Lalic L, Roughley P, Glorieux FH. Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. J Bone Miner Res. 2010;25:1367–74. doi: 10.1359/jbmr.091109. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, et al. Safety and efficacy of denosumab in children with osteogenesis imperfecta - a first prospective trial. J Musculoskelet Neuronal Interact. 2016;16:24–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Misof BM, Roschger P, Klaushofer K, Rauch F, Ma J, Mack DR, et al. Increased bone matrix mineralization in treatment-naive children with inflammatory bowel disease. Bone. 2017 doi: 10.1016/j.bone.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Misof BM, Roschger P, McMillan HJ, Ma J, Klaushofer K, Rauch F, et al. Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with Duchenne muscular dystrophy:A paired transiliac biopsy study. J Bone Miner Res. 2016;31:1060–9. doi: 10.1002/jbmr.2756. [DOI] [PubMed] [Google Scholar]
- 23.Ward LM, Rauch F, Matzinger MA, Benchimol EI, Boland M, Mack DR. Iliac bone histomorphometry in children with newly diagnosed inflammatory bowel disease. Osteoporos Int. 2010;21:331–7. doi: 10.1007/s00198-009-0969-z. [DOI] [PubMed] [Google Scholar]
- 24.Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012;27:1462–70. doi: 10.1002/jbmr.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gossai N, Hilgers MV, Polgreen LE, Greengard EG. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer. 2015;62:1078–80. doi: 10.1002/pbc.25393. [DOI] [PubMed] [Google Scholar]
- 26.Setsu N, Kobayashi E, Asano N, Yasui N, Kawamoto H, Kawai A, et al. Severe hypercalcemia following denosumab treatment in a juvenile patient. J Bone Miner Metab. 2016;34:118–22. doi: 10.1007/s00774-015-0677-z. [DOI] [PubMed] [Google Scholar]


