Abstract
Adrenarche, defined as a prepubertal increase in adrenal androgen secretion resulting from zona reticularis (ZR) maturation, is thought to occur only in humans and some Great Apes. In the rhesus macaque, studies of circulating dehydroepiandrosterone (DHEA) or its sulpho-conjugate (DHEAS) have failed to demonstrate a prepubertal rise typical of human adrenarche, but available data are cross-sectional and include few neonatal or early infant samples. However, ZR maturation is complete in rhesus infants by 3 months of age based on morphological and biochemical analyses. Furthermore, preliminary longitudinal study from birth through infancy of castrated males, and intact males and females, suggests for the first time that there is a transient, prepubertal elevation of adrenal androgen in rhesus macaques. Serum DHEAS concentration increased, peaking between 6 and 8 weeks of age in castrate males, and intact males and females, then declined. These longitudinal profiles add endocrinological support to the morphological and biochemical evidence that adrenarche occurs in a narrow developmental window in infant rhesus macaques. Adrenarche in any species should be defined only after careful longitudinal hormone analysis have been conducted in stages of development that are suggested by morphological and biochemical evidence of ZR maturation.
Keywords: Adrenarche, human, non-human primate, rhesus, androgen, adrenal cortex, zona reticularis, dehydroepiandrosterone, DHEA, dehydroepiandrosterone sulphate, DHEAS, castrate, prepubertal, neonate, infant, longitudinal sampling
1. Introduction - The Definition of Adrenarche
The appearance of axillary and pubic hair in boys and girls represents a response to the increased secretion of C19 steroids by the zona reticularis (ZR) of the developing adrenal cortex years before the prepubertal increase in gonadal steroid secretion (ALBRIGHT, 1947). The increase in the so-called “adrenal androgens”, dehydroepiandrosterone (DHEA) or its sulphate (DHEAS), is known as adrenarche (Havelock et al., 2004;Rainey and Nakamura, 2008;Miller, 2009), and it is an event thought to be unique to humans (evident by 6-9 years of age) and certain Great Apes (Arlt et al., 2002). However, there is scant definitive evidence with which to establish the occurrence of adrenarche in either Great Apes or other non-human primates (Nguyen and Conley, 2008). The general view of which species do, is for obvious reasons not based on the appearance of axillary hair, but it is seldom based on definitive evidence of adrenal development either. Instead, conclusions about the occurrence of adrenarche in non-human primates rest on a few studies that have measured circulating concentrations of DHEA and DHEAS. Circulating steroid concentrations reflect the balance between synthesis and metabolism, and are subject to considerable individual (Orentreich et al., 1984), diurnal and even seasonal variation (Garde et al., 2000). In the case of the rhesus macaque, where adrenal morphological development has been best studied (Mesiano and Jaffe, 1997), these endocrine data are entirely cross-sectional and especially limited in observations from neonatal and infant subjects (Koritnik et al., 1983;Seron-Ferre et al., 1986;Seron-Ferre et al., 1983). Since puberty is initiated in the rhesus macaque between two and three years of age (Plant and Witchel, 2006), an “adrenarche” preceding puberty by years may occur at a very young age, perhaps in the first months of life.
Concentrations of adrenal androgens in rhesus newborns are reportedly twice those in neonatal and infant rhesus (Seron-Ferre et al., 1983), but longitudinal studies of adrenal androgen secretion have yet to be reported for this species. Relevant hormonal data covering this early developmental window are lacking among non-human primates in all but the baboon (Ducsay et al., 1991). Progress in understanding the processes that regulate adrenarche specifically, and adrenal androgen secretion in general, may be slowed as a result of the current reliance on endocrine data alone as the sole defining criterion, and of the narrow perspective it engenders. Still, a broad assessment of adrenarche that encompasses endocrinological, morphological and biochemical facets of the phenomenon is yet to be completed for any primate. Data will be reviewed herein, emphasizing recent evidence from all three aspects that together support and define the occurrence of adrenarche in the rhesus monkey, discussing how it differs from the event as we know it in humans.
2. Adrenarche Defined Morphologically
Notwithstanding the logistical constraints in gathering such data, the definition of adrenarche should rest as much on the morphological and biochemical aspects of adrenal development, as it now does on the hormones that reflect those biological processes. The increase in adrenal androgen output associated with adrenarche in human children coincides with morphological development and differentiation of the innermost adrenocortical zone, the ZR (Suzuki et al., 2000). The human foetal zone disappears over the course of the first year of life (Benner, 1940;Lanman, 1953;Sucheston and Cannon, 1968;Dhom, 1973), and the ZR, first recognizable as early as three years of age (Dhom, 1973), reaches morphological maturity in the second decade (Sucheston and Cannon, 1968;Dhom, 1973). The human adrenal develops increased functional capacity for androgen secretion with the expression of requisite enzymes (Hui et al., 2009;Narasaka et al., 2001;Suzuki et al., 2000), especially of cytochrome b5 (Yanase et al., 1998), a well known positive regulator of androgen synthesis (Katagiri et al., 1982;Onoda and Hall, 1982;Auchus et al., 1998;Miller et al., 1997;Miller and Auchus, 2000). Human adrenarche has a functional, morphological signature consistent with the production of androgens by a maturing ZR.
The adrenal cortex of the adult rhesus has a distinct ZR, essentially identical in its enzymatic differentiation (Mapes et al., 1999) to that of the human ZR (Nguyen and Conley, 2008), and its development follows collapse of an adrenal foetal zone (Mesiano and Jaffe, 1997;Seron-Ferre et al., 1986;Seron-Ferre and Jaffe, 1981), much as in humans (Hill, 1930;Mesiano and Jaffe, 1997;Lanman, 1957). However, development and differentiation of the ZR proceeds considerably faster in rhesus monkeys than humans, over a period of months rather than years (McNulty, 1981). Recent studies have characterized the ontogeny of steroidogenic enzyme expression during morphological adrenarche in this primate (Nguyen et al., 2008). The establishment of the ZR through expansion the “dense band” that separates the foetal and definitive zones of the developing rhesus adrenal gland was highlighted by the prominent expression of cytochrome b5 (Figure 1[b1]). Based on the establishment of a continuous band of cytochrome b5-expressing cells at the cortico-medullary junction, maturation of the rhesus ZR was essentially complete by three months of age, during which there was also regression of the foetal zone (Nguyen et al., 2008). Thus, the interval encompassing morphological adrenarche in the rhesus is very rapid by comparison with the human, but follows a similar differentiation process with respect to the expression of steroidgenic and associated enzymes.
Figure 1.
Morphological maturation of the adrenal glands from rhesus macaques ranging in age from one day to three months of age marked by cytochrome b5 expression. Cytochrome b5 expression (red/brown chromagen) marks the differentiating zona reticularis (ZR) which becomes better defined with age in tissue sections from 1 day old, 4, 8 and 12 week old neonatal rhesus macaques (ages shown above each panel) detected by immunohistochemistry. Note also the developing expression of cytochrome b5 (arrow) between the definitive zone (DZ) abutting the capsule and the foetal zone (FTZ) toward the medulla.. Bars = 50μm. Micrographs are reproduced from published data (Nguyen et al., 2008) with permission from the publisher.
3. Adrenarche Defined Biochemically
The synthesis of DHEA, and thereby DHEAS, results directly from the 17,20-lyase activity of the enzyme 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17) (Hall, 1991;Zuber et al., 1986) and human adrenarche is thought to result from a selective increase in 17,20-lyase activity (Rich et al., 1981;Kelnar and Brook, 1983). Cytochrome b5 selectively augments 17,20-lyase over 17α-hydroxylase activity (Katagiri et al., 1982;Onoda and Hall, 1982;Lee-Robichaud et al., 1995;Auchus et al., 1998;Brock and Waterman, 1999;Sakai et al., 1993). Based on the functional morphology of human ZR development described above (Suzuki et al., 2000;Narasaka et al., 2001;Hui et al., 2009;Nakamura et al., 2009), cytochrome b5 is thought to be a key factor augmenting adrenal androgen secretion during adrenarche (Miller, 2009). Couch et al. investigated 17,20-lyase activity in human adrenal tissues, but found no significant increase during the adrenarche (Couch et al., 1986). However, biochemical evidence of adrenarche was investigated in rhesus adrenal gland tissues focusing on the window of ZR development between birth and three months of age established by the functional morphological studies described above (Nguyen et al., 2009). Three important observations were made for the first time in any primate. First, 17,20-lyase activity in rhesus adrenal microsomal protein was shown to increase with age (and ZR development), from a level of 2.9nmol/mg/hr in a specimen from a 5 day old perinatal subject to a peak average of 22.9 nmol/mg/hr in two specimens from 8 week old infants. Thereafter, 17,20-lyase activity declined on average in specimens collected from juveniles at 26 weeks of age (Figure 2, dashed line). Second, there was a concomitant and positively correlated increase in cytochrome b5 expression during this developmental window. The levels of expression of cytochrome b5 peaked in specimens from 8 and 12 week old infants and steadily decreasing in specimens taken from juveniles over a year of age (Figure 2, solid line[b2]). Third, adrenal microsomal 17,20-lyase activity was increased significantly by up to 250% with addition of purified recombinant cytochrome b5, and the less endogenous cytochrome b5 adrenal microsomes contained, the more activity was stimulated (Nguyen et al., 2009). These data directly support the belief that adrenarche is brought about by an increase in 17,20-lyase activity in the developing ZR, due in large part by increased expression of cytochrome b5.
Figure 2.
Biochemical evidence of adrenarche in rhesus adrenal tissues collected from birth through one year of age. Shown are plots of 17,20-lyase enzyme activity (nmol/mg/hr; dashed line) and expression levels of cytochrome b5 (pmol/μg; solid line and symbols) in microsomal protein. Graphs are reproduced from published data (Nguyen et al., 2009) with permission from the publisher.
4. Adrenarche Defined Endocrinologically
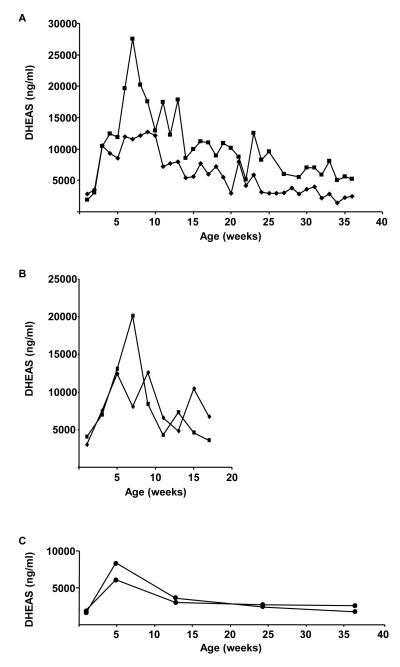
The post-natal increase in circulating DHEAS, as first characterized in humans (Rosenfield and Eberlein, 1969), begins in infancy, peaks between the second and third decade of life, and declines progressively thereafter (de Peretti and Forest, 1976;Parker et al., 1977;Sulcova et al., 1997). No such rise akin to this has been observed in rhesus monkeys, though they develop a ZR post-natally and are well known to experience a similar age-related decline (Lane et al., 1997;Kemnitz et al., 2000). However, a transient rise in immuno-reactive androgen seen in castrated male rhesus infants (Plant, 1985;Plant and Zorub, 1984) is not detectable in adrenalectomized castrates (Plant and Zorub, 1984) suggesting an adrenal source. Therefore, preliminary studies were initiated to examine the concentrations of DHEAS in castrated as well as gonad-intact males. Comparable data from ovary-intact female rhesus monkeys from a previous study was generated using commercial radio-immunoassay. Plasma samples were collected longitudinally from one or two weeks of age through infancy and analyzed for DHEAS using liquid chromatography tandem mass spectrometry (see supplementary files for detailed methods). Concentrations of DHEAS rose from less than 3 μg/ml at 1 week of age in the two castrate males to peaks at over 25 μg/ml in one and over 10 μg/ml in the other at 7 and 9 weeks of age, and a consistent decline followed thereafter (Figure 3A). Though sampled less frequently over shorter intervals, similar profiles of systemic DHEAS concentrations were observed in two intact males sampled to 17 weeks of age (Figure 3B) and two intact females sampled to 36 weeks of age (Figure 3C[b3]). The concentrations of DHEAS were much lower overall in the two females than those seen in either intact or castrate males, peaking in the samples taken at 4 weeks of age at around 6 and 8 μg/ml, and declined steadily thereafter to less than 3 μg/ml by the final sampling at 36 weeks of age. These data have been confirmed in additional castrated (and gonad-intact) males that exhibited a similarly timed increase in DHEAS, and a correlated increase in DHEA (Conley, unpublished observations). The pattern of DHEAS in the male castrates corresponds well with the previously reported increase in immuno-reactive androgen in castrate males (Plant and Zorub, 1984;Plant, 1985) and within the ranges for DHEAS determined previously for gonad-intact males and females (Koritnik et al., 1983;Seron-Ferre et al., 1983;Seron-Ferre et al., 1986). To the best of our knowledge however, these data are the first to document a transient increase in concentrations of DHEAS between birth and puberty (adrenarche as most often defined in humans) in any non-human primate.
Figure 3.
Dehydroepiandrosterone sulphate (DHEAS, ng/ml) concentrations in longitudinal samples of peripheral blood of rhesus macaques from birth through infancy. A. Two males castrated at 1 week of age and bled weekly up to 40 weeks of age. B. Two gonad-intact males bled on alternate weeks to 17 weeks of age. C. Two gonad-intact females sampled one day after birth and then at 4, 12, 24 and 36 weeks of age.
The often stated view that adrenarche occurs in the Great Apes but not Old World primates is based on fragmentary evidence, almost exclusively reliant on circulating DHEAS. Most conclude that rhesus macaques do not experience an adrenarche based on an absence of evidence from prior studies (Havelock et al., 2004;Arlt et al., 2002;Miller and Auchus, 2010). All previous studies in infant rhesus macaques have used cross-sectional sampling, and only a decline in circulating DHEAS concentrations was observed that began from high and variable concentrations in neonates and early infants (Koritnik et al., 1983;Seron-Ferre et al., 1986;Seron-Ferre et al., 1983). The elevated DHEAS concentrations seen in late gestation (Seron-Ferre et al., 1986;Seron-Ferre et al., 1983) and pre-term (McNulty et al., 1981) rhesus fetuses decays slowly postpartum (Seron-Ferre et al., 1986). Thus, doubt was expressed previously that the decreasing secretion of DHEAS by the foetal zone might obscure increasing DHEAS production by the developing rhesus ZR (Nguyen et al., 2008). However, despite the narrow and overlapping developmental window of these two events, foetal zone regression and ZR differentiation appear to be distinguishable from each other based on circulating androgen concentrations if frequent longitudinal samples are examined. As suggested here, DHEAS concentrations begin to increase from two weeks of age to a peak around six to eight weeks of age in castrate males, intact males and intact female rhesus macaques, then subsequently decline. Limited data available for neonatal rhesus monkeys accord well with more extensive results of studies conducted in foetuses, all consistent with the ability of ACTH and luteinizing hormone as stimulatory of adrenal androgen secretion (Jaffe et al., 1981). Still, the regulation of neonatal and infant adrenal growth and differentiation have not received adequate attention, most to date remaining focused on the foetus. The endocrine data reported herein, though preliminary, correlate closely with biochemical (Nguyen et al., 2009) and morphological (Nguyen et al., 2008) observations summarized here for neonates and infants, supporting the contention that adrenarche is initiated in the first three months of life in this species. Thus, three independent lines of evidence concur, and all correspond with similar events that comprise adrenarche in humans (Rainey et al., 2002;Auchus and Rainey, 2004;Havelock et al., 2004;Belgorosky et al., 2008;Miller, 2009)
The baboon is the only other non-human primate that has been studied in appreciable detail for an adrenarche. Yet, neither cross-sectional (Castracane et al., 1981) nor careful longitudinal (Ducsay et al., 1991) sampling of newborn baboons has provided evidence of a transient, post-natal increase in DHEAS concentrations. DHEAS concentrations remain elevated relative to late gestation levels for several days, then decline markedly from one week of age, despite growth of the adrenals (Ducsay et al., 1991). These authors described concurrent development of the ZR as the foetal zone regressed morphologically. However, it is generally agreed that Great Apes experience adrenarche. Though cross-sectional data indicate that DHEAS concentrations increase progressively in pre- and post-pubertal chimpanzees and gorillas (Winter et al., 1980;Collins et al., 1981;Copeland et al., 1985;Cutler et al., 1978;Smail et al., 1982), it is not clear when (or if) it reaches a peak and declines thereafter. Moreover, there are no data from Great Apes on morphological maturation of the ZR or its biochemical capacity for androgen synthesis as exists for the rhesus (Nguyen et al., 2009;Nguyen et al., 2008). If the definition of adrenarche relies on a demonstrated post-pubertal peak and subsequent decline in DHEAS, there is no such evidence of adrenarche in chimpanzees or any other non-human primate.
5. Non-human Primate Models of Adrenal Androgen Secretion
There is a clear need to develop animal models for human adrenal physiology and disease, especially for adrenal androgen secretion that provides precursors for local biopotent androgen and estrogen production whether or not gonads are functional (Labrie et al., 2003). The potential for adrenal C19 steroids to be converted to biopotent androgens and estrogens in target tissues makes diseases such as prostate and breast cancers more difficult to manage (Mostaghel and Nelson, 2008;Tworoger et al., 2006), and otherwise contributes to hyper-androgenism (Yildiz and Azziz, 2007). The propensity for androgen synthesis by the ZR is a characteristic that appears to be exhibited almost exclusively by Old World primates (Nguyen and Conley, 2008;Pattison et al., 2009). Still, there has been reluctance to use the rhesus monkey as a model for adrenal androgen synthesis because of the perception that this species does not exhibit an adrenarche (Arlt et al., 2002).
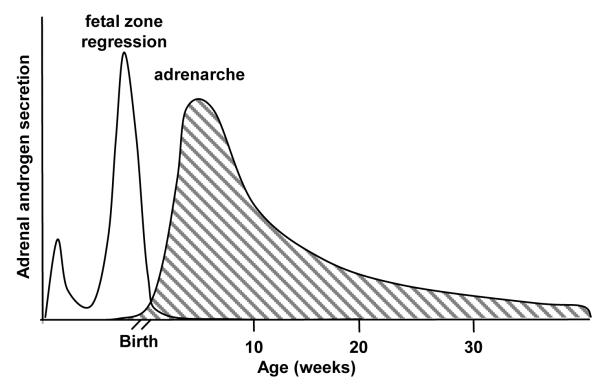
The profiles of adrenal androgen secretion across the lifespan of humans and that of the rhesus macaque as described here share some similarities. Both exhibit a prenatal rise associated with development of a foetal zone (Mapes et al., 2002;Narasaka et al., 2001). As demonstrated here, both share a second subsequent prepubertal peak marking ZR differentiation (Suzuki et al., 2000;Nguyen et al., 2008), after which adrenal androgen secretion appears to decline with age, progressively and without interruption (Parker, Jr., 1999) (Figure 4[b4]), at least in men. In addition, recent studies in women have identified a third, transient peak in DHEAS that coincides with the menopausal transition (Crawford et al., 2009;Lasley et al., 2002), and there may be a similar peri-menopausal peak in aging female rhesus monkeys (Shideler et al., 2001). The occurrence of adrenarche in male and female rhesus infants means that it still precedes puberty by two or three years (Plant and Witchel, 2006). It is notable that longitudinal urinary monitoring of children indicates that adrenal androgen secretion begins to increase far earlier than can be detected in plasma (Remer et al., 2005). Therefore, the major excursions of DHEAS secretion seen during human development and aging appear to have counterparts in the rhesus macaque.
Figure 4.
Schematic of adrenal androgen synthesis and secretion through foetal and early post-natal development showing contributions from the foetal zone and the developing zona reticularis representing “adrenarche” in the rhesus macaque.
There are also notable differences between the species with respect to peak concentrations of adrenal androgens as well as in timing. The concentrations of DHEAS are many fold higher in the rhesus (Seron-Ferre et al., 1983) than in human fetuses at term (Parker, Jr., 1999) and rhesus macaques experience higher peak concentrations (Koritnik et al., 1983;Seron-Ferre et al., 1986;Seron-Ferre et al., 1983) than humans (Sulcova et al., 1997), as confirmed by the current data. This might reflect differences in clearance rates (Schut et al., 1978;Leblanc et al., 2003) as much as it may synthesis. The most obvious disparity in the events surrounding adrenarche in the rhesus and human would appear to relate to the pace with which they take place and when the peak of adrenal androgen secretion itself occurs. The data presented here concur with the observations of others (Lane et al., 1997;Kemnitz et al., 2000) that adrenal androgen secretion begins a slow progressive decline from infancy, years before puberty is initiated. Although adrenarche is most commonly described as the initiation of adrenal androgen secretion in infancy, the peak of concentrations are reached over decades in humans (Parker, Jr., 1999), not months as shown here for the rhesus monkey. Adrenarche is not thought to be different in boys and girls, even though peak DHEAS concentrations occur later and are higher in men than in women (Orentreich et al., 1984;Sulcova et al., 1997). It was unclear whether or not female rhesus, like women, have lower peak concentration of DHEAS than males, though the preliminary results presented here are consistent with that possibility. Sex differences in concentrations of DHEAS were not observed among rhesus infants aged 4-8 weeks (Koritnik et al., 1983), but the morphological, biochemical and endocrine data reviewed above illustrate how dynamic this interval of adrenal development actually is. Differences in DHEAS concentrations between males and females would best be determined at their secretory peaks which would require sequential sampling to verify. Similarities such as these, if verified, would further suggest that adrenal androgen secretion in human and non-human primates may be more conserved than previously thought. Clearly, there are differences in adrenarche in humans and the rhesus macaque. However, these differences appear to involve issues of timing, not whether or not the rhesus experiences adrenarche at all. Given the dynamic nature of the phenomenon in the rhesus, it seems likely that much could be learned about human adrenarche from investigating the process in this monkey.
6. Conclusion
The data summarized here establish the occurrence of adrenarche in infant rhesus macaques based on corroborating evidence from endocrine, biochemical and morphological studies. Regular longitudinal sampling allowed the detection of an increase in adrenal androgen output in the first few weeks of life. These data suggest that the need for intensive, longitudinal sampling may explain the long-held misconception concerning the absence of adrenarche in the rhesus. Although a steady increase in secretion of adrenal androgens occurs over almost two decades in humans, that process appears to be completed in only a couple of months in the rhesus macaque. The realization that the rhesus experiences an adrenarche supports the conclusion that this species is a suitable and accessible model for studies into the biology and regulation of adrenal development and function.
Supplementary Material
ACKNOWLEDGEMENTS
The authors express gratitude to Jo Corbin for technical assistance, Dr. Alice Tarantal for sharing female serum samples, Dr Suresh Ramaswamy and the staff of the Primate Core of the Center for Research in Reproductive Physiology (Mr. Michael Cicco and Ms Rachel Rosland) for conducting the surgical procedures and for collecting of sequential plasma samples from the agonadal male monkeys, and Amber Edwards and Tammie Frost at the Wisconsin National Primate Research Center for technical assistance in sampling of intact males. Partial support was provided from grants HD13254 and HD08160 (TMP).
Footnotes
Abbreviations: zona reticularis (ZR), dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulphate (DHEAS), 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17), definitive zone (DZ), foetal zone (FTZ), adrenocorticotropic hormone (ACTH)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- ALBRIGHT F. Osteoporosis. Ann Intern Med. 1947;27:861–882. doi: 10.7326/0003-4819-27-6-861. [DOI] [PubMed] [Google Scholar]
- Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: structural and functional analysis of p450c17 from four primate species. Endocrinology. 2002;143:4665–4672. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Rainey WE. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Belgorosky A, Baquedano MS, Guercio G, Rivarola MA. Adrenarche: postnatal adrenal zonation and hormonal and metabolic regulation. Horm Res. 2008;70:257–267. doi: 10.1159/000157871. [DOI] [PubMed] [Google Scholar]
- Benner MC. Studies on the involution of the fetal cortex of the adrenal glands. American Journal of Pathology. 1940;16:787–798. [PMC free article] [PubMed] [Google Scholar]
- Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38:1598–1606. doi: 10.1021/bi9821059. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Cutler GB, Jr., Loriaux DL. Pubertal endocrinology of the baboon: adrenarche. Am J Physiol. 1981;241:E305–E309. doi: 10.1152/ajpendo.1981.241.4.E305. [DOI] [PubMed] [Google Scholar]
- Collins DC, NADLER RD, Preedy JRK. Adrenarche in the Great Apes. Am J Primatol. 1981;1:344. (abstract) [Google Scholar]
- Copeland KC, Eichberg JW, Parker CR, Jr., Bartke A. Puberty in the chimpanzee: somatomedin-C and its relationship to somatic growth and steroid hormone concentrations. J Clin Endocrinol Metab. 1985;60:1154–1160. doi: 10.1210/jcem-60-6-1154. [DOI] [PubMed] [Google Scholar]
- Couch RM, Muller J, Winter JS. Regulation of the activities of 17-hydroxylase and 17,20-desmolase in the human adrenal cortex: kinetic analysis and inhibition by endogenous steroids. J Clin Endocrinol Metab. 1986;63:613–618. doi: 10.1210/jcem-63-3-613. [DOI] [PubMed] [Google Scholar]
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler GJ, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978;103:2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- de Peretti E, Forest MG. Unconjugated dehydroepiandrosterone plasma levels in normal subjects from birth to adolescence in human: the use of a sensitive radioimmunoassay. J Clin Endocrinol Metab. 1976;43:982–991. doi: 10.1210/jcem-43-5-982. [DOI] [PubMed] [Google Scholar]
- Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche) Beitr Pathol. 1973;150:357–377. doi: 10.1016/s0005-8165(73)80086-1. [DOI] [PubMed] [Google Scholar]
- Ducsay CA, Hess DL, McClellan MC, Novy MJ. Endocrine and morphological maturation of the fetal and neonatal adrenal cortex in baboons. J Clin Endocrinol Metab. 1991;73:385–395. doi: 10.1210/jcem-73-2-385. [DOI] [PubMed] [Google Scholar]
- Garde AH, Hansen AM, Skovgaard LT, Christensen JM. Seasonal and biological variation of blood concentrations of total cholesterol, dehydroepiandrosterone sulfate, hemoglobin A(1c), IgA, prolactin, and free testosterone in healthy women. Clin Chem. 2000;46:551–559. [PubMed] [Google Scholar]
- Hall PF. Cytochrome P-450 C21scc: one enzyme with two actions: hydroxylase and lyase. Journal of Steroid Biochemistry and Molecular Biology. 1991;40:527–532. doi: 10.1016/0960-0760(91)90272-7. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22:337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- Hill WCO. Observations on the growth of the suprarenal cortex. Journal of Anatomy. 1930;64:479–502. [PMC free article] [PubMed] [Google Scholar]
- Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203:241–252. doi: 10.1677/JOE-09-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe RB, Seron-Ferre M, Crickard K, Koritnik D, Mitchell BF, Huhtaniemi IT. Regulation and function of the primate fetal adrenal gland and gonad. Recent Prog Horm Res. 1981;37:41–103. doi: 10.1016/b978-0-12-571137-1.50007-4. [DOI] [PubMed] [Google Scholar]
- Katagiri M, Suhara K, Shiroo M, Fujimura Y. Role of cytochrome b5 in the cytochrome P-450-mediated C21-steroid 17,20-lyase reaction. Biochem Biophys Res Commun. 1982;108:379–384. doi: 10.1016/0006-291x(82)91877-0. [DOI] [PubMed] [Google Scholar]
- Kelnar CJ, Brook CG. A mixed longitudinal study of adrenal steroid excretion in childhood and the mechanism of adrenarche. Clin Endocrinol (Oxf) 1983;19:117–129. doi: 10.1111/j.1365-2265.1983.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Roecker EB, Haffa AL, Pinheiro J, Kurzman I, Ramsey JJ, MacEwen EG. Serum dehydroepiandrosterone sulfate concentrations across the life span of laboratory-housed rhesus monkeys. J Med Primatol. 2000;29:330–337. doi: 10.1034/j.1600-0684.2000.290504.x. [DOI] [PubMed] [Google Scholar]
- Koritnik DR, Laherty RF, Rotten D, Jaffe RB. A radioimmunoassay for dehydroepiandrosterone sulfate in the circulation of rhesus monkeys. Steroids. 1983;42:653–667. doi: 10.1016/0039-128x(83)90129-0. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–2096. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- Lanman JT. The Fetal Zone of the Adrenal Gland - Its Developmental Course, Comparative Anatomy, and Possible Physiologic Functions. Medicine. 1953;32:389–430. doi: 10.1097/00005792-195312000-00001. [DOI] [PubMed] [Google Scholar]
- Lanman JT. The adrenal fetal zone: its occurrence in primates and a possible relationship to chorionic gonadotropin. Endocrinology. 1957;61:684–691. doi: 10.1210/endo-61-6-684. [DOI] [PubMed] [Google Scholar]
- Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87:3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- Leblanc M, Labrie C, Belanger A, Candas B, Labrie F. Bioavailability and pharmacokinetics of dehydroepiandrosterone in the cynomolgus monkey. J Clin Endocrinol Metab. 2003;88:4293–4302. doi: 10.1210/jc.2003-022012. [DOI] [PubMed] [Google Scholar]
- Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17 alpha-hydroxylase-17,20-lyase CYP17 by cytochrome b5: endocrinological and mechanistic implications. Biochemical Journal. 1995;308(Pt 3):901–908. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapes S, Corbin CJ, Tarantal A, Conley A. The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17alpha-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3beta-hydroxysteroid dehydrogenase/delta5-4 isomerase (3beta-HSD) J Clin Endocrinol Metab. 1999;84:3382–3385. doi: 10.1210/jcem.84.9.6105. [DOI] [PubMed] [Google Scholar]
- Mapes S, Tarantal AF, Parker CR, Moran FM, Bahr JM, Pyter L, Conley AJ. Adrenocortical cytochrome b5 expression during fetal development of the rhesus macaque. Endocrinology. 2002;143:1451–1458. doi: 10.1210/endo.143.4.8718. [DOI] [PubMed] [Google Scholar]
- McNulty WP. Postnatum evolution of the adrenal glands of rhesus macaques. In: Novy MJ, Resko JA, editors. Fetal Endocrinology. Academic Press; New York: 1981. pp. 53–64. [Google Scholar]
- McNulty WP, Novy MJ, Walsh SW. Fetal and postnatal development of the adrenal glands in Macaca mulatta. Biol Reprod. 1981;25:1079–1089. doi: 10.1095/biolreprod25.5.1079. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- Miller WL. Androgen synthesis in adrenarche. Reviews in Endocrine & Metabolic Disorders. 2009;10:3–17. doi: 10.1007/s11154-008-9102-4. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. Role of cytochrome b5 in the 17,20-lyase activity of P450c17. J Clin Endocrinol Metab. 2000;85:1346. doi: 10.1210/jcem.85.3.6434-3. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr Rev. 2010 doi: 10.1210/er.2010-0013. DOI er.2010-0013 [pii];10.1210/er.2010-0013 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids. 1997;62:133–142. doi: 10.1016/s0039-128x(96)00172-9. [DOI] [PubMed] [Google Scholar]
- Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22:243–258. doi: 10.1016/j.beem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94:2192–2198. doi: 10.1210/jc.2008-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol. 2001;174:111–120. doi: 10.1016/s0303-7207(00)00445-7. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ. The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques. Endocrinology. 2009;150:1748–1756. doi: 10.1210/en.2008-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AD, Mapes SM, Corbin CJ, Conley AJ. Morphological adrenarche in rhesus macaques: development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J Endocrinol. 2008;199:367–378. doi: 10.1677/JOE-08-0337. [DOI] [PubMed] [Google Scholar]
- Onoda M, Hall PF. Cytochrome b5 stimulates purified testicular microsomal cytochrome P-450 (C21 side-chain cleavage) Biochem Biophys Res Commun. 1982;108:454–460. doi: 10.1016/0006-291x(82)90850-6. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Parker CR., Jr. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids. 1999;64:640–647. doi: 10.1016/s0039-128x(99)00046-x. [DOI] [PubMed] [Google Scholar]
- Parker L, Sack J, Fisher D, Odell W. Adrenarche - Prolactin, Gonadotropins, Adrenal Androgens and Cortisol. Clinical Research. 1977;25:A173. doi: 10.1210/jcem-46-3-396. [DOI] [PubMed] [Google Scholar]
- Pattison JC, Abbott DH, Saltzman W, Conley AJ, Bird IM. Plasticity of the zona reticularis in the adult marmoset adrenal cortex: voyages of discovery in the New World. J Endocrinol. 2009;203:313–326. doi: 10.1677/JOE-08-0554. [DOI] [PubMed] [Google Scholar]
- Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in Nonhuman Primates and Humans. In: Neill JD, Challis JR, De Kretser DM, Pfaff D, Richards JS, Plant TM, Wassarman PM, editors. Knobil and Neill’s Physiology of Reproduction. Raven Press; St. Louis, MO: 2006. pp. 2177–2230. [Google Scholar]
- Plant TM, Zorub DS. A study of the role of the adrenal glands in the initiation of the hiatus in gonadotropin secretion during prepubertal development in the male rhesus monkey (Macaca mulatta) Endocrinology. 1984;114:560–565. doi: 10.1210/endo-114-2-560. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3-18 years. J Clin Endocrinol Metab. 2005;90:2015–2021. doi: 10.1210/jc.2004-1571. [DOI] [PubMed] [Google Scholar]
- Rich BH, Rosenfield RL, Lucky AW, Helke JC, Otto P. Adrenarche: changing adrenal response to adrenocorticotropin. J Clin Endocrinol Metab. 1981;52:1129–1136. doi: 10.1210/jcem-52-6-1129. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Eberlein WR. Plasma 17-ketosteroid levels during adolescence. J Pediatr. 1969;74:932–936. doi: 10.1016/s0022-3476(69)80228-3. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Yanase T, Takayanagi R, Nakao R, Nishi Y, Haji M, Nawata H. High expression of cytochrome b5 in adrenocortical adenomas from patients with Cushing’s syndrome associated with high secretion of adrenal androgens. J Clin Endocrinol Metab. 1993;76:1286–1290. doi: 10.1210/jcem.76.5.8496319. [DOI] [PubMed] [Google Scholar]
- Schut HA, Pepe GJ, Townsley JD. Clearance and production of dehydroepiandrosterone and its sulfate in female baboons. Am J Physiol. 1978;235:E74–E77. doi: 10.1152/ajpendo.1978.235.1.E74. [DOI] [PubMed] [Google Scholar]
- Seron-Ferre M, Hess DL, Lindholm U, Jaffe RB. Persistence of fetal zone function in the infant rhesus monkey adrenal gland. J Clin Endocrinol Metab. 1986;62:460–465. doi: 10.1210/jcem-62-3-460. [DOI] [PubMed] [Google Scholar]
- Seron-Ferre M, Jaffe RB. The fetal adrenal gland. Annu Rev Physiol. 1981;43:141–162. doi: 10.1146/annurev.ph.43.030181.001041. [DOI] [PubMed] [Google Scholar]
- Seron-Ferre M, Taylor NF, Rotten D, Koritnik DR, Jaffe RB. Changes in fetal rhesus monkey plasma dehydroepiandrosterone sulfate: relationship to gestational age, adrenal weight and preterm delivery. J Clin Endocrinol Metab. 1983;57:1173–1178. doi: 10.1210/jcem-57-6-1173. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JS. Further studies on adrenarche in nonhuman primates. Endocrinology. 1982;111:844–848. doi: 10.1210/endo-111-3-844. [DOI] [PubMed] [Google Scholar]
- Sucheston ME, Cannon MS. Development of zonular patterns in the human adrenal gland. J Morphol. 1968;126:477–491. doi: 10.1002/jmor.1051260408. [DOI] [PubMed] [Google Scholar]
- Sulcova J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocrinol. 1997;154:57–62. doi: 10.1677/joe.0.1540057. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Missmer SA, Eliassen AH, Spiegelman D, Folkerd E, Dowsett M, Barbieri RL, Hankinson SE. The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:967–971. doi: 10.1158/1055-9965.EPI-05-0976. [DOI] [PubMed] [Google Scholar]
- Winter JS, Faiman C, Hobson WC, Reyes FI. The endocrine basis of sexual development in the chimpanzee. J Reprod Fertil Suppl. 1980;(Suppl 28):131–138. [PubMed] [Google Scholar]
- Yanase T, Sasano H, Yubisui T, Sakai Y, Takayanagi R, Nawata H. Immunohistochemical study of cytochrome b5 in human adrenal gland and in adrenocortical adenomas from patients with Cushing’s syndrome. Endocr J. 1998;45:89–95. doi: 10.1507/endocrj.45.89. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord. 2007;8:331–342. doi: 10.1007/s11154-007-9054-0. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Simpson ER, Waterman MR. Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic COS 1 cells. Science. 1986;234:1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.