Abstract
Pelvic fracture urethral injury (PFUI) management in male adults and children is controversial. The jury is still out on the best way to manage these injuries in the short and long-term to minimise complications and optimise outcomes. There is also little in the urological literature about pelvic fractures themselves, their causes, grading systems, associated injuries and the mechanism of PFUI. A review of pelvic fracture and male PFUI literature since 1757 was performed to determine pelvic fracture classification, associated injuries and, PFUI classification and management. The outcomes of; suprapubic catheter (SPC) insertion alone, primary open surgical repair (POSR), delayed primary open surgical repair (DPOSR), primary open realignment (POR), primary endoscopic realignment (PER), delayed endoscopic treatment (DET) and delayed urethroplasty (DU) in male adults and children in all major series have been reviewed and collated for rates of restricture (RS), erectile dysfunction (ED) and urinary incontinence (UI). For SPC, POSR, DPOSR, POR, PER, DET and DU; (I) mean RS rate was 97.9%, 53.9%, 18%, 58.3%, 62.0%, 80.2%, 14.4%; (II) mean ED rate was 25.6%, 22.5%, 71%, 37.2%, 23.6%, 31.9%, 12.7%; (III) mean UI rate was 6.7%, 13.6%, 0%, 14.5%, 4.1%, 4.1%, 6.8%; (IV) mean FU in months was 46.3, 29.4, 12, 61, 31.4, 31.8, 54.9. For males with PFUI restricture and new onset ED is lowest following DU whilst UI is lowest following DPOSR. On balance DU offers the best overall outcomes and should be the treatment of choice for PFUI.
Keywords: Pelvic fracture, urethral injury, classification, treatment, outcomes
Introduction
Pelvic fracture urethral injury (PFUI) management is a source of significant debate. Primary surgical repair (1-4), delayed primary repair (5), primary open realignment (POR) (6-10), primary endoscopic realignment (PER) (11-19), suprapubic cystostomy with delayed endoscopic management (20-22) or delayed urethroplasty (DU) (23-28) all have their advocates. We present a contemporary review of PFUI analysing all literature since 1757 with particular reference to the long-term incidence of recurrent strictures (RS), erectile dysfunction (ED) and urinary incontinence (UI). The current orthopaedic classification of pelvic fracture is also detailed to provide clarity on the subject (29-32).
Pelvic fracture classification
The three commonly used orthopaedic pelvic fracture classifications are: Young-Burgess (29), Tile (30) and AO/OTA (31). They all stem from the 1990 Young-Burgess classification system (29,32). This system is based upon mechanism of injury and associated injuries. There are 4 major categories; 2 of which have subdivisions according to severity (Table 1) (Figure 1).
Table 1. Young-Burgess pelvic fracture classification.
| Young-Burgess classification | Type of fracture | Associated injuries |
|---|---|---|
| Lateral compression | ||
| Type I | Force is directed posteriorly | Minimal problems with resuscitation |
| Sacral crush and ipsilateral horizontal pubic rami fracture | ||
| Stable | ||
| Type II | Force is directed anteriorly | Often associated head and intra-abdominal injuries |
| Horizontal pubic rami fractures, anterior sacral crush and disruption of either the posterior sacro-iliac joints or fractures through the iliac wing | ||
| Ipsilateral injury | ||
| Vertical stability is maintained | ||
| Type IIII | Force is anteriorly directed and continued across the pelvis | Often associated head and intra-abdominal injuries |
| Type I or II ipsilateral fracture and an external rotation component to the contralateral hemi-pelvis opening the sacro-iliac joint posteriorly and disrupting the sacrotuberous and spinous ligaments | ||
| Anteroposterior compression | ||
| Type I | Force is antero-posteriorly directed | Minimal problems with resuscitation |
| <2.5 cm diastasis | ||
| Vertical fracture of 1 or both pubic rami | ||
| Or disruption of symphysis, opening the pelvis | ||
| Posterior ligaments are intact | ||
| Stable | ||
| Type II | Continuation of type I with disruption of posterior ligaments | Minimal problems with resuscitation |
| >2.5 cm diastasis | ||
| Opening of sacroiliac joints | ||
| Vertical stable | ||
| Rotational instability | ||
| Type III | Complete disruption anteriorly and posteriorly | Brain, abdominal, visceral, pelvic vascular |
| Significant sacral diastasis or displacement of vertical pelvic rami fracture | Increased risk of shock, sepsis and ARDS | |
| Completely unstable or vertical instability | ||
| Vertical shear | Force is directed vertically or at right angles to support structures of pelvis | Often associated head and intra-abdominal injuries |
| Vertical fractures of all rami and disruption of all ligaments | ||
| Completely unstable and rotationally unstable | ||
| Combined mechanism of injury | Any combination of the above | – |
| Unstable injury | ||
Figure 1.
Young-Burgess pelvic fracture classification. LC, lateral compression; APC, anteroposterior compression; VS, vertical shear.
Lateral compression (LC): these are implosion injuries from lateral impact to the innominate bone. The pelvis on the side of the impact rotates toward the midline. The anterior pelvic ligaments are shortened. The anterior pattern of LC fracture may be unilateral, contralateral or bilateral involving one or more sets of pubic rami, one set of which will always have a transverse fracture. It is the extent of the posterior pathology that differentiates the subsets. LC fractures tend to close the pelvic cavity and death, if it occurs, is due to associated non-pelvic injuries (33).
Anteroposterior compression (APC): all have pubic symphysis diastasis or anterior vertical fracture of the rami. There is no cephalad shift of the hemi-pelvis. Again, it is the extent of the posterior pathology that defines the subsets. APC fractures tend to open up the pelvic cavity and death, if it occurs, is most commonly secondary to bleeding and its complications (33).
Vertical shear (VS): a symphyseal diastasis or a vertical fracture pattern of the rami occurs anteriorly. VS fractures are distinguished by vertical displacement of the hemi-pelvis and are more likely to be unstable (33).
Mixed (CM): a combination of fracture patterns and grades (33).
The incidence of pelvic fracture is approximately 20/100,000 for men and 29/100,000 for women. 50% of women have uncomplicated pubic rami fractures (34,35). The majority (90%) of patients with pelvic fractures have associated injuries (36-38). The male to female ratio is 2:1 for young adults, falling to 1:3 for the over 50’s. They mainly occur in the first 4 decades of life (modal age 31–33 years) (3,34,39,40). The commonest cause is motor vehicle accidents with motorist(s) affected in 54–71% and pedestrians in 12–18%. Falls and crush injuries constitute the majority of the remainder (29,38,39,41,42).
Mortality, associated injuries and resuscitation requirements may be predicted by the Young-Burgess classification (29,32,43). The most frequently occurring pelvic fracture categories are: LC1 (48.8%), APCII (11.1%), VS (5.6%) and CM (6.8%) (29,32). Orthopaedic management of unstable injuries comprises external and/or internal pelvic fixation to control fracture related bleeding, immobilise the fracture, reduce pain and facilitate mobilisation and rehabilitation (44,45). Stable fractures are generally treated similarly to facilitate recovery (46,47).
Injuries associated with pelvic fractures
Pelvic fractures resulting in PFUI are high impact injuries with mortality rates between 5–33% (4,29,32,36,45,48-53). Other associated injuries are common and include: intracranial (40–66.1%), splenic (9.3–37%), colorectal (6.8–29.1%), bladder (2.5–28%), chest (6–16.6%), liver (5.6–19%), lower limb fracture(s) (17%), pulmonary (9.3%), upper limb fracture(s) (3%) and diaphragmatic rupture (3–21%) (4,32,33,39,52,54-58). Initial medical management should concentrate on resuscitating and stabilising the patient, and then on identifying all associated injuries.
Urethral injuries associated with pelvic fractures
PFUI occurs in 1.6% to 25% of pelvic fractures; giving a frequency of 0.32–5/100,000 for men and 0.46–7.25/100,000 for women (4,41,52,54,55,59-72). Straddle fractures (fractures of all 4 pubic rami) with or without distraction of the sacro-iliac joint, fractures of the inferior pubic ramus with a widened pubic symphysis and Malgaigne fractures [double pelvic ring break fracture dislocations (73)] are most commonly associated with PFUI (39,41,59,67,73-75). Combined urethral and bladder injury occur in 1–33% of patients (16,54,59,63,73,76,77). Bladder injury is more commonly extra-peritoneal (56–85%) than intra-peritoneal (17–39%) but can be both (63,72,77-80).
Early theories on the mechanism of PFUI postulated a horizontal or shearing force through the membranous urethra at the point where it was fixed by the urogenital diaphragm (54,65,66,81). More recently the concept of a urogenital diaphragm has been rejected and it is suggested that PFUI is caused by an avulsion of the membranous urethra from the bulbar urethra at the point where they meet at the perineal membrane (24,82,83). The original term “Pelvic Fracture Urethral Distraction Defect” has duly been amended to PFUI. It was also previously thought to be a complete defect of the urethra but is now known to be a partial or complete disruption of the urethra; hence the change of terminology (84,85). The relative frequency of partial and complete disruption varies in most series from 11–90% for partial and 6–100% for complete (24,25,54,64,69,80,86-90). These wide variations may be due to variability in the use of urethrography for diagnosis and the limitations of subsequent interpretation.
The usual result of complete PFUI is traumatic disruption of urethral continuity at the bulbomembranous junction with little or no loss of urethral length, variable displacement of the two ends of the urethra and some degree of damage in many instances to the urethral sphincter (91). Unusual injuries include: longitudinal tears through the bladder base and neck down into the prostatic or bulbar urethra; avulsion of the anterior prostate and/or transection above and below the prostate. Injuries to the bladder neck and prostatic urethra are seen more commonly in children (39,92-94). The presence of concomitant bladder neck or rectal injury dictate immediate laparotomy and primary repair ± defunctioning colostomy (80). Women with PFUI mainly suffer anterior longitudinal tearing of the urethra resulting in UI rather than urethral stricture (95,96).
Historical considerations
PFUI was uniformly fatal due to urinary outflow obstruction, extravasation, secondary sepsis and uraemia until Verguin determined how to perform suprapubic cystostomy with antegrade-retrograde railroading of a perineal catheter into the bladder in 1757 (97). Survival was ad hoc for the next 150 years until management of associated injuries and imaging improved—such that mortality dropped from close to 100% in 1757 to 78% in 1907 and 23% in 1942 (98).
Initial assessment
In the acute situation, management of PFUI should wait until the patient is stable, as 90–97% of patients will have associated injuries (38,99,100). A urethral injury should be suspected if one or more of the following are noted: blood at the meatus (present in 37–93%, this may take at least 1 hour to appear), difficulty or inability to void, a palpable bladder, a high riding prostate (often unreliable due to the presence of fracture haematoma) or a pelvic fracture with displacement of pubic rami. Butterfly bruising of the perineum due to haematoma confined to Colles fascia is a late finding and indicates rupture of the perineal membrane (64,66,67,101,102). Classical findings may be absent in 29–76% and a high index of suspicion should be maintained (60,103).
At this stage one gentle attempt at urethral catheterisation is reasonable, even if blood is seen at the meatus (16,54,67,104). The fear that urethral catheterisation may convert a partial tear into a complete tear (24,54,61,105) does not seem realistic, especially as a urethral catheter passes easily into the bladder in 50% of patients with partial injuries (62,106). If the catheter does not pass with ease or does not drain clear urine it should be removed immediately (107).
Early diagnosis of PFUI and prompt urinary diversion will prevent infection of extravasated blood and urine, which can lead to abscess formation. This may extend along fascial planes and across anatomical compartmental barriers into the abdomen, chest, perineum and medial thighs. This can result in urethrocutaneous fistula, peri-urethral diverticula, necrotising fasciitis and even death (9,10,102,108). If urethral catheterisation fails, a suprapubic catheter (SPC) should be inserted using ultrasound guidance or via open cystostomy (109).
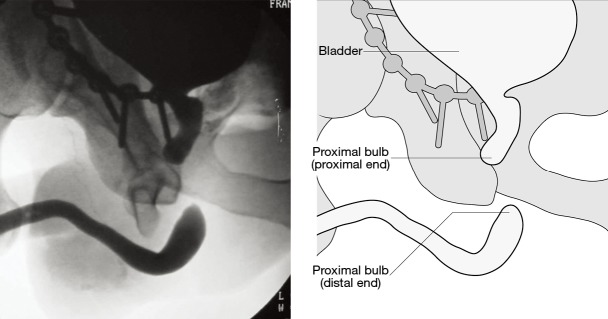
In a stable patient urethrography (retrograde and/or antegrade) is the gold standard to diagnose urethral trauma. This is usually performed with 20–30 mL of water-soluble contrast media using an aseptic technique with intravenous antibiotic prophylaxis to prevent contamination of fracture haematoma. The best images are obtained with the patient in a 30o oblique position (16,66,110,111). Extravasation of contrast from the urethra without filling of the bladder is interpreted as a complete disruption (Figure 2), whilst extravasation of contrast from the urethra with partial filling of the bladder is interpreted as a partial disruption (Figure 3) (104,112,113). It is not possible to differentiate a complete from a partial disruption on urethrography in all patients, as some patients with a partial tear may have concomitant sphincter spasm preventing passage of contrast into the bladder (12,90). There is a tendency to over-diagnose complete rupture on urethrography alone. Other imaging modalities have been investigated especially prior to elective delayed repair including CT and MRI (114,115). Contrast MRI urethrography is the most promising as it shows 3D urethral anatomy and allows for advanced preoperative planning (116).
Figure 2.

Complete PFUI. PFUI, pelvic fracture urethral injury.
Figure 3.

Partial PFUI. PFUI, pelvic fracture urethral injury.
A variety of classification systems have been proposed for PFUI based on urethrographic findings (81,94,117-120). The difficulties in differentiating partial from complete disruption on urethrography prevent accurate utilisation of these classifications. The EAU classification is the most commonly used and is generic for all urethral trauma not just PFUI (119).
Management of PFUI
This remains controversial due to the paucity of comparative studies and because there is little long-term follow-up for many of the treatments. Late stricture recurrence (>10 years post treatment) and new stricture formation also occur, to confuse the issue (121).
Management options can be divided into primary versus delayed repair techniques. Both primary and delayed techniques include a range of options including; open or endoscopic repair or realignment and urethroplasty.
Primary surgical (open) repair
Young originally described primary anastomotic repair of PFUI in 1929 (1). The rationale was to evacuate the pelvic haematoma and produce a watertight repair of the urethra thus preventing urine extravasation and subsequent infection and death, which were otherwise inevitable (1,122,123).
This approach has high complication rates with RS in @54% (0–100%), ED in @23% (range, 0–100%) and UI in @14% (range, 0–50%) (1,2,4,36,61,62,80,92,124-137) (Table 2). It is associated with significant intra-operative blood loss (>3 L on average) and prolonged hospital stay (>28 days on average). It is now rarely used unless there is a simultaneous bladder neck or rectal injury requiring definitive reconstruction or diverting sigmoid colostomy (1,4,73,80,126,138).
Table 2. Outcomes of primary open repair.
| Author | Number | Age, mean (range) | Follow up, mean (range) | Stricture, % (N) | Erectile dysfunction, % (N) | Incontinence, % (N) | Catheter Time | Comments |
|---|---|---|---|---|---|---|---|---|
| Young 1929 (1) | 1 | 23 y | Ua | 0 (0/1) | 100 (1/1) | 0 (0/1) | Ua | 1st Report AP BPA |
| Kishev 1964 (124) | 3 | Ua | ≥12 m | 0 (0/3) | 0 (0/3) | 0 (0/3) | Ua | AP BPA |
| Ragde 1969 (125) | 8 | Ua | (25 d–4 m) | 0 (0/8) | Ua | Ua | 10–18 d | Vietnamese war victims A BPA |
| Pierce 1972 (126) | 4 | Ua | Ua | 50 (2/4) | Ua | Ua | Ua | A BPA |
| Janosz 1975 (2) | 34 | Ua | Ua | 50 (17/34) | Ua | Ua | Ua | A BPA |
| Coffield 1977 (61) | 9 | Ua | Ua | 77 (7/9) | 33 (3/9) | 22 (2/9) | – | A BPA |
| Glass 1978 (62) | 71 | Ua | Ua | 93 (66/71) | Ua | Ua | Ua | A BPA |
| Cass 1978 (135) | 3 | (<20–>60 y) | 14 m (1–87 m) | 100 (3/3) | 50 (1/2) | 0 (0/2) | Ua | A BPA |
| Weems 1979 (127) | 9 | Ua | Ua | 78 (7/9) | 33 (3/9) | 22 (2/9) | Ua | A BPA |
| Muhlbauer 1980 (128) | 1 | 25 y | 12 m | 0 (0/1) | 0 (0/1) | 0 (0/1) | 3 m | A BPA |
| Webster 1983 (80) | 4 | Ua | (8 m–17 y) | 75 (3/4) | 50 (2/4) | 50 (2/4) | Ua | A BPA |
| Cass 1984 (36) | 4 | Ua | Ua | 75 (3/4) | 50 (1/2) | 0 (0/2) | Ua | A BPA |
| Reinberg 1989 (136) | 6 | 9.5 y (3–17 y) | 27.9 m | 17 (1/6) | Ua | Ua | Ua | A BPA |
| Zingg 1990 (129) | 13 | Ua | Ua | 38 (5/13) | Ua | Ua | Ua | |
| Tryfanos 1990 (137) | 6 | 8.25 y (3.5–12 y) | 4.45 y (6 m–8 y) | 67 (4/6) | 20 (1/5) | 0 (0/6) | 2 w | Suprapubic BPA |
| Boone 1992 (92) | 8 | (children) | (>15 y) | 75 (6/8) | 75 (6/8) | 25 (2/8) | Ua | >2 L blood loss; all UI had BN injury |
| Gadhvi 1993 (130) | 16 | 32.5 y (22–43 y) | 12.4 m (7–14 m) | 12.5 (2/16) | 6.25 (1/16) | 0 (0/16) | 2 w | Lateral perineal BPA |
| Koraitim 1996 (131) | 4 | Ua | Ua | 50 (2/4) | 50 (2/4) | 0 (0/4) | Ua | A BPA |
| Podesta 1997 (4) | 6 | Ua | (3–17 y) | 67 (4/6) | Ua | 50 (3/6) | Ua | BPA |
| Upadhyaya 2002 (132) | 5 boys | (18 m–11 y) | (6 m–10 y) | 40 (2/5) | 0 (0/4) | 0 (0/4) | 3 w | Transpubic BPA |
| Onen 2005 (133) | 8 | (4–17 y) | Ua | 25 (2/8) | 12.5 (1/8) | 12.5 (1/8) | Ua | BPA |
| Qu 2014 (134) | 35 boys | Ua | 58 m (6–192 m) | 9 (3/35) | 8.6 (3/35) | 11.9 (4/35) | Ua | Perineal BPA |
Ua, un-assessed; A BPA, abdominal bulbo-prostatic anastomotic urethroplasty; AP BPA, abdomino-perineal bulbo-prostatic anastomotic urethroplasty.
Delayed acute primary repair
Originally described by Mundy in 1991 for the “pie in the sky” bladder (Figure 4) (5). The principle was that with a severe urethral injury, recovery was likely to be slow and the net result of conservative treatment would be a long stricture making subsequent surgery difficult and its success limited. Bulboprostatic anastomosis was performed 7–10 days after the injury to evacuate the haematoma and bring the ends of the urethral together at a time after acute bleeding had stopped and the patient was stable from their other injuries.
Figure 4.

Pie in the sky bladder.
The aim was not to prevent a stricture but to ensure that should a stricture develop it would be easily treatable. 17 patients were reported in this series; with RS in 18%, ED in 71% and UI in 0% at 12 months (5) (Table 3). Whether success is attributable to the repair of the urethra or drainage of the haematoma accelerating recovery and decreasing fibrosis is unknown (28). The relatively high ED rate could be attributed to the severity of the injury rather than the repair. To date no other series on this technique have been published.
Table 3. Outcome of delayed primary open repair.
| Author | Number | Age, mean (range) | Follow up, mean (range) | Stricture, % (N) | Erectile dysfunction, % (N) | Incontinence, % (N) | Catheter time | Comments |
|---|---|---|---|---|---|---|---|---|
| Mundy 1991 (5) | 17 | (16–42 y) | ≥12 m | 18 (3/17) | 71 (12/17) | 0 (0/17) | 4–5 w | A BPA |
Primary open realignment (POR)
First reported by Ormond and Cothran in 1934 (3,6) as an easier alternative to Young’s primary open repair, POR became popular because of its ease. Primary realignment after open cystostomy can be achieved in a number of ways: retrograde catheter placement under direct vision, sound to sound, sound to finger and combined antegrade-catheter guided retrograde catheter placement (7,8,107). Originally, traction was applied to the urethral catheter to encourage realignment (7), however ischaemic damage to the bladder neck sphincter mechanism secondary to pressure from the catheter balloon resulted in UI in some (82). Alternative techniques trialled include transprostatic Vest sutures (9,10,139) and traction-free open realignment (8,93,140). Depending on the technique used varying degrees of peri-urethral mobilisation occurs. Following all methods of POR the urethral catheter remains in situ for 4–8 weeks prior to urethrogram and trial of void if healed (8).
POR does not actually produce anatomical realignment of the urethra; at best it re-establishes urethral continuity. Actual 3-dimensional urethral realignment requires fluoroscopic guidance to keep the proximal and distal urethra in the same cephalocaudal axis (15) and is rarely achieved. Other disadvantages of POR are increased blood loss and the potential to worsen the urethral injury. There are concerns that this technique may increase the incidence of ED and UI (80,141), although recent studies have shown similar ED and UI rates to those of DU (8,140).
The RS rate following POR is @58% (8.5–100%) (7,25,62,129,132, 142-162), the ED rate is @37% (0–79.5%) and the UI rate is @15% (0–44%) (2,8,20,25,36,92,104,107,131,133,135,140) (Table 4).
Table 4. Outcomes of primary open realignment.
| Author | Number | Age, mean (range) | Follow up, mean (range) | Stricture, % (N) | Erectile dysfunction, % (N) | Incontinence, % (N) | Catheter time | Comments |
|---|---|---|---|---|---|---|---|---|
| Myers 1972 (151) | 22 | 7–71 y | ≥18 m | 59 (13/22) | Ua | Ua | 4–6 w | Urethral traction |
| Jackson 1974 (152) | 63 (27 FU) | 32.3 y (15–65 y) | Ua | 74 (20/27) | 46 (23/50) | Ua | Ua | Railroading |
| Gibson 1974 (149) | 44 | 37 y (13–72 y) | 6 y (3–12 y) | 73 (32/44) | 32 (14/44) | Ua | 3–4 w | – |
| Janknegt 1975 (153) | 7 | Ua | Ua | 0 (0/3) | Ua | Ua | Ua | Prostatic vest suture traction |
| Janosz 1975 (2) | 34 | 38 y (13–69 y) | 5 y (96 m–12 y) | 44 (15/34) | 62 (21/34) | Ua | Ua | – |
| Crassweller 1977 (161) | 38 | Ua | (6 m–10 y) | 100 (38/38) | Ua | Ua | Ua | 100% required dilation |
| De Weerd 1977 (7) | 28 (22 FU) | (7–71 y) | (>18 m) | 100 (22/22) | Ua | 10 (2/20) | 5 w | 100% periodic sounding |
| Malek 1977 (162) | 7 | (6–15 y) | 14 y (8–22 y) | 57 (4/7) | 0 (0/7) | 0 (0/7) | Ua | Functionally significant stricture |
| Glass 1978 (62) | 8 | Ua | Ua | 50 (4/8) | Ua | Ua | Ua | – |
| Guba 1978 (154) | 2 | Ua | Ua | 0 (0/2) | Ua | 0 (0/2) | Ua | – |
| Islam 1978 (155) | 3 | 45.5 y (19–61 y) | 4.3 y (4–6 y) | 100 (3/3) | 0 (0/3) | 0 (0/3) | Ua | – |
| Cass 1978 (135) | 35 (16 FU) | (<20–>60 y) | 14 m (1–87 m) | 62 (11/16) | 38 (3/7) | 21 (3/14) | Ua | Railroading |
| Morehouse Mackinnon 1980 (25) | 54 | Ua | Ua | 100 (54/54) | 43 (23/54) | 44 (24/54) | Ua | – |
| Patterson 1983 (156) | 34 (29 FU) | (68–72 y) | Ua | 38 (11/29) | 15 (4/27) | 3 (1/29) | Ua | Functionally significant stricture |
| Al-Ali 1983 (159) | 16 (14 FU) | Ua | (6 w–6 y) | 36 (5/14) | 40 (2/5) | 14 (2/14) | 2 w | Only 5>12 m |
| Cass 1984 (36) | 24 (14 FU) | Ua | Ua | 79 (11/14) | 75 (6/8) | 11 (1/9) | Ua | – |
| Fowler 1986 (157) | 12 | 45 y (18–83 y) | 4 y (1–10 y) | 100 (12/12) | 17 (2/12) | 17 (2/12) | 2–8 w | Traction |
| 100% bougiage | ||||||||
| Murshidi 1988 (158) | 3 | 30.7 y (20–33 y) | 1.7 y (1–2 y) | 100 (3/3) | Ua | Ua | 4 w | All DIU |
| Morehouse 1988 (104) | 128 | Ua | Ua | 11 (14/128) | 38 (33/87) | 23 (21/92) | Ua | – |
| Zingg 1990 (129) | 21 (20 FU) | Ua | Ua | 60 (12/20) | Ua | Ua | Ua | – |
| Husmann 1990 (143) | 17 | Ua | Ua | 94 (16/17) | 65 (11/17) | 12 (2/17) | Ua | – |
| Follis 1992 (8) | 20 | 28 y (4–65 y) | 42 m (1–360 m) | 15 (3/20) | 20 (4/20) | 0 (0/20) | Ua | 10% significant haemorrhage |
| Boone 1992 (92) | 7 | (children) | (>15 y) | 43 (3/7) | 14 (1/7) | 0 (0/7) | Ua | High blood loss |
| El-Abd 1995 (20) | 44 | Ua | 24 m | 100 (44/44) | 79.5 (35/44) | 2 (1/44) | Ua | Railroading |
| Koraitim 1996 (131) | 23 | Ua | Ua | 56 (12/23) | 28 (5/18) | 4 (1/23) | Ua | Sounds |
| Routt 1996 (150) | 9 | Ua | Ua | 44 (4/9) | 16.7 (1/6) | Ua | Ua | – |
| Kotkin 1996 (140) | 20 | Ua | 51 m (13–115 m) | 50 (9/18) | 24 (4/18) | 17 (3/18) | 4–8 w | Railroading |
| 3 bladder stones | ||||||||
| Elliott, Barrett 1997 (107) | 53 | 31 y | 10.5 y | 66 (35/53) | 21 (11/53) | 3.7 (2/53) | Ua | Railroading |
| Asci 1999 (144) | 12 | Ua | 39 m | 45 (5/12) | 20 (2/10) | 10 (1/10) | Ua | – |
| Khan 2000 (160) | 32 | 28 y (3–81 y) | 6.5 y (9 m–46 y) | 78 (25/32) | 27 (8/30) | 6 (2/32) | 6–8 w | Railroad |
| Upadhyaya 2002 (132) | 4 | (18 m–10 y) | (6 m–10 y) | 75 (3/4) | Ua | Ua | Ua | – |
| Balkan 2005 (148) | 12 | 7.4 y (5–10 y) | 4.92±2.36 y | 66.7 (8/12) | Ua | 8.3 (1/12) | 3–4 w | Quoted stricture rate 16.7% (did not count UD or DVIU as failure) |
| Mouraviev 2005 (142) | 57 | Ua | Ua | 49 (28/57) | 34 (20/57) | 18 (10/57) | Ua | Interlocking sounds |
| NB all did urethral hydrodilation | ||||||||
| Onen 2005 (133) | 22 | (4–17 y) | Ua | 22.7 (5/22) | 22.7 (5/22) | 18.1 (4/22) | Ua | – |
Ua, un-assessed; DVIU, direct visual internal urethrotomy; DIU, direct internal urethrotomy.
Primary endoscopic realignment (PER)
PER includes: antegrade or retrograde catheter insertion over a guidewire at flexible cystoscopy and rendezvous procedures for catheter insertion over a guidewire (11-13,163). Most rendezvous procedures involve passing a ureteric catheter or guidewire antegradely via a suprapubic tract through the lumen of a Goodwin sound or cystoscope so it can be retrieved by a cystoscope in the distal urethra. This catheter is used as a guide to pass a Foley catheter retrogradely into the bladder (11,15,17,18,163-166). This can also be achieved radiologically using multiplane fluoroscopy (167) and has been described using magnetic catheters(14). The mean operative time is reported a 55.5–78 minutes but it can be up to 280 minutes (87,88,168). In the majority of series a SPC was initially placed and PER performed on average 2 days (0–19 days) after this (15,87,88,169).
The rationale for early endoscopic realignment is to avoid the “morbidity” of prolonged suprapubic catheterisation and to prevent/shorten any consequent strictures (12,93,147,170). As per open realignment the term realignment is a misnomer as the result is restoration of urethral continuity without actual 3D realignment (171). The theoretical advantages of endoscopic realignment are not apparent in clinical practice. Most series of urethroplasty for PFUI report increased surgical difficulty following PER because of an epithelial-lined cavity at the site of the disruption (Figure 5), which must be excised prior to repair and may compromise results (4,26,80,109,172-174). Tausch et al. [2015] and Johnsen et al. [2015] noted an increase in delay to definitive treatment for PER patients and an increase in the number of interval procedures (173,175). Conversely, Koraitim found primary realignment patients were significantly more likely to have a stricture <2 cm than those managed solely with SPC diversion (176). Experimentally, in a dog model of complete urethral transection, the stricture rate was the same (17%) following suprapubic catheterisation alone or combined suprapubic and urethral catheterisation (177). Duration of catheterisation post-endoscopic realignment varies; Seo et al. removed catheters at day 7 for partial and day 14 for complete PFUI (87) whilst the majority of surgeons remove catheters at 3 weeks for partial and 6 weeks for complete PFUI (163). In reality the average period of catheterisation is 8 weeks (range, 2–16 weeks) (12,88), which is only slightly shorter than in patients having delayed repair at 3 months.
Figure 5.

Epithelial lined cavity following primary realignment.
Success in terms of PFUI treatment should be defined as absence of; symptoms, reduced flow rate and urethrographic or cystographic evidence of stricture. Any need for further intervention, including “office” urethral dilation or clean intermittent self-catheterisation, denotes failure. This reduces the reported success rates in some studies from 50% to 0% as all patients required further intervention (13). Overall the RS is @62% (range, 10–100%) (87,136,138,140,150,164,167-169,175,178-184,185), the ED rate is @24% (0–100%) and the UI rate is @4% (0–20%) (4,11-15,17,88,140,150,168,169,178-184,186-188) (Table 5). There is only one study with long term follow-up (>5 years), in which the RS rate was 39.2% (87).
Table 5. Outcome of primary endoscopic realignment.
| Author | Number | Age, mean (range) | Follow up, mean (range) | Stricture, % (N) | Erectile dysfunction, % (N) | Incontinence, % (N) | Catheter Time | Comments |
|---|---|---|---|---|---|---|---|---|
| Lieberman 1982 (138) | 4 | Ua | (2–9 m) | 25 (1/4) | Ua | Ua | Ua | DVIU |
| 1 req recurrent DVIU + 6 weekly UD | ||||||||
| Towler, Eisen 1987 (178) | 4 | 41.3 y (27–58 y) | 1.9 y (8 m–4 y) | 75 (3/4) | Ua | 0 (0/5) | 4–6 w | 2 DIU |
| 1 U Dilation | ||||||||
| Chiou 1988 (164) | 8 | Ua | Ua | 100 (8/8) | Ua | Ua | Ua | 2–3 urethrotomies/pt in first 12m |
| Gelbard 1989 (17) | 7 (6 FU) | 35.4 y (9–60 y) | 9.6 m (2–24 m) | 50 (3/6) | 17 (1/6) | 0 (0/7) | 4 w | 2 DIU |
| 1 U dilation | ||||||||
| Cohen 1991 (15) | 5 | (17–41 y) | ≤18 m | 100 (5/5) | 50 (2/4) | 20 (1/5) | 6–13 w | All had 3m CISC |
| Guille 1991 (179) | 5 | 43 y (19–57 y) | 12 m | 75 (3/4) | 20 (1/5) | 0 (0/5) | 3 w | – |
| Yasuda 1991 (180) | 17 | Ua | 3.7 y (1–8 y) | 100 (17/17) | 41 (7/17) | 12 (2/17) | 6 w | All required UD/sounds in 1st 6m |
| Herschorn 1992 (12) | 16 | Ua | 27 m (13–83 m) | 63 (10/16) | 42 (5/12) | Ua | 8 w | – |
| Kotkin 1996 (140) | 12 | Ua | 38 m (12–98 m) | 10 (1/10) | 30 (3/10) | 20 (2/10) | 1–4 w | 2 stretch, 2 partial |
| Routt 1996 (150) | 23 (9 FU) | 35.3 y (17–63 y) | 15.5 m (3–36 m) | 44 (4/9) | 17 (3/18) | 20 (1/5) | Ua | – |
| Londergan 1997 (167) | 5 | Ua | (1–35 m) | 80 (4/5) | Ua | Ua | Ua | – |
| Porter 1997 (14) | 13 (10 FU) | 24.8 y (10–47 y) | 6.1 m (2–31 m) | 50 (5/10) | 10 (1/10) | 0 (0/10) | 8–10 w | Magnetic catheters |
| Podesta 1997 (4) | 10 | Ua | (3–17 y) | 100 (10/10) | 10 (1/10) | 0 (0/10) | Ua | Urethral catheter |
| Gheiler 1997 (11) | 3 | (25–36 y) | 6 m | 100 (3/3) | 0 (0/3) | 0 (0/3) | 6 w | Flexi insertion with guidewire, 100% CISC |
| Rehman 1998 (181) | 6 | Ua | Ua | 67 (4/6) | 17 (1/6) | 0 (0/6) | Ua | Fluoroscopic realignment |
| Reinberg 1998 (136) | 3 | 9.5 y (3–17 y) | 27.9 m | 100 (3/3) | Ua | Ua | Ua | – |
| Jepson 1999 (182) | 8 | (16–64 y) | 50.4 m (35–85 m) | 75 (6/8) | 37.5 (3/8) | 12.5 (1/8) | 6–9 w | Above and below guidewire |
| 1 colovesical fistula | ||||||||
| Moudouni 2001 (168) | 29 | 36 y (17–70 y) | 68 m (18–155 m) | 54 (16/29) | 14 (4/29) | 0 (0/29) | Ua | Antegrade and retrograde cystoscopy |
| Kielb 2001 (13) | 10 (6 FU) | 36.8 y | 18 m (9–27 m) | 100 (6/6) | 100 (6/6) | 17 (1/6) | Minimum 6 w | 100% CISC |
| Flexi insertion with guidewire | ||||||||
| Tazi 2003 (183) | 36 | Ua | 34 m (12–72 m) | 41.7 (15/36) | 19.4 (7/36) | 0 (0/36) | Ua | – |
| Healy 2007 (184) | 10 | Ua | 41.4 m | 50 (4/8) | 50 (4/8) | 0 (0/8) | Ua | 2 patients failed endoscopic alignment and required delayed urethroplasty |
| Hadjizacharia 2008 (185) | 14 | 30 y | 7 m (14 d–1.7 y) | 43 (6/14) | Ua | Ua | Ua | – |
| Olapade-Olaopa 2010 (186) | 10 | Ua | 36.6 m | 100 (10/10) | 0 (0/10) | 0 (0/10) | Ua | – |
| Sofer 2010 (187) | 11 | 32 y (20–62 y) | 4.3 y (2–7 y) | 45 (5/11) | 45 (5/11) | 0 (0/11) | 4 w | – |
| Leddy 2012 (88) | 19 | 36 y (21–73 y) | 40 m (10–80 m) | 78.9 (15/19) | 0 (0/19) | 0 (0/19) | Minimum 3 w | ED + SUI in failures |
| Seo 2012 (87) | 51 | 47.8 y (23–70 y) | 89.1 m (60–176 m) | 39.2 (20/51) | Ua | Ua | Mean 22 d (6–63 d) | – |
| Kim 2013 (188) | 15 | Ua | 31.8 m | 53.3 (8/15) | 46.7 (7/15) | 20 (3/15) | Ua | – |
| Shrestha 2013 (169) | 20 | Ua | 6 m | 100 (20/20) | 5 (1/20) | 0 (0/20) | Ua | All pt CISC for 3 m. 25% re-stricture afterwards |
| Johnsen 2015 (175) | 27 | 39 y (±14 y) | 39 m (±40 m) | 63.6 (17/27) | Ua | Ua | 60.9 d (±36.2 d) | – |
Ua, un-assessed; DVIU, direct visual internal urethrotomy; ED, erectile dysfunction; UD, urethral dilation; DU, delayed urethroplasty; CISC, clean intermittent self catheterisation; SUI, stress urinary incontinence.
Whether the PFUI is complete or partial is not recorded in most series. It may be more difficult to treat complete PFUI with PER. Failure or no attempt due to lack of patient suitability has been reported in up to 20% of cases (13,14,168). Other complications include perineal abscess and urethral fistulae, related to extravasation of irrigation fluid or contrast into the haematoma with consequent infection (8,15,17,189,190).
Primary SPC insertion and delayed endoscopic stricture management
This technique has been reported infrequently but is utilised commonly in general urological practice (191-193). A suprapubic cystostomy is performed at time of PFUI and then any subsequent stricture is managed by direct visual internal urethrotomy (DVIU), laser urethrotomy or “core-through” (194,195). RS developed in the DVIU series in @82% (range, 12.5–100%) (20-22). There is a 19% failure rate for initial attempt at DVIU; all such failures will require a core-through procedure (20). Eventual RS is almost inevitable at 95.8–100% (20,21,196,197) with 39–41.8% requiring urethroplasty at a follow-up of 24–43 months.
The commonest delayed endoscopic method used is the “cut to the light” technique whereby the proximal end of the PFUI is illuminated via suprapubic tract cystoscopy and an antegrade stiff guidewire is passed to guide a second operator to perform DVIU from the urethral end (198). Operative times range from 45–120 minutes (20). Laser has also been used, with a failure rate of Nd: YAG laser core through of 8% and RS rates of 12.5% at a mean follow-up of 30 months. However all “successful” patients needed to perform CISC, meaning in reality the failure rate was 100% (21).
Endoscopic skin graft urethroplasty has been reported with a skin patch held dorsally following DVIU using a special retaining catheter (199-201). Early reports revealed RS rates of 25% at 2 years when performed within 3 weeks of PFUI increasing to 60–67% when performed for established PFUI. This technique has not been widely adopted (199-201).
Complications associated with delayed endoscopic management of PFUI stricture include primary and secondary haemorrhage, urinary tract infection and extravasation of irrigating fluids (20,22). Evaluating all techniques of delayed endoscopic management RS occurs in @80% (27,143,192,195,199,202-219), ED in @32% (range, 0–64%) and UI in @4% (0–40%) (8,20-22,138,164-166,180,196-198,202-205,207-210,211,213,215-217) (Table 6).
Table 6. Outcome of delayed endoscopic treatment.
| Author | Number | Age, mean (range) | Follow up, mean (range) | Stricture, % (N) | Erectile dysfunction, % (N) | Incontinence, % (N) | Catheter time | Comments |
|---|---|---|---|---|---|---|---|---|
| Lieberman 1982 (138) | 4 | 31.5 y (20–53 y) | 4.1 m (2–9 m) | 100 (4/4) | Ua | 25 (1/4) | 1–3 w | 100% dilation |
| Gonzalez 1983 (202) | 3 | 49 y (21–74 y) | 17.6 m (11–28 m) | 100 (3/3) | 0 (0/2) | 0 (0/2) | Ua | Mean 3 DIU each |
| Chiou 1985 (165) | 1 | 45 | Ua | 100 (1/1) | 0 (0/1) | 0 (0/1) | 3 w | DIU |
| Gupta 1986 (203) | 10 | (20–58 y) | 16.2 m (6–24 m) | 100 (10/10) | 0 (0/10) | 40 (4/10) | 4–8 m | All >2 DIU |
| McCoy 1987 (204) | 12 | 20–64 y | 22 m (6–35 m) | 100 (11/11) | 0 (0/5) | 25 (3/12) | 3–7 m | 6 DIU 12 U Dilation |
| 58% (7/12) ED post injury | ||||||||
| Fishman 1987 (195) | 1 | Ua | Ua | 100 (1/1) | Ua | Ua | Ua | Goodwin sound guided DIU |
| Marshall 1987 (205) | 5 | 29.8 y (13–48 y) | Ua | 100 (5/5) | 50 (2/4) | 0 (0/5) | 6–7 m | All CISC and or DIU |
| Peterson 1987 (206) | 5 | 31.4 y (24–45 y) | 28.8 m (18–42 m) | 100 (5/5) | Ua | Ua | Ua | Forceful perforation with sound |
| All CISC, 3DIU | ||||||||
| Chiou 1988a (164) | 9 (8 FU) | 38 y (21–74 y) | 3.6 m (1–6.6 m) | 100 (8/8) | 0 (0/4) | 14 (1/7) | 3–4 w | Thin trocar puncture All 2–3 DIU |
| Chiou 1988b (166) | 3 | 53 y (39–68 y) | 21.5 m (12–31 m) | 67 (2/3) | 33 (1/3) | 0 (0/3) | 3 w | Endourethroplasty |
| FTSG prepuce | ||||||||
| Lim 1989 (207) | 20 | 36 y (20–75 y) | 4 y (4–12 y) | 100 (20/20) | 5 (1/20) | 10 (2/20) | Ua | 100% dilation, mean of 5 each |
| Marshall 1989 (208) | 10 | 5 y | NA | 100 (10/10) | 40 (4/10) | 0 (0/10) | 4–6 w | 100% further DIU + CISC |
| Barry 1989 (198) | 12 | Ua | (1.5–85 m) | 100 (12/12) | 58.3 (7/12) | 25 (3/12) | 12–24 w | 100% CISC + U Dilation |
| Leonard 1990 (209) | 7 | (20–75 y) | 31 m (13–51 m) | 57 (4/7) | 14 (1/7) | 14 (1/7) | 4 m | Cut to the light |
| Kernohan 1990 (210) | 7 | 25.8 y (7–54 y) | 51.9 m (25–180 m) | 100 (7/7) | Ua | 29 (2/7) | Ua | Cut to the light |
| Husmann 1990 (143) | 17 | Ua | 3 y (6 m–4 y) | 53 (9/17) | Ua | Ua | Ua | DIU |
| Yasuda 1991 (180) | 17 | 41.1 y (20–70 y) | 3.7 y (1–8 y) | 100 (17/17) | 55 (6/11) | 18 (3/17) | 5–7 m | 41% ED post injury |
| 17 OPD dilation 6m | ||||||||
| Wu 1992 (211) | 15 | (10–61 y) | 33.5 m (1.5–5.5 y) | 27 (4/15) | 7 (1/15) | Ua | 3–7 m | – |
| Jenkins 1992 (212) | 33 | 28 y (16–81 y) | 8 y (1–22 y) | 33 (11/33) | Ua | Ua | Ua | Short PFUDD DIU |
| Follis 1992 (8) | 24 (20 FU) | 28 y (4–65 y) | 42 m (1–360 m) | 35 (7/20) | 20 (4/20) | 10 (2/20) | 4–6 w | 4 failed initial attempts |
| Spirnak 1993 (213) | 5 | 32 y (7–74 y) | 31 m | 100 (5/5) | 0 (0/4) | Ua | 5–10 m | 100% CISC and 100% DIU |
| 20% ED post injury | ||||||||
| Quint 1993 (196) | 10 | 40 y (7–78 y) | 43 m (7–108 m) | 100 (10/10) | 0 (0/5) | 10 (1/10) | 4 m | Ante and retro fluoroscopic guided DIU and TUR |
| 100% balloon dilation and sounds | ||||||||
| Wu 1994 (214) | 10 | Ua | 24.4 m | 60 (6/10) | Ua | Ua | Ua | Perforation with sound, dilation and TUR |
| White 1994 (215) | 4 | 21.8 y (18–26 y) | 10.5 m (5–17 m) | 100 (4/4) | 25 (1/4) | 0 (0/4) | 4–8 w | Retro DIU and dilation |
| All CISC 3 DIU | ||||||||
| Koraitim 1995 (27) | 12 | (3–58 y) | Ua | 42 (5/12) | Ua | Ua | 3 m | – |
| El-Abd 1996 (20) | 352 | – | 24 m | 96 (338/352) | 37.5 (132/352) | 1 (4/352) | – | DIU |
| Al-Ali 1997 (22) | 154 | 36 y (18–54 y) | 31.5 m (3–60 m) | 65 (100/154) | Ua | 0.6 (1/154) | 12 w | Core through DIU |
| Goel 1997 (197) | 13 | (25–45 y) | 17.7 m (11–24 m) | 100 (13/13) | Ua | 0 (0/13) | 2–5 w | Core-through DIU, 100% DIU + CISC |
| Naude 1998 (199) | 16 (15 FU) | Ua | (2 y) | 60 (9/15) | Ua | Ua | 7 m | Established PFUDD |
| Naude 1998 (199) | 10 (8 FU) | Ua | (3 y) | 25 (2/8) | Ua | Ua | 5 w | Recent PFUDD |
| Sahin 1998 (216) | 5 | 32 y (18–32 y) | 31 m (21–53 m) | 100 (5/5) | 0 (0/4) | 20 (1/5) | 3–8 m | Core-through DIU |
| 100% CISC | ||||||||
| 4 DIU | ||||||||
| 20% ED posy injury | ||||||||
| Dogra 1999 (217) | 8 | 27.5 y (18–44 y) | 10.25 m (7–14 m) | 12.5 (1/8) | 0 (0/8) | 0 (0/8) | 4–6 w post op | <2 cm PFUDD |
| No ED | ||||||||
| Levine 2001 (218) | 6 | 48 y (17–78 y) | 7 y (7–14 y) | 100 (6/6) | Ua | Ua | 7–8 m | Cut to the light |
| Minimum 3 DIU each of | ||||||||
| Dogra 2002 (21) | 65 | (5–62 y) | 30 m | 100 (65/65) | Ua | 3 (2/65) | Ua | NdYAG core through |
| 100% CISC | ||||||||
| Ravichandran 2003 (219) | 25 | Ua | 24 m | 100 (25/25) | Ua | Ua | Ua | 9 failed initial attempts |
| 15 CISC | ||||||||
| 2 urosepsis | ||||||||
| Islam 2010 (192) | 45 | (20–60 y) | (3–12 m) | 31.4 (14/45) | Ua | Ua | Ua | All did CISC for 1–3 m |
Ua, un-assessed; ED, erectile dysfunction; CISC, clean intermittent self catheterisation; DIU, direct internal urethrotomy; PFUDD, pelvic fracture urethral distraction defect (old terminology for PFUI).
Primary SPC insertion and DU
Suprapubic cystostomy and DU was initially proposed by Johanson in 1953 (23). It successfully achieves urinary diversion whilst avoiding entry into the fracture haematoma and consequent infection and blood loss (8). A well-planned elective surgical procedure can then be performed for any resultant stricture (Figure 6) in a stable healthy patient at a later date by a urologist experienced in urethral surgery. The disadvantage is the 3–6 months wait with a SPC in situ. There is often pressure from orthopaedic colleagues to achieve urethral drainage and avoid a SPC at the time of pelvic fracture fixation. This is prompted by the fear that an SPC will be an infection risk (220). There is no documented evidence that an SPC presents a greater infection risk than a urethral catheter over and above the nature of the associated injuries and the duration and difficulty of the pelvic fracture fixation (220-222).
Figure 6.
Stricture post SPC placement prior to delayed urethroplasty. SPC, suprapubic catheter.
Following suprapubic cystostomy alone RS occurs in @97% (80–100%) (8,80,131,142-144,164,185,223,224). ED occurs in @39% (2.5–75%) and UI in @5% (2.1–8%) (93,131,144). ED and UI following SPC insertion alone are consequent to the injury itself and are therefore the baseline against which all post-procedure ED and UI rates should be compared.
DU is performed a minimum of 3 months following PFUI to allow resolution of haematoma and any other injury (9,10,24,25,61,64,225-227). The operation now most commonly performed is transperineal bulbo-prostatic anastomotic urethroplasty (BPA) (26,228). The technique of BPA is based on the progressive perineal approach first clearly described as such by Webster (26) and the work of other authors notably Young, Marion, Turner-Warwick and Waterhouse (9,10,23,122,229,230) (Figure 7).
Figure 7.
Method for progressive perineal bulboprosatatic anastomotic urethroplasty repair for PFUI. (A) Urethra divided at site of stricture, which is excised. All of urethra anterior to stricture is mobilised to the level of the suspensory ligament (BPA Step 1); (B) midline raphe between the corpora cavernosi bilaterally is divided anteriorly until the corpora coalesce (BPA Step 2); (C) inferior wedge pubectomy is performed (Step 3); (D) the distal urethra is rerouted under one corpora cavernosus (Step 4); (E) the bulboprostatic anastomosis is performed using interrupted small calibre absorbable sutures; (F) the final appearance. BPA, bulbo-prostatic anastomotic. PFUI, pelvic fracture urethral injury.
The technique capitalises on the elasticity of the urethra and the ability to straighten out the natural perineal curve of the bulbar urethra to allow a tension-free anastomosis (26,109). The length of stricture on pre-operative urethrogram is not predictive of what manoeuvres may be necessary. MRI appears to accurately predict the length of the PFUI and 3D displacement but has not as yet been correlated with operative requirement (231). Koraitim [2009] found that the “gapometry/urethrometry index” (length of urethral gap/length of bulbar urethra) was a significant predictor for technique required. A value of <0.35 predicted that a simple perineal approach could be used (232).
BPA urethroplasty RS rates are @14% (4,26,27,28,61,86,92,99,104,113,121,133,134,160,170,173,174,212,218,219,225,228,233-235) (range, 0–67%). Studies vary in length of follow-up but success rate appears to be maintained out to 22 years (28,236-258). Early stricture recurrence is due to technical failure or ischaemia, with recurrence rates higher in patients with ED (162,228,245,259-269). New onset ED occurs in @13% (0–72%) (4,27,28,61,92,99,104,113,121,133,134,160,212,225,228,234,237,238,240,242,246,251,252,253,255) and UI in @7% (0–20%) (164,233,241,250,257,258,261-265,269) (Table 7).
Table 7. Outcomes of suprapubic catheter insertion ± delayed urethroplasty.
| Author | Number | Age, mean (range) | Follow up, mean (range) | Stricture, % (N) | Erectile dysfunction, % (N) | Incontinence, % (N) | Catheter time | Other complications |
|---|---|---|---|---|---|---|---|---|
| Johanson 1953 (23) | 120 | Ua | Ua | 100 (120/120) | 3.3 (4/120) | 0 (0/120) | Ua | SPC alone |
| Netto 1973 (254) | 15 | Ua | (2–42 m) | 33 (5/15) | Ua | Ua | Ua | Pullthrough |
| Allen 1975 (233) | 3 | 28 y (21–33 y) | Ua | 0 (0/3) | 0 (0/2) | 33 (1/3) | Ua | Transpubic BPA |
| 33% (1/3) ED post injury | ||||||||
| Chatelain 1975 (121) | 39 | (adult) | (<1–16 y) | 50 (19/38) | 27 (7/26) | 0 (0/38) | Ua | Perineal BPA |
| Chatelain 1975 (234) | 7 | 26 y (19–40 y) | (4–16 m) | 14 (1/7) | 20 (1/5) | 14 (1/7) | 25–50 d | Transpubic BPA |
| Waterhouse 1976 (235) | 7 | 8.7 y (5–13 y) | Ua | 17 (1/6) | Ua | 0 (0/6) | Ua | Transpubic BPA |
| Children | ||||||||
| De La Pena Zayas 1979 (236) | 7 | 29 y (21–59 y) | 24 m | 0 (0/7) | 0 (0/5) | 14 (1/7) | Ua | Transpubic BPA |
| Coffield 1977 (61) | 11 | Ua | Ua | 0 (0/11) | 0 (0/11) | 0 (0/11) | Ua | Scrotal inlay urethroplasty |
| Morehouse, Mackinnon 1980 (25) | 41 | Ua | Ua | 0 (0/41) | 10 (4/40) | 3 (1/40) | Ua | Transpubic BPA |
| 2 patients with ED >75 y | ||||||||
| Harshman 1981 (255) | 7 | 10 y (4–20 y) | 29.8 m (3–68 m) | 14 (1/7) | 14 (1/7) | 57 (4/7) | Ua | 2AP BPA, 2 BPA, 3 Skin Inlay |
| McAninch 1981 (64) | 21 (14 FU) | 33 y (14–76 y) | Ua | 7 (1/14) | 14(2/14) | 0 (0/14) | Ua | Transpubic BPA |
| Webster 1983 (80) | 11 | Ua | (6 m–17 y) | 0 (0/11) | 36 (4/11) | 14 (1/7) | Ua | Transpubic BPA |
| Hayden 1984 (256) | 7 | 9.3 y (7–14 y) | 64 m (42–84 m) | Ua | Ua | Ua | 2–3 w post op | – |
| Netto 1985 (257) | 66 (10 children; 56 adults) | (5–64 y) | 9 y* (1–11 y) | 20 (2/10) child; 34 (19/56) adult | 0 (0/24) | 0 (0/28) | Ua | Pull through |
| Netto 1985 (257) | 28 (9 children; 19 adults) | (5–40 y) | 6 y* (1–9 y) | 11 (1/9) child; 31 (6/19) adult | 0 (0/22) | 2 (1/28) | Ua | Transpubic BPA |
| Koraitim 1985 (258) | 76 | (3–54 y) | (6 m–25 y) | 9 (5/54) BPA; 19 (4/21) AP BPA | 69 (22/32) | Ua | Ua | – |
| Patil 1986 (259) | 30 (5 FU) | 9.4 y (7–10 y) | 9.2 y (6–11 y) | 0 (0/5) | 0 (0/5) | 0 (0/5) | 4–6 m | Transpubic BPA. Children |
| Zvara 1986 (260) | 10 | 22.6 y (9–36 y) | (29–54 m) | 10 (1/10) | 25 (1/4) | 10 (1/10) | Ua | Transpubic BPA |
| Gait disturbance (1/10) | ||||||||
| Chiou 1988 (164) | 6 | 18 y (11–24 y) | – | 67 (4/6) | 0 (0/5) | 50 (3/6) | Ua | Transpubic BPA |
| Morehouse 1988a (104) | 36 | Ua | Ua | 14 (5/36) | 11 (4/36) | 0 (0/36) | Ua | Perineal and Transpubic BPA |
| Morehouse 1988b (113) | 92 | Ua | Ua | 0 (0/92) | 10 (9/92) | 2 (2/92) | Ua | 2 stage |
| Redman 1988 (237) | 16 | 29 y (17–62 y) | Ua | 6.25 (1/16) | 25 (2/8) | 0 (0/8) | 7 m | Perineal and Transpubic BPA |
| Singh 1988 (223) | 45 | Ua | Ua | 100 (45/45) | 44.4 (20/45) | Ua | Ua | 36 req urethroplasty |
| 6 –BPA | ||||||||
| 30 –transpubic | ||||||||
| Dhabuwala 1990 (225) | 26 | 34 y (7–62 y) | ≥18 m | Ua | 56 (15/26) | Ua | Ua | Transperineal BPA |
| Webster 1990 (26) | 20 | Ua | Ua | 5 (1/20) | Ua | Ua | Ua | 14 BPA, 1 PIPS, 5 staged skin inlaies |
| Husmann 1990 (143) | 64 | Ua | Ua | 95 (61/64) | 52 (33/64) | 12.5 (8/64) | Ua | SPC alone (eventually underwent BPA) |
| Webster 1991 (249) | 74 | Ua | (2–10 y) | 10.8 (8/74) | 0 | 0 | Ua | 1.4% gained potency |
| BPA | ||||||||
| Al Rifae 1991 (250) | 20 | (2–15 y) | (1–7 y) | 10 (2/20) | Ua | 25 (4/20) all associated BN injury | Ua | 16 Transpubic BPA 4 Perineal BPA |
| Jenkins 1992 (212) | 73 | Ua | Ua | 20 (15/72) | 38.9 (28/72) | Ua | Ua | Skin inlay urethroplasty |
| Follis 1992 (8) | 13 | 31 y (18–64 y) | 37 m (1–147 m) | 100 (13/13) | 46 (6/13) | Ua | Ua | Stricture rate SPC alone |
| Boone 1992 (92) | 7 | (4–16 y) | 14 (1/7) | 14 (1/7) | 0 (0/7) | – | – | |
| Flah 1992 (251) | 4 | 5.75 y (3–9 y) | (3–22 m) | 0 (0/4) | 0 (0/4) | 0 (0/4) | Ua | Posterior sagittal approach |
| Baskin 1993 (261) | 7 | 10 y (4–16 y) | 2.2 y (0.8–5 y) | 14 (1/7) | 0 (0/6) | 0 (0/7) | Ua | A BPA |
| Perineal BPA | ||||||||
| Corriere 1994 (252) | 50 | 34 y (15–61 y) | ≥12 m | 36 (18/50) | 32 (16/50) | 14 (7/50) | 10 (5/50) CISC due to areflexic bladder | Transpubic and perineal BPA |
| 8 recovered potency post BPA | ||||||||
| 48% ED post injury | ||||||||
| Koraitim 1995 (27) | 80 | (3–58 y) | Ua | 5 (4/80) | 17.5 (14/80)* combined evaluation of perineal and Transpubic approach | Ua | 7 m | Perineal BPA |
| 40% (32/80) ED post injury | ||||||||
| Koraitim 1995 (27) | 32 | (3–58 y) | Ua | 3 (1/32) | See above | Ua | 7 m | Transpubic BPA |
| 40% (32/80) ED post injury | ||||||||
| Koraitim 1995 (27) | 23 | (3–58 y) | Ua | 57 (13/23) | Ua | Ua | 7 m | Scrotal inlay |
| 40% (32/80) ED post injury | ||||||||
| Mark 1995 (238) | 113 (92 FU) | Ua | 12 m | 15 (12/92) | 6 (6/92) | 2 (2/92) | Ua | 6% regained potency post op perineal BPA |
| ED post PFUDD 68% (62/92) | ||||||||
| Mundy 1996 (228) | 82 | Ua | 10 y | 12 (10/82) | 7 (6/82) | 0 (0/82) | Ua | BPA |
| Koraitim 1996 (131) | 73 | (3–62 y) | (4–15 y) | 97 (71/73) | 18 (9/50) | 2/7 (2/73) | Ua | SPC alone |
| Ennemoser 1997 (262) | 42 | 46.4 y | 42 m | 0 (0/42) | 4.7 (2/42) | 0 (0/42) | Ua | – |
| Koraitim 1997 (239) | 42 | 3–15 y | Ua | 7 (3/42) | Ua | Ua | Ua | BPA |
| Koraitim 1997 (239) | 24 | 3–15 y | Ua | 9 (2/24) | Ua | Ua | Ua | Transpubic BPA |
| Koraitim 1997 (239) | 13 | 3–15 y | Ua | 54 (6/13) | Ua | Ua | Ua | 2 stage scrotal |
| Martinez-Pineiro 1997 (240) | 150 (57 FU) | 35.7 y (5–73 y) | 44.4 m (3–168 m) | 5.6 (3/54) | 19 (28/150) | 1.3 (2/150) | Ua | 12 A BPA |
| Rest perineal BPA | ||||||||
| Morey 1997 (263) | 82 | Ua | 12 m | 15 (12/82) | 0 (0/82) | 5 (2/82) | Ua | BPA/transpubic (30) 16% regained potency postop |
| Podesta 1997 (4) | 19 | Ua | (3–17) | 15 (3/19) | 8.5 (1/12) | 16 (3/19) | Ua | BPA |
| Podesta 1998 (241) | 15 | 9.2 y* (5.8–15 y) | 6 y* (2.6–14 y) | 27 (4/15) | Ua | 6.7 (1/15) | 6 m | Perineal BPA |
| Podesta 1998 (241) | 15 | 8.2 y* (3.8–15.4 y) | 10 y* (2–15 y) | 0 (0/15) | Ua | 20 (3/15) | 6–12 m | AP BPA |
| Asci 1999 (144) | 18 | Ua | 37 m | 83.3 (15/18) | 17.6 (3/18) | 5.6 (1/18) | Ua | SPC alone |
| 44.4% req BPA | ||||||||
| Khan 2000 (160) | 10 | 28 y (3–81 y) | 6.5 y (9 m–46 y) | 40 (4/10) | 0 (0/2) | 20 (2/10) | Ua | AP BPA |
| Tunc 2000 (242) | 77 | 24.2 y (7–62 y) | 47 m (15 m–14 y) | 5.2 (4/77) | 16.2 (7/43) | 9.1 (7/77) | 12 m | BPA |
| 1 regained potency pot BPA | ||||||||
| Corriere 2001 (99) | 60 | 35 y (15–61 y) | 12 m | 38.3 (23/60) | 35 (21/60) | 20 (12/60) | 3–4 w + 6–26 w preop | 15% regained potency post op |
| Levine 2001 (218) | 9 | 37 y (19–72 y) | (3–6 y) | 22.2 (2/9) | Ua | Ua | Ua | BPA |
| Basiri 2002 (243) | 10 | 6 (4–13 y) | 2.5 y (0.5–4 y) | 0 (0/10) | 0 (0/10) | 0 (0/10) | Ua | Transpubic BPA |
| Flynn 2003 (244) | 120 (11 boys) | 32 y (6–82 y) | 64 m (9–128 m) | 7.5 (9/120) | Ua | Ua | 3.4 w | All strictures in 1st yr post–op |
| Ku 2002 (264) | 20 | Ua | Ua | 65 (13/20) | 15 (3/20) | 5 (1/20) | Ua | – |
| Andrich 2003 (28) | 100 | 3.6y | Ua | 5 (5/100) | 0 (0/100) | 0 (0/100) | Ua | 42% ED after PFUDD BPA |
| Ravichandran 2003 (219) | 25 | Ua | 24 m | 24 (6/25) | Ua | Ua | Ua | – |
| Koraitim 2005 (246) | 155 | 3–58 y (21 y) | 13 y (1–22 y) | 9.6 (11/115) perineal BPA 2 (1/40) Perineo-abdominal | 15.4 (17/110) | 1.3 (2/110) | 2–3 w post-op | 115 perineal BPA |
| 40 perineo-abdominal | ||||||||
| Mouraviev 2005 (142) | 39 | Ua | 8.8 y (1–22 y) | 100 (39/39) | 42 (16/39) | 25 (10/39) | 3 m | Stricture rate for SPC alone |
| 18 eventually had BPA—no outcomes given | ||||||||
| Onen 2005 (133) | 16 | (4–17 y) | Ua | 25 (4/16) | 18.8 (3/16) | 18.8 (3/16) | Ua | Transpubic and perineal BPA |
| Culty 2007 (247) | 23 | Ua | (>10 y) | 53 (12/23) | Ua | Ua | Ua | BPA |
| Hadjizacharia 2008 (185) | 7 | 29 y (±14 y) | 7 m (±6 m) | 100 (7/7) | Ua | Ua | 6 m (±3 m) |
SPC alone |
| All req BPA eventually | ||||||||
| Lumen 2009 (265) | 61 | 34 y (14–68 y) | 67 m (19–173 m) | 14.8 (9/61) | 32.8 (20/61) | 1.6 (1/61) | <3 m | Inf Pubectomy in 4 |
| 21 had prev intervention | ||||||||
| Koraitim 2010 (248) | 64 | (5–40 y) | (1–24 y) | 1.6 (1/64) | 3.1 (2/64) | Ua | Ua | Partial transpubic BPA with wedge pubectomy |
| Singh 2010 (174) | 58 | 27 y (6–65 y) | 31.5m (6–146 m) | 25.9 (15/58) | Ua | Ua | Ua | If previous intervention failure rate 50% |
| Onofre 2011 (266) | 11 | 11 y (1.5–23 y) | 41 m (10 m–10 y 9 m) | 18.2 (2/11) | Ua | Ua | Ua | 1 inf pubectomy 10 BPA |
| Sunay 2011 (267) | 75 | 12.3 y (6–17 y) | 43.2 m (12–94 m) | 30.7 (23/75) | Ua | Ua | Ua | BPA 54 pts |
| Pull through 20pts | ||||||||
| Graft 1pt | ||||||||
| Voelzke 2012 (268) | 18 | 15 y | 2.9 y | 10.5 (2/18) | Ua | Ua | Ua | BPA |
| Fu 2013 (253) | 573 | 36 y (7–71 y) | 36 m (18–47 m) | 12 (69/573) | <0.1 (5/573) | 9.1 (52/573) | Ua | 537 had previous interventions |
| Qu Y 2014 (134) | 142 boys | Ua | 58 m (6–192 m) | 11.9 (17/142) | 21.8 (31/142) | 17.7 (25/142) | 6 m | – |
| Fu 2015 (170) | 186 | 35.3 y (4–79 y) | >12 m | 19.4 (36/186) | Ua | Ua | Ua | BPA |
| Koraitim 2015 (86) | 86 | 23 y (5–50 y) | 5.5 y (2–8 y) | 12 (10/86) | Ua | Ua | Ua | Previous interventions in 44% |
| All presented with SPC in situ | ||||||||
| Podesta 2015 (269) | 49 boys | 9.6 y (3.5–17.5 y) | 6.5 y (5–22 y) | 10.2 (5/49) | 20 (9/49) | 6.1 (3/49) | Ua | ED present before urethroplasty |
| 4 cases UI resolved at puberty | ||||||||
| Tausch 2015 (173) | 23 | Ua | 35 m (5–64 m) | 7 (2/23) | Ua | Ua | Ua | BPA |
Ua, un-assessed; BPA, bulbo-prostatic anastomotic; ED, erectile dysfunction; SPC, suprapubic catheter.
Interestingly 6–20% of patients have been reported to recover erectile function after BPA urethroplasty (238,249,252,270,271). New onset ED may be reduced by the newer bulbar artery sparing techniques (272).
Occasionally an abdominoperineal or transpubic approach may be required, particularly in children, in whom the perineal approach is unsuccessful in 10–26% (244), and for complex strictures, normally following failed previous surgery, severe injury or war injuries (82,109,230,248,273). The main indications for these approaches are; to improve visualisation, remove fistulous tracts or cavities, repair bladder neck sector defects and allow a tension-free BPA urethroplasty when this cannot be achieved transperineally. Some authors quote a stricture length of >2.5 cm as requiring a transpubic approach (82,232,274). This approach can cause problems with the penis dropping back into the gap in the pubis created by wedge pubectomy, gait abnormalities and pelvic girdle pain (138,260,275,276). RS rates for this procedure are 0–12% and outcomes appear durable for up to 24 years (82,248,273).
The presence or absence of pre- or post-injury ED should be recorded prior to delayed repair. Whether or not ED is present, nocturnal penile tumescence (NPT) studies should ideally be performed to document erectile function (for medicolegal reasons). Absence of erections on NPT studies should prompt a search for a surgically correctable vascular lesion by penile arterial Doppler studies followed by pudendal arteriography if indicated (277).
UI occurs rarely and is due to concomitant bladder neck injury at time of pelvic fracture. Prior to delayed BPA urethroplasty an ascending and micturating cystourethrogram or urethroscopic and suprapubic cystoscopy are useful although, in a patient with PFUI and a long-term SPC, the bladder neck does not behave necessarily in the same way as it will after urethroplasty (81,278-280). Koraitim found that of 21 patients with known bladder neck injury, 12 became continent after urethroplasty alone (280).
Abdalla described a posterior sagittal pararectal approach for BPA urethroplasty to improve access and vision during the procedure with RS rates of 14% at 13 months (281). Substitution urethroplasty is contraindicated as a primary procedure in PFUI (228).
As PER becomes more popular there are now series of patients undergoing PFUI urethroplasty after failed endoscopic realignment. There is no consensus as to whether or not previous treatment has an effect on the success of future urethroplasty—some series quote good results with RS rates of 9.6–24% whilst others indicate higher RS rates of 57% dropping to 37% at 10 years (170,247,253,265,282).
Post-pelvic fracture ED
ED following pelvic fracture with or without associated urethral injury occurs in @56% (2.5–80%) of patients (8,25,39,131,238,263,270,271,277,283-287). The wide range of incidence of ED is related to the variability in the complexity and severity of injury and the variability in the definition of ED. Generally post-pelvic fracture ED (PPF-ED) is a consequence of the injury causing the PFUI rather than its treatment (140). ED may be neurogenic, vasculogenic or psychogenic—alone or in combination. Vasculogenic ED can be further divided into; arterial insufficiency, venous leak or both. Reporting of the aetiology of PPF-ED is also variable due to the differing diagnostic techniques (284). PPF-ED is due to vascular injury in @45% (11–80%) and neurological injury in @71% (20–89%) (238,263,277,283,284,288,289).
The majority of cases of PPF-ED are associated with vascular or neural injury at the apex of the prostate following complete urethral rupture and prostatic dislocation (238,277,283,289,290). Neurogenic ED can occur following damage at any point from the S2–S4 nerve roots, via the pelvic plexuses to the cavernous nerves (93,277,290). ED can occur following pelvic fracture without associated PFU, although this is rare, occurring in only @5% (2–27.7%) (62,290,291).
Arteriogenic ED may occur from fracture-related injury to the main trunk of the internal pudendal artery, to the penile artery as it passes through the perineal membrane, or to the accessory pudendal artery. Venogenic ED is consequent to damage to the corporal bodies resulting in corpora-veno occlusive dysfunction and/or penile venous leakage (231,284,289,292,293).
Factors that appear to significantly increase the risk of ED are pubic diastasis, lateral prostatic displacement and a long urethral gap (294-296). Spontaneous recovery from ED has been reported in up to 23% of patients following injury (93,225,271,283,290). This recovery may be secondary to the development of arterial collaterals or the regeneration of nerves. Arterial collateral development has been shown to occur experimentally in a dog model (297). In some cases, recovery of erectile function is delayed by a “psychogenic” component.
Blaschko et al. found a base rate of ED after PFUI of 34%. This dropped to 16% for patients having primary realignment (endoscopic or open), most likely due to the tendency to treat less severe urethral injuries in this way. In patients undergoing DU the ED rate increased to 37% (285). The authors postulated that there is significant under-reporting of ED in this cohort (285). It is unusual for patients who have adequate erections before urethroplasty for PFUI to develop ED post-operatively although a small number do (<7%) (28,239,270,285,288). Conversely 6–20% of patients have recovery following BPA urethroplasty (99,290).
ED is far commoner in children with PFUI and is present in 31–75% (92,294). This may be attributable to the greater degree of force required to cause PFUI in a child and the higher incidence of supraprostatic or transprostatic injury (92,225,238,271).
Oral therapy for ED in the PFUI patients is successful in 46.4–81%. There is no difference in response rates between neurogenic or vasculogenic ED (287,298-300). Neurogenic ED can be successfully treated with self-injection therapy (80–100% success) or a vacuum constriction device (225,289). Vasculogenic ED does not respond to self-injection therapy but has been managed by revascularisation in selected cases (84.6% success) or penile prosthesis (301,302).
Problems with emission and/or ejaculation often present as infertility and occur in up to 90% of post-PFUI patients despite the majority of patients have antegrade ejaculation (161,270,303-305). This is usually due to damage to the lumbar sympathetic nerves and the hypogastric and pelvic plexus in those with loss of emission, or secondary to reduced perineal muscle function in those with ejaculatory problems (161,271).
UI after PFUI
UI after PFUI is often related to concomitant bladder neck injury and more severe trauma (4,144). The urethral sphincter mechanism may be overtly destroyed or poorly-functional and continence depends on bladder neck function (82), although some patients do have preservation of urethral sphincter function (91,306).
If bladder neck and detrusor function are normal the patient will be continent following re-establishment of urethral continuity although a degree of stress and/or urge incontinence may be present, especially when the patient has a full bladder and in the first few months after urethroplasty (228,252).
UI occurs in @5% (2.1–8%) of men following PFUI (39,99,131,144). A further 8.3% report mild urgency UI and 7.8% report mild stress UI (99,162). Conversely @8% (1.5–10%) of men are unable to void post-PFUI, secondary to sacral nerve damage, and need to perform clean intermittent self-catheterisation (99,162,252).
Bladder neck injury at the time of original trauma should be repaired as soon as possible (80). Bladder neck injury is more common in children, secondary to the relative intra-abdominal position of the bladder and immature prostate (92,109,144,239). UI is also more common in children with high (supra-prostatic) injuries than in those with a standard PFUI (92,109,236,239).
An open bladder neck on a pre-operative cystourethrogram does not predict post-BPA incontinence. It may be open due to a generalised detrusor contraction or fibrosis around the bladder neck rather than bladder neck injury (278,307). The average length of proximal urethra seen associated with bladder neck opening is significantly longer in incontinent patients (>1.5 cm) (278). Despite the poor association, an open bladder neck at rest on cystourethrogram should result in antegrade cystoscopy to assess the bladder neck prior to BPA urethroplasty. If circumferential integrity of the bladder neck is confirmed then continence is maintained after BPA urethroplasty. If a deficiency is noted then urinary continence is less likely (307) although even in patients with bladder neck injury, some do become continent after BPA urethroplasty (57%) (280).
Treatment options for incontinent patients include bladder neck reconstruction or bladder neck artificial urinary sphincter (AUS) (278,280). In general, if there is a clearly defined sector defect in the bladder neck, then this should be reconstructed. In all other cases BPA urethroplasty should proceed and post-operative UI managed by subsequent bladder neck AUS insertion.
Male children and PFUI
PFUI is rare in boys, with a reported frequency of less than 2/year in the UK, and occurs in association with 0–3.5% of pelvic fractures in this age group (308-312). Pelvic fractures in children have the same mechanism of injury as in adults but LCII/III and APCI (the fractures that are most commonly associated with PFUI) occur more commonly (29,32). Children have more proximal PFUIs than adults and a higher frequency of concomitant bladder neck injury (7–33%) (39,92,109,239,241,250,269,280). Childhood PFUIs occur at the level of the bladder neck or through the prostate in 15–57%, although the majority are still below the level of the verumontanum (92,133,250,255,269,313,314). The more proximal the injury, the greater is the risk of UI, ED and stricture.
The results of treatment of PFUI appear to be worse in children with RS rates between 0–31% (134,255,256,267,268). The complication rates after supraprostatic and transprostatic injury are significantly higher than after lower injuries with ED in 75%, UI in 25–100%, and RS in 75% (92,250,269,314). ED is difficult to assess in children and may be consequent to the primary injury rather than the treatment. It is more likely when the urethral gap length is >2.5 cm and with lateral prostatic displacement (294). ED in children after PFUI is arteriogenic in the majority and in 75% it is secondary to PFUI proximal to the prostatomembranous region. Both ED and UI may present at puberty (92,294).
Delayed BPA urethroplasty is the most popular operative technique (133). Perineal BPA can be difficult due to the small and relatively inelastic urethra in children (314,315). A transpubic or perineo-abdominal route is required in 10–42% of children (269,316,317). As a result, the success rates for childhood DU (by any technique) are not as good as in those for adults with 62.5–85% 5-year stricture free rates (4,28,133,243,250,269,318,319). An anterior sagittal transanorectal approach has been reported with good results (82% success) but only in 1 series with small numbers (266).
Conclusions
Acute PFUI is a rare urological emergency. The mean re-stricture, ED and UI rates after SPC only are 97.9%, 25.6% and 6.7% at a mean of 46.3 months follow-up, those after primary open surgical repair (POSR) are 53.9%, 22.5% and 13.6% at a mean of 29.4 months follow-up, those after delayed primary open surgical repair (DPOSR) are 18%, 71% and 0% at a mean of 12 months follow-up, those after POR are 58.3%, 37.2% and 14.5% at a mean of 61 months follow-up, those after PER are 62%, 23.6% and 4.1% at a mean of 31.4 months follow-up, those after delayed endoscopic treatment (DET) are 80.2%, 31.9% and 4.1% at a mean of 31.8 months follow-up and those after DU are 14.4%, 12.7% and 6.8% at a mean of 54.9 months follow-up (Table 8).
Table 8. Overall PFUI treatment outcomes.
| Treatment | Number | Age, mean (range) | Follow up, mean (range) | Stricture, % (N) | Erectile dysfunction, % (N) | Incontinence, % (N) |
|---|---|---|---|---|---|---|
| SPC alone | 379 | 30 y (3–64 y) | 46.3 m (1 m–15 y) | 97.9 (371/379) | 25.6 (91/349) | 6.7 (21/314) |
| Primary open repair | 258 | 19.6 y (3–>60 y) | 29.4 m (25 d–17 y) | 53.9 (139/258) | 22.5 (25/111) | 13.6 (16/118) |
| Delayed primary open repair | 17 | (16–42 y) | >12 m | 18 (3/17) | 71 (12/17) | 0 (0/17) |
| Primary open realignment | 883 | 32.4 y (18 m–83 y) | 61 m (1 m–46 y) | 58.3 (484/830) | 37.2 (238/640) | 14.5 (83/571) |
| Primary endoscopic realignment | 401 | 34.4 y (3–73 y) | 31.4 m (2 m–17 y) | 62.0 (232/374) | 23.6 (62/263) | 4.1 (10/245) |
| Delayed endoscopic treatment | 955 | 33.9 y (4–74 y) | 31.8 m (2–360 m) | 80.2 (759/946) | 31.9 (160/501) | 4.1 (31/748) |
| Delayed urethroplasty* | 3,520 | 23 y (2–82 y) | 54.9 m (3–46 y) | 14.4 (480/3,334) | 12.7 (292/2,304) | 6.8 (148/2,166) |
*, NB. BPA urethroplasty alone stricture rate, 367/2,563 (14.3%).
Where stricture is defined as any clinical, endoscopic or radiological evidence of stricture or any requirement for further intervention (including CISC, dilatation or calibration); ED as the inability to achieve an erection sufficient for penetrative sexual intercourse without requiring medical intervention; and UI as any involuntary leak of urine of whatever volume, delayed repair with BPA urethroplasty appears to be the most successful mode of PFUI treatment with the least side effects.
Take home message
Suprapubic cystostomy is a relatively simple procedure, familiar to all urologists and other surgeons, that allows diversion of urine away from the PFUI and safeguards against associated complications from this and should be the initial treatment of choice for PFUI unless complicated by bladder neck or rectal injury. Following recovery from all other associated injuries the patient should be referred to a centre of excellence for expert delayed BPA urethroplasty and can then expect to achieve long-term excellent results.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Young HH. Treatment of complete rupture of the posterior urethra, recent or ancient, by anastomosis. J Urol 1929;21:417-50. 10.1016/S0022-5347(17)73112-6 [DOI] [Google Scholar]
- 2.Janosz F, Zielinski J, Szkodny A, et al. Surgical technique and results of primary repair in recent urethral injuries: a review of 49 consecutive cases. Eur Urol 1975;1:278-81. [PubMed] [Google Scholar]
- 3.Koraitim MM. Pelvic fracture urethral injuries: the unresolved controversy. J Urol 1999;161:1433-41. 10.1016/S0022-5347(05)68918-5 [DOI] [PubMed] [Google Scholar]
- 4.Podestá ML, Medel R, Castera R, et al. Immediate management of posterior urethral disruptions due to pelvic fracture: therapeutic alternatives. J Urol 1997;157:1444-8. 10.1016/S0022-5347(01)65016-X [DOI] [PubMed] [Google Scholar]
- 5.Mundy AR. The role of delayed primary repair in the acute management of pelvic fracture injuries of the urethra. Br J Urol 1991;68:273-6. 10.1111/j.1464-410X.1991.tb15322.x [DOI] [PubMed] [Google Scholar]
- 6.Pierce JM., Jr Management of dismemberment of the prostatic-membranous urethra and ensuing stricture disease. J Urol 1972;107:259-64. 10.1016/S0022-5347(17)60997-2 [DOI] [PubMed] [Google Scholar]
- 7.DeWeerd JH. Immediate realignment of posterior urethral injury. Urol Clin North Am 1977;4:75-80. [PubMed] [Google Scholar]
- 8.Follis HW, Kock MO, McDougal WS. Immediate management of prostatomembranous urethral disruptions. J Urol 1992;147:1259-62. 10.1016/S0022-5347(17)37534-1 [DOI] [PubMed] [Google Scholar]
- 9.Turner-Warwick R. A personal view of the immediate management of pelvic fracture urethral injuries. Urol Clin North Am 1977;4:81-93. [PubMed] [Google Scholar]
- 10.Turner-Warwick R. A personal view of the management of traumatic posterior urethral strictures. Urol Clin North Am 1977;4:111-24. [PubMed] [Google Scholar]
- 11.Gheiler EL, Frontera JR. Immediate primary realignment of prostatomembranous urethral disruptions using endourological techniques. Urology 1997;49:596-9. 10.1016/S0090-4295(97)80002-7 [DOI] [PubMed] [Google Scholar]
- 12.Herschorn S, Thijssen A, Radmoski S. The value of immediate or early catheterisation of the traumatised posterior urethra. J Urol1 1992;148:1428-31. [DOI] [PubMed]
- 13.Kielb SJ, Voeltz ZL, Wolf JJ. Evaluation and management of traumatic posterior urethral disruption with flexible cystourethroscopy. J Trauma 2001;50:36-40. 10.1097/00005373-200101000-00006 [DOI] [PubMed] [Google Scholar]
- 14.Porter JR, Takayama JK, Defalco AJ. Traumatic posterior urethral injury and early realignment using magnetic catheters. J Urol 1997;158:425-30. 10.1016/S0022-5347(01)64494-X [DOI] [PubMed] [Google Scholar]
- 15.Cohen JK, Berg G, Carl GH, et al. Primary endoscopic realignment following posterior urethral disruption. J Urol 1991;146:1548-50. 10.1016/S0022-5347(17)38162-4 [DOI] [PubMed] [Google Scholar]
- 16.Clark SS, Prudencio RF. Lower urinary tract injuries associated with pelvic fractures. Diagnosis and management. Surg Clin North Am 1972;52:183-201. 10.1016/S0039-6109(16)39642-6 [DOI] [PubMed] [Google Scholar]
- 17.Gelbard MK, Heyman AM, Weintraub P. A technique for immediate realignment and catheterisation of the disrupted prostatomembranous urethra. J Urol 1989;142:52-5. 10.1016/S0022-5347(17)38659-7 [DOI] [PubMed] [Google Scholar]
- 18.Melekos MD, Pantazakos A, Daauaher H, et al. Primary endourological reestablishment of urethral continuity after disruption of prostatomembranous urethra. Urology 1992;39:135-8. 10.1016/0090-4295(92)90269-3 [DOI] [PubMed] [Google Scholar]
- 19.Gross AJ, Seseke F, Kugler A, et al. Transurethral catheterisation under visual control in patients with urethral injury. Br J Urol 1998;82:130-1. 10.1046/j.1464-410x.1998.00732.x [DOI] [PubMed] [Google Scholar]
- 20.el-Abd SA. Endoscopic treatment of prostraumatic urethral obliteration: experience with 396 patients. J Urol 1995;153:67-71. 10.1097/00005392-199501000-00025 [DOI] [PubMed] [Google Scholar]
- 21.Dogra PN, Nabi G. Corethrough urethrotomy using Neodymium:YAG laaser for obliterative urethral stricture after traumatic disruption and or distraction defects: long term outcome. J Urol 2002;167:543-6. 10.1016/S0022-5347(01)69082-7 [DOI] [PubMed] [Google Scholar]
- 22.al-Ali M, al-Shukry M. Endoscopic repair in 154 cases of urethral occlusion: the promise of guided optical urethral reconstruction. J Urol 1997;157:129-31. 10.1016/S0022-5347(01)65304-7 [DOI] [PubMed] [Google Scholar]
- 23.Johanson B. Reconstruction of the male urethra in strictures. In: Riches E, ed. Modern Trends in Urology. London: Butterworths; 1953:5-102. [Google Scholar]
- 24.Mitchell JP. Injuries to the urethra. Br J Urol 1968;40:649-70. 10.1111/j.1464-410X.1968.tb11861.x [DOI] [PubMed] [Google Scholar]
- 25.Morehouse DD, Mackinnon KJ. Management of prostatomembranous urethral disruption: 13-year experience. J Urol 1980;123:173-4. 10.1016/S0022-5347(17)55839-5 [DOI] [PubMed] [Google Scholar]
- 26.Webster GD, Ramon J, Kreder KJ. Salvage posterior urethroplasty after failed initial repair of pelvic fracture membranous urethral defects. J Urol 1990;144:1370-2. 10.1016/S0022-5347(17)39744-6 [DOI] [PubMed] [Google Scholar]
- 27.Koraitim MM. The lessons of 145 posttraumatic posterior urethral strictures treated in 17 years. J Urol 1995;153:63-6. 10.1097/00005392-199501000-00024 [DOI] [PubMed] [Google Scholar]
- 28.Andrich DE, Dunglison N, Greenwell TJ, et al. The long-term results of urethroplasty. J Urol 2003;170:90-2. 10.1097/01.ju.0000069820.81726.00 [DOI] [PubMed] [Google Scholar]
- 29.Dalal SA, Burgess AR, Siegel JH, et al. Pelvic fracture in multiple trauma: classification by mechanism is key to pattern of organ injury, resuscitative requirements, and outcome. J Trauma 1989;29:981-1000; discussion 1000-2. 10.1097/00005373-198907000-00012 [DOI] [PubMed] [Google Scholar]
- 30.Pennal GF, Tile M, Waddell JP, et al. Pelvic disruption: assessment and classification. Clin Orthop Relat Res 1980;(151):12-21. [PubMed] [Google Scholar]
- 31.Tile M. Pelvis. In: Muller M, Allgower M, Schneider R, eds. Manual of Internal Fixation. Berlin: Springer Verlag; 1995:488-91. [Google Scholar]
- 32.Burgess AR, Eastridge BJ, Young JW, et al. Pelvic ring disruptions: effective classification system and treatment protocols. J Trauma 1990;30:848-56. 10.1097/00005373-199007000-00015 [DOI] [PubMed] [Google Scholar]
- 33.Siegel JH, Dalal SA, Burgess AR, et al. Pattern of organ injuries in pelvic fracture: impact force implications for survival and death in motor vehicle injuries. Accid Anal Prev 1990;22:457-66. 10.1016/0001-4575(90)90040-R [DOI] [PubMed] [Google Scholar]
- 34.Lüthje P, Nurmi I, Kataja M, et al. Incidence of pelvic fractures in Finland in 1988. Acta Orthop Scand 1995;66:245-8. 10.3109/17453679508995533 [DOI] [PubMed] [Google Scholar]
- 35.Balogh Z, King KL, Mackay P, et al. The epidemiology of pelvic ring fractures: a population-based study. J Trauma 2007;63:1066-73; discussion 1072-3. 10.1097/TA.0b013e3181589fa4 [DOI] [PubMed] [Google Scholar]
- 36.Cass AS. Urethral injury in the multiple-injured patient. J Trauma 1984;24:901-6. 10.1097/00005373-198410000-00006 [DOI] [PubMed] [Google Scholar]
- 37.Giannoudis PV, Grotz MRW, Tzioupis C, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma 2007;63:875-83. 10.1097/01.ta.0000242259.67486.15 [DOI] [PubMed] [Google Scholar]
- 38.Poole GV, Ward EF, Muakkassa FF, et al. Pelvic fracture from major blunt trauma. Outcome is determined by associated injuries. Ann Surg 1991;213:532-8; discussion 538-9. 10.1097/00000658-199106000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koraitim MM, Marzouk ME, Atta MA, et al. Risk factors and mechanism of urethral injury in pelvic fractures. Br J Urol 1996;77:876-80. 10.1046/j.1464-410X.1996.01119.x [DOI] [PubMed] [Google Scholar]
- 40.Poole GV, Ward EF, Griswold JA, et al. Complications of pelvic fractures from blunt trauma. Am Surg 1992;58:225-31. [PubMed] [Google Scholar]
- 41.Aihara R, Blansfield JS, Millham FH, et al. Fracture locations influence the likelihood of rectal and lower urinary tract injuries in patients sustaining pelvic fractures. J Trauma 2002;52:205-8; discussion 208-9. 10.1097/00005373-200202000-00001 [DOI] [PubMed] [Google Scholar]
- 42.Barbagli G, Sansalone S, Romano G, et al. The spectrum of pelvic fracture urethral injuries and posterior urethroplasty in an Italian high-volume centre, from 1980 to 2013. Arab J Urol 2015;13:32-6. 10.1016/j.aju.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manson T, O’Toole RV, Whitney A, et al. Young-Burgess classification of pelvic ring fractures: does it predict mortality, transfusion requirements, and non-orthopaedic injuries? J Orthop Trauma 2010;24:603-9. 10.1097/BOT.0b013e3181d3cb6b [DOI] [PubMed] [Google Scholar]
- 44.Tile M, Pennal GF. Pelvic disruption: principles of management. Clin Orthop Relat Res 1980;(151):56-64. [PubMed] [Google Scholar]
- 45.Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br 1988;70:1-12. 10.1302/0301-620X.70B1.3276697 [DOI] [PubMed] [Google Scholar]
- 46.Tile M. Pelvic fractures: operative versus nonoperative treatment. Orthop Clin North Am 1980;11:423-64. [PubMed] [Google Scholar]
- 47.Demetriades D, Karaiskakis M, Toutouzas K, et al. Pelvic fractures: epidemiology and predictors of associated abdominal injuries and outcomes. J Am Coll Surg 2002;195:1-10. 10.1016/S1072-7515(02)01197-3 [DOI] [PubMed] [Google Scholar]
- 48.Evers BM, Cryer HM, Miller FB. Pelvic fracture hemorrhage. Priorities in management. Arch Surg 1989;124:422-4. 10.1001/archsurg.1989.01410040032006 [DOI] [PubMed] [Google Scholar]
- 49.Cryer HM, Miller FB, Evers BM, et al. Pelvic fracture classification: correlation with hemorrhage. J Trauma 1988;28:973-80. 10.1097/00005373-198807000-00011 [DOI] [PubMed] [Google Scholar]
- 50.McMurtry R, Walton D, Dickinson D, et al. Pelvic disruption in the polytraumatized patient: a management protocol. Clin Orthop Relat Res 1980;(151):22-30. [PubMed] [Google Scholar]
- 51.Peltier LF. Treatment of trauma. The fractured pelvis. Med Times 1976;104:76-8. [PubMed] [Google Scholar]
- 52.Pajenda GS, Seitz H, Mousavi M, et al. Concomitant intra-abdominal injuries in pelvic trauma. Wien Klin Wochenschr 1998;110:834-40. [PubMed] [Google Scholar]
- 53.Sharpe JP, Magnotti LJ, Gobbell WC, et al. Impact of early operative pelvic fixation on long-term self-reported outcome following severe pelvic fracture. J Trauma Acute Care Surg 2017;82:444-50. 10.1097/TA.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 54.Sandler CM, Harris JHJ, Corriere JNJ, et al. Posterior urethral injuries after pelvic fracture. AJR Am J Roentgenol 1981;137:1233-7. 10.2214/ajr.137.6.1233 [DOI] [PubMed] [Google Scholar]
- 55.Bjurlin MA, Fantus RJ, Mellett MM, et al. Genitourinary injuries in pelvic fracture morbidity and mortality using the National Trauma Data Bank. J Trauma 2009;67:1033-9. 10.1097/TA.0b013e3181bb8d6c [DOI] [PubMed] [Google Scholar]
- 56.Gao JM, Wei GB, He P, et al. Management of severe pelvic fracture associated with injuries of viscera. Zhonghua Wai Ke Za Zhi 2005;43:232-4. [PubMed] [Google Scholar]
- 57.Ooi CK, Goh HK, Tay SY, et al. Patients with pelvic fracture: what factors are associated with mortality? Int J Emerg Med 2010;3:299-304. 10.1007/s12245-010-0224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pejcić T, Loncar Z, Rafailovic D, et al. Genitourinary injuries associated with pelvic fractures. Acta Chir Iugosl 2013;60:117-20. 10.2298/ACI1302117P [DOI] [PubMed] [Google Scholar]
- 59.Palmer JK, Benson GS, Corriere JNJ. Diagnosis and initial management of urological injuries associated with 200 consecutive pelvic fractures. J Urol 1983;130:712-4. 10.1016/S0022-5347(17)51420-2 [DOI] [PubMed] [Google Scholar]
- 60.Lowe MA, Mason JT, Luna GK, et al. Risk factors for urethral injuries in men with traumatic pelvic fractures. J Urol 1988;140:506-7. 10.1016/S0022-5347(17)41703-4 [DOI] [PubMed] [Google Scholar]
- 61.Coffield KS, Weems WL. Experience with management of posterior urethral injury associated with pelvic fracture. J Urol 1977;117:722-4. 10.1016/S0022-5347(17)58601-2 [DOI] [PubMed] [Google Scholar]
- 62.Glass RE, Flynn JT, King JB, et al. Urethral injury and fractured pelvis. Br J Urol 1978;50:578-82. 10.1111/j.1464-410X.1978.tb06216.x [DOI] [PubMed] [Google Scholar]
- 63.Pokorny M, Pontes JE, Pierce JMJ. Urological injuries associated with pelvic trauma. J Urol 1979;121:455-7. 10.1016/S0022-5347(17)56822-6 [DOI] [PubMed] [Google Scholar]
- 64.McAninch JW. Traumatic injuries to the urethra. J Trauma 1981;21:291-7. 10.1097/00005373-198104000-00006 [DOI] [PubMed] [Google Scholar]
- 65.Devine PC, Devine CJJ. Posterior urethral injuries associated with pelvic fractures. Urology 1982;20:467-70. 10.1016/0090-4295(82)90114-5 [DOI] [PubMed] [Google Scholar]
- 66.Colapinto V. Trauma to the pelvis: urethral injury. Clin Orthop Relat Res 1980;(151):46-55. [PubMed] [Google Scholar]
- 67.Fallon B, Wendt JC, Hawtrey CE. Urological injury and assessment in patients with fractured pelvis. J Urol 1984;131:712-4. 10.1016/S0022-5347(17)50592-3 [DOI] [PubMed] [Google Scholar]
- 68.Antoci JP, Schiff MJ. Bladder and urethral injuries in patients with pelvic fractures. J Urol 1982;128:25-6. 10.1016/S0022-5347(17)52734-2 [DOI] [PubMed] [Google Scholar]
- 69.Andrich DE, Day AC, Mundy AR. Proposed mechanisms of lower urinary tract injury in fractures of the pelvic ring. BJU Int 2007;100:567-73. 10.1111/j.1464-410X.2007.07020.x [DOI] [PubMed] [Google Scholar]
- 70.Lückhoff C, Mitra B, Cameron PA, et al. The diagnosis of acute urethral trauma. Injury 2011;42:913-6. 10.1016/j.injury.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 71.Basta AM, Blackmore CC, Wessells H. Predicting urethral injury from pelvic fracture patterns in male patients with blunt trauma. J Urol 2007;177:571-5. 10.1016/j.juro.2006.09.040 [DOI] [PubMed] [Google Scholar]
- 72.Dobrowolski ZF, Weglarz W, Jakubik P, et al. Treatment of posterior and anterior urethral trauma. BJU Int 2002;89:752-4. 10.1046/j.1464-410X.2002.02719.x [DOI] [PubMed] [Google Scholar]
- 73.Malgaigne J. A Treatise on Fracture. Philedelphia: JB Lippincott; 1859. [Google Scholar]
- 74.Flaherty JJ, Kelley R, Burnett B, et al. Relationship of pelvic bone fracture patterns to injuries of urethra and bladder. J Urol 1968;99:297-300. 10.1016/S0022-5347(17)62696-X [DOI] [PubMed] [Google Scholar]
- 75.Patterson BM. Pelvic ring injury and associated urologic trauma: an orthopaedic perspective. Semin Urol 1995;13:25-33. [PubMed] [Google Scholar]
- 76.Milenković S, Mitković M, Bašić D, et al. Urinary tract injury associated with pelvic fractures. Acta Chir Iugosl 2014;61:9-15. 10.2298/ACI1401009M [DOI] [PubMed] [Google Scholar]
- 77.Corriere JNJ, Sandler CM. Mechanisms of injury, patterns of extravasation and management of extraperitoneal bladder rupture due to blunt trauma. J Urol 1988;139:43-44. 10.1016/S0022-5347(17)42284-1 [DOI] [PubMed] [Google Scholar]
- 78.Kaiser TF, Farrow FC. Injury of the bladder and prostatomembranous urethra associated with fracture of the bony pelvis. Surg Gynecol Obstet 1965;120:99-112. [PubMed] [Google Scholar]
- 79.Carlin BI, Resnick MI. Indications and techniques for urologic evaluation of the trauma patient with suspected urologic injury. Semin Urol 1995;13:9-24. [PubMed] [Google Scholar]
- 80.Webster GD, Mathes GL, Selli C. Prostatomembranous urethral injuries: a review of the literature and a rational approach to their management. J Urol 1983;130:898-902. 10.1016/S0022-5347(17)51561-X [DOI] [PubMed] [Google Scholar]
- 81.Colapinto V, McCallum RW. Injury to the male posterior urethra in fractured pelvis: a new classification. J Urol 1977;118:575-80. 10.1016/S0022-5347(17)58110-0 [DOI] [PubMed] [Google Scholar]
- 82.Turner-Warwick R. Prevention of complications resulting from pelvic fracture urethral injuries--and from their surgical management. Urol Clin North Am 1989;16:335-8. [PubMed] [Google Scholar]
- 83.Lupu AN, Farrer JH, Smith RB, et al. Urethral gap in complete disruption of membranous urethra. Urology 1987;29:378-82. 10.1016/0090-4295(87)90500-0 [DOI] [PubMed] [Google Scholar]
- 84.Turner-Warwick R. Principles of urethral reconstruction. In: Wesbter G, Kirby R, King L, eds. Reconstructive Urology. Oxford: Blackwell Scientific Publications; 1993:609-42. [Google Scholar]
- 85.Gómez RG, Mundy T, Dubey D, et al. SIU/ICUD Consultation on Urethral Strictures: Pelvic fracture urethral injuries. Urology 2014;83:S48-58. 10.1016/j.urology.2013.09.023 [DOI] [PubMed] [Google Scholar]
- 86.Koraitim MM, Kamel MI. Perineal repair of pelvic fracture urethral injury: in pursuit of a successful outcome. BJU Int 2015;116:265-70. 10.1111/bju.12679 [DOI] [PubMed] [Google Scholar]
- 87.Seo IY, Lee JW, Park SC, et al. Long-term outcome of primary endoscopic realignment for bulbous urethral injuries: risk factors of urethral stricture. Int Neurourol J 2012;16:196-200. 10.5213/inj.2012.16.4.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leddy LS, Vanni AJ, Wessells H, et al. Outcomes of endoscopic realignment of pelvic fracture associated urethral injuries at a level 1 trauma center. J Urol 2012;188:174-8. 10.1016/j.juro.2012.02.2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Black PC, Miller EA, Porter JR, et al. Urethral and bladder neck injury associated with pelvic fracture in 25 female patients. J Urol 2006;175:2140-4; discussion 2144. 10.1016/S0022-5347(06)00309-0 [DOI] [PubMed] [Google Scholar]
- 90.Glassberg KI, Kassner EG, Haller JO, et al. The radiographic approach to injuries of the prostatomembranous urethra in children. J Urol 1979;122:678-83. 10.1016/S0022-5347(17)56555-6 [DOI] [PubMed] [Google Scholar]
- 91.Andrich DE, Mundy AR. The nature of urethral injury in cases of pelvic fracture urethral trauma. J Urol 2001;165:1492-5. 10.1016/S0022-5347(05)66334-3 [DOI] [PubMed] [Google Scholar]
- 92.Boone TB, Wilson WT, Husmann DA. Postpubertal genitourinary function following posterior urethral disruptions in children. J Urol 1992;148:1232-4. 10.1016/S0022-5347(17)36869-6 [DOI] [PubMed] [Google Scholar]
- 93.Devine CJJ, Jordan GH, Devine PC. Primary realignment of the disrupted prostatomembranous urethra. Urol Clin North Am 1989;16:291-5. [PubMed] [Google Scholar]
- 94.Al Rifaei M, Eid NI, Al Rifaei A. Urethral injury secondary to pelvic fracture: anatomical and functional classification. Scand J Urol Nephrol 2001;35:205-11. 10.1080/003655901750291971 [DOI] [PubMed] [Google Scholar]
- 95.Venn SN, Greenwell TJ, Mundy AR. Pelvic fracture injuries of the female urethra. BJU Int 1999;83:626-30. 10.1046/j.1464-410x.1999.00001.x [DOI] [PubMed] [Google Scholar]
- 96.Perry MO, Husmann DA. Urethral injuries in female subjects following pelvic fractures. J Urol 1992;147:139-43. 10.1016/S0022-5347(17)37162-8 [DOI] [PubMed] [Google Scholar]
- 97.Chopart F. Traite des maldies des voies urinaires. Vol.2 Paris: Chez Remont et Fils Libraries, 1821:239-40 [Google Scholar]
- 98.McCague EJ, Semans JH. The management of traumatic rupture of the urethra and bladder complicating fracture of the pelvis. J Urol 1944;52:36-41 10.1016/S0022-5347(17)70229-7 [DOI] [Google Scholar]
- 99.Corriere JN. 1-Stage delayed bulboprostatic anastomotic repair of posterior urethral rupture: 60 patients with 1-year followup. J Urol 2001;165:404-7. 10.1097/00005392-200102000-00012 [DOI] [PubMed] [Google Scholar]
- 100.Coburn M. Damage control for urologic injuries. Surg Clin North Am 1997;77:821-34. 10.1016/S0039-6109(05)70587-9 [DOI] [PubMed] [Google Scholar]
- 101.Johnson MH, Chang A, Brandes SB. The value of digital rectal examination in assessing for pelvic fracture-associated urethral injury: what defines a high-riding or nonpalpable prostate? J Trauma Acute Care Surg 2013;75:913-5. 10.1097/TA.0b013e3182a68668 [DOI] [PubMed] [Google Scholar]
- 102.Turner-Warwick R. Observations on the treatment of traumatic urethral injuries and the value of the fenestrated urethral catheter. Br J Surg 1973;60:775-81. 10.1002/bjs.1800601007 [DOI] [PubMed] [Google Scholar]
- 103.Malangoni MA, Botner BK, Amin EA, et al. Blunt urethral injury: results of initial management. Am Surg 1988;54:181-4. [PubMed] [Google Scholar]
- 104.Morehouse DD. Injuries to the urethra and urinary bladder associated with fractures of the pelvis. Can J Surg 1988;31:85-8. [PubMed] [Google Scholar]
- 105.Mitchell JP. Trauma to the urethra. Injury 1975;7:84-8. 10.1016/0020-1383(75)90003-0 [DOI] [PubMed] [Google Scholar]
- 106.Dixon C. Diagnosis and acute management of posterior urethral disruptions. In: McAninch J, ed. Traumatic and Reconstructive Urology. Philadelphia: Saunders; 1996:347-56. [Google Scholar]
- 107.Elliott DS, Barrett DM. Long-term followup and evaluation of primary realignment of posterior urethral disruptions. J Urol 1997;157:814-6. 10.1016/S0022-5347(01)65051-1 [DOI] [PubMed] [Google Scholar]
- 108.Blandy JP. Urethral stricture. Postgrad Med J 1980;56:383-418. 10.1136/pgmj.56.656.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mundy AR. Reconstruction of the urethra after pelvic trauma. Acta Urol Belg 1998;66:48. [PubMed] [Google Scholar]
- 110.Spirnak JP. Pelvic fracture and injury to the lower urinary tract. Surg Clin North Am 1988;68:1057-69. 10.1016/S0039-6109(16)44636-0 [DOI] [PubMed] [Google Scholar]
- 111.Ingram MD, Watson SG, Skippage PL, et al. Urethral injuries after pelvic trauma: evaluation with urethrography. Radiographics 2008;28:1631-43. 10.1148/rg.286085501 [DOI] [PubMed] [Google Scholar]
- 112.Sandler CM, Corriere JNJ. Urethrography in the diagnosis of acute urethral injuries. Urol Clin North Am 1989;16:283-9. [PubMed] [Google Scholar]
- 113.Morehouse DD. Management of posterior urethral rupture: a personal view. Br J Urol 1988;61:375-81. 10.1111/j.1464-410X.1988.tb06578.x [DOI] [PubMed] [Google Scholar]
- 114.Marks W, Dawid S, Lasek J, et al. Posterior urethra rupture: contrast-enhanced computed tomography scan and urethrocystography demonstrations. Case Rep Urol 2012;2012:109589. 10.1155/2012/109589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.da Silva Gaspar SR, Ferreira ND, Oliveira T, et al. Magnetic resonance imaging and pelvic fracture urethral injuries. Urology 2017;110:9-15. 10.1016/j.urology.2017.06.041 [DOI] [PubMed] [Google Scholar]
- 116.Joshi PM, Desai DJ, Shah D, et al. Injury in pelvic fracture urethral injury is membranobulbar: Fact or myth. Urology 2017;102:e9-10. 10.1016/j.urology.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 117.Goldman SM, Sandler CM, Corriere JNJ, et al. Blunt urethral trauma: a unified, anatomical mechanical classification. J Urol 1997;157:85-9. 10.1016/S0022-5347(01)65291-1 [DOI] [PubMed] [Google Scholar]
- 118.Moore EE, Cogbill TH, Malangoni MA, et al. Organ injury scaling. Surg Clin North Am 1995;75:293-303. 10.1016/S0039-6109(16)46589-8 [DOI] [PubMed] [Google Scholar]
- 119.Martínez-Piñeiro L, Djakovic N, Plas E, et al. EAU Guidelines on Urethral Trauma. Eur Urol 2010;57:791-803. 10.1016/j.eururo.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 120.Chapple C, Barbagli G, Jordan G, et al. Consensus statement on urethral trauma. BJU Int 2004;93:1195-202. 10.1111/j.1464-410x.2004.04805.x [DOI] [PubMed] [Google Scholar]
- 121.Chatelain C, Jardin A, Le Guillou M, et al. Segmental urethrectomy and urethrorrhaphy for treatment of fresh and late traumatic urethral lesions. Eur Urol 1975;1:126-8. [PubMed] [Google Scholar]
- 122.Trafford HS. Traumatic rupture of the posterior urethra; with a review of thirty-two civilian cases. Br J Urol 1955;27:165-71. 10.1111/j.1464-410X.1955.tb03461.x [DOI] [PubMed] [Google Scholar]
- 123.Simpson-Smith A. A Traumatic rupture of the urethra: eight personal cases and a review of 381 recorded ruptures. Br J Surg 1936;24:309-32. 10.1002/bjs.1800249412 [DOI] [Google Scholar]
- 124.Kishev S. Our operative technique for repairing and avulsed prostate or a subsequent obliteration of the posterior urethra. Urol Int 1964;17:364-88. 10.1159/000279134 [DOI] [PubMed] [Google Scholar]
- 125.Ragde H, McInnes GF. Transpubic repair of the severed prostatomembranous urethra. J Urol 1969;101:335-7. 10.1016/S0022-5347(17)62338-3 [DOI] [PubMed] [Google Scholar]
- 126.Pierce JMJ. Management of dismemberment of the prostatic-membranous urethra and ensuing stricture disease. J Urol 1972;107:259-64. 10.1016/S0022-5347(17)60997-2 [DOI] [PubMed] [Google Scholar]
- 127.Weems WL. Management of genitourinary injuries in patients with pelvic fractures. Ann Surg 1979;189:717-23. 10.1097/00000658-197906000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muhlbauer S, Bard RH. Early definitive urologic management of patient with crush pelvic trauma and deep perineal laceration. Urology 1980;15:56-9. 10.1016/0090-4295(80)90542-7 [DOI] [PubMed] [Google Scholar]
- 129.Zingg EJ, Casanova GA, Isler B, et al. Pelvic fractures and traumatic lesions of the posterior urethra. Eur Urol 1990;18:27-32. 10.1159/000463861 [DOI] [PubMed] [Google Scholar]
- 130.Gadhvi NP. Further study of Sushruta’s lateral perineal approach for primary repair of the ruptured posterior urethra. Br J Urol 1993;72:939-40. 10.1111/j.1464-410X.1993.tb16303.x [DOI] [PubMed] [Google Scholar]
- 131.Koraitim MM. Pelvic fracture urethral injuries: evaluation of various methods of management. J Urol 1996;156:1288-91. 10.1016/S0022-5347(01)65571-X [DOI] [PubMed] [Google Scholar]
- 132.Upadhyaya M, Freeman NV. Management of traumatic urethral disruption in children: Oman experience, 1988-2000. J Pediatr Surg 2002;37:1451-5. 10.1053/jpsu.2002.35410 [DOI] [PubMed] [Google Scholar]
- 133.Onen A, Ozturk H, Kaya M, et al. Long-term outcome of posterior urethral rupture in boys: a comparison of different surgical modalities. Urology 2005;65:1202-7. 10.1016/j.urology.2005.01.039 [DOI] [PubMed] [Google Scholar]
- 134.Qu Y, Zhang W, Sun N, et al. Immediate or delayed repair of pelvic fracture urethral disruption defects in young boys: twenty years of comparative experience. Chin Med J (Engl) 2014;127:3418-22. [PubMed] [Google Scholar]
- 135.Cass AS, Godec CJ. Urethral injury due to external trauma. Urology 1978;11:607-11. 10.1016/0090-4295(78)90013-4 [DOI] [PubMed] [Google Scholar]
- 136.Reinberg O, Yazbeck S. Major perineal trauma in children. J Pediatr Surg 1989;24:982-4. 10.1016/S0022-3468(89)80197-6 [DOI] [PubMed] [Google Scholar]
- 137.Tryfonas G, Georgiou G, Gavopoulos S, et al. Injury to the posterior urethra: management in children. Pediatr Surg Int 1990;5:266-9. 10.1007/BF00169667 [DOI] [Google Scholar]
- 138.Lieberman SF, Barry JM. Retreat from transpubic urethroplasty for obliterated membranous urethral strictures. J Urol 1982;128:379-81. 10.1016/S0022-5347(17)52938-9 [DOI] [PubMed] [Google Scholar]
- 139.Turner-Warwick R. Complex traumatic posterior urethral strictures. J Urol 1977;118:564-74. 10.1016/S0022-5347(17)58109-4 [DOI] [PubMed] [Google Scholar]
- 140.Kotkin L, Koch MO. Impotence and incontinence after immediate realignment of posterior urethral trauma: result of injury or management? J Urol 1996;155:1600-3. 10.1016/S0022-5347(01)66140-8 [DOI] [PubMed] [Google Scholar]
- 141.Lucey DT, Smith MJ, Koontz WWJ. Modern trends in the management of urologic trauma. J Urol 1972;107:641-6. 10.1016/S0022-5347(17)61102-9 [DOI] [PubMed] [Google Scholar]
- 142.Mouraviev VB, Coburn M, Santucci RA. The treatment of posterior urethral disruption associated with pelvic fractures: comparative experience of early realignment versus delayed urethroplasty. J Urol 2005;173:873-6. 10.1097/01.ju.0000152145.33215.36 [DOI] [PubMed] [Google Scholar]
- 143.Husmann DA, Wilson WT, Boone TB, et al. Prostatomembranous urethral disruptions: management by suprapubic cystostomy and delayed urethroplasty. J Urol 1990;144:76-8. 10.1016/S0022-5347(17)39371-0 [DOI] [PubMed] [Google Scholar]
- 144.Aşci R, Sarikaya S, Buyukalpelli R, et al. Voiding and sexual dysfunctions after pelvic fracture urethral injuries treated with either initial cystostomy and delayed urethroplasty or immediate primary urethral realignment. Scand J Urol Nephrol 1999;33:228-33. 10.1080/003655999750015826 [DOI] [PubMed] [Google Scholar]
- 145.Firmanto R, Irdam GA, Wahyudi I. Early realignment versus delayed urethroplasty in management of pelvic fracture urethral injury: a meta-analysis. Acta Med Indones 2016;48:99-105. [PubMed] [Google Scholar]
- 146.Warner JN, Santucci RA. The management of the acute setting of pelvic fracture urethral injury (realignment vs. suprapubic cystostomy alone). Arab J Urol 2015;13:7-12. 10.1016/j.aju.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barrett K, Braga LH, Farrokhyar F, et al. Primary realignment vs suprapubic cystostomy for the management of pelvic fracture-associated urethral injuries: a systematic review and meta-analysis. Urology 2014;83:924-9. 10.1016/j.urology.2013.12.031 [DOI] [PubMed] [Google Scholar]
- 148.Balkan E, Kilic N, Dogruyol H. The effectiveness of early primary realignment in children with posterior urethral injury. Int J Urol 2005;12:62-6. 10.1111/j.1442-2042.2004.00978.x [DOI] [PubMed] [Google Scholar]
- 149.Gibson GR. Urological management and complications of fractured pelvis and ruptured urethra. J Urol 1974;111:353-5. 10.1016/S0022-5347(17)59964-4 [DOI] [PubMed] [Google Scholar]
- 150.Routt ML, Simonian PT, Defalco AJ, et al. Internal fixation in pelvic fractures and primary repairs of associated genitourinary disruptions: a team approach. J Trauma 1996;40:784-90. 10.1097/00005373-199605000-00018 [DOI] [PubMed] [Google Scholar]
- 151.Myers RP, Deweerd JH. Incidence of stricture following primary realignment of the disrupted proximal urethra. J Urol 1972;107:265-8. 10.1016/S0022-5347(17)60998-4 [DOI] [PubMed] [Google Scholar]
- 152.Jackson DH, Williams JL. Urethral injury: a retrospective study. Br J Urol 1974;46:665-6. 10.1111/j.1464-410X.1974.tb08902.x [DOI] [PubMed] [Google Scholar]
- 153.Janknegt RA. Management of complete disruption of the posterior urethra. Br J Urol 1975;47:305-8. 10.1111/j.1464-410X.1975.tb03971.x [DOI] [PubMed] [Google Scholar]
- 154.Guba T, Boer PW, Bruinen CL. Sutureless repair of ruptured urethra. Acta Chir Acad Sci Hung 1978;19:103-9. [PubMed] [Google Scholar]
- 155.Islam M. Posterior urethral trauma and strictures: an attempt to solve a controversy. J Urol 1978;119:418-21. 10.1016/S0022-5347(17)57507-2 [DOI] [PubMed] [Google Scholar]
- 156.Patterson DE, Barrett DM, Myers RP, et al. Primary realignment of posterior urethral injuries. J Urol 1983;129:513-6. 10.1016/S0022-5347(17)52209-0 [DOI] [PubMed] [Google Scholar]
- 157.Fowler JW, Watson G, Smith MF, et al. Diagnosis and treatment of posterior urethral injury. Br J Urol 1986;58:167-73. 10.1111/j.1464-410X.1986.tb09020.x [DOI] [PubMed] [Google Scholar]
- 158.Murshidi MS. The place of abdominal safety line in the treatment of posterior urethral injury. Int Urol Nephrol 1988;20:623-9. 10.1007/BF02549495 [DOI] [PubMed] [Google Scholar]
- 159.Al-Ali IH, Husain I. Disrupting injuries of the membranous urethra--the case for early surgery and catheter splinting. Br J Urol 1983;55:716-20. 10.1111/j.1464-410X.1983.tb03412.x [DOI] [PubMed] [Google Scholar]
- 160.Khan MS, Thornhill JA, Grainger R, et al. Rupture of the male membranous urethra. Ir J Med Sci 2000;169:208-10. 10.1007/BF03167698 [DOI] [PubMed] [Google Scholar]
- 161.Crassweller PO, Farrow GA, Robson CJ, et al. Traumatic rupture of the supramembranous urethra. J Urol 1977;118:770-1. 10.1016/S0022-5347(17)58188-4 [DOI] [PubMed] [Google Scholar]
- 162.Malek RS, O’Dea MJ, Kelalis PP. Management of ruptured posterior urethra in childhood. J Urol 1977;117:105-9. 10.1016/S0022-5347(17)58358-5 [DOI] [PubMed] [Google Scholar]
- 163.Leddy L, Voelzke B, Wessells H. Primary realignment of pelvic fracture urethral injuries. Urol Clin North Am 2013;40:393-401. 10.1016/j.ucl.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 164.Chiou RK, Gonzalez R, Ortlip S, et al. Endoscopic treatment of posterior urethral obliteration: long-term followup and comparison with transpubic urethroplasty. J Urol 1988;140:508-11. 10.1016/S0022-5347(17)41704-6 [DOI] [PubMed] [Google Scholar]
- 165.Chiou RK, Gonzalez R. Endoscopic treatment of complete urethral obstruction using thin trocar. Urology 1985;25:475-8. 10.1016/0090-4295(85)90454-6 [DOI] [PubMed] [Google Scholar]
- 166.Chiou RK. Endourethroplasty in the management of complicated posterior urethral strictures. J Urol 1988;140:607-10. 10.1016/S0022-5347(17)41735-6 [DOI] [PubMed] [Google Scholar]
- 167.Londergan TA, Gundersen LH, van Every MJ. Early fluoroscopic realignment for traumatic urethral injuries. Urology 1997;49:101-3. 10.1016/S0090-4295(96)00429-3 [DOI] [PubMed] [Google Scholar]
- 168.Moudouni SM, Patard JJ, Manunta A, et al. Early endoscopic realignment of post-traumatic posterior urethral disruption. Urology 2001;57:628-32. 10.1016/S0090-4295(00)01068-2 [DOI] [PubMed] [Google Scholar]
- 169.Shrestha B, Baidya JL. Early endoscopic realignment in posterior urethral injuries. J Nepal Health Res Counc 2013;11:62-5. [PubMed] [Google Scholar]
- 170.Fu Q, Zhang Y, Barbagli G, et al. Factors that influence the outcome of open urethroplasty for pelvis fracture urethral defect (PFUD): an observational study from a single high-volume tertiary care center. World J Urol 2015;33:2169-75. 10.1007/s00345-015-1533-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mundy AR, Andrich DE. Urethral trauma. Part II: Types of injury and their management. BJU Int 2011;108:630-50 10.1111/j.1464-410X.2011.10340.x [DOI] [PubMed] [Google Scholar]
- 172.Mundy AR. Transperineal bulbo-prostatic anastomotic urethroplasty. World J Urol 1998;16:164-70. 10.1007/s003450050046 [DOI] [PubMed] [Google Scholar]
- 173.Tausch TJ, Morey AF. The case against primary endoscopic realignment of pelvic fracture urethral injuries. Arab J Urol 2015;13:13-6. 10.1016/j.aju.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Singh BP, Andankar MG, Swain SK, et al. Impact of prior urethral manipulation on outcome of anastomotic urethroplasty for post-traumatic urethral stricture. Urology 2010;75:179-82. 10.1016/j.urology.2009.06.081 [DOI] [PubMed] [Google Scholar]
- 175.Johnsen N V, Dmochowski RR, Mock S, et al. Primary Endoscopic Realignment of Urethral Disruption Injuries--A Double-Edged Sword? J Urol 2015;194:1022-6. 10.1016/j.juro.2015.03.112 [DOI] [PubMed] [Google Scholar]
- 176.Koraitim MM. Effect of early realignment on length and delayed repair of postpelvic fracture urethral injury. Urology 2012;79:912-5. 10.1016/j.urology.2011.11.054 [DOI] [PubMed] [Google Scholar]
- 177.Raney AM, Scott MP, Brownstein PK, et al. Urethral injury: experimental study. Urology 1977;9:281-3. 10.1016/0090-4295(77)90346-6 [DOI] [PubMed] [Google Scholar]
- 178.Towler JM, Eisen SM. A new technique for the management of urethral injuries. Br J Urol 1987;60:162-6. 10.1111/j.1464-410X.1987.tb04955.x [DOI] [PubMed] [Google Scholar]
- 179.Guille F, Cipolla B, Leveque JM, et al. Early endoscopic realignment of complete traumatic rupture of the posterior urethra. Br J Urol 1991;68:178-80. 10.1111/j.1464-410X.1991.tb15290.x [DOI] [PubMed] [Google Scholar]
- 180.Yasuda K, Yamanishi T, Isaka S, et al. Endoscopic re-establishment of membranous urethral disruption. J Urol 1991;145:977-9. 10.1016/S0022-5347(17)38505-1 [DOI] [PubMed] [Google Scholar]
- 181.Rehman J, Samadi D, Ricciardi RJ, et al. Early endoscopic realignment as primary therapy for complete posterior urethral disruptions. J Endourol 1998;12:283-9. 10.1089/end.1998.12.283 [DOI] [PubMed] [Google Scholar]
- 182.Jepson BR, Boullier JA, Moore RG, et al. Traumatic posterior urethral injury and early primary endoscopic realignment: evaluation of long-term follow-up. Urology 1999;53:1205-10. 10.1016/S0090-4295(99)00003-5 [DOI] [PubMed] [Google Scholar]
- 183.Tazi H, Ouali M, Lrhorfi MH, et al. Endoscopic realignment for post-traumatic rupture of posterior urethra. Prog Urol 2003;13:1345-50. [PubMed] [Google Scholar]
- 184.Healy CE, Leonard DS, Cahill R, et al. Primary endourologic realignment of complete posterior urethral disruption. Ir Med J 2007;100:488-9. [PubMed] [Google Scholar]
- 185.Hadjizacharia P, Inaba K, Teixeira PG, et al. Evaluation of immediate endoscopic realignment as a treatment modality for traumatic urethral injuries. J Trauma 2008;64:1443-9; discussion 1449-50. 10.1097/TA.0b013e318174f126 [DOI] [PubMed] [Google Scholar]
- 186.Olapade-Olaopa EO, Atalabi OM, Adekanye AO, et al. , Early endoscopic realignment of traumatic anterior and posterior urethral disruptions under caudal anaesthesia - a 5-year review. Int J Clin Pract 2010;64:6-12. 10.1111/j.1742-1241.2007.01481.x [DOI] [PubMed] [Google Scholar]
- 187.Sofer M, Mabjeesh NJ, Ben-Chaim J, et al. Long-term results of early endoscopic realignment of complete posterior urethral disruption. J Endourol 2010;24:1117-21. 10.1089/end.2010.0069 [DOI] [PubMed] [Google Scholar]
- 188.Kim FJ, Pompeo A, Sehrt D, et al. Early effectiveness of endoscopic posterior urethra primary alignment. J Trauma Acute Care Surg 2013;75:189-94. 10.1097/TA.0b013e31829bb7c8 [DOI] [PubMed] [Google Scholar]
- 189.Arora R, John NT, Kumar S. Vesicourethral fistula after retrograde primary endoscopic realignment in posterior urethral injury. Urology 2015;85:e1-2. 10.1016/j.urology.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 190.Salehipour M, Khezri A, Askari R, et al. Primary realignment of posterior urethral rupture. Urol J 2005;2:211-5. [PubMed] [Google Scholar]
- 191.Greenwell T, Castle C, Nicol D. Is Endoscopic management overutilised in the treatment of urethral stricture disease. Eur Urol Supp 2003;2:149 10.1016/S1569-9056(03)80590-0 [DOI] [Google Scholar]
- 192.Islam M, Anwar F, Ahmed S, et al. Optical urethrotomy in strictures following fracture pelvis. J Ayub Med Coll Abbottabad 2010;22:106-8. [PubMed] [Google Scholar]
- 193.Kitahara S, Sato R, Yasuda K, et al. Surgical treatment of urethral distraction defect associated with pelvic fracture: a nationwide survey in Japan. Int J Urol 2008;15:621-4; quiz 624. 10.1111/j.1442-2042.2008.02064.x [DOI] [PubMed] [Google Scholar]
- 194.Fellows GJ, Maranya GA, Kaggwa S, et al. Sutureless membranous urethroplasty. Br J Urol 1996;77:307-9. 10.1046/j.1464-410X.1996.92627.x [DOI] [PubMed] [Google Scholar]
- 195.Fishman IJ, Hirsch IH, Toombs BD. Endourological reconstruction of posterior urethral disruption. J Urol 1987;137:283-6. 10.1016/S0022-5347(17)43981-4 [DOI] [PubMed] [Google Scholar]
- 196.Quint HJ, Stanisic TH. Above and below delayed endoscopic treatment of traumatic posterior urethral disruptions. J Urol 1993;149:484-7. 10.1016/S0022-5347(17)36124-4 [DOI] [PubMed] [Google Scholar]
- 197.Goel MC, Kumar M, Kapoor R. Endoscopic management of traumatic posterior urethral stricture: early results and followup. J Urol 1997;157:95-7. 10.1016/S0022-5347(01)65295-9 [DOI] [PubMed] [Google Scholar]
- 198.Barry JM. Visual urethrotomy in the management of the obliterated membranous urethra. Urol Clin North Am 1989;16:319-24. [PubMed] [Google Scholar]
- 199.Naudé JH. Endoscopic skin-graft urethroplasty. World J Urol 1998;16:171-4. 10.1007/s003450050047 [DOI] [PubMed] [Google Scholar]
- 200.deVries CR, Anderson RU. Endoscopic urethroplasty: an improved technique. J Urol 1990;143:1225-6. 10.1016/S0022-5347(17)40232-1 [DOI] [PubMed] [Google Scholar]
- 201.Petterson S, Lundstam S. Endourethral urethroplasty: a simple method for treating urethral retraction. J Urol Nephrol (Paris) 1977;83 Suppl 2:659-62. [PubMed] [Google Scholar]
- 202.Gonzalez R, Chiou RK, Hekmat K, et al. Endoscopic re-establishment of urethral continuity after traumatic disruption of the membranous urethra. J Urol 1983;130:785-7. 10.1016/S0022-5347(17)51462-7 [DOI] [PubMed] [Google Scholar]
- 203.Gupta NP, Gill IS. Core-through optical internal urethrotomy in management of impassable traumatic posterior urethral strictures. J Urol 1986;136:1018-21. 10.1016/S0022-5347(17)45193-7 [DOI] [PubMed] [Google Scholar]
- 204.McCoy GB, Barry JM, Lieberman SF, et al. Treatment of obliterated membranous and bulbous urethras by direct vision internal urethrotomy. J Trauma 1987;27:883-6. 10.1097/00005373-198708000-00006 [DOI] [PubMed] [Google Scholar]
- 205.Marshall FF, Chang R, Gearhart JP. Endoscopic reconstruction of traumatic membranous urethral transection. J Urol 1987;138:306-9. 10.1016/S0022-5347(17)43129-6 [DOI] [PubMed] [Google Scholar]
- 206.Peterson NE. Perforation-reconstitution of proximal urethral obliteration. J Urol 1987;137:507-10. 10.1016/S0022-5347(17)44092-4 [DOI] [PubMed] [Google Scholar]
- 207.Lim PH, Chng HC. Initial management of acute urethral injuries. Br J Urol 1989;64:165-8. 10.1111/j.1464-410X.1989.tb05980.x [DOI] [PubMed] [Google Scholar]
- 208.Marshall FF. Endoscopic reconstruction of traumatic urethral transections. Urol Clin North Am 1989;16:313-8. [PubMed] [Google Scholar]
- 209.Leonard MP, Emtage J, Perez R, et al. Endoscopic management of urethral stricture: “cut to the light” procedure. Urology 1990;35:117-20. 10.1016/0090-4295(90)80056-S [DOI] [PubMed] [Google Scholar]
- 210.Kernohan RM, Anwar KK, Johnston SR. Complete urethral stricture of the membranous urethra: a different perspective. Br J Urol 1990;65:51-4. 10.1111/j.1464-410X.1990.tb14661.x [DOI] [PubMed] [Google Scholar]
- 211.Wu YA, Huang CH, Liu JH. Endoscopic management for traumatic occlusion of posterior urethra. Chin Med J (Engl) 1992;105:940-3. [PubMed] [Google Scholar]
- 212.Jenkins BJ, Badenoch DF, Fowler CG, et al. Long-term results of treatment of urethral injuries in males caused by external trauma. Br J Urol 1992;70:73-5. 10.1111/j.1464-410X.1992.tb15667.x [DOI] [PubMed] [Google Scholar]
- 213.Spirnak JP, Smith EM, Elder JS. Posterior urethral obliteration treated by endoscopic reconstitution, internal urethrotomy and temporary self-dilation. J Urol 1993;149:766-8. 10.1016/S0022-5347(17)36202-X [DOI] [PubMed] [Google Scholar]
- 214.Wu YA, Huang CH, Liu JH. Transurethral resection in children with urethral stricture and occlusion. J Endourol 1994;8:69-71. 10.1089/end.1994.8.69 [DOI] [PubMed] [Google Scholar]
- 215.White JL, Hirsch IH, Bagley DH. Endoscopic urethroplasty of posterior urethral avulsion. Urology 1994;44:100-5. 10.1016/S0090-4295(94)80017-0 [DOI] [PubMed] [Google Scholar]
- 216.Sahin H, Bircan MK, Akay AF, et al. Endoscopic treatment of complete posterior urethral obliteration. Acta Urol Belg 1998;66:21-4. [PubMed] [Google Scholar]
- 217.Dogra PN, Aron M, Rajeev TP. Core through urethrotomy with the neodymium:YAG laser for posttraumatic obliterative strictures of the bulbomembranous urethra. J Urol 1999;161:81-4. 10.1016/S0022-5347(01)62071-8 [DOI] [PubMed] [Google Scholar]
- 218.Levine J, Wessells H. Comparison of open and endoscopic treatment of posttraumatic posterior urethral strictures. World J Surg 2001;25:1597-601. 10.1007/s00268-001-0156-7 [DOI] [PubMed] [Google Scholar]
- 219.Ravichandran S, Nambirajan T, Athmalingham G. A randomised study of core through urethrotomy and anastomotic urethroplasty. BJU Int 2003;91:20. [Google Scholar]
- 220.Mayher BE, Guyton JL, Gingrich JR. Impact of urethral injury management on the treatment and outcome of concurrent pelvic fractures. Urology 2001;57:439-42. 10.1016/S0090-4295(00)01038-4 [DOI] [PubMed] [Google Scholar]
- 221.Taffet R. Management of pelvic fractures with concomitant urologic injuries. Orthop Clin North Am 1997;28:389-96. 10.1016/S0030-5898(05)70296-0 [DOI] [PubMed] [Google Scholar]
- 222.Matta J. Anterior exposure with the inguinal approach. In: Mears DC, Rubens HE, eds. Pelvic and Acetabular Fractures. New Jersey: Slack, Thorofare; 1986:224-31. [Google Scholar]
- 223.Singh PB, Karmakar D, Gupta RC, et al. Result of suprapubic cystostomy only as primary management of posterior urethral rupture following pelvic fracture. Int Surg 1988;73:59-62. [PubMed] [Google Scholar]
- 224.Chang PC, Hsu YC, Shee JJ, et al. Early endoscopic primary realignment decreases stricture formation and reduces medical costs in traumatic complete posterior urethral disruption in a 2-year follow-up. Chang Gung Med J 2011;34:179-85. [PubMed] [Google Scholar]
- 225.Dhabuwala CB, Hamid S, Katsikas DM, et al. Impotence following delayed repair of prostatomembranous urethral disruption. J Urol 1990;144:677-8. 10.1016/S0022-5347(17)39552-6 [DOI] [PubMed] [Google Scholar]
- 226.Koraitim MM. Optimising the outcome after anastomotic posterior urethroplasty. Arab J Urol 2015;13:27-31. 10.1016/j.aju.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Morehouse DD, Belitsky P, Mackinnon K. Rupture of the posterior urethra. J Urol 1972;107:255-8. 10.1016/S0022-5347(17)60996-0 [DOI] [PubMed] [Google Scholar]
- 228.Mundy AR. Urethroplasty for posterior urethral strictures. Br J Urol 1996;78:243-7. 10.1046/j.1464-410X.1996.11617.x [DOI] [PubMed] [Google Scholar]
- 229.Marion G. Traite D’Urologie. Masson edi. (Masson, ed.). Paris; 1928. [Google Scholar]
- 230.Waterhouse K, Abrahams JI, Caponegro P, et al. The transpubic repair of membranous urethral strictures. J Urol 1974;111:188-90. 10.1016/S0022-5347(17)59923-1 [DOI] [PubMed] [Google Scholar]
- 231.Dixon CM, Hricak H, McAninch JW. Magnetic resonance imaging of traumatic posterior urethral defects and pelvic crush injuries. J Urol 1992;148:1162-5. 10.1016/S0022-5347(17)36849-0 [DOI] [PubMed] [Google Scholar]
- 232.Koraitim MM. Predictors of surgical approach to repair pelvic fracture urethral distraction defects. J Urol 2009;182:1435-9. 10.1016/j.juro.2009.06.022 [DOI] [PubMed] [Google Scholar]
- 233.Allen TD. The transpubic approach for strictures of the membranous urethra. J Urol 1975;114:63-8. 10.1016/S0022-5347(17)66944-1 [DOI] [PubMed] [Google Scholar]
- 234.Chatelain C, Le Guillou M, Petit M, et al. Symphysiotomy or transpubic approach to traumatic strictures of the posterior urethra. Eur Urol 1975;1:140-4. [PubMed] [Google Scholar]
- 235.Waterhouse K. The surgical repair of membranous urethral strictures in children. J Urol 1976;116:363-5. 10.1016/S0022-5347(17)58816-3 [DOI] [PubMed] [Google Scholar]
- 236.de la Peña Zayas E, Esteva JF, Diaz RP, et al. Pubectomy for repair of membranous urethral strictures. J Urol 1979;121:170-2. 10.1016/S0022-5347(17)56708-7 [DOI] [PubMed] [Google Scholar]
- 237.Redman JF. Surgical management of post-traumatic prostatomembranous urethral strictures: a ten year experience. J Ark Med Soc 1988;85:45-7. [PubMed] [Google Scholar]
- 238.Mark SD, Keane TE, Vandemark RM, et al. Impotence following pelvic fracture urethral injury: incidence, aetiology and management. Br J Urol 1995;75:62-4. 10.1111/j.1464-410X.1995.tb07234.x [DOI] [PubMed] [Google Scholar]
- 239.Koraitim MM. Posttraumatic posterior urethral strictures in children: a 20-year experience. J Urol 1997;157:641-5. 10.1016/S0022-5347(01)65239-X [DOI] [PubMed] [Google Scholar]
- 240.Martínez-Piñeiro JA, Cárcamo P, Garcia Matres MJ, et al. Excision and anastomotic repair for urethral stricture disease: experience with 150 cases. Eur Urol 1997;32:433-41. [PubMed] [Google Scholar]
- 241.Podestá ML. Use of the perineal and perineal-abdominal (transpubic) approach for delayed management of pelvic fracture urethral obliterative strictures in children: long-term outcome. J Urol 1998;160:160-4. 10.1016/S0022-5347(01)63078-7 [DOI] [PubMed] [Google Scholar]
- 242.Tunc HM, Tefekli AH, Kaplancan T, et al. Delayed repair of post-traumatic posterior urethral distraction injuries: long-term results. Urology 2000;55:837-41. 10.1016/S0090-4295(00)00464-7 [DOI] [PubMed] [Google Scholar]
- 243.Basiri A, Shadpour P, Moradi MR, et al. Symphysiotomy: a viable approach for delayed management of posterior urethral injuries in children. J Urol 2002;168:2166-9; discussion 2169. 10.1016/S0022-5347(05)64345-5 [DOI] [PubMed] [Google Scholar]
- 244.Flynn BJ, Delvecchio FC, Webster GD. Perineal repair of pelvic fracture urethral distraction defects: experience in 120 patients during the last 10 years. J Urol 2003;170:1877-80. 10.1097/01.ju.0000091642.41368.f5 [DOI] [PubMed] [Google Scholar]
- 245.Andrich DE, O’Malley KJ, Summerton DJ, et al. The type of urethroplasty for a pelvic fracture urethral distraction defect cannot be predicted preoperatively. J Urol 2003;170:464-7. 10.1097/01.ju.0000076752.32199.40 [DOI] [PubMed] [Google Scholar]
- 246.Koraitim MM. On the art of anastomotic posterior urethroplasty: a 27-year experience. J Urol 2005;173:135-9. 10.1097/01.ju.0000146683.31101.ff [DOI] [PubMed] [Google Scholar]
- 247.Culty T, Boccon-Gibod L. Anastomotic urethroplasty for posttraumatic urethral stricture: previous urethral manipulation has a negative impact on the final outcome. J Urol 2007;177:1374-7. 10.1016/j.juro.2006.11.092 [DOI] [PubMed] [Google Scholar]
- 248.Koraitim MM. Transpubic urethroplasty revisited: total, superior, or inferior pubectomy? Urology 2010;75:691-4. 10.1016/j.urology.2009.09.026 [DOI] [PubMed] [Google Scholar]
- 249.Webster GD, Ramon J. Repair of pelvic fracture posterior urethral defects using an elaborated perineal approach: experience with 74 cases. J Urol 1991;145:744-8. 10.1016/S0022-5347(17)38442-2 [DOI] [PubMed] [Google Scholar]
- 250.al-Rifaei MA, Gaafar S, Abdel-Rahman M. Management of posterior urethral strictures secondary to pelvic fractures in children. J Urol 1991;145:353-6. 10.1016/S0022-5347(17)38337-4 [DOI] [PubMed] [Google Scholar]
- 251.Flah LM, Alpuche JO, Castro RS. Repair of posttraumatic stenosis of the urethra through a posterior sagittal approach. J Pediatr Surg 1992;27:1465-70. 10.1016/0022-3468(92)90201-H [DOI] [PubMed] [Google Scholar]
- 252.Corriere JN, Jr, Rudy DC, Benson GS. Voiding and erectile function after delayed one-stage repair of posterior urethral disruptions in 50 men with a fractured pelvis. J Trauma 1994;37:587-9; discussion 589-90. 10.1097/00005373-199410000-00011 [DOI] [PubMed] [Google Scholar]
- 253.Fu Q, Zhang J, Sa YL, et al. Recurrence and complications after transperineal bulboprostatic anastomosis for posterior urethral strictures resulting from pelvic fracture: a retrospective study from a urethral referral centre. BJU Int 2013;112:E358-63. 10.1111/bju.12171 [DOI] [PubMed] [Google Scholar]
- 254.Netto R, Jr, Goes GM, Freire JG. A pull-through operation for the treatment of lesions of the posterior and bulbar urethra. Int Urol Nephrol 1973;5:383-91 10.1007/BF02083386 [DOI] [PubMed] [Google Scholar]
- 255.Harshman MW, Cromie WJ, Wein AJ, et al. Urethral stricture disease in children. J Urol 1981;126:650-4. 10.1016/S0022-5347(17)54675-3 [DOI] [PubMed] [Google Scholar]
- 256.Hayden LJ, Koff SA. One-stage membranous urethroplasty in childhood. J Urol 1984;132:311-2. 10.1016/S0022-5347(17)49606-6 [DOI] [PubMed] [Google Scholar]
- 257.Netto NRJ. The surgical repair of posterior urethral strictures by the transpubic urethroplasty or pull-through technique. J Urol 1985;133:411-2. 10.1016/S0022-5347(17)49000-8 [DOI] [PubMed] [Google Scholar]
- 258.Koraitim M. Experience with 170 cases of posterior urethral strictures during 7 years. J Urol 1985;133:408-10. 10.1016/S0022-5347(17)48997-X [DOI] [PubMed] [Google Scholar]
- 259.Patil UB. Long-term results of transpubic prostatomembranous urethroplasty in children. J Urol 1986;136:286-7. 10.1016/S0022-5347(17)44843-9 [DOI] [PubMed] [Google Scholar]
- 260.Zvara V, Hornak M. Long-term results of treatment of urethral strictures by transpubic urethroplasty. Czech Med 1986;9:1-8. [PubMed] [Google Scholar]
- 261.Baskin LS, McAninch JW. Childhood urethral injuries: perspectives on outcome and treatment. Br J Urol 1993;72:241-6. 10.1111/j.1464-410X.1993.tb00696.x [DOI] [PubMed] [Google Scholar]
- 262.Ennemoser O, Colleselli K, Reissigl A, et al. Posttraumatic posterior urethral stricture repair: anatomy, surgical approach and long-term results. J Urol 1997;157:499-505. 10.1016/S0022-5347(01)65187-5 [DOI] [PubMed] [Google Scholar]
- 263.Morey AF, McAninch JW. Reconstruction of traumatic posterior urethral strictures. Tech Urol 1997;3:103-7. [PubMed] [Google Scholar]
- 264.Ku JH, Jeon YS, Kim ME, et al. Comparison of long-term results according to the primary mode of management and type of injury for posterior urethral injuries. Urol Int 2002;69:227-32. 10.1159/000063947 [DOI] [PubMed] [Google Scholar]
- 265.Lumen N, Hoebeke P, Troyer B, De, et al. Perineal anastomotic urethroplasty for posttraumatic urethral stricture with or without previous urethral manipulations: a review of 61 cases with long-term followup. J Urol 2009;181:1196-200. 10.1016/j.juro.2008.10.170 [DOI] [PubMed] [Google Scholar]
- 266.Onofre LS, Leao JQ de S, Gomes AL, et al. Pelvic fracture urethral distraction defects in children managed by anterior sagittal trans anorectal approach: a facilitating and safe access. J Pediatr Urol 2011;7:349-55. 10.1016/j.jpurol.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 267.Sunay M, Karabulut A, Dadali M, et al. Single-institution outcomes of open reconstruction techniques for management of pediatric and adolescent post-traumatic urethral strictures. Urology 2011;77:706-10. 10.1016/j.urology.2010.07.476 [DOI] [PubMed] [Google Scholar]
- 268.Voelzke BB, Breyer BN, McAninch JW. Blunt pediatric anterior and posterior urethral trauma: 32-year experience and outcomes. J Pediatr Urol 2012;8:258-63. 10.1016/j.jpurol.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Podesta M, Podesta MJ. Delayed surgical repair of posttraumatic posterior urethral distraction defects in children and adolescents: long-term results. J Pediatr Urol 2015;11:67.e1-6. 10.1016/j.jpurol.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 270.El-Assmy A, Harraz AM, Benhassan M, et al. Erectile dysfunction post-perineal anastomotic urethroplasty for traumatic urethral injuries: analysis of incidence and possibility of recovery. Int Urol Nephrol 2015;47:797-802. 10.1007/s11255-015-0945-9 [DOI] [PubMed] [Google Scholar]
- 271.Gibson GR. Impotence following fractured pelvis and ruptured urethra. Br J Urol 1970;42:86-8. 10.1111/j.1464-410X.1970.tb11912.x [DOI] [PubMed] [Google Scholar]
- 272.Gomez RG, Campos RA, Velarde LG. Reconstruction of pelvic fracture urethral injuries with sparing of the bulbar arteries. Urology 2016;88:207-12. 10.1016/j.urology.2015.09.032 [DOI] [PubMed] [Google Scholar]
- 273.Koraitim MM. The combined perineo-abdominal transpubic urethroplasty. Arab J Urol 2015;13:24-6. 10.1016/j.aju.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Koraitim MM. Failed posterior urethroplasty: lessons learned. Urology 2003;62:719-22. 10.1016/S0090-4295(03)00573-9 [DOI] [PubMed] [Google Scholar]
- 275.Lenzi R, Selli C, Stomaci N, et al. Bladder herniation after transpubic urethroplasty. J Urol 1983;130:778-80. 10.1016/S0022-5347(17)51458-5 [DOI] [PubMed] [Google Scholar]
- 276.Bissada NK, Barry JM, Morcos R, et al. Hernias after transpubic urethroplasty. J Urol 1986;135:1010-1. 10.1016/S0022-5347(17)45959-3 [DOI] [PubMed] [Google Scholar]
- 277.Shenfeld OZ, Kiselgorf D, Gofrit ON, et al. The incidence and causes of erectile dysfunction after pelvic fractures associated with posterior urethral disruption. J Urol 2003;169:2173-6. 10.1097/01.ju.0000067660.51231.05 [DOI] [PubMed] [Google Scholar]
- 278.Iselin CE, Webster GD. The significance of the open bladder neck associated with pelvic fracture urethral distraction defects. J Urol 1999;162:347-51. 10.1016/S0022-5347(05)68557-6 [DOI] [PubMed] [Google Scholar]
- 279.MacDiarmid S, Rosario D, Chapple CR. The importance of accurate assessment and conservative management of the open bladder neck in patients with post-pelvic fracture membranous urethral distraction defects. Br J Urol 1995;75:65-7. 10.1111/j.1464-410X.1995.tb07235.x [DOI] [PubMed] [Google Scholar]
- 280.Koraitim MM. Assessment and management of an open bladder neck at posterior urethroplasty. Urology 2010;76:476-9. 10.1016/j.urology.2009.11.043 [DOI] [PubMed] [Google Scholar]
- 281.Abdalla MA. A posterior sagittal pararectal approach for repair of posterior urethral distraction injuries. Eur Urol 2008;53:191-6. 10.1016/j.eururo.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 282.Tausch TJ, Morey AF. Con: bulbomembranous anastomotic urethroplasty for pelvic fracture urethral injuries. Transl Androl Urol 2015;4:79-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chambers HL, Balfour J. The incidence of impotence following pelvic fracture with associated urinary tract injury. J Urol 1963;89:702-3. 10.1016/S0022-5347(17)64628-7 [DOI] [PubMed] [Google Scholar]
- 284.Guan Y, Wendong S, Zhao S, et al. The vascular and neurogenic factors associated with erectile dysfunction in patients after pelvic fractures. Int Braz J Urol 2015;41:959-66. 10.1590/S1677-5538.IBJU.2014.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Blaschko SD, Sanford MT, Schlomer BJ, et al. The incidence of erectile dysfunction after pelvic fracture urethral injury: A systematic review and meta-analysis. Arab J Urol 2015;13:68-74. 10.1016/j.aju.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Koraitim MM. Predictors of erectile dysfunction post pelvic fracture urethral injuries: a multivariate analysis. Urology 2013;81:1081-5. 10.1016/j.urology.2012.12.040 [DOI] [PubMed] [Google Scholar]
- 287.Shenfeld OZ, Gofrit ON, Gdor Y, et al. The role of sildenafil in the treatment of erectile dysfunction in patients with pelvic fracture urethral disruption. J Urol 2004;172:2350-2. 10.1097/01.ju.0000145249.24842.b6 [DOI] [PubMed] [Google Scholar]
- 288.Tang CY, Fu Q, Cui RJ, et al. Erectile dysfunction in patients with traumatic urethral strictures treated with anastomotic urethroplasty: a single-factor analysis. Can J Urol 2012;19:6548-53. [PubMed] [Google Scholar]
- 289.Armenakas NA, McAninch JW, Lue TF, et al. Posttraumatic impotence: magnetic resonance imaging and duplex ultrasound in diagnosis and management. J Urol 1993;149:1272-5. 10.1016/S0022-5347(17)36365-6 [DOI] [PubMed] [Google Scholar]
- 290.Al-Rifaei MA, Zaghloul S, Al-Rifaei AM. Bulboprostatic anastomotic urethroplasty with preservation of potency: anatomical study, operative approach and clinical results. Scand J Urol Nephrol 2005;39:163-8. 10.1080/00365590310019972 [DOI] [PubMed] [Google Scholar]
- 291.King J. Impotence after fractures of the pelvis. J Bone Joint Surg Am 1975;57:1107-9. 10.2106/00004623-197557080-00013 [DOI] [PubMed] [Google Scholar]
- 292.Pandian RM, John NT, Eapen A, et al. Does MRI help in the pre - operative evaluation of pelvic fracture urethral distraction defect? - A pilot study. Int Braz J Urol 2017;43:127-33. 10.1590/s1677-5538.ibju.2016.0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Narumi Y, Hricak H, Armenakas NA, et al. MR imaging of traumatic posterior urethral injury. Radiology 1993;188:439-43. 10.1148/radiology.188.2.8327694 [DOI] [PubMed] [Google Scholar]
- 294.Koraitim MM. Predicting risk of erectile dysfunction after pelvic fracture urethral injury in children. J Urol 2014;192:519-23. 10.1016/j.juro.2014.02.094 [DOI] [PubMed] [Google Scholar]
- 295.Malavaud B, Mouzin M, Tricoire JL, et al. Evaluation of male sexual function after pelvic trauma by the International Index of Erectile Function. Urology 2000;55:842-6. 10.1016/S0090-4295(00)00492-1 [DOI] [PubMed] [Google Scholar]
- 296.Metze M, Tiemann AH, Josten C. Male sexual dysfunction after pelvic fracture. J Trauma 2007;63:394-401. 10.1097/01.ta.0000241145.02748.df [DOI] [PubMed] [Google Scholar]
- 297.Aboseif S, Lue T. Impotence after urethral injury. In: McAninch J, ed. Traumatic and Reconstructive Urology. Philadelphia: Saunders; 1998:445-62. [Google Scholar]
- 298.Peng J, Zhang Z, Gao B, et al. Effect of daily sildenafil on patients with absent nocturnal erections due to pelvic fracture urethral disruption: a single-centre experience. Andrologia 2016;48:1120-4. 10.1111/and.12548 [DOI] [PubMed] [Google Scholar]
- 299.Peng J, Zhang Z, Cui W, et al. Role of nocturnal penile erection test on response to daily sildenafil in patients with erectile dysfunction due to pelvic fracture urethral disruption: a single-center experience. Urology 2014;84:1389-94. 10.1016/j.urology.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 300.Fu Q, Sun X, Tang C, et al. An assessment of the efficacy and safety of sildenafil administered to patients with erectile dysfunction referred for posterior urethroplasty: a single-center experience. J Sex Med 2012;9:282-7. 10.1111/j.1743-6109.2011.02470.x [DOI] [PubMed] [Google Scholar]
- 301.Zuckerman JM, McCammon KA, Tisdale BE, et al. Outcome of penile revascularization for arteriogenic erectile dysfunction after pelvic fracture urethral injuries. Urology 2012;80:1369-73. 10.1016/j.urology.2012.07.059 [DOI] [PubMed] [Google Scholar]
- 302.Morales A, Condra MS, Owen JE, et al. Oral and transcutaneous pharmacologic agents in the treatment of impotence. Urol Clin North Am 1988;15:87-93. [PubMed] [Google Scholar]
- 303.El-Assmy A, Harraz AM, Benhassan M, et al. Erectile function after anastomotic urethroplasty for pelvic fracture urethral injuries. Int J Impot Res 2016;28:139-42. 10.1038/ijir.2016.18 [DOI] [PubMed] [Google Scholar]
- 304.Anger JT, Sherman ND, Webster GD. Ejaculatory profiles and fertility in men after posterior urethroplasty for pelvic fracture-urethral distraction defect injuries. BJU Int 2008;102:351-3. 10.1111/j.1464-410X.2008.07657.x [DOI] [PubMed] [Google Scholar]
- 305.El-Assmy A, Benhassan M, Harraz AM, et al. Ejaculatory function after anastomotic urethroplasty for pelvic fracture urethral injuries. Int Urol Nephrol 2015;47:497-501. 10.1007/s11255-015-0923-2 [DOI] [PubMed] [Google Scholar]
- 306.Whitson JM, McAninch JW, Tanagho EA, et al. Mechanism of continence after repair of posterior urethral disruption: evidence of rhabdosphincter activity. J Urol 2008;179:1035-9. 10.1016/j.juro.2007.10.081 [DOI] [PubMed] [Google Scholar]
- 307.Turner-Warwick R. The anatomical basis of functional reconstruction of the urethra. In: Droller M, ed. Surgical Anatomy. New York: Mosby Year Book; 1991:Chap 61. [Google Scholar]
- 308.Reichard SA, Helikson MA, Shorter N, et al. Pelvic fractures in children--review of 120 patients with a new look at general management. J Pediatr Surg 1980;15:727-34. 10.1016/S0022-3468(80)80272-7 [DOI] [PubMed] [Google Scholar]
- 309.Tarman GJ, Kaplan GW, Lerman SL, et al. Lower genitourinary injury and pelvic fractures in pediatric patients. Urology 2002;59:123-6; discussion 126. 10.1016/S0090-4295(01)01526-6 [DOI] [PubMed] [Google Scholar]
- 310.McAleer IM, Kaplan GW, Scherz HC, et al. Genitourinary trauma in the pediatric patient. Urology 1993;42:563-7; discussion 567-8. 10.1016/0090-4295(93)90274-E [DOI] [PubMed] [Google Scholar]
- 311.Devereux MH, Williams DI. The treatment of urethral stricture in boys. J Urol 1972;108:489-93. 10.1016/S0022-5347(17)60783-3 [DOI] [PubMed] [Google Scholar]
- 312.Musemeche CA, Fischer RP, Cotler HB, et al. Selective management of pediatric pelvic fractures: a conservative approach. J Pediatr Surg 1987;22:538-40. 10.1016/S0022-3468(87)80216-6 [DOI] [PubMed] [Google Scholar]
- 313.Morra MN, Das S. Traumatic stricture of prostatic urethra in ten-year-old boy. Urology 1991;38:552-3. 10.1016/0090-4295(91)80177-9 [DOI] [PubMed] [Google Scholar]
- 314.Kardar AH, Sundin T, Ahmed S. Delayed management of posterior urethral disruption in children. Br J Urol 1995;75:543-7. 10.1111/j.1464-410X.1995.tb07280.x [DOI] [PubMed] [Google Scholar]
- 315.Brock WA, Kaplan GW. Use of the transpubic approach for urethroplasty in children. J Urol 1981;125:496-501. 10.1016/S0022-5347(17)55085-5 [DOI] [PubMed] [Google Scholar]
- 316.Trachta J, Moravek J, Kriz J, et al. Pediatric bulbar and posterior urethral injuries: operative outcomes and long-term follow-Up. Eur J Pediatr Surg 2016;26:86-90. [DOI] [PubMed] [Google Scholar]
- 317.Orabi S. Transpubic posterior urethroplasty via perineal approach in children: a new technique. J Pediatr Urol 2012;8:393-400. 10.1016/j.jpurol.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 318.Aggarwal SK, Sinha SK, Kumar A, et al. Traumatic strictures of the posterior urethra in boys with special reference to recurrent strictures. J Pediatr Urol 2011;7:356-62. 10.1016/j.jpurol.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 319.Singh A, Panda SS, Bajpai M, et al. Our experience, technique and long-term outcomes in the management of posterior urethral strictures. J Pediatr Urol 2014;10:40-4. 10.1016/j.jpurol.2013.05.018 [DOI] [PubMed] [Google Scholar]