Abstract
Objective
To investigate the effect of subspecialty practice and experience on the relationship between annual volume and inpatient mortality following hepatic resection.
Summary Background Data
The impact of annual surgical volume on post-operative outcomes has been extensively examined. However, the impact of cumulative surgeon experience and specialty training on this relationship warrants investigation.
Methods
The New York Statewide Planning and Research Cooperative System inpatient database was queried for patients ≥18 years who underwent wedge hepatectomy or lobectomy from 2000-2014. Primary exposures included annual surgeon volume, surgeon experience (early vs. late career), and surgical specialization – categorized as general surgery (GS), surgical oncology (SO), and transplant (TS). Primary endpoint was inpatient mortality. Hierarchical logistic regression was performed accounting for correlation at the level of the surgeon and the hospital, and adjusting for patient demographics, comorbidities, presence of cirrhosis, and annual surgical hospital volume.
Results
13,467 cases were analyzed. Overall inpatient mortality was 2.35%. On unadjusted analysis, late career surgeons had a mortality rate of 2.62% versus 1.97% for early career surgeons. GS had a mortality rate of 2.98% compared to 1.68% for SO and 2.67% for TS. Once risk-adjusted, annual volume was associated with reduced mortality only among early-career surgeons (OR 0.82, p=0.001) and general surgeons (OR 0.69, p=0.01). No volume effect was seen among late-career or specialty-trained surgeons.
Conclusion
Annual volume alone likely contributes only a partial assessment of the volume-outcome relationship. In patients undergoing hepatic resection, increased annual volume did not confer a mortality benefit on subspecialty surgeons or late career surgeons.
Introduction
Annual surgeon volume has been identified as a major predictor of mortality after major operations, including hepatectomy.1 Although studies have demonstrated a survival benefit for patients undergoing major resection at high volume hospitals by high volume surgeons,2–4 debate has arisen on the potential unintended consequences of restricting cases above individual surgeon volume thresholds and whether annual volume alone is the best marker to allow for optimization of patient outcomes.5–8
Utilizing annual surgeon volume offers an easy to measure proxy for quality;9 however, annual surgeon volume alone may not appropriately capture the underlying phenomena that differentiates high performing and low performing surgeons. One can imagine that beyond volume, there are other measures of a surgeon’s expertise or proficiency, which can contribute beneficially to patient outcomes. Supporting such a hypothesis, recent work has demonstrated that other factors, such as surgeon procedure specialization, can be predictive of mortality independent of annual surgeon volume.10
In addition to specialization, experience over time, a cumulative volume effect, may have a role in predicting outcomes following surgery.11, 12 The goal of our study was to investigate the effect of cumulative experience over an entire career, as well as subspecialty practice, on the relationship between volume and inpatient mortality following hepatic resection.
Methods
Patient Database
The New York (NY) Statewide Planning and Research Cooperative System (SPARCS) inpatient database was utilized to capture patients undergoing surgery from 2000-2014. SPARCS is a database for the state of NY that captures all patients and payers and collects information on patients, treatments, and providers for every emergency department admission, inpatient admission/hospital discharge, outpatient visit, and ambulatory surgery. SPARCS also contains a unique physician identifier that allows for identification of each patient’s physicians for an episode of care. Unique physician identifiers in SPARCS correspond to identifiers maintained in the New York Physician License database, which contains information on physician identity, including year of medical school graduation.
Study Population
All patients over the age of 18 years who underwent wedge hepatectomy or lobectomy were identified and included using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) procedure codes (50.3, 50.22). Patients who underwent trauma or recipient (i.e. transplant) hepatectomy were excluded. Each procedure was attributed to an individual surgeon by a unique surgeon identifier within the dataset.
Main Exposures
Our main exposures were individual surgeon annual volume, surgeon experience, and surgeon specialty.
Surgeon Identification by Annual Volume
Using the above specified procedure codes, annual hepatic volumes were determined per surgeon, across the study period. Surgeon volume was included in the models both as a continuous variable and a categorical variable based on quartiles of annual volume.
Surgeon Identification by Career Stage
Surgeon status as “early career” vs. “late career” was determined by calculating years since medical school graduation. Surgeons with fewer than twenty years out from graduation were labeled as early career and those twenty years or greater from graduation were labeled as late career. Twenty years was selected as the cut-off for an early career surgeon under the assumption that surgical residency lasts five to seven years and fellowship training (if applicable) lasts one to two years. Thus, a twenty-year cut-off could be reasonably expected to capture the first 10-12 years of a surgeon’s independent practice.
Surgeon Identification by Specialty
Liver transplant centers were identified using the Scientific Registry of Transplant Recipients (SRTR). Institutional websites of liver transplant centers were mined for liver transplant faculty affiliation and linked to the New York Physicians License database to allow linking of transplant surgeon (TS) status to SPARCS data. The Society of Surgical Oncology (SSO) physician database was utilized to identify surgeons with a SSO affiliation and linked to the New York Physicians License database to allow for linking of SSO affiliation to SPARCS data. These surgeons are subsequently referred to as surgical oncologists (SO). SO status was cross-referenced against institutional websites for verification of surgeon specialty. All other surgeons were categorized as general surgeons (GS).
Outcome Measures
The primary outcome measure was inpatient mortality (i.e. death on index admission for hepatectomy). Death was determined based on a mortality variable coded into the SPARCS dataset.
Statistical Analysis
Baseline demographic characteristics were analyzed. Unadjusted analysis was performed to compare patients who were operated on by early versus late career surgeons, and those operated on by GS versus SO vs. TS.
Several multilevel logistic regressions were performed to investigate the volume-specialty and volume-experience relationship. We accounted for clustering of surgeon and hospital by using a nested multilevel model. We adjusted for age, year of operation, race, payer status, Charlson Comorbidity Index (CCI), need for biliary-enteric reconstruction, presence of hepatic malignancy (primary vs. secondary tumor), hepatic necrosis, and presence of one or more complications (e.g. postoperative shock, hemorrhage, cardiac complication, wound complication, postoperative infection, respiratory/ventilator-associated complications, anastomotic leak, and urinary complication) and annual hospital volume. Annual individual surgeon volume was initially examined as a continuous variable.
Subset analysis was performed to assess for the role of annual surgeon volume on mortality in early career and late career surgeons, as well as in general, oncologic, and transplant surgeons. Additionally, patients were divided into quartiles based on their surgeon’s annual volume to enable visualization of the relationship of experience and specialization across sub-groups of annual volume. Adjusted mortality rates were calculated from odds ratios using the marginal standardization form of predictive margins.13 Significance level was set at alpha=0.05.
Statistical analysis was performed using STATA 14/IC (StataCorp, College Station, TX).
Results
A total of 13,467 hepatectomies were performed from 2000-2014 in the state of NY with a crude inpatient mortality rate of 2.35%. The majority of patients were Caucasian, and half were female. Patients of TS tended to be racial minorities, had a diagnosis of cirrhosis, and were more likely to undergo lobectomy than wedge hepatectomy, compared to patients of GS and SO (Table 1).
Table 1.
Patient demographics, incidence of comorbidities, and hepatectomy technique by surgeon career status and specialization.
| Experience | Specialization | ||||||
|---|---|---|---|---|---|---|---|
| Early Career (n=5639) | Late Career (n=7828) | p | General (n=4540) | Oncologic (n=5778) | Transplant (n=3149) | p | |
|
Age Mean (SD) |
58.2 (14) | 57.8 (14.5) | 0.184 | 57.9 (14.6) | 59.6 (13.5) | 55.1 (14.8) | <0.001 |
| Gender | 51.3% | 50% | 0.142 | 54.6% | 49.5% | 46.5% | <0.001 |
| Race | |||||||
| White | 69.8% | 71% | 0.125 | 70.8% | 81.4% | 50.4% | <0.001 |
| Black | 9.5% | 7.6% | <0.001 | 8.6% | 6.5% | 11.6% | <0.001 |
| Asian | 10.3% | 7.9% | <0.001 | 5.6% | 6.5% | 18.2% | <0.001 |
| Other | 10.1% | 13.3% | <0.001 | 15% | 5.6% | 19.8% | <0.001 |
| Ethnicity, (%) | |||||||
| Hispanic | 8.1% | 6.1% | <0.001 | 6.7% | 5.6% | 9.9% | <0.001 |
| Comorbidities | |||||||
| CCIMedian (IQR) | 6 (3,8) | 6 (3,9) | 0.088 | 6.2 (3.4) | 6.9 (3.0) | 4.2 (3.2) | <0.001 |
| Cirrhosis | 18.8% | 16.7% | 0.002 | 10.6% | 19.2% | 24.8% | <0.001 |
| Technique | |||||||
| Lobectomy | 25.4% | 29.6% | <0.001 | 24.4% | 25.3% | 37.7% | <0.001 |
A total of 909 individual surgeons were identified. The majority of surgeons were GS (n=850, 93.5%), of which most were late career (Table 2). Late career surgeons were more likely to perform a hepatic lobectomy than early career surgeons (Table 1). One third of patients were operated on by GS, one quarter by TS, and the remaining patients by SO.
Table 2.
Surgeon level information by experience and subspecialty.
| Experience | Specialization | ||||
|---|---|---|---|---|---|
| Early Career | Late Career | General | Oncologic | Transplant | |
| Number of Surgeons | 300 | 609 | 850 | 33 | 26 |
| Academic Affiliation (%) | 62.7% | 60.9% | 58.8% | 75.8% | 100% |
| Early Career (%) | N/A | N/A | 33% | 33% | 38% |
| Median Surgeon Annual Volume (IQR) | 1 (1,4) | 1 (1,3) | 1 (1,2) | 12 (4,34) | 12 (3,24) |
On unadjusted analysis, ate career surgeons had a mortality rate of 2.62% versus 1.97% for early career surgeons (p<0.014). GS had a mortality rate of 2.98% compared to 1.68% for SO and 2.67% for TS.
After risk adjustment, patient age greater than 60 years, Medicare payer status, primary liver tumor, need for biliary-enteric reconstruction, and lobectomy were significant predictors of inpatient mortality (Table 3, all p<0.02). Early career status and surgeon specialization were not significant predictors of mortality. In the overall patient cohort, annual surgeon volume was not a significant predictor for inpatient mortality (OR 0.94, 95% CI 0.85-1.05, p=0.259).
Table 3.
Overall risk-adjusted multilevel, multi-effect logistic regression model of all patients to determine independent predictors of inpatient mortality after hepatectomy. P-values in bold are significant predictors.
| OR | 95% CI | p-value | |
|---|---|---|---|
| Annual surgeon volume (per 10 additional cases/year) | 0.94 | 0.85, 1.05 | 0.259 |
| Early career surgeon (vs. late) | 0.99 | 0.71, 1.38 | 0.971 |
| Surgeon Specialization (vs. General Surgeon) | |||
| Surgical Oncologist | 0.75 | 0.43, 1.29 | 0.3 |
| Transplant Surgeon | 1.07 | 0.6, 1.92 | 0.816 |
| Patient Age (vs. 18-40 years) | |||
| 41-50 years | 1.77 | 0.78, 4.02 | 0.172 |
| 51-60 years | 1.83 | 0.85, 3.92 | 0.122 |
| 61-70 years | 2.65 | 1.24, 5.63 | 0.012 |
| 71-80 years | 3.13 | 1.41, 6.92 | 0.005 |
| 81-90 years | 5.51 | 2.33,13.01 | <0.001 |
| >90 years | 16.2 | 2.78, 94.1 | 0.002 |
| Female (vs. male) | 0.71 | 0.54, 0.92 | 0.011 |
| Race (vs. white) | |||
| Black | 0.97 | 0.59, 1.58 | 0.891 |
| Native American | 1.8 | 0.27, 11.9 | 0.543 |
| Asian | 0.98 | 0.6, 1.6 | 0.941 |
| Other/Unknown | 1.14 | 0.77, 1.69 | 0.527 |
| Hispanic (vs. non-hispanic) | 1.13 | 0.67, 1.88 | 0.652 |
| Charlson Comorbidity Index ≥3 (vs. CCI <3) | 1.43 | 0.95, 2.16 | 0.088 |
| Insurer (vs. private) | |||
| Medicare | 1.78 | 1.24, 2.54 | 0.002 |
| Medicaid | 1.25 | 0.79, 2.0 | 0.342 |
| Self-Pay | 1.32 | 0.48, 3.62 | 0.584 |
| Year (2001–14 vs. 2000) | 0.94 | 0.91, 0.98 | 0.001 |
| Primary tumor (vs. other indication) | 2.21 | 1.66, 2.95 | <0.001 |
| Necrotic liver (vs. non-necrotic) | 22.96 | 15.69, 33.6 | <0.001 |
| Biliary reconstruction (vs. no reconstruction) | 2.35 | 1.65, 3.35 | <0.001 |
| Lobectomy (vs. wedge) | 1.76 | 1.35, 2.3 | <0.001 |
On subset analysis, annual surgeon volume was a significant predictor of inpatient mortality for early career surgeons and GS. For every ten additional cases performed per year there were significantly decreased odds of inpatient mortality for early career surgeons (OR 0.81, 95% CI 0.73-0.92, p=0.001) and general surgeons (OR 0.65, 95% CI 0.5-0.85, p=0.002). Annual surgeon volume was not a significant factor in prediction of inpatient mortality for late career surgeons (OR 0.97, 95% CI 0.89-1.06, p=0.5), SO (OR 1.08, 95% CI 0.92-1.28, p=0.3), or TS (OR 0.92, 95% CI 0.8-1.04, p=0.2).
Adjusted mortality rate by annual volume quartiles demonstrates a significantly higher mortality rate for early career surgeons in the bottom volume quartile (p=0.008) (Figure 1). In addition, adjusted mortality rate of GS was significantly higher in the bottom volume quartile (p=0.04) (Figure 2). No significant differences in adjusted mortality rate were seen across quartiles for late career surgeons, SO, or TS.
Figure 1.
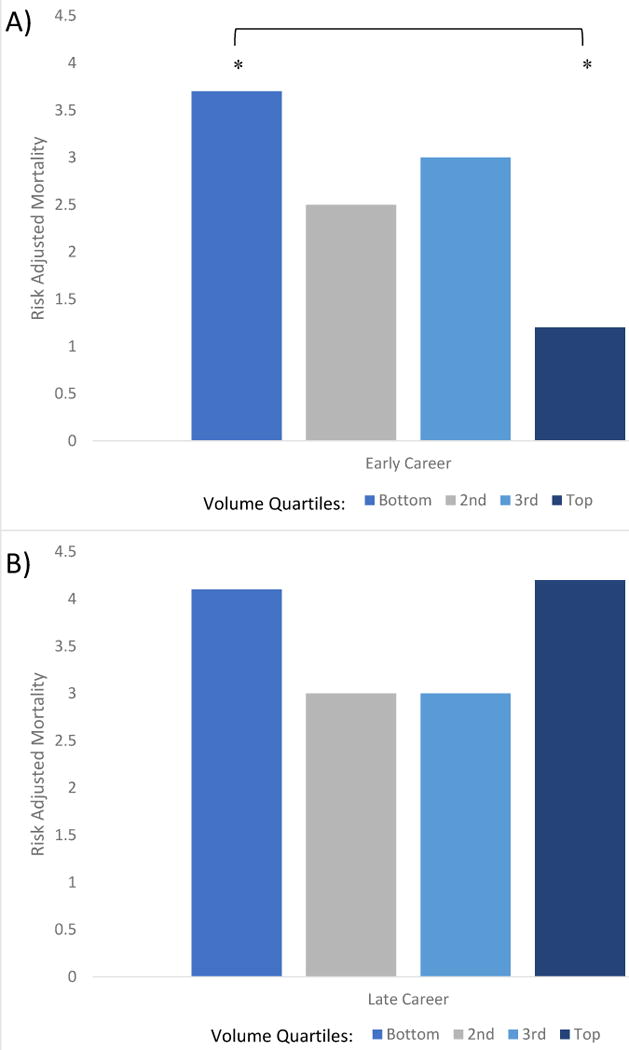
A) Risk adjusted mortality rate of early career surgeons by quartile of annual volume. *p<0.05 B) Risk adjusted mortality rate of late career surgeons by quartile of annual volume.
Figure 2.
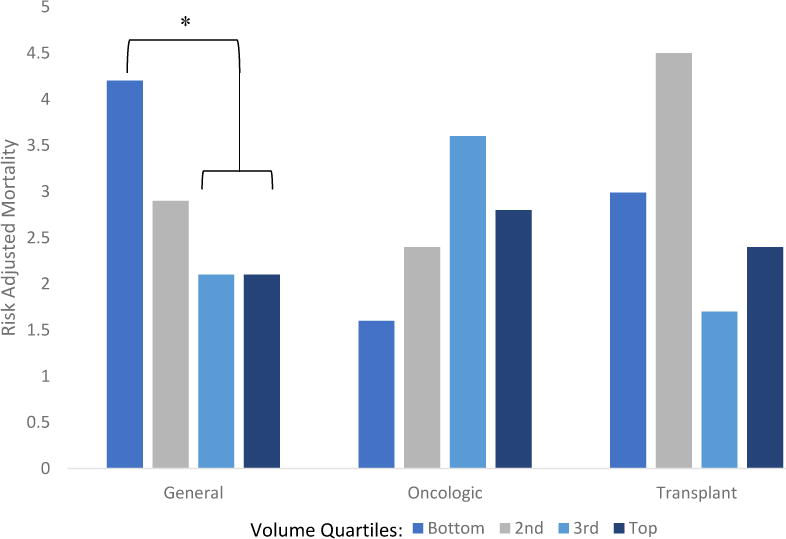
Risk adjusted mortality rate by surgeon specialty and quartile of annual volume. Quartiles are compared within each specialty. * p<0.05
Discussion
The results of this study demonstrate that annual surgical volume is associated with lower inpatient mortality following hepatic resection performed by early career surgeons and general surgeons. No significant effect of annual volume was seen on predicted mortality for late career surgeons or surgeons who specialize in liver surgery (i.e. SO and TS). These findings suggest that annual surgical volume alone may not be predictive of decreased inpatient mortality for all surgeons. Our work suggests that the need for surgeons to maintain high annual volume may be more relevant for some groups of surgeons and not others. Accrual of cumulative experience (as reflected by years since medical school graduation) or specialization in liver surgery may obviate the need for surgeons to maintain high annual volume in order to optimize their mortality rate after hepatectomy.
Cumulative experience is a difficult metric to capture as no central database captures longitudinal data of all surgeons and their patient outcomes across states; thus, alternative methods have been utilized to attempt to determine whether cumulative experience is predictive of operative outcomes. For example, surgeon age has been investigated as a surrogate marker with mixed findings depending on case complexity.14 Using a different technique for assessment of surgeon experience, Yeo et al (2017) found, similar to our results, that cumulative experience matters. The authors calculated case volumes over a 5-year period to estimate cumulative experience and found cumulative experience, in conjunction with annual volume, to be a significant predictor of decreased complications after rectal surgery.12 We utilized years since graduation from medical school as a surrogate marker for cumulative experience and found significant predictive effect of annual volume in early – but not late – career surgeons. The latter suggests that experienced surgeons may not need to maintain high annual volume to optimize postoperative mortality rates after hepatectomy.
Prior research has suggested that surgeon specialization is more likely to yield improved operative outcomes, though the effect varies by type of operation.15 Sahni et al (2016) demonstrated in a Medicare population that surgeon specialization was a predictor of operative mortality independent of annual volume for coronary artery bypass grafting, valve replacement, carotid endarterectomy, abdominal aortic aneurysm repair, cystectomy, and lung resection. Their work further suggested that surgeon specialization may account for part of the volume-outcome relationship in operative mortality.10 Through a different approach, our findings suggest a similar phenomenon in hepatectomy. For surgeons who specialize in liver surgery, annual volume thresholds may not be the best mechanism to optimize postoperative mortality rates after hepatectomy.
This study has several limitations – many of which arise from the utilization of a single state database. However, many of the limitations of using this single state database may be offset by its relative strengths. While utilization of a single state database raises concern about the generalizability of the results, SPARCS is one of the only all-payer databases that allows for identification of individual surgeons over a longitudinal period. Furthermore, it is the only all-payer database that allows for additional data enhancement such as identification of surgeons’ subspecialty practice. Use of a single state database does not allow for complete data capture of specific cumulative case volume that a surgeon performs over their career, unless a surgeon spends their entire career in that single state. Thus, years since medical school graduation are used as a surrogate marker for cumulative experience. Alternative methods, such as capturing cumulative case volume over a pre-determined five-year time frame, may not be representative of the cumulative case volume of an entire career.11, 12 Use of institutional and society affiliation may not capture fellowship training but is more likely to include surgical subspecialists who may not have been fellowship eligible at the start of their career but whose practices are predominantly hepatobiliary in nature (i.e. “grandfathered” into a specialty).
Despite these limitations, our findings have policy implications related to work surrounding the volume-outcome relationship and can help influence the practice of surgeons. Our work suggests that implementing surgeon annual volume cutoffs for hepatectomy may not result in improvements in inpatient mortality rate for experienced or subspecialist surgeons. Furthermore, low volume early career surgeons and general surgeons may consider partnering with more experienced surgeons or subspecialists when performing hepatectomy to allow for the greatest benefit in operative risk reduction. Such mentoring relationships likely already informally exist at many institutions, and studies of formal mentoring programs suggest it is an effective strategy to optimize outcomes.16 Similarly, partnership between hospitals that were high and low volume in hepatobiliary surgery has resulted in improvements to postoperative outcomes;17 however, such partnerships may come across regulatory obstacles depending on the local political and economic environment. Therefore, additional work should evaluate the effectiveness of pairing low volume early career or general surgeons with experienced mentors to determine what types of mentoring partnerships are most beneficial. Finally, future research should assess whether similar relationships between experience and outcomes exist in other types of operations.
Conclusion
While annual individual surgeon volume matters, annual volume alone likely gives a partial picture of the volume-outcome relationship. In hepatectomy, increased annual volume does not confer a mortality benefit on late career or subspecialty surgeons; thus, experienced surgeons may not have to maintain high annual volume of hepatectomy to optimize inpatient mortality rate. Additional research should further investigate other characteristics which may modify the volume-outcome relationship and whether similar phenomena are noted in other major operations.
Acknowledgments
Daniel Hashimoto is financially supported by the NIH National Institute of Diabetes and Digestive and Kidney Diseases (Grant #: T32 DK007754-16A1) and by the Massachusetts General Hospital Department of Surgery Edward D. Churchill Research Fellowship. Yanik Bababekov is supported by the Massachusetts General Hospital Department of Surgery Marshall K. Bartlett Research Fellowship. Winta Mehtsun received funding for this work from the National Cancer Institute (Grant #: R25CA92203). Sahael Stapleton is supported by the Massachusetts General Hospital Department of Surgery Ernest A. Codman Research Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Eppsteiner RW, Csikesz NG, Simons JP, et al. High volume and outcome after liver resection: surgeon or center? J Gastrointest Surg. 2008;12(10):1709–16. doi: 10.1007/s11605-008-0627-3. discussion 1716. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 4.Dimick JB, Cowan JA, Jr, Knol JA, et al. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138(2):185–91. doi: 10.1001/archsurg.138.2.185. [DOI] [PubMed] [Google Scholar]
- 5.Lillemoe KD. Surgical Volume/Outcome Debate. Ann Surg. 2017;265(2):270. doi: 10.1097/SLA.0000000000002110. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz DM, Fong ZV, Warshaw AL, et al. The Hidden Consequences of the Volume Pledge: “No Patient Left Behind”? Ann Surg. 2017;265(2):273–274. doi: 10.1097/SLA.0000000000001833. [DOI] [PubMed] [Google Scholar]
- 7.Pronovost P, Higgins RS. Mastery of Care-toward Communitarian Regulation. Ann Surg. 2017;265(2):271–272. doi: 10.1097/SLA.0000000000001813. [DOI] [PubMed] [Google Scholar]
- 8.Funk LM, Gawande AA, Semel ME, et al. Esophagectomy outcomes at low-volume hospitals: the association between systems characteristics and mortality. Ann Surg. 2011;253(5):912–7. doi: 10.1097/SLA.0b013e318213862f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha AK. Back to the future: volume as a quality metric. Jama. 2015;314(3):214–215. doi: 10.1001/jama.2015.7580. [DOI] [PubMed] [Google Scholar]
- 10.Sahni NR, Dalton M, Cutler DM, et al. Surgeon specialization and operative mortality in United States: retrospective analysis. Bmj. 2016;354:i3571. doi: 10.1136/bmj.i3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abelson JS, Spiegel JD, Afaneh C, et al. Evaluating cumulative and annual surgeon volume in laparoscopic cholecystectomy. Surgery. 2017;161(3):611–617. doi: 10.1016/j.surg.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Yeo HL, Abelson JS, Mao J, et al. Surgeon Annual and Cumulative Volumes Predict Early Postoperative Outcomes after Rectal Cancer Resection. Ann Surg. 2017;265(1):151–157. doi: 10.1097/SLA.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 13.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. The STATA Journal. 2012;12(2):308–331. [Google Scholar]
- 14.Waljee JF, Greenfield LJ, Dimick JB, et al. Surgeon age and operative mortality in the United States. Ann Surg. 2006;244(3):353–62. doi: 10.1097/01.sla.0000234803.11991.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury M, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. British journal of surgery. 2007;94(2):145–161. doi: 10.1002/bjs.5714. [DOI] [PubMed] [Google Scholar]
- 16.Miskovic D, Wyles SM, Ni M, et al. Systematic review on mentoring and simulation in laparoscopic colorectal surgery. Annals of surgery. 2010;252(6):943–951. doi: 10.1097/SLA.0b013e3181f662e5. [DOI] [PubMed] [Google Scholar]
- 17.Ravaioli M, Pinna AD, Francioni G, et al. A partnership model between high- and low-volume hospitals to improve results in hepatobiliary pancreatic surgery. Ann Surg. 2014;260(5):871–5. doi: 10.1097/SLA.0000000000000975. discussion 875–7. [DOI] [PubMed] [Google Scholar]


