Abstract
Background
Various techniques are used to enhance the results of mechanical thrombectomy with stent-retrievers, including proximal arrest with balloon guide catheter (BGC), conventional large bore proximal catheter (CGC), or in combination with local aspiration through a large-bore catheter positioned at the clot interface (Aspiration-Retriever Technique for Stroke [ARTS]). We evaluated the impact of ARTS in the North American Solitaire Acute Stroke (NASA) registry.
Summary
Data on the use of the aspiration technique were available for 285 anterior circulation patients, of which 29 underwent ARTS technique, 131 CGC, and 125 BGC. Baseline demographics were comparable, except that ARTS patients are less likely to have hypertension or atrial fibrillation. The ARTS group had more ICA occlusions (41.4 vs. 22% in the BGC, p = 0.04 and 26% in CGC, p = 0.1) and less MCA/M1 occlusions (44.8 vs. 68% in BGC and 62% in CGC). Time from arterial puncture to reperfusion or end of procedure with ARTS was shorter than with CGC (54 vs. 91 min, p = 0.001) and was comparable to the BGC time (54 vs. 67, p = 0.11). Final degree of reperfusion was comparable among the groups (TICI [modified Thrombolysis in Cerebral Infarction] score 2b or higher was 72 vs. 70% for CGC vs. 78% for BGC). Procedural complications, mortality, and good clinical outcome at 90 days were similar between the groups.
Key Messages
The ARTS mechanical thrombectomy in acute ischemic stroke patients appears to yield better results as compared to the use of CGCs with no significant difference when compared to BGC. This early ARTS technique NASA registry data are limited by the earlier generation distal large bore catheters and small sample size. Future studies should focus on the comparison of ARTS and BGC techniques.
Keywords: Stroke, Stent retriever, Thrombectomy, Aspiration technique
Introduction
Recent studies have shown that endovascular reperfusion therapies for acute ischemic stroke caused by proximal large vessel occlusion are safe and improve patient clinical outcome when compared to best medical therapy without endovascular intervention [1, 2, 3, 4]. Previous studies of these endovascular rescue therapies had demonstrated that successful reperfusion and the time to successful reperfusion correlate with good clinical outcome [5, 6, 7, 8, 9].
Published multicenter series of endovascular stroke therapy have reported procedural or reperfusion times of 96–126 min with earlier endovascular stroke devices and strategies (defined as beginning with groin puncture to end of procedure) [10, 11, 12, 13]. The time has improved to 36–48 min (although now defined to begin with guide catheter placement in TREVO 2 and STAR and with first guide catheter angiogram in SWIFT to TICI [modified Thrombolysis in Cerebral Infarction] score 2b reperfusion) with more recent series reporting stent retriever technologies [7, 13, 14, 15, 16, 17]. Still, these procedural reperfusion times can represent 38% of the overall time elapsed from symptom onset to reperfusion [10] and so a therapeutic or technical strategy which significantly decreases these procedural reperfusion times may translate into clinically significant decreases in the overall time of ischemia.
Endovascular clot removal with stent retrievers can be performed without aspiration. Clot retrieval combined with aspiration techniques can be performed through a conventional large bore proximal catheter (CGC) positioned with its tip in the cervical carotid (internal carotid or common carotid), or through a balloon guide catheter (BGC) positioned with its tip in the cervical carotid, [18] or through a large bore distal access catheter positioned with its tip intracranially at the proximal clot interface (Aspiration-Retriever Technique for Stroke “ARTS” (also previously referred to as “Solumbra technique”; Fig. 1). Anecdotal reports suggest this latter technique is gaining popularity in real-world use, but there are few other reports in the literature measuring its technical and clinical outcomes [19]. We sought to evaluate the impact of local clot aspiration in a substudy of the North American Solitaire Acute Stroke (NASA) registry.
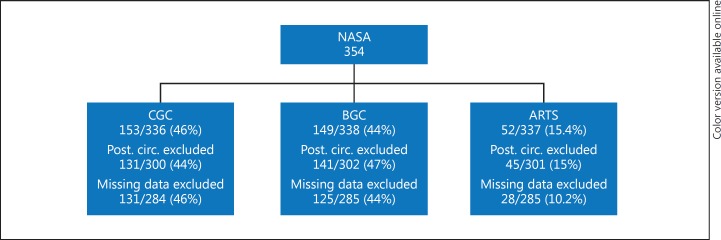
Fig. 1.
ARTS NASA substudy flow chart.
Methods
NASA Design
The investigator-initiated, retrospective NASA registry collected demographic, clinical presentation, procedural details, and site-adjudicated angiographic and clinical outcome data on consecutive acute ischemic stroke patients treated with the Solitaire FR device at the 24 participating North American centers (see Appendix). Local institutional review board approval was obtained for the study protocol and retrospective data collection at each site. The stent retriever procedure was performed utilizing local protocols. Procedural details, clinical events, and outcomes were collected and reported utilizing standardized definitions provided by the registry [20].
The primary comparative groups in this substudy were the combined aspiration-retriever technique in stroke (ARTS), balloon guide catheter adjunctive technique (BGC), and conventional large bore proximal catheter (CGC) groups. The exclusion criteria (Fig. 1) were posterior circulation group, and missing data from the participating site to reach any conclusion about the most likely adjunctive technique that was used.
Procedure reperfusion time was defined as time of groin puncture to revascularization or end of procedure if revascularization of the occluded vessel was not achieved. The primary angiographic outcome was successful reperfusion defined as TICI scores of ≥2b. Symptomatic ICH was defined as any intracranial hemorrhage associated with neurological deterioration of 4 points or more on the NIHSS at 24 h. Clinical outcomes also included modified Rankin Scale at 90 days and mortality.
Statistical Analysis
The NASA Registry cohort was dichotomized into those undergoing local aspiration or no local aspiration and restricted to occlusion in the ICA and MCA territories only. Baseline characteristics were compared using the Fisher exact (when small cell size) or χ2 tests for categorical variables and Student t tests for continuous variables. The data were stored and analyzed by the central coordinating site, the Medical College of Wisconsin. Descriptive, univariate statistics were performed using JMP 11 statistical software (SAS Institute, Cary, NC, USA).
Results
There were 354 patients included in the NASA registry. Data on the combined aspiration-retrieval technique in acute ischemic stroke were available for 285 anterior circulation vessel occlusion patients in this analysis, of which 29 patients underwent the ARTS technique, 131 CGC and 125 BGC. Of those undergoing ARTS, the mean age was 63 ± 17.1 years and mean National Institute of Health Stroke Score (NIHSS) was 16.6 ± 5.9, which were comparable to the no local aspiration cohorts of BGC and CGC (BGC 68.9 ± 14.4, 17.3 ± 6, and CGC 68.3 ± 15.5, 18.2 ± 6) (Table 1). Patients undergoing ARTS technique were less likely to have a history of hypertension and atrial fibrillation, and were more likely to harbor ICA occlusions compared to patients with BGC or CGC (41.4 vs. 22.4% in BGC, p = 0.04 and 26% in the CGC, p = 0.10). Rates of intravenous tPA administration prior to the endovascular procedure were not different between the groups (Table 1). Time from symptom onset to groin puncture was significantly shorter in the ARTS group (252.6 ± 103.1 vs. 355.7 ± 234 min in the BGC group, p = 0.001, and 381.4 ± 254 in the CGC group, p < 0.001). There was less intra-arterial tPA use (13.8 vs. 26.4%, p = 0.23, and 40.5%, p = 0.002 in the BGC and CGC groups; respectively) and a trend towards less endovascular adjuvant rescue therapy in the ARTS group (13.8 vs. 18.4%, p = 0.6, in BGC group, and 31.3%, p = 0.058, in the CGC group).
Table 1.
Baseline characteristics of the NASA ARTS versus BGC and CGC cohorts
| ARTS aspiration (N = 29) % (n/N) | BGC (N = 125) % (n/N) | p BGC vs. ARTS | CGC (N = 131) % (n/N) | p CGC vs. ARTS | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | |||||
| Mean (SD) | 63 (17.1) | 68.9 (14.4) | 0.09 | 68.3 (15.5) | 0.13 |
| Median (IQR, R) | 67 (49–76.3) | 71 (60–81) | 70.5 (57–81) | ||
| Gender, female | 50 (14/28) | 50.4 (63/125) | 1 | 52.7 (69/131) | 0.68 |
| Race, white | 64.3 (18/28) | 76 (92/121) | 0.2 | 74.6 (97/130) | 0.4 |
| Risk factors | |||||
| Hypertension | 55.2 (16/29) | 82.3 (102/124) | 0.003 | 77.7 (101/130) | 0.02 |
| Atrial fibrillation | 27.6 (8/29) | 54.8 (68/124) | 0.01 | 35.1 (46/131) | 0.4 |
| Diabetes mellitus | 20.7 (6/29) | 23.4 (29/124) | 1 | 28.2 (37/131) | 0.64 |
| Hyperlipidemia | 51.7 (15/29) | 56.5 (70/124) | 0.84 | 48.1 (63/131) | 0.84 |
| Smoking history | 48.3 (14/29) | 29.8 (37/124) | 0.08 | 30.2 (39/129) | 0.12 |
| Coronary artery disease | 31 (9/29) | 36.3 (45/124) | 0.83 | 27.5 (36/131) | 1 |
| Clinical presentation | |||||
| Initial NIHSS, mean (SD) | 16.6 (5.9) | 17.3 (6) | 0.47 | 18.2 (6) | 0.21 |
| Occlusion site | |||||
| MCA/M1 | 44.8 (13/29) | 68 (85/125) | 0.02 | 62 (81/131) | 0.09 |
| ICA terminus | 41.4 (12/29) | 22.4 (28/125) | 0.04 | 26 (34/131) | 0.10 |
| Initial systolic BP, mean (SD) | 142.1 (26.5) | 142.6 (25.7) | 0.86 | 146 (30.8) | 0.53 |
| Initial diastolic BP, mean (SD) | 76.1 (18.3) | 77.8 (15.1) | 0.67 | 77.8 (19.6) | 0.66 |
| IV tPA | 39.3 (11/28) | 52 (65/125) | 0.41 | 39.2 (51/130) | 1.00 |
Final degree of reperfusion was comparable among the groups (TICI 2b or higher was 72 vs. 70% for CGC vs. 78% for BGC) (Table 2). Time from arterial puncture to reperfusion or end of procedure with ARTS was shorter than with CGC (54 vs. 91 min, p = 0.001) and was comparable to the BGC time (54 vs. 67, p = 0.11). Distal emboli, emboli to new territories, symptomatic hemorrhage, mortality, and good clinical outcome at 90 days were not different between the ARTS and the other 2 cohorts (Table 2). Figure 2 is an illustrative case of the ARTS technique showing complete recanalization of the dominant right MCA inferior division occlusion with accompanying cartoon illustration.
Table 2.
Procedural characteristics and outcomes of the NASA ARTS versus BGC and CGC cohorts
| ARTS aspiration (N = 29) % (n/N) | BGC (N = 125) % (n/N) | p BGC vs. ARTS | CGC (N = 131) % (n/N) | p CGC vs. ARTS | |
|---|---|---|---|---|---|
| Procedural factors | |||||
| Time from onset to puncture, mean (SD), min | 252.6 (103.1) | 355 (234) | 0.001 | 381.4 (25) | <0.001 |
| Fluoroscopic time, mean (SD), min | 37.4 (20.1) | 26.7 (18) | 0.03 | 33.4 (29.5) | 0.52 |
| Time to revascularization or end of procedure, mean (SD), min | 53.5 (36.1) | 67.1 (102) | 0.11 | 90.9 (108) | 0.001 |
| General anesthesia | 64.3 (18/28) | 84.4 (81/96) | 0.04 | 54 (61/113) | 0.25 |
| IA tPA | 13.8 (4/29) | 26.4 (33/125) | 0.23 | 40.5 (53/131) | 0.002 |
| Rescue therapy | 13.8 (4/29) | 18.4 (23/125) | 0.6 | 31.3 (41/131) | 0.058 |
| Angiographic outcome | |||||
| TIMI ≥2 | 86.2 (25/29) | 88.8 (111/125) | 1 | 83.1 (109/131) | 0.17 |
| TICI ≥2a | 93.1 (27/29) | 90.4 (113/125) | 1 | 88.6 (116/131) | 0.40 |
| TICI ≥2b | 72.4 (21/29) | 77.6 (97/125) | 0.60 | 70.2 (92/131) | 0.98 |
| Distal embolization | 23.1 (6/26) | 17.5 (21/120) | 0.58 | 15.1 (19/126) | 0.23 |
| Embolization into new territory | 7.7 (2/27) | 6.4 (8/125) | 0.38 | 5.7 (7/124) | 0.48 |
| Clinical outcome | |||||
| mRS ≤2 at 90 days | 34.6 (9/26) | 54.4 (56/103) | 0.10 | 36.13 (43/119) | 0.62 |
| NIHSS at 90 days | |||||
| Mean (SD) | 23.4 (19.6) | 19 (12.5) | 0.43 | 26 (18.3) | 0.62 |
| Median (IQR, R) | 30 (2–42) | 14 (1–42) | 42 (4–42) | ||
| Mortality at 90 days | 34.6 (9/26) | 25.24 (26/103) | 0.1 | 28.6 (34/119) | 0.34 |
| sICH | 11 (3/28) | 12.9 (14/124) | 0.75 | 9.9 (13/131) | 0.53 |
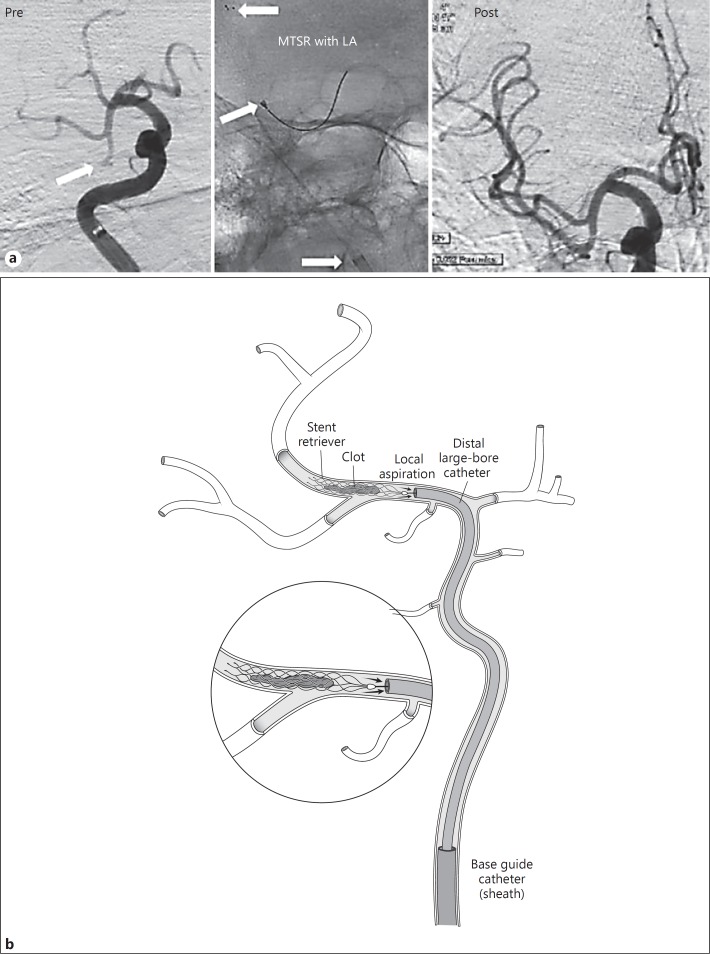
Fig. 2.
a Illustrative case of mechanical thrombectomy with stent-retriever (MTSR) with local aspiration (LA) technique showing complete recanalization of the dominant right MCA inferior division occlusion. Pre-MTSR image, arrow indicates site of occlusion. MTSR with LA image, arrows (inferior to superior) indicate tip of base catheter, tip of local aspiration catheter, and tip of stent retriever. b Illustration of the ARTS technique.
Discussion
Aspiration (or, in the vertebrobasilar system, alternative methods of achieving flow reversal) during withdrawal of a clot capture device has been proposed as an important step of the endovascular clot retrieval procedure since the earliest reported experiences with these devices [21, 22, 23, 24, 25, 26]. More recent studies have demonstrated that aspiration through a BGC in stent retriever procedures is associated with superior recanalization results, decreased need for rescue therapy, shorter procedure times and improved clinical outcome [18, 27]. The mechanism for these benefits of BGC aspiration is thought to be a reduced incidence of the clot or its fragments being displaced from the stent retriever during its withdrawal and reembolization into the same or a new intracranial vascular territory. However, studies have been inconsistent in demonstrating a decreased incidence of distal embolization or embolization into a new territory [17, 18, 28].
ARTS, with its local clot aspiration through a distal large bore catheter placed with its tip intracranially at the clot interface, has several theoretical advantages over aspiration through either a BGC or CGC positioned in the cervical carotid. First, the collapsible nature of the cervical vessels can work against the CGC/BGC aspiration strategy in 2 ways: complete collapse of the cervical vessel distal to the guide catheter tip but proximal to the clot being withdrawn eliminates the suction transmitted to the clot, while partial collapse of the cervical vessel reduces the diameter of the cervical vessel through which the clot must be withdrawn, ideally without fragmenting. Second, the tortuosity of the cervical vessels in elderly stroke patients often results in temporary kinks forming between the tip of the CGC/BGC and the skull base, again decreasing the diameter of the vessel at the kink through which the clot must be withdrawn. Third, there may be underlying atheroma or other baseline stenosis between the CGC/BGC tip and the target thrombus, again decreasing the diameter of the vessel to be navigated by the thrombus during withdrawal. Fourth, the CGC/BGC tip is usually separated from the target thrombus by 10–20 cm of vessel, and this intervening segment may have numerous branch vessels (petrous and cavernous branches, ophthalmic artery, posterior communicating artery, anterior choroidal artery) which can reverse flow during CGC/BGC aspiration, providing new inflow of blood into the carotid between the CGC/BGC and the intracranial thrombus with the result that there is no residual suction effect or reversal of flow at the downstream clot interface. Fifth, if the thrombus can be withdrawn leaving the large bore distal access catheter positioned immediately proximal to the original site of occlusion, there is no opportunity for the thrombus to embolize to a new territory. Sixth, local aspiration provides real-time monitoring of blood return into the distal access catheter, allowing recognition during stent retriever device withdrawal of clot “corking” in the catheter tip, such that the clot is then retrieved with a combination of both physical adherence to the stent retriever and also aspiration as a “corked” thrombus. Seventh, if the clot does not cork and the distal access catheter can be maintained at the clot interface, then any required successive passes with the stent retriever are quicker as there is no need to repeat navigation of the stent retriever delivery microcatheter through the petrous, cavernous, and supra-clinoid carotid segments of the carotid artery; the stent delivery microcatheter is simply readvanced through the already positioned distal access catheter.
Earlier arguments against ARTS techniques were largely based on the decreased chance of withdrawing a thrombus intact through distal access catheters, which had inner diameters (IDs) dramatically smaller than the IDs of BGCs. With newer technologies, however, it is possible to safely navigate distal large bore catheters from several manufacturers into the middle cerebral artery. These newer catheter technologies have distal IDs up to 0.070′′, IDs which are more than 80% of the IDs of commonly used BGCs in the earlier reports.
The effect of aspiration strategy on distal embolization upon mechanical thrombectomy for large-vessel occlusion rates has been studied in vitro [28, 29]. In contrast, previous clinical reports addressing either local clot aspiration, base catheter aspiration, or nonaspiration techniques have not allowed direct comparisons of these techniques [30, 31, 32, 33].
To our knowledge, this is the first multicenter report comparing the technical and clinical results of stent retriever use with ARTS with a contemporaneous cohort undergoing stent retriever use without ARTS. This report, which compares the 2 techniques within a single registry, may allow a comparison benefitting from contemporaneous multicenter enrollment and standardization of the definitions for the results and outcomes. These distal-access large-bore catheter technologies have improved since the NASA registry, and we expect to see higher rates than our 10% utilization of ARTS in future studies.
Limitations
The NASA registry [20, 34, 35, 36] was an investigator-initiated, retrospective registry of consecutive acute ischemic stroke patients treated with the Solitaire FR device at 24 participating North American centers. The stent retriever procedure was performed utilizing local protocols.
Limitations of this nonrandomized registry include a heterogeneous stroke population and site adjudication of angiographic and other imaging results. Variation between the participating institutions in choice of aspiration techniques may be linked to variation between institutions in patient selection criteria and other procedural variables. As such, the clinical impact of the ARTS technique in this retrospective registry would require confirmation in a prospective, multicenter, randomized trial with more homogeneous patient selection criteria and standardization of procedural protocols. We do not, however, believe these limitations of a retrospective registry are likely to bias actual or reported differences in procedural reperfusion times between the aspiration techniques.
There are imbalances in the 2 groups that should be addressed: patients undergoing ARTS technique had less hypertension and atrial fibrillation (known to adversely affect outcomes) and had more ICA occlusions (known to be more challenging with worse prognosis) compared to patients without ARTS.
Conclusion
Stent retriever use with ARTS in a cohort that had more ICA occlusions was associated with shorter time to reperfusion and less use of intra-arterial tPA than CGC, with a similar risk profile in comparison to stent retriever use with alternative aspiration through a CGC or a nonaspiration technique. ARTS appears to lead to better results as compared to the use of aspiration through CGCs or nonaspiration techniques. Future studies should focus on the comparison of ARTS and BCG techniques.
Disclosure Statement
Dr. Malisch, Covidien (DSMB service for SWIFT Trial) and Stryker Neurovascular (CEC service for DAWN Trial); Dr. Taqi, Stryker Neurovascular (consultant) and Penumbra Inc. (consultant); Dr. Abraham, Stryker Neurovascular (consultant), Boehringer Ingelheim (speaker's bureau); Dr. Nogueira, Stryker Neurovascular (PI for Trevo-2 Trial, PI for DAWN Trial), Covidien (Steering Committee for SWIFT Trial, Steering Committee for SWIFT-PRIME Trial, Core Lab for STAR Trial), Penumbra Inc. (Executive Committee for 3-D Separator Trial); Dr. Mueller-Kronast, Medtronic Neurovascular (consultant); Dr. Yoo, Penumbra Inc. (consultant) and Neuravi Inc. (consultant). The other authors report no conflicts.
Author Contributions
All authors listed contributed data to this registry and participated in the writing of the manuscript.
Acknowledgement
We thank Ginger Reilly ANP-BC, Alexian Brothers Medical Center, for her support in this study, and Kristen W. Marzejon, for her illustration of the mechanical thrombectomy with stent retriever and local aspiration technique.
Appendix
Site Investigators/Team
Mercy Health St Vincent Hospital Neuroscience Institute, Toledo, Ohio: Osama O. Zaidat, Alicia C. Castonguay; Emory University School of Medicine, Atlanta, GA: Rishi Gupta, Raul G. Nogueira, Chung-Huan Johnny Sun; St. Luke's Kansas City, Kansas City, MO: Coleman O. Martin, William E. Holloway; Delray Medical Center, Delray Beach, FL: Nils Mueller-Kronast; California Pacific Medical Center, San Francisco, CA: Joey English; Baptist Cardiac and Vascular Institute, Miami, FL: Italo Linfante, Guilherme Dabus, Eugene Lin, Edgar Samaniego; Alexian Brothers Medical Center, Elk Grove Village, IL: Tim W. Malisch, Franklin Marden; Oregon Health and Science University, Portland, OR: Hormozd Bozorgchami; Wayne State University School of Medicine, Detroit, MI: Andrew Xavier; West Virginia University Hospital, Morgantown, WV: Ansaar Rai, Jennifer Domico; Vanderbilt University Medical Center, Nashville, TN: Michael T. Froehler; University of Iowa, Iowa City, IA: Jeri Sieren, Heena Olalde; Provena Saint Joseph Medical Center, Joliet, IL: Aamir Badruddin; Boston Medical Center, Boston, MA: Thanh N. Nguyen, Alexander M. Norbash, Hesham Masoud, Judith Clark; Desert Regional Medical Center, Palm Springs, CA: Muhammad A. Taqi, Tom Wolfe, Ajeet Sodhi; University of Kansas Medical Center, Kansas City, KS: Michael G. Abraham; Texas Stroke Institute, Plano, TX: Vallabh Janardhan; University of Texas Health Science Center, Houston, TX: Hashem Shaltoni; UT Southwestern Medical Center, Dallas, TX: Roberta Novakovic, G. Lee Pride, Jr., Kim L. Rickert, Babu G. Welch, Jonathan A. White; Massachusetts General Hospital, Boston, MA: Albert J. Yoo, Thabele M. Leslie-Mazwi, Joshua A. Hirsch; University of Louisville Medical School, Louisville, KY: Alex Abou-Chebl; University of Texas, Houston, TX: Peng Roc Chen, Aditya Sanzgiri; Methodist Neurological Institute, Houston, TX: Gavin Britz; Duke University Medical Center, Durham, NC: Abhishek Agrawal; Saint Louis University, St. Louis, MO: Ritesh Kaushal; University of Missouri, Columbia, MO: Ashish Nanda.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, Eesa M, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA, IMS I and II Investigators Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatri P, Yeatts SD, Mazighi M, Broderick JP, Liebeskind DS, Demchuk AM, Amarenco P, Carrozzella J, Spilker J, Foster LD, Goyal M, Hill MD, Palesch YY, Jauch EC, Haley EC, Vagal A, Tomsick TA, IMS III Trialists Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM, Penumbra Pivotal Stroke Trial Investigators CSP Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2001;42:93–97. doi: 10.1161/STROKEAHA.110.594481. [DOI] [PubMed] [Google Scholar]
- 8.Almekhlafi MA, Eesa M, Menon BK, Demchuck AM, Goyal M. Ultrashort imaging to reperfusion time interval arrests core expansion in endovascular therapy for acute ischemic stroke. J Neurointerv Surg. 2013;5((suppl 1)):158–161. doi: 10.1136/neurintsurg-2012-010486. [DOI] [PubMed] [Google Scholar]
- 9.Zaidat OO, Suarez JI, Sunshine JL, Tarr RW, Alexander MJ, Smith TP, Enterline DS, Selman WR, Landis DM. Thrombolytic therapy of acute ischemic stroke: correlation of angiographic recanalization with clinical outcome. AJNR Am J Neuroradiol. 2005;26:880–884. [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal M, Almekhlafi MA, Fan L, Menon BK, Demchuk AM, Yeatts SD, Hill MD, Tomsick T, Khatri P, Zaidat OO, Jauch EC, Eesa M, Jovin TG, Broderick JP. Evaluation of interval times from onset to reperfusion in patients undergoing endovascular therapy in the IMS III Trial. Circulation. 2014;130:265–272. doi: 10.1161/CIRCULATIONAHA.113.007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP, MERCI Trial Investigators Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 12.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 13.Almekhlafi MA, Menon BK, Freiheit EA, Demchuk AM, Goyal M. A meta-analysis of observational intra-arterial stroke therapy studies using the merci device, penumbra system and retrievable stents. AJNR Am J Neuroradiol. 2013;34:140–145. doi: 10.3174/ajnr.A3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguiera RG, Lutsep HLl, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS, TREVO 2 Trialists TREVO versus Merci retrievers for thrombectomy revascularization of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomized trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO, SWIFT Trialists Solitaire flow restoration device versus the MERCI retriever in patients with acute ischaemic stroke (SWIFT): a randomized, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 16.Davalos A, Pereira VM, Chapt R, Bonafe A, Andersson T, Gralla J. Retrospective multicenter study of Solitaire FR for revascularization in the treatment of acute ischemic stroke. Stroke. 2012;43:2699–2705. doi: 10.1161/STROKEAHA.112.663328. [DOI] [PubMed] [Google Scholar]
- 17.Pereira VM, Gralla J, Davalos A, Bonafé A, Castaño C, Chapot R, Liebeskind DS, Nogueira RG, Arnold M, Sztajzel R, Liebig T, Goyal M, Besselmann M, Moreno A, Schroth G. Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire flow restoration in acute ischemic stroke. Stroke. 2013;44:2802–2807. doi: 10.1161/STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TN, Malisch T, Castonguay AC, Gupta R, Sun CH, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Linfante I, Dabus G, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Taqi M, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Abou-Chebl A, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Masoud H, Nogueira RG, Norbash AM, Zaidat OO. Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of NASA registry. Stroke. 2014;45:141–145. doi: 10.1161/STROKEAHA.113.002407. [DOI] [PubMed] [Google Scholar]
- 19.Turk AS, Turner R, Spiotta A, Vargas J, Holmstedt C, Ozark S, Chalela J, Turan T, Adams R, Jauch EC, Battenhouse H, Whitsitt B, Wain M, Chaudry MI. Comparison of endovascular treatment approaches for acute ischemic stroke: cost effectiveness, technical success and clinical outcomes. J Neurointerv Surg. 2015;7:666–670. doi: 10.1136/neurintsurg-2014-011282. [DOI] [PubMed] [Google Scholar]
- 20.Zaidat OO, Castonguay AC, Gupta R, Sun CJ, et al. North American Solitaire stent retriever acute stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg. 2014;6:584–588. doi: 10.1136/neurintsurg-2013-010895. [DOI] [PubMed] [Google Scholar]
- 21.Smith WS, for the Multi MERCI Investigators Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the Multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) Trial, Part 1. Am J Neuroradiol. 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 22.Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, Pile-Spellman J, Segal A, Vinuela F, Saver JL. MERCI 1: A phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke. 2004;35:2848–2854. doi: 10.1161/01.STR.0000147718.12954.60. [DOI] [PubMed] [Google Scholar]
- 23.Imai K, Mori T, Izumoto H, Watanbe M. Successful thrombectomy in acute terminal internal carotid occlusion using a basket type microsnare in conjunction with temporary proximal occlusion: a case report. Am J Neuroradiol. 2005;26:1395–1398. [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer TE, Hamann GF, Brueckmann HJ. Treatment of basilar artery embolism with a mechanical extraction device. Necessity of flow reversal. Stroke. 2002;33:2232–2235. doi: 10.1161/01.str.0000024524.71680.c6. [DOI] [PubMed] [Google Scholar]
- 25.Henkes H, Reinartz J, Lowens S, Miloslavski E, Roth C, Reith W, Kühne D. A device for fast mechanical clot retrieval from intracranial arteries (Phenox clot retriever) Neurocrit Care. 2006;05:134–140. doi: 10.1385/NCC:5:2:134. [DOI] [PubMed] [Google Scholar]
- 26.Kerber CW, Barr JD, Berger RM, Chopko BW. Snare retrieval of intracranial thrombus in patients with acute stroke. J Vasc Interv Radiol. 2002;13:1269–1274. doi: 10.1016/s1051-0443(07)61978-2. [DOI] [PubMed] [Google Scholar]
- 27.Davalos A, Gralla J, Bonafe A, Chapot R, Andersson T, Mendes V. A retrospective multicenter study of Solitaire FR for revascularization in the treatment of acute ischemic stroke. New Orleans: Int Stroke Conf; 2012. January 31 to February 3. [DOI] [PubMed] [Google Scholar]
- 28.Chueh J-Y, Kuhn AL, Puri AS, Wilson SD, Wakhloo AK, Gounis MJ. Reduction in distal emboli with proximal flow control during mechanical thrombectomy. A quantitative in vitro study. Stroke. 2013;44:1396–1401. doi: 10.1161/STROKEAHA.111.670463. [DOI] [PubMed] [Google Scholar]
- 29.Chueh J-Y, Puri AS, Wakhloo AK, Counis MJ. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J Neurointerv Surg. 2016;8:197–202. doi: 10.1136/neurintsurg-2014-011491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphries W, Hoit D, Doss VT, Elijovich L, Frei D, Loy D, Dooley G, Turk AS, Chaudry I, Turner R, Mocco J, Morone P, Fiorella D, Siddiqui A, Mokin M, Arthur AS. Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J Neurointerv Surg. 2015;7:90–94. doi: 10.1136/neurintsurg-2013-010986. [DOI] [PubMed] [Google Scholar]
- 31.Mokin M, Ionita CN, Nagesh SVS, Rudin S, Levy EI, Siddiqui AH. Primary stentriever versus combined stentriever plus aspiration thrombectomy approaches: in vitro stroke model comparison. J Neurointerv Surg. 2015;7:453–457. doi: 10.1136/neurintsurg-2014-011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jankowitz B, Grandhi R, Horev A, Aghaebrahim A, Jadhav A, Linares G, Jovin T. Primary manual aspiration thrombectomy (MAT) for acute ischemic stroke: safety, feasibility and outcomes in 112 consecutive patients. J Neurointerv Surg. 2015;7:27–31. doi: 10.1136/neurintsurg-2013-011024. [DOI] [PubMed] [Google Scholar]
- 33.Spiotta AM, Chaudry MI, Hui FK, Turner RD, Kellogg RT, Turk AS. Evolution of thrombectomy approaches and devices for acute stroke: a technical review. J Neurointerv Surg. 2015;7:2–7. doi: 10.1136/neurintsurg-2013-011022. [DOI] [PubMed] [Google Scholar]
- 34.Linfante I, Walker GR, Castonguay AC, Dabus G, et al. Predictors of mortality in acute ischemic stroke intervention. Stroke. 2015;46:2305–2308. doi: 10.1161/STROKEAHA.115.009530. [DOI] [PubMed] [Google Scholar]
- 35.Castonguay AC, Zaidat O, Novakovic R, Nguyen T, et al. Influence of age on clinical and revascularization outcomes in the North American SOLITAIRE Acute Stroke Registry. Stroke. 2014;45:3631–3636. doi: 10.1161/STROKEAHA.114.006487. [DOI] [PubMed] [Google Scholar]
- 36.Linfante I, Starosciak AK, Walker GR, Dabus G, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg. 2016;8:224–229. doi: 10.1136/neurintsurg-2014-011525. [DOI] [PubMed] [Google Scholar]