Abstract
High impact exercise programmes can improve bone strength, but little is known about whether this type of training further benefits fracture risk by improving physical function in older people.
Objectives:
This study investigated the influence of high impact exercise on balance, muscle function and morphology in older men.
Methods:
Fifty, healthy men (65-80 years) were assigned to a 6-month multidirectional hopping programme (TG) and twenty age and physical activity matched volunteers served as controls (CG). Before and after training, muscle function (hop performance, leg press and plantar- and dorsiflexion strength) and physiological determinants (muscle thickness and architecture) as well as balance control (sway path, one leg stance duration) were measured. Resting gastrocnemius medialis (GM) muscle thickness and architecture were assessed using ultrasonography.
Results:
Significant improvements in hop impulse (+12%), isometric leg-press strength (+4%) and ankle plantarflexion strength (+11%), dorsiflexor strength (+20%) were found in the TG compared to the CG (ANOVA interaction, P<0.05) and unilateral stance time improved over time for TG. GM muscle thickness indicated modest hypertrophy (+4%), but muscle architecture was unchanged.
Conclusion:
The positive changes in strength and balance after high impact and odd impact training would be expected to improve physical function in older adults.
Keywords: High Impact Exercise, Neuromuscular Function, Balance Control, Postural Sway
Introduction
High impact plyometric exercise programmes (e.g. involving jumping and hopping) are recommended for improving bone mineral density (BMD) and structure through exposing bone to mechanical strain[1]. This type of exercise was feasible and effective among older men, who demonstrated good adherence (>90% over a 12 month period) and increased femoral neck BMD, with localised skeletal adaptation across the proximal femur[2-3]. It is still unknown whether high impact exercise programmes can further reduce fracture risk by improving fall risk factors, or physical function in older adults.
Lower-limb muscle weakness and imbalances predispose older adults to an increased risk of falls[4]. The loss in neuromuscular function (strength and power) with age[5] and functional impairments may also eventually affect everyday activities such as ascending stairs or standing from a chair[6-7]. The loss of strength in the ankle plantarflexors with age in particular has been associated with poor balance[8] and an increased risk of falls[9]. More recently, ankle plantarflexor strength has been shown as a stronger predictor of mobility than knee extension strength or power in older people[10]. The age-related reduction in joint moment and power at the ankle, but not at knee or hip, during walking and running also suggests that the propulsive deficit of the ankle contributes most to locomotor decline with age[11].
High impact, plyometric exercises involve extensive ankle plantarflexion activity[12-13] so this type of training may have beneficial effects on functional performance capacity and balance control in older people. However, very few studies have investigated the effects of high impact training on muscle function and balance control in this population and results are inconsistent across studies[14-17]. Some studies have reported improvements in dynamic balance, leg-extensor power[14] and chair-stand performance[15], when high impact jumps are combined with additional training components (such as aerobic step, strength or gymnastic exercises), while others have reported no change in ankle plantar flexor strength, leg-extensor power[16] or balance[17] following high impact jump/hop training alone.
High impact exercises performed in both anteroposterior and mediolateral directions may provide a further challenge to balance control and stimulate adaptations that may benefit postural stability in both mediolateral and anteroposterior directions. The magnitude of the changes in neuromuscular function and physiological adaptations, aswell as implications for postural balance, resulting from multidirectional high impact, plyometric exercise are currently unknown. A greater understanding of these adaptations will be invaluable in optimising the improvements in neuromuscular function and balance following high impact exercise regimes. Therefore the aim of this study was to investigate the influence of a programme of multidirectional, high impact exercise on muscle function (hop performance, leg press and plantar- and dorsiflexion strength) and morphological/architectural features (thickness, pennation angle, fascicle length) as well as balance control (sway path, one leg stance duration) in older men.
Methods
Participants
Seventy healthy men aged between 65 and 80 yrs, were recruited from the local community to form a training group (TG [n=50]) and a control group (CG [n=20]). The bone response to 12 months unilateral high impact exercise in the TG has been reported previously[2-3]. All men had no history of strength training or recent (previous 12-months) involvement in strength, power or weight-bearing endurance exercise for more than 1 hr/wk. Men that had previous or existing injuries to the lower limbs or a history (previous 12-months) of diagnosed symptomatic diseases, as well as current medical or surgical problems likely to influence or provide contraindications to neuromuscular function were excluded from taking part in the study. All participants completed health and lifestyle questionnaires and provided written informed consent. The study was approved by the Loughborough University ethical advisory committee and the UK National Research Ethics Service.
Study design
Participants in the TG completed a high impact exercise intervention trial, specifically multi-directional hopping intervention over a 6-month period. Neuromuscular and balance measurements were conducted unilaterally on a randomly assigned trained leg in the TG and on a randomly chosen leg in the CG, before (pre-intervention) and one day after the 6-month training/control period (post-intervention). The familiarisation session and pre and post-intervention measurement sessions consisted of a standing balance test, a series of maximal hops, maximum isometric contractions involving the leg and ankle plantar- and dorsiflexor muscles, and ultrasound recordings of the resting gastrocnemius medialis (GM). All measurements were taken in this order and conducted at a consistent time of day. Height was measured to the nearest 0.001 m using a wall mounted stadiometer (Holtain Ltd, Crymych UK) and body mass was recorded the nearest 0.1 kg using a beam balance scale (Avery Ltd, Fairmont, MN USA) before the start of each session. All participants were requested to maintain a consistent lifestyle, with no unaccustomed exercise or diet during the study period.
High impact exercise programme
The exercise programme was performed with one randomly allocated leg of participants in the TG, 7 days a week, for a 6 month period. Each training session consisted of mobilisation exercises for the knee extensor/flexor and ankle plantar flexor/dorsi flexor muscle groups followed by ~5 minutes of high impact work. The high impact exercises involved very brief bursts (5 sets x 10 repetitions, with a 15 s rest period between each set) of one-legged hopping exercises that were performed in variety of directions on a hard, even surface in barefoot. Training sessions were completed at home for the 6-month training period and during supervised sessions at the start of training programme. Each training session was recorded in a log book. The progression of the training programme for the first 11 weeks was consistent with that described previously[2]. Subjective ratings of the exercise programme’s delivery and acceptability were assessed by an exercise evaluation form at the end of the study by participants in the TG.
Static balance
Balance was recorded with a force plate (9286AA, Kistler Instruments Ltd, London, UK), connected to a PC utilising Bioware Software (Kistler Instruments Ltd, London, UK) set to sample vertical and horizontal ground reaction forces at 100 Hz[18]. Participants stood in the centre of the force plate in an upright and relaxed position with feet shoulder width apart. They were instructed to keep their eyes open with their vision fixed on a visual reference point at eye level, 3 m in front of them. Following 1 practice attempt, participants performed 3 trials (each separated by >30 s) of unilateral balance for 30 s by placing their hands on hips and lifting up their uninvolved leg to 90° of knee flexion. Stance time was recorded with a stop watch and termination or a failed test was recorded if their uninvolved foot touched the standing leg or the floor, or they removed their hands from their hips.
Static balance analysis: duration and postural sway
Duration of standing unilateral balance was defined as the average time in seconds over three 30 s trials for which participants could stand on one leg. The Ax (amplitude of postural sway in the anteroposterior [AP] direction) and Ay (amplitude of sway in the mediolateral [ML] direction) measurements of the centre of pressure were recorded during 30 s of unilateral stance and were then low pass filtered with a cut off frequency at 10 Hz using a Butterworth filter. The cut off frequency at 10 Hz was determined using residual analysis. Sway path was calculated in millimetres as the sum of the displacement between measurement time points of the centre of pressure during the 30 s time period. The stance with the lowest sway path from three 30 s trials was used for further analysis.
Muscle function
Hop performance
Hop performance was recorded with a force plate (9286AA, Kistler Instruments Ltd, London, UK), connected to a PC utilising Spike 2 software (version 7.02a; CED, Cambridge, UK) that sampled vertical ground reaction force (Fz) at 2000 Hz. Sampling was initiated prior to the participant standing on the force plate. Participants stood in the centre of the force plate and were instructed to stand upright and still with their shoulders back and arms by their sides for ≥5 s. This was to ensure that the vertical force (Fz) signal at the start of the hop was equal to the participant’s body weight and that initial velocity was zero. From stationary standing, participants were instructed to lift up their contralateral leg (to 90° of knee flexion) and then hop as high as possible on the allocated training leg for the TG and measured leg for the CG. Following 1-2 practice attempts, participants completed 3 maximal hops (each separated by >30 s) with hands placed on their hips. All hops were performed with a countermovement, at a self-selected depth and rate. A hop was repeated if the participant: (i) did not take off or land successfully in the centre of the force plate on one leg; (ii) felt that their attempt was not maximal or; (iii) their hands came off the hips at any time during the movement (take-off, flight and landing).
Hop performance analysis
A stable 1 s period of Fz during quiet standing (≥5 s) was used to calculate body mass. The start of the hop movement was identified visually and defined as a stable period of Fz prior to deflection away from the baseline force due to body mass. Net impulse (IMP) (a determinant of hop height[19-20]) was calculated for each hop from the start of the movement to the instant of take-off using the force due to body mass as the datum. Relative impulse (IMPbm) was determined by dividing impulse by body mass. The highest impulse from three hops was used for further analysis.
Leg-press strength
Isometric leg-press strength was measured on a modified recumbent leg-press machine. A detailed account of this leg-press set-up by our group has been described previously[5]. Briefly, the leg-press machine was instrumented with a force plate mounted on the foot plate (9286AA, Kistler Instruments Ltd, London, UK) which recorded the vertical ground reaction force at 2000 Hz on a PC utilising Spike 2 software (Spike2 5.21, CED, Cambridge). Maximum isometric strength was assessed at -8% of standing height defined relative to full knee and hip extension (i.e. leg straight) for both pre and post measurements. Once supine in the leg-press dynamometer, participants were instructed to position their foot on the force plate, hold onto arm supports for stability and hold the opposite leg in a relaxed position without support. Following a 2 minute warm up of sub-maximal contractions, participants completed 3-4 maximum voluntary contractions (MVCs) by trying to extend the hip, knee and ankle joints as forcefully as possible for ~4 s with ≥30 s rest between each contraction. Biofeedback and verbal encouragement were provided between each contraction. The force signal was low-pass filtered at 100 Hz in both directions using a 2nd order Butterworth digital filter and the highest instantaneous force achieved from 3 contractions was defined as maximum isometric strength.
Ankle plantar and dorsiflexion strength
Isometric ankle plantar and dorsiflexion strength were recorded unilaterally while participants lay prone on a calibrated isokinetic dynamometer (Lumex Inc, New York, USA) with the knee at full extension and the lateral malleolus aligned with the dynamometer’s axis of rotation. The foot of each participant was firmly secured to a modified dynamometer foot adapter. Modifications included a deeper, narrower heel bracket and additional strapping across the dorsum of the foot. These modifications enabled participants to perform maximal contractions comfortably whilst their bare foot was firmly secured to the footplate. To localise plantar/dorsiflexion and avoid synergistic contribution, participants were restrained at the waist and distal part of the thigh. To aid stability, participants were instructed to hold onto the long handles either side of the dynamometer. Following a 3 min warm up of sub-maximal isometric plantarflexion contractions, participants completed 3-4 MVCs at a joint angle of 0° (plantar surface of the foot perpendicular to the tibial shaft) by plantarflexing as forcefully as possible against the foot plate for 3 s, with a 60 s rest between each contraction. The same procedure was followed for dorsiflexion contractions except participants were instructed to dorsiflex the foot as forcefully as possible. The torque output was displayed on an additional monitor positioned so that participants had biofeedback during each contraction and they were enthusiastically encouraged to achieve a higher peak torque with each subsequent contraction. The highest instantaneous torque achieved from 3-4 contractions was defined as isometric ankle plantar / dorsiflexion strength.
Gastrocnemius medialis muscle thickness and architecture
Participants laid supine for ~15 minutes before the collection of muscle thickness ultrasound images in order to minimise any fluid shifts following functional measurements. Images of the gastrocnemius medialis (GM) were taken using B-Mode real-time ultrasonography (Toshiba, Power Vision 6000, SSA-37OA; Mount International Ultrasound Services Ltd) with a scanning width and depth of 60 and 50 mm respectively, whilst participants lay prone on the isokinetic dynamometer (as described above). The ultrasound images were taken at 30% of the tibial length, defined as the distance from the popliteal crease to the midpoint of the lateral malleolus. The linear array probe (7.5-MHz B-mode) was positioned perpendicular to the longitudinal axis of the GM muscle, at the midpoint of the GM muscle belly; identified as 50% of the length between the popliteal fossa and Achilles insertion using a tape measure. Each measurement was marked on the skin and an echo-absorbent marker (elastic band) was taped to the skin beneath the ultrasound probe to standardised positioning. A water soluble gel (Aquasonic 100 Ultrasound Transmission Gel) was coated over the probe to assist with wave transmission and thus image clarity. The ultrasound images were recorded at 39 Hz on miniDV tapes via a camcorder (Sony Walkman, GV-D900E, Tokyo, Japan) interfaced with the ultrasound machine, and then imported to image analysis software (ImageJ 1.46r; National Institutes of Health, Bethesda, MD, USA). The video frame most clearly displaying the muscle fascicles and deep and superficial aponeuroses was analysed for each participant. Muscle thickness (MT) was measured as the mean of the distance between the superficial and deep aponeurosis in the proximal, central and distal regions of the image recorded. Two to three fascicles in the same video frame were analysed for pennation angle (θ) the angle of insertion of the muscle fascicles into the deep aponeurosis, and fascicle length (Lf) the length of the fascicles between the deep and superficial aponeuroses. In images where the full length of the fascicles was not visible, it was necessary to linearly extrapolate the fascicle length using trigonometry[18]. Multiple measurements were averaged to provide a representative value.
Statistical analysis
Statistical analysis for all variables was conducted using PASW Statistics (21.0; SPSS Inc, Chicago, IL, USA). Unpaired t-tests were used to determine pre-intervention differences between the two groups (TG vs. CG) for all variables. For variables meeting assumptions for parametric testing, two-way factorial analysis of variance (ANOVA) was used to identify any group (TG vs. CG) x time (Pre vs. Post) interactions. For variables that were not normally distributed Wilcoxon matched pairs test was used to compare pre and post exercise values for both the TG and CG. For all statistical analyses, the significance level was set at P<0.05 and results are expressed as mean±SEM, unless otherwise noted.
Results
Exercise adherence and evaluation
Thirty-nine men out of fifty men in the TG completed the 6-month exercise programme and eighteen out of twenty men in the CG completed post-intervention measurements. Reasons for participant withdrawal in the CG were due to illness (n=1) and loss of interest (n=1). Reasons for participant withdrawal in the TG were due to discomfort from exercise due to the reoccurrence of injury experienced more than 12 months before enrolment into the study (knee pain, n=1, sciatic pain n=1), time commitments (n=2) and health problems or injuries that were unrelated to the exercise intervention (n=7). Amongst the TG participants that completed the intervention, adherence to the prescribed 6-month exercise programme was 92% (135±16 sessions completed out of 147 prescribed sessions. Thirty-two men in the TG completed the exercise evaluation form at the end of the study. Almost all participants (94%) reported that they found it easy to remember to perform the exercises at home, and 38% reported that they preferred to carry out exercises at home than in supervised group sessions. More than half of participants (53%) reported that they felt their balance or coordination in everyday activities had improved, and 66% of participants reported that they would be willing to take up new exercises after the study.
Physical characteristics
At baseline, there were no differences between groups for physical characteristics (body mass, BMI or duration of habitual physical activity [unpaired t-test, All, P≥0.434; Table 1) or for muscle function, balance or muscle thickness and architecture (unpaired t-test, All, P≥0.431; Table 2). There were no significant changes pre- to post-intervention in the TG and CG for body mass or BMI (ANOVA interaction, All, P≥0.111).
Table 1.
Physical characteristics of participants in the TG and CG prior to the exercise programme.
| TG (n=39) | CG (n=18) | |
|---|---|---|
| Age (y) | 70.2±3.8 | 70.1±4.1 |
| Height (cm) | 176.4±6.6 | 174.9±6.7 |
| Body mass (kg) | 81.6±8.7 | 82.1±14.6 |
| BMI (kg/m2) | 26±2 | 27±4 |
| Habitual physical activity duration (hrs/wk) | 1.8±2 | 1.5±1.4 |
Values are presented as mean ± SD.
Table 2.
Muscle function and balance in the TG (n=39) and CG (n=18) of older men pre and post intervention.
| TG | CG | ||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ANOVA Interaction P | |
| Hop performance | |||||
| Impulse (N.s) | 76±3 | 84±3 | 79±4 | 76±6 | 0.002* |
| Impulse relative to body mass (N.s/kg) | 0.09±0.04 | 0.10±0.04 | 0.10±0.06 | 0.09±0.07 | 0.004* |
| Isometric strength | |||||
| Leg press force (N) | 1622±61 | 1664±74 | 1637±94 | 1480±91 | 0.018* |
| Leg press force (N.kg-1) | 20.1±0.8 | 20.4±1.0 | 20.5±1.4 | 18.6±1.2 | 0.020* |
| Ankle plantarflexion torque (Nm) | 92.7±3.7 | 100.6±3.2 | 96.7±4.0 | 94.7±5.6 | 0.030* |
| Ankle plantarflexion torque (Nm.kg-1) | 1.14±0.05 | 1.23±0.05 | 1.20±0.06 | 1.16±0.05 | 0.001* |
| aAnkle dorsiflexion torque (Nm) | 28.4±1.8 | 32.3±1.5 | 29.8±1.9 | 30.8±1.9 | 0.075 |
| aAnkle dorsiflexion torque (Nm.kg-1) | 0.35±0.02 | 0.41±0.02 | 0.37±0.03 | 0.38±0.02 | 0.037* |
| Postural sway | |||||
| b30 s Anterior-posterior swaypath (mm) | 1381.4±135.7 | 1283.4±77.5 | 1302.3±98.0 | 1317.5±134.0 | 0.441 |
| b30 s Mediolateral swaypath (mm) | 1392.1±79.4 | 1318.4±55.8 | 1389.9±96.8 | 1446.0±99.7 | 0.320 |
| b30 s Total swaypath (mm) | 2169.0±153.7 | 2030.2±93.7 | 2113.2±142.0 | 2159.6±172.5 | 0.314 |
Values are mean ± SEM.
TG data n=23,
TG data n=23; CG data n=9.
Static balance
The stance time (average score from 3 x 30 s trials) increased in the TG (+4.0±0.9s, Wilcoxon, P=0.000), but not the CG (+0.2±1.1s, Wilcoxon P=0.753) (Figure 1).
Figure 1.
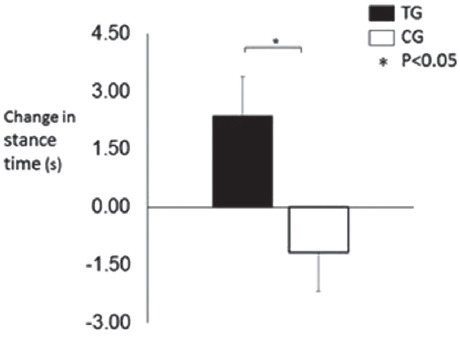
Absolute change in stance time in response to the hopping exercise training/control period.
Seventeen men in the TG and nine men in the CG were unable to maintain unilateral balance for 30 seconds in any of the three trials and were therefore excluded from the analysis of postural sway. The modest declines in total sway, anterior-posterior sway and medio-lateral sway in the TG over the 30 second period (-2.6%, -1.4%, -1.3%) were not significantly different from changes in the CG (+2.1%, +0.5%, +4.0%) (ANOVA interaction, P>0.05; Table 2).
Muscle function
Absolute and normalised to body mass hop impulse, isometric leg-press strength and ankle plantar flexor strength increased in the TG compared to the CG (All, ANOVA interaction, P<0.020, Table 2). The magnitude of change in the TG compared to the CG was +11.7 vs.-4.5% for absolute hop impulse, +10.7 vs. -2.6% for absolute ankle plantar flexor strength (Figure 2), and +4.0 vs. -9.0% for absolute leg-press strength.
Figure 2.
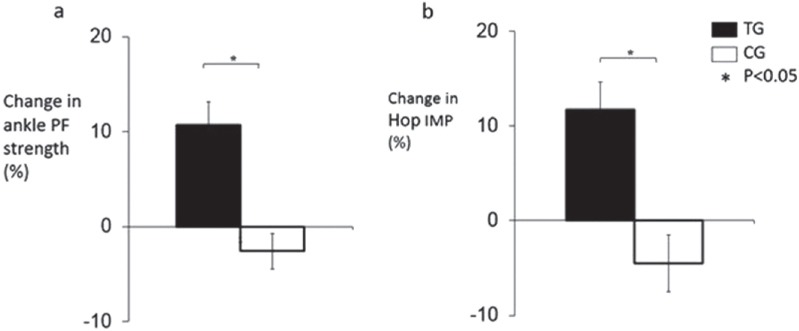
Percentage change in: a) ankle plantarflexion (PF) strength and b) hop impulse (IMP) for the TG and CG in response to the hopping exercise training/control period.
Dorsiflexor strength data were available for twenty-three men out of the thirty-nine men in the TG who completed the exercise programme. Expressed relative to body mass, dorsiflexor strength also increased in the TG (20%) compared to the CG (6%; ANOVA interaction, P=0.037; Table 2), although changes in absolute dorsiflexor strength showed only a tendency for a group x time interaction effect (P=0.075; Table 2).
Gastrocnemius medialis muscle thickness and architecture
Due to technical problems with the ultrasound equipment, ultrasound images were recorded on twenty participants in the TG and fifteen participants in the CG. Poor quality images on three further CG participants were subsequently also excluded from further analysis.
The average between session intra-observer coefficients of variation (based on seven repeat analyses) for muscle thickness, pennation angle and fascicle length were 1.7%, 3.7% and 4.0%, respectively. Muscle thickness increased in the TG compared to the CG (ANOVA interaction, P=0.003; Table 3; Figure 2) by +4.3% vs. -6.9%. There were no significant effects of the exercise programme on pennation angle and changes in fascicle length showed only a tendency for a group x time interaction effect (ANOVA interaction, P=0.082; Table 3).
Table 3.
GM muscle thickness and architectural properties before and after 6-month exercise/control period.
| TG (n=20) | CG (n=15) | ANOVA | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Interaction P | |
| Muscle Thickness (cm) | 1.62±0.09 | 1.68±0.08 | 1.68±0.09 | 1.55±0.07 | 0.003* |
| Pennation angle (°) | 24.9±1.2 | 24.8±1.0 | 24.8±1.0 | 24.4±1.1 | 0.873 |
| Fascicle Length (cm) | 3.83±0.18 | 4.00±0.17 | 3.98±0.19 | 3.80±0.23 | 0.082 |
Values are presented as mean ± SEM.
Discussion
This is the first intervention study to investigate the influence of a high impact exercise programme on balance and muscle function and morphological/architectural features in older men. We found modest improvements in hop performance, lower-limb strength and balance after a high impact exercise intervention with changes differing significantly from those in a control group. Therefore, high impact activity produces beneficial changes in neuromuscular function and sensorimotor control that may benefit physical function and fall risk, in addition to the positive skeletal adaptations previously documented[2-3].
A number of studies have demonstrated that high impact exercises such as hopping involves extensive activity of the plantarflexors[12-13]. Hop performance (measured by net-impulse) and ankle plantarflexor strength showed the greatest magnitude of improvement after the training (11-12%), demonstrating that high impact, unilateral plyometric (hopping) exercise produces changes in neuromuscular function specific to the training task. These findings are consistent with previous studies that have documented task specific muscle function, neural and hypertrophic changes to other types of exercise programmes (i.e. explosive strength training) in young adults[21]. We also observed a small improvement in leg-press strength after the training (+4.0%). As the plantar surface of the foot remains in contact with the foot plate throughout high-load action, this seems likely to reflect improvements in knee and/or hip extension components of the leg press action and indicate that adaptations were not isolated to the plantarflexors. Dorsiflexor strength expressed relative to body mass also increased more in the training than control group. These findings are consistent with a previous study that documented improvements in overall lower limb performance (chair-stand) after a low-impact plyometric exercise intervention in older women[15].
We also investigated whether the muscle morphological or architectural parameters were responsive to the 6 month high impact exercise programme. Thickness of the GM muscle followed similar patterns to the functional changes, with an increase found in the TG that seems to indicate modest hypertrophy. Muscle architecture did not change significantly in the current investigation, which might be expected given the limited hypertrophy that we have documented. A previous study also reported no changes in muscle architecture in response to 12 weeks of repetitive hopping (on balls of feet only)[17]. In addition to hypertrophy it is likely that neural adaptations also contributed to the improvements in function we have documented.
The inability to maintain a one-leg stance has been shown to independently predict injurious falls in community-dwelling older people[22-23] and is routinely used in clinical assessment of frailty in older adults[24]. We found that unilateral stance time improved substantially in the TG compared to the CG, although this improvement was not accompanied by statistically significant changes in sway amplitude (total sway path or multidirectional sway path). Our findings are consistent with previous studies that have found no change in postural sway following a 3 month repetitive hopping programme[17] and 12 month jumping combined with aerobic exercise in older adults[14]. The ability to stand on one leg may depend on a number of factors other than postural adjustments, including; vision, reaction time, vestibular function, peripheral sensation and muscular strength[6]. It is therefore likely that sway path does not change in response to high and odd impact exercise, but with greater plantarflexor strength (and possibly general foot strength) after training, older adults are better able to compensate/correct for the sway without losing balance and therefore manage a longer duration of unilateral stance.
Despite the novel findings of the present study, the study has some limitations. We were unable to randomise participants to training and control groups or blind participants to the intervention, so we cannot discount that motivational differences between these groups may have contributed to some of the changes observed in the present study, particularly declines in the control group. Some of the observed changes were relatively modest (e.g. muscle thickness 4%) and future studies would benefit from more rigorous methods (e.g. MRI) and procedures (e.g. better control for possible fluid shifts). Furthermore, the modest sample size, which was further reduced for some parameters, may mean that the study was not statistically powered to detect smaller effects.
Potential applications
To have sustained benefits, exercise must be acceptable, safe and feasible to maintain in the longer term. The intervention was feasible to fit into everyday life, with 92% able to sustain the intervention for 6 months, which has been identified by older adults as an important facilitator to the adoption of high impact activity in the long-term[25]. The intervention was demonstrated to participants in laboratory sessions, and individually progressed. One concern about hopping exercises is the risk of falls and injury during exercise, but no accidental injuries sustained during exercise were reported in this study. Two participants demonstrated recurrence of a previous injury although in one case this resolved spontaneously on ceasing the intervention. A bilateral intervention consisting of hops on both legs, and/or multidirectional jumping and skipping may be more acceptable to participants and produce greater benefit.
Conclusion
In conclusion, this study demonstrated that a high impact exercise programme improved unilateral stance time, muscle function and muscle thickness in older men. Therefore, high and odd impact exercises, that are required to produce beneficial changes in bone, were also effective for counteracting the age-related decline in lower-limb neuromuscular function and sensorimotor control. In addition to preventing fragility fracture, this type of exercise could potentially offer a way to maintain or improve muscle function and balance of older people, and thus overall physical function. As these adaptations are recognised fall risk factors, simple high impact exercises may benefit fracture risk through improved fall risk as well as increased bone density.
Acknowledgements
The authors are extremely grateful to the participants for their time and commitment given to this project.
Footnotes
The authors have no conflict of interest.
Edited by: A. Ireland
References
- 1.Bolam KA, van Uffelen JG, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men:A systematic review. Osteoporos Int. 2013;24:2749–62. doi: 10.1007/s00198-013-2346-1. [DOI] [PubMed] [Google Scholar]
- 2.Allison SJ, Folland JP, Rennie WJ, Summers GD, Brooke-Wavell K. High impact exercise increased femoral neck bone mineral density in older men:a randomised unilateral intervention. Bone. 2013;53:321–328. doi: 10.1016/j.bone.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 3.Allison SJ, Poole KES, Treece GM, Gee HA, Tonkin C, Rennie WJ, Folland JP, Summers GD, Brooke-Wavell K. The influence of high impact exercise on cortical and trabecular bone mineral content and 3D distribution across the proximal femur in older men:a randomised controlled unilateral intervention. J Bone Miner Res. 2015;30:1709–1716. doi: 10.1002/jbmr.2499. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 5.Allison SJ, Folland JP, Brooke-Wavell K. Multiple joint muscle function with ageing:The force-velocity and power-velocity relationships in young and older men. Aging Clin Exp Res. 2013;25:159–66. doi: 10.1007/s40520-013-0024-y. [DOI] [PubMed] [Google Scholar]
- 6.Granacher U, Muehlbauer T, Gruber M. A qualitative review of balance and strength performance in healthy older adults:impact for testing and training. J Aging Res. 2012;708905 doi: 10.1155/2012/708905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne C, Faure C, Keene DJ, Lamb SE. Ageing, Muscle Power and Physical Function:A Systematic Review and Implications for Pragmatic Training Interventions Sports Med. 2016;46:1311–32. doi: 10.1007/s40279-016-0489-x. [DOI] [PubMed] [Google Scholar]
- 8.Spink MJ, Fotoohabadi MR, Wee E, Hill KD, Lord SR, Menz HB. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Arch Phys Med Rehabil. 2011;92:68–75. doi: 10.1016/j.apmr.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Cattagni T, Scaglioni G, Laroche D, Van Hoecke J, Gremeaux V, Martin A. Ankle muscle strength discriminates fallers from non-fallers. Front Aging Neurosci. 2014;19:336. doi: 10.3389/fnagi.2014.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenroth L, Sillanpää E, McPhee JS, Narici MV, Gapeyeva H, Pääsuke M, Barnouin Y, Hogrel JY, Butler-Browne G, Bijlsma A, Meskers CG, Maier AB, Finni T, Sipilä S. Plantarflexor Muscle-Tendon Properties are Associated With Mobility in Healthy Older Adults. J Gerontol A Biol Sci Med Sci. 2015;70:996–1002. doi: 10.1093/gerona/glv011. [DOI] [PubMed] [Google Scholar]
- 11.Kulmala JP, Korhonen MT, Kuitunen S, Suominen H, Heinonen A, Mikkola A, Avela J. Which muscles compromise human locomotor performance with age? J R Soc Interface. 2014;11(100):20140858. doi: 10.1098/rsif.2014.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffrén M, Ishikawa M, Rantalainen T, Avela J, Komi PV. Age-related muscle activation profiles and joint stiffness regulation in repetitive hopping. J Electromyogr Kinesiol. 2011;21:483–91. doi: 10.1016/j.jelekin.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Moritani T, Oddsson L, Thorstensson A. Phase-dependent preferential activation of the soleus and gastrocnemius muscles during hopping in humans. J Electromyogr Kinesiol. 1991;1:34–40. doi: 10.1016/1050-6411(91)90024-Y. [DOI] [PubMed] [Google Scholar]
- 14.Uusi-Rasi K, Kannus P, Cheng S, Sievänen H, Pasanen M, Heinonen A, Nenonen A, Halleen J, Fuerst T, Genant H, Vuori I. Effect of alendronate and exercise on bone and physical performance of postmenopausal women:a randomized controlled trial. Bone. 2003;33:132–43. doi: 10.1016/s8756-3282(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 15.Sáez de Villarreal E, Requena B, Arampatzi F, Salonikidis K. Effect of plyometric training on chair-rise, jumping and sprinting performance in three age groups of women. J Sports Med Phys Fitness. 2010;50:166–73. [PubMed] [Google Scholar]
- 16.Bassey EJ, Rothwell MC, Littlewood JJ, Pye DW. Pre- and postmenopausal women have different bone mineral responses to the same high-impact exercise. J Bone Miner Res. 1998;13:1805–13. doi: 10.1359/jbmr.1998.13.12.1805. [DOI] [PubMed] [Google Scholar]
- 17.Rantalainen T, Hoffren M, Linnamo V, Heinonen A, Komi PV, Avela J, Nindi BC. Three-month bilateral hopping intervention is ineffective in initiating bone biomarker response in healthy elderly men. Eur J Appl Physiol. 2011;111:2155–62. doi: 10.1007/s00421-011-1849-8. [DOI] [PubMed] [Google Scholar]
- 18.Ruhe A, Fejer R, Walker BF. The test-retest reliability of centre of pressure measures in bipedal static task conditions:a systematic review of the literature. Gait Posture. 2010;32(4):436–45. doi: 10.1016/j.gaitpost.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Kirby TJ, McBride JM, Haines TL, Dayne AM. Relative net vertical impulse determines jumping performance. J Appl Biomech. 2011;27:207–14. doi: 10.1123/jab.27.3.207. [DOI] [PubMed] [Google Scholar]
- 20.Winter E. Jumping:Power or Impulse? Med Sci Sports Exerc. 2005;37:523. [Google Scholar]
- 21.Balshaw TG, Massey GJ, Maden-Wilkinson TM, Tillin NA, Folland JP. Training-specific functional, neural, and hypertrophic adaptations to explosive- vs. sustained-contraction strength training. J Appl Physiol. 2016;120:1364–73. doi: 10.1152/japplphysiol.00091.2016. [DOI] [PubMed] [Google Scholar]
- 22.Chang CJ, Chang YS, Yang SW. Using single leg standing time to predict the fall risk in elderly. Conf Proc IEEE Eng Med Biol Soc. 2013:7456–8. doi: 10.1109/EMBC.2013.6611282. [DOI] [PubMed] [Google Scholar]
- 23.Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45:735–8. doi: 10.1111/j.1532-5415.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 24.Vellas BJ, Rubenstein LZ, Ousset PJ, Faisant C, Kostek V, Nourhashemi F, Allard M, Albarede JL. One-leg standing balance and functional status in a population of 512 community-living elderly persons. Aging. 1997;9:95–8. doi: 10.1007/BF03340133. [DOI] [PubMed] [Google Scholar]
- 25.Simmonds BA, Hannam KJ, Fox KR, Tobias JH. An exploration of barriers and facilitators to older adults'participation in higher impact physical activity and bone health:a qualitative study. Osteoporos Inter. 2016;27:979–87. doi: 10.1007/s00198-015-3376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


