Abstract
Background
To investigate the outcomes and mechanisms of low-intensity extracorporeal shock wave therapy (Li-ESWT) on stress urinary incontinence (SUI) in a vaginal balloon dilation (VBD) rat model.
Methods
Thirty Sprague-Dawley rats were randomly grouped into normal controls, VBD only, and VBD with Li-ESWT. Li-ESWT was administered twice per week for 3 weeks. Afterward, all 30 rats were assessed with functional and histological studies. To explore the acute effect of Li-ESWT, another 25 rats, given intraperitoneal 5-ethynyl-2-deoxyuridine (EdU) at birth, were treated with Li-ESWT followed by assessment of vascular endothelial growth factor (VEGF) expression and endogenous progenitor cells distribution at 24 hours or 1 week after the last Li-ESWT therapy. Additionally, rat myoblast L6 cells were used for myotube formation assay in vitro.
Results
Functional analysis with leak-point pressure (LPP) testing showed that rats treated with Li-ESWT following VBD had significantly higher LPP relative to those receiving VBD only (44.8±3.2 versus 27.0±2.9 cmH2O, P<0.01). Histological examinations showed increased urethral sphincter regeneration in Li-ESWT group. The rats treated with Li-ESWT also had increased vascularity, which was confirmed by immunohistochemistry of rat endothelial cell antigen, while reverse-transcriptase polymerase chain reaction (RT-PCR) showed VEGF expression was significantly enhanced. Additionally, there were significantly increased EdU+ cells in Li-ESWT treated rats at 24 hours. In vitro, Li-ESWT promoted myotube formation from L6 cells.
Conclusions
Li-ESWT ameliorated SUI by promoting angiogenesis, progenitor cell recruitment, and urethral sphincter regeneration in a rat model induced by VBD. Li-ESWT represents a potential novel non-invasive therapy for SUI.
Keywords: Low-intensity Extracorporeal Shock Wave Therapy (Li-ESWT), stress urinary incontinence (SUI), vaginal balloon dilation (VBD), leak-point pressure (LPP), 5-ethynyl-2-deoxyuridine (EdU)
Introduction
More than 25% of women suffer from stress urinary incontinence (SUI) in the United States and the annually cost related to management of this condition is high (1,2). Current treatments for SUI, including oral medications, urethral bulking agents, and urethral sling surgeries, provide limited efficacy or can incur short-term and long-term complications (3-5). Moreover, these therapies treat only the symptoms, not the underlying pathology. Therefore, novel treatments to restore normal urethral function are urgently needed (6).
Stem cell (SC) therapy for SUI was first published in 2002 (7) and has since advanced to several clinical trials (6). In 2010 we firstly published a preclinical study in which adipose-derived stem cells (ADSCs) were used for SUI therapy (8). As ADSC can be more easily, abundantly, and safely obtained than other SC types, it has become the choice cell type in many recent studies (6,9). Clinically, the use of SC therapy is still very limited due to safety and efficacy concerns. Recently, low-intensity extracorporeal shock wave therapy (Li-ESWT) has been applied in regenerative medicine to stimulate skeletal muscle growth and accelerate repair (10). Li-ESWT has been shown to mobilize endogenous SCs to injury sites (11,12), and to activate local stem/progenitor cells for the treatment of various urological disorders including erectile dysfunction (ED) and chronic pelvic pain syndrome (13-16). Recently, we also demonstrated that Li-ESWT is able to activate penile endogenous SCs (17,18) and promote myogenesis in vitro (19). In addition, Li-ESWT therapy has also been shown to promote host tissue angiogenesis and neuroregeneration (13,20,21).
Multiple animal studies examining tissue grafts, skin flaps, and myocardium have shown the increased angiogenesis and re-vascularization effects of Li-ESWT (22-24). In a rat diabetic ED model (25), we also found that Li-ESWT was able to significantly reverse diabetes-associated deficits in penile muscle, endothelium, and nerve contents. In addition, we obtained evidence that Li-ESWT was able to mobilize endogenous SCs to injury sites—a phenomenon previously observed in hind limb ischemia animal models (11,12). Li-ESWT represents a potential safe and effective treatment for various trauma-associated disorders, including SUI related to birth trauma.
Methods
Animals and overview
All experimental protocols were approved by the Institutional Animal Care and Use Committee at our institution. Thirty female Sprague-Dawley rats (12 weeks) were obtained from Charles River Laboratories (Wilmington, MA, USA), and randomly divided into 3 groups including normal controls (10 rats), vaginal balloon dilation (VBD) only (9 rats), and VBD with Li-ESWT treatment (11 rats). VBD and subsequent ovariectomy was performed as previously described (26,27). Briefly, after rats were anesthetized, VBD was performed via vaginal insertion of a 16-Fr latex Foley catheter, with subsequent filling of the catheter balloon to 4 mL of fluid. The balloon was left in place for 4 hours to simulate prolonged labor. One week later, the rats were again anesthetized; and both ovaries were excised with a midline incision. Li-ESWT was administered twice per week for 3 weeks under anesthesia. One week wash-out following completion of Li-ESWT therapy, approximately 5 weeks after initial VBD, leak-point pressures (LPP) were measured for functional evaluation, and then the rats were sacrificed with the urethras harvested for histological analysis.
An additional set of twenty-five 8-week-old female Sprague-Dawley rats that had been injected with 5-ethynyl-2-deoxyuridine (EdU, Invitrogen, Carlsbad, CA, USA) at birth as previously described, were divided into 3 groups including normal controls (6 rats), VBD only (7 rats), and 1 week of Li-ESWT (twice a week) following VBD with sacrifice at 24 hours (6 rats) or 1 week (6 rats) after the last Li-ESWT. These rats were used for checking vascular endothelial growth factor (VEGF) expression either via histological analysis or via reverse-transcriptase polymerase chain reaction (RT-PCR) analysis (28). This was done to assess for early and potentially transient signaling following Li-ESWT. Rats that were used for histological analysis, given that they were treated with EdU at birth, could potentially allow for tracking of progenitor cells as previous reported.
Application of Li-ESWT and treatment protocol
While conventional shockwave treatment in Urology is performed as a focused high energy pulse for urinary stone disease, Li-ESWT disperses considerably less energy at 0.03–0.06 mJ/mm2, is unfocused, and fewer shocks are administered. Application of Li-ESWT was performed using the MTS Dermagold (Atlanta, GA, USA). The shockwave probe was applied directly to the shaved pelvis of the rat, overlying the area of the urethra and surrounding pelvis, and was coupled to the skin using ultrasound gel with energy flux density (EFD) of 0.06 mJ/mm2, and 300 shocks at 3 Hz during each session.
Measurement of LPP
Under anesthesia with urethane, the LPP measurement was conducted as we reported previously (29). In brief, a polyethylene-90 (PE-90) tube (Clay-Adams, Parsippany, NJ, USA) was inserted into the bladder dome. The rat’s bladder was slowly filled with warmed phosphate buffered saline, and the bladder capacity was determined by the point at which leakage of urine was noted. This procedure was repeated 3 times and the average bladder capacity was used. The LabView 6.0 software (National Instruments, Austin, TX, USA) was used to record the intravesical pressure. The bladder was filled to 40% of capacity, and increasing manual extravesical pressure was applied until leakage was noted. This procedure was repeated 6 times, and the average pressure at which the rat leaked urine served as the LPP for that particular rat. The rat was subsequently sacrificed, and organs harvested.
Histological staining
Cryosections of urethral tissue samples were prepared, and Immunohistochemical staining was performed as previously described (25,30,31) using rat endothelial cell antibody (RECA, 1:500, AbD Serotec, Raleigh, NC, USA) to label blood vessels and fluorescence staining of Alexa-488–conjugated phalloidin (Invitrogen, Carlsbad, CA, USA) to label urethral smooth and striated muscular bundles. In the second set of rats receiving EdU injection at birth, sections were assessed for EdU labeling using the Click-iT reaction cocktail (Invitrogen, Carlsbad, CA, USA). The entire urethral section for each rat was surveyed and the number of EdU labeled cells counted. Urethral tissue sections were also prepared for Masson’s trichrome staining as we reported previously (1). In brief, the tissue sections were immersed in Bouin solution for 15 min followed by Weigert Hematoxylin staining for 10 min, Biebrich scarlet—acid fuchsin for 3 min, and aniline blue for 3 min.
Images were captured with A Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY, USA) and analyzed with Image-Pro Plus image software.
Myodifferentiation of rat myoblast L6 cells in vitro
Rat myoblast L6 cells were used in this experiment and were divided into 4 groups: (I) control; (II) induction; (III) Li-ESWT; and (IV) induction + Li-ESW. Control and Induction groups were cells cultured in DMEM containing 15% FBS and 2% horse serum, respectively. Li-ESW groups were cells treated with Li-ESW at 0.03 mJ/mm2 for 200 shocks. All groups were stained for Myosin heavy chain (MHC) (1:500, Abcam Inc., MA, USA) and Myogenin (1:500, Abcam Inc., MA, USA), followed by microscopy and photography at 7 days.
Statistical analysis
Data were analyzed with Prism 5 (GraphPad Software, San Diego, CA, USA). One way analysis of variance (ANOVA) followed by the Tukey-Kramer test for post hoc comparisons. The difference was considered significant when P<0.05. All data are shown as mean ± standard deviation (SD).
Results
Li-ESWT improves urethral LPP
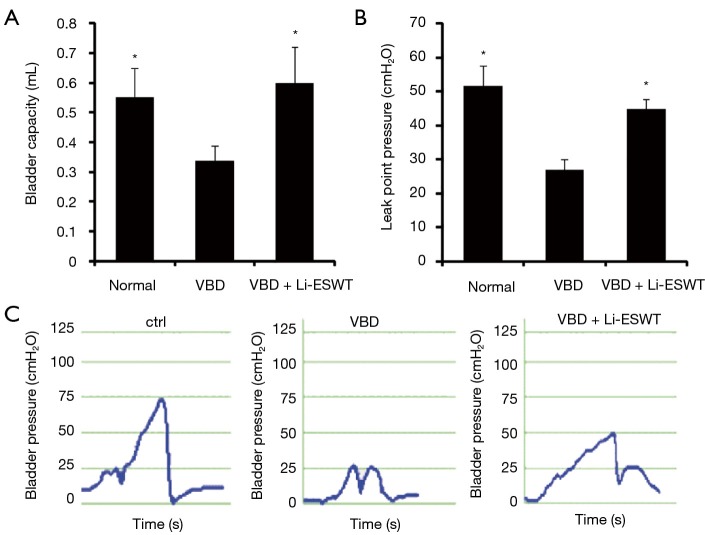
At 5 weeks following VBD, rats underwent functional evaluations with bladder capacity measurement and LPP analysis. Average bladder capacity across groups is shown in Figure 1A. The rats that underwent VBD had a decreased average bladder capacity compared to normal controls. In the group of VBD with Li-ESWT, bladder capacity trended towards being greater (improved) than that of the VBD group, though the difference is not statistically significant (0.6±0.10 versus 0.3±0.05 mL, P=0.15).
Figure 1.
Functional parameters. (A) Average bladder capacity (mL) (*, P=0.15 compared to VBD); (B) average LPP (cmH2O) (**, P<0.01 compared to VBD). There is no significant difference of average LPPs between the groups of VBD with Li-ESWT and normal controls (44.8±3.2 versus 51.5±6 cmH2O, P=0.51); (C) representative LPP curves. VBD, vaginal balloon dilation; LPP, leak-point pressure; Li-ESWT, low-intensity extracorporeal shock wave therapy.
Average LPPs across groups is shown in Figure 1B. The average LPPs for rats receiving VBD was significantly deceased compared to normal controls (27.0±2.9 versus 51.5±6 cmH2O, P<0.01). In the group of Li-ESWT, the average LPPs were significantly improved compared to those of VBD only (44.8±3.2 versus 27.0±2.9 cmH2O, P<0.01). Characteristic LPP curves are shown in Figure 1C.
Li-ESWT increased EdU + progenitor cells in the urethra
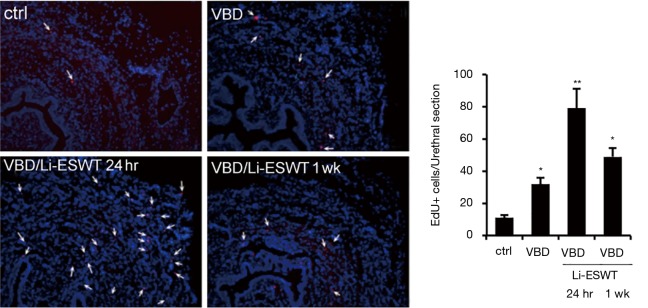
To track the endogenous progenitor cells, we modified the label-retaining cell (LRC) method by replacing 5-bromo-2-deoxyuridine (BrdU) with EdU (32,33), which is much easier to detect and the resulting signal is much more reliable (34). EdU was intraperitoneally injected into newborn rats, and VBD was performed when the rats were 3 months old, Li-ESWT were applied 1 week after VBD. Analysis of the urethras at 24 hours and 1 week after the last Li-ESWT therapy showed significantly increased EdU+ endogenous progenitor cells in the muscle layer, particularly the striated muscle (Figure 2).
Figure 2.
Li-ESWT increased recruitment of EdU LRCs. Rats with EdU-labeled LRCs were divided into control (ctrl), VBD, and VBD + Li-ESWT. The ctrl, VBD, and VBD + Li-ESWT rats were sacrificed 24 hours and 1 week later. Their urethras were stained for EdU (red) and DAPI (blue) (×100). *, P<0.05 vs. ctrl; **, P<0.05 vs. VBD and ctrl. LRC, label-retaining cell; VBD, vaginal balloon dilation; LPP, leak-point pressure; Li-ESWT, low-intensity extracorporeal shock wave therapy; EdU, 5-ethynyl-2-deoxyuridine; DAPI, 4',6-diamidino-2-phenylindole.
Li-ESWT increases urethral angiogenesis by promoting VEGF expression
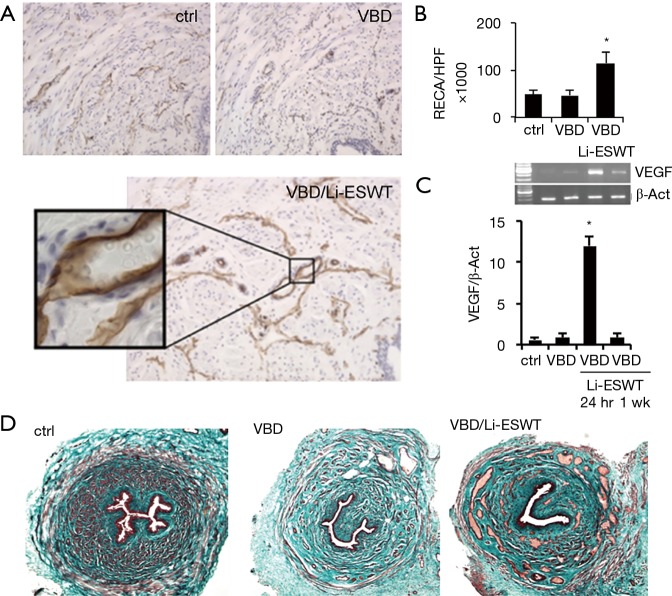
With Masson’s trichrome staining, it was found that rats treated with Li-ESWT after VBD consistently had an increase in venous channels involving all layers of the urethra compared to VBD only and normal control groups (Figure 3A,B). Expressions of VEGF in urethral wall were checked with RT-PCR. Compared to the 2 groups of normal control and VBD only, the Li-ESWT treated rats, sacrificed at 24 hours, showed significantly increased expression of VEGF. However, the VEGF expression decreased by 1 week after Li-ESWT. This suggests that in the first 24 hours after Li-ESWT, VEGF is up-regulated, and that this effect diminishes to baseline within 1 week after Li-ESWT (Figure 3C). Those results were further confirmed With Masson’s trichrome staining (Figure 3D).
Figure 3.
Urethral angiogenesis affected by Li-ESWT. (A,B) Expression of RECA were significantly increased in the VBD + Li-ESWT group (*, P<0.01) (×100); (C) RT-PCR of rat urethras shows increased VEGF expression at 24 hours following Li-ESWT (*, P<0.01); (D) increased venous channels seen on trichrome staining in the VBD + Li-ESWT group (×40).
Li-ESWT evokes urethral muscle regeneration in vivo
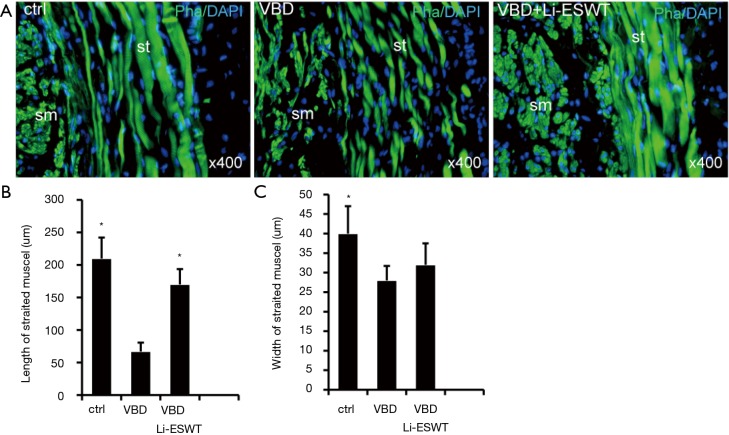
Urethral staining with phalloidin revealed that the striated muscle layer was attenuated in rats receiving VBD only, appearing thinner and less well organized. These impaired muscle layers showed improvement in the group of Li-ESWT after VBD compared to the VBD only group, characterized by longer and wider myofibers (Figure 4) (*P<0.05), suggesting muscle regeneration associated with Li-ESWT.
Figure 4.
Li-ESWT promoted urethral sphincter regeneration. The urethral striated muscle fibers (A), as measured by length (B) and width (C) (*, P<0.05 vs. VBD). Both smooth (sm) and striated (st) muscles stained green with phalloidin while cell nuclei stained blue with DAPI. VBD, vaginal balloon dilation; Li-ESWT, low-intensity extracorporeal shock wave therapy; DAPI, 4',6-diamidino-2-phenylindole.
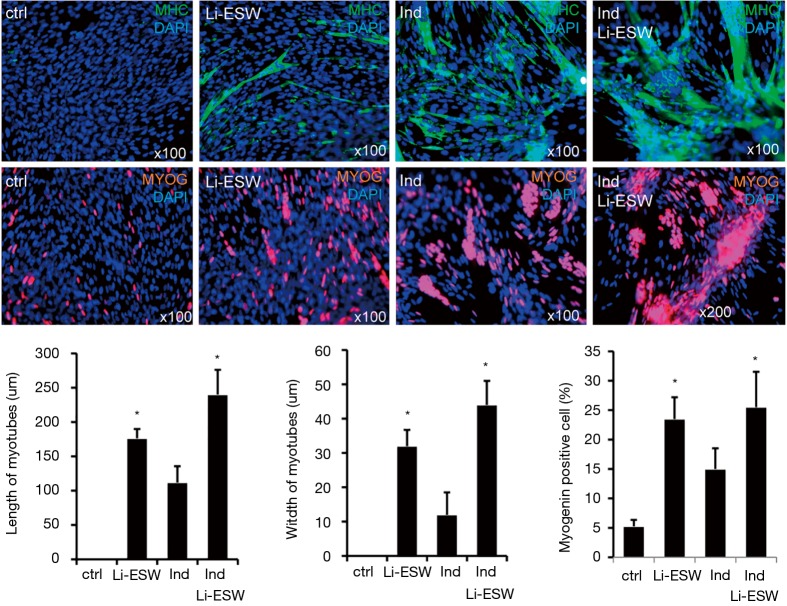
Li-ESW promotes myotube formation from rat myoblast L6 in vitro
To further test our hypothesis that Li-ESW can induce muscle regeneration, we checked the effect of Li-ESW on rat myoblast myotube formation in vitro. In medium supplemented with 15% FBS, these L6 cells could be propagated without differentiation and myotube formation. However, in medium containing 2% horse serum (induction medium), the formed myotubes stained positive for MHC and myogenin. Importantly, when treated with Li-ESWT, the induced myotubes grew larger and more robust (Figure 5), suggesting satellite cell myogenic differentiation as a possible mechanism for the therapeutic effects of Li-ESWT.
Figure 5.
Li-ESW induces myodifferentiation. Urethral satellite cells were untreated (ctrl), treated with 2% horse serum (Ind), Li-ESW, or Ind + Li-ESW. Seven days later, the cells were stained for MHC (green) and myogenin (red), and the myotubes’ length and width determined. *, P<0.05 vs. ctrl & Ind. Li-ESW, low-intensity extracorporeal shock wave; MHC, Myosin heavy chain.
Discussion
Anatomically, the urethral supportive and sphincteric system are critical to maintain urinary continence (35). Proper sphincteric function is dependent mostly on the urethral striated muscles, but the smooth muscle, mucosa and vascular elements also play a significant role. Urethral sphincter deficiency is the primary culprit in female SUI (36). We and others have confirmed that smooth muscle distributes throughout the entire length of the urethra, while the striated muscle is confined to the proximal half of the structure similar to the urethra in women (1,37,38). As seen in this study and prior study of rat models of SUI after VBD, there is significant diminution in the quality and completeness of urethral striated muscle fibers following VBD (39). Tissue hypoxia may be, in part, the etiology of this breakdown in striated muscle fibers (40). The findings of increased vascularity in our Li-ESWT treated group may thus lead to a reduction in tissue hypoxia, and may mitigate urethral damage.
In 1998, we established an SUI rat model that mimics closely the female human pathology of SUI (41). This model, best known as VBD, has since been the most widely used SUI model (42). It has been confirmed that in this SUI animal model, both striated and smooth muscles are significantly reduced. Treatment of such models with SCs partially can restore both striated and smooth muscles, but the underlying mechanism remains unknown (8,39,41,43).
In the past decade, despite significant advances in the management of SUI, there are still no therapeutic options capable of reversing the pathophysiologic deficit in the urethral sphincter. The potential of Li-ESWT for the treatment of various urological diseases has been reported in recent years (13,14). As far as we know, this report is the first to investigate the therapeutic effects of Li-ESWT in SUI by using a well-established VBD-induced rat model. Our results showed that Li-ESWT significantly improved LPP in VBD-induced SUI rats. More importantly, we demonstrated that Li-ESWT significantly promoted angiogenesis and progenitor cell activation in urethra.
Although our functional and histological results demonstrated the therapeutic effects of Li-ESWT in SUI, the mechanism of Li-ESWT in improving urethral function is not understood. As seen in this study and prior study of rat models of SUI via VBD, there is significant diminution in the shape and size of urethral striated muscle fibers following VBD (39). These altered striated muscle fibers appear to have recovered partially following Li-ESWT in the current study. In this VBD induced SUI model, tissue hypoxia has been proposed to be, in part, the cause of breakdown in striated muscle fibers. Multiple prior studies with Li-ESWT in other organ systems have shown benefit through increased angiogenesis (22-24). Our findings of increased vascularity in Li-ESWT treated group might lead to improved tissue oxygenation and thus mitigate urethral damages. Based on these findings, increased vasculature in the urethra may represent one of the therapeutic mechanisms of Li-ESWT in the SUI rat model.
The involvement of progenitor cells in the therapeutic effects of Li-ESWT has been observed in previous studies using rat models of chronic hind limb ischemia and bone defects. Li-ESWT was found to enhance recruitment of endothelial progenitor cells in the ischemic tissue, and also to result in the recruitment of mesenchymal SCs with increased expression of VEGF in the defect tissues. In the current result, we found Li-ESWT also increased the number of progenitor cells in urethra likely due to activation of local progenitor cells as shown in our studies in the penis (17). Our data provide evidence for both theories, promotion of angiogenesis and in situ activation of progenitor cells. The former is supported by our finding of increased VEGF expression during the first 24 hours after Li-ESWT, and by our findings of increased vasculature within the Li-ESWT treated rats. The latter is supported by the increase in progenitor cells (labeled with EdU) found in the urethras of the rats sacrificed within 24 hours after Li-ESWT. Thus, it is conceivable that the tissue effects of Li-ESWT on urethra as observed in the present study might have a progenitor cell activation component.
Another significant effect from Li-ESWT on urethra is the regeneration of sphincter, especially urethral striated muscles. This in vivo effect was further confirmed by tube formation form rat myoblast L6 cells in vitro. Li-ESWT enhanced the formation of myotube induced by 2% HS in vitro, and, more impressively, these myotubes are wider and stronger compared to induction only. Thus, the underlying mechanism for how Li-ESWT effects SUI is likely related to recovery and repair of urethral sphincteric tissues.
In summary, the present study shows that Li-ESWT was able to significantly restore urethral closure function for in a VBD rat model of SUI. Furthermore, we also showed that these beneficial effects of Li-ESWT were possibly mediated by increased angiogenesis and activation of progenitor cells and sphincter regeneration in urethra. However, this study represents a preliminary study and requires further validation. As such, there are several limitations of this study. First, given that we do not fully understand the mechanism of action or its time course, the Li-ESWT protocol likely requires future adjustment and optimization. Second, the effect of Li-ESWT on the vascular supply to the urethra should be further investigated; and Western blot analysis should be employed to more accurately quantify the endothelial and smooth muscle contents. Third, relatively small numbers of rats, particularly in the set used for EdU and RT-PCR analysis, may limit the significance of our findings.
Conclusions
Li-ESWT can ameliorate SUI by promoting angiogenesis, progenitor cells activation, and sphincter regeneration of urethra in a rat model induced by VBD, which represents a potential novel non-invasive therapy for SUI in a preclinical setting. To improve our understandings of Li-ESWT therapeutic mechanism and feasibility, more study is needed.
Acknowledgements
Funding: Research reported in this publication was supported by NIDDK of the National Institutes of Health under award number R01 DK069655, R56DK105097 and R01DK105097. Opinions, interpretations, conclusions and recommendations are those of the author and do not necessarily represent the official views of the National Institutes of Health.
Ethical Statement: All experimental protocols were approved by the Institutional Animal Care and Use Committee at our institution (approval ID No.: AN109665).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Zhang X, Alwaal A, Lin G, et al. Urethral musculature and innervation in the female rat. Neurourol Urodyn 2016;35:382-9. 10.1002/nau.22722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong EC, Khan AA, Anger JT. The financial burden of stress urinary incontinence among women in the United States. Curr Urol Rep 2011;12:358-62. 10.1007/s11934-011-0209-x [DOI] [PubMed] [Google Scholar]
- 3.Chermansky CJ, Winters JC. Complications of vaginal mesh surgery. Curr Opin Urol 2012;22:287-91. 10.1097/MOU.0b013e32835480b2 [DOI] [PubMed] [Google Scholar]
- 4.Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2012;2:CD003881. [DOI] [PubMed] [Google Scholar]
- 5.Shamliyan TA, Kane RL, Wyman J, et al. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med 2008;148:459-73. 10.7326/0003-4819-148-6-200803180-00211 [DOI] [PubMed] [Google Scholar]
- 6.Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: a critical review. Stem Cells Dev 2012;21:834-43. 10.1089/scd.2011.0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yiou R, Dreyfus P, Chopin DK, et al. Muscle precursor cell autografting in a murine model of urethral sphincter injury. BJU Int 2002;89:298-302. 10.1046/j.1464-4096.2001.01618.x [DOI] [PubMed] [Google Scholar]
- 8.Lin G, Wang G, Banie L, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy 2010;12:88-95. 10.3109/14653240903350265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thaker H, Sharma AK. Regenerative medicine based applications to combat stress urinary incontinence. World J Stem Cells 2013;5:112-23. 10.4252/wjsc.v5.i4.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zissler A, Steinbacher P, Zimmermann R, et al. Extracorporeal Shock Wave Therapy Accelerates Regeneration After Acute Skeletal Muscle Injury. Am J Sports Med 2017;45:676-84. 10.1177/0363546516668622 [DOI] [PubMed] [Google Scholar]
- 11.Aicher A, Heeschen C, Sasaki K, et al. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 2006;114:2823-30. 10.1161/CIRCULATIONAHA.106.628623 [DOI] [PubMed] [Google Scholar]
- 12.Tepeköylü C, Wang FS, Kozaryn R, et al. Shock wave treatment induces angiogenesis and mobilizes endogenous CD31/CD34-positive endothelial cells in a hindlimb ischemia model: implications for angiogenesis and vasculogenesis. J Thorac Cardiovasc Surg 2013;146:971-8. 10.1016/j.jtcvs.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 13.Li H, Matheu MP, Sun F, et al. Low-energy Shock Wave Therapy Ameliorates Erectile Dysfunction in a Pelvic Neurovascular Injuries Rat Model. J Sex Med 2016;13:22-32. 10.1016/j.jsxm.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Xu L, Zhao Y, et al. Endogenous Stem Cells Were Recruited by Defocused Low-Energy Shock Wave in Treating Diabetic Bladder Dysfunction. Stem Cell Rev 2017;13:287-98. 10.1007/s12015-016-9705-1 [DOI] [PubMed] [Google Scholar]
- 15.Gruenwald I, Appel B, Vardi Y. Low-intensity extracorporeal shock wave therapy--a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med 2012;9:259-64. 10.1111/j.1743-6109.2011.02498.x [DOI] [PubMed] [Google Scholar]
- 16.Vardi Y, Appel B, Kilchevsky A, et al. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol 2012;187:1769-75. 10.1016/j.juro.2011.12.117 [DOI] [PubMed] [Google Scholar]
- 17.Lin G, Reed-Maldonado AB, Wang B, et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J Sex Med 2017;14:493-501. 10.1016/j.jsxm.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 18.Lu Z, Lin G, Reed-Maldonado A, et al. Low-intensity Extracorporeal Shock Wave Treatment Improves Erectile Function: A Systematic Review and Meta-analysis. Eur Urol 2017;71:223-33. 10.1016/j.eururo.2016.05.050 [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Zhou J, Banie L, et al. Low-intensity extracorporeal shock wave therapy promotes myogenesis through PERK/ATF4 pathway. Neurourol Urodyn 2017. [Epub ahead of print]. 10.1002/nau.23380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito K, Fukumoto Y, Shimokawa H. Extracorporeal shock wave therapy for ischemic cardiovascular disorders. Am J Cardiovasc Drugs 2011;11:295-302. 10.2165/11592760-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 21.Mense S, Hoheisel U. Shock wave treatment improves nerve regeneration in the rat. Muscle Nerve 2013;47:702-10. 10.1002/mus.23631 [DOI] [PubMed] [Google Scholar]
- 22.Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 2004;110:3055-61. 10.1161/01.CIR.0000148849.51177.97 [DOI] [PubMed] [Google Scholar]
- 23.Meirer R, Brunner A, Deibl M, et al. Shock wave therapy reduces necrotic flap zones and induces VEGF expression in animal epigastric skin flap model. J Reconstr Microsurg 2007;23:231-6. 10.1055/s-2007-981506 [DOI] [PubMed] [Google Scholar]
- 24.Stojadinovic A, Elster EA, Anam K, et al. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis 2008;11:369-80. 10.1007/s10456-008-9120-6 [DOI] [PubMed] [Google Scholar]
- 25.Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 2013;10:738-46. 10.1111/jsm.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sievert KD, Emre Bakircioglu M, Tsai T, et al. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol 2001;166:311-7. 10.1016/S0022-5347(05)66151-4 [DOI] [PubMed] [Google Scholar]
- 27.Resplande J, Gholami SS, Graziottin TM, et al. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol 2002;168:323-30. 10.1016/S0022-5347(05)64915-4 [DOI] [PubMed] [Google Scholar]
- 28.Bella AJ, Lin G, Garcia MM, et al. Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signalling pathway in the major pelvic ganglion of the rat after cavernous nerve transection. Eur Urol 2007;52:574-80. 10.1016/j.eururo.2006.10.043 [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Lin G, Lee YC, et al. Transgenic animal model for studying the mechanism of obesity-associated stress urinary incontinence. BJU Int 2017;119:317-24. 10.1111/bju.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin G, Garcia M, Ning H, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 2008;17:1053-63. 10.1089/scd.2008.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin G, Qiu X, Fandel TM, et al. Improved penile histology by phalloidin stain: circular and longitudinal cavernous smooth muscles, dual-endothelium arteries, and erectile dysfunction-associated changes. Urology 2011;78:970. e1-8. [DOI] [PMC free article] [PubMed]
- 32.Lin G, Huang YC, Shindel AW, et al. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy 2009;11:864-73. 10.3109/14653240903180084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Lin G, Qiu X, et al. Label retaining and stem cell marker expression in the developing rat urinary bladder. Urology 2012;79:746.e1-6. 10.1016/j.urology.2011.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CS, Xin ZC, Dai J, et al. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol Histopathol 2013;28:1109-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashton-Miller JA, Howard D, DeLancey JO. The functional anatomy of the female pelvic floor and stress continence control system. Scand J Urol Nephrol Suppl 2001;(207):1-7; discussion 106-25. 10.1080/003655901750174773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YC, Lin G, Wang G, et al. Impaired contractility of the circular striated urethral sphincter muscle may contribute to stress urinary incontinence in femal zucker fatty rats. Neurourol Urodyn 2017;36:1503-10. 10.1002/nau.23165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim RJ, Kerns JM, Liu S, et al. Striated muscle and nerve fascicle distribution in the female rat urethral sphincter. Anat Rec (Hoboken) 2007;290:145-54. 10.1002/ar.20420 [DOI] [PubMed] [Google Scholar]
- 38.Lim SH, Wang TJ, Tseng GF, et al. The distribution of muscles fibers and their types in the female rat urethra: cytoarchitecture and three-dimensional reconstruction. Anat Rec (Hoboken) 2013;296:1640-9. 10.1002/ar.22740 [DOI] [PubMed] [Google Scholar]
- 39.Cannon TW, Wojcik EM, Ferguson CL, et al. Effects of vaginal distension on urethral anatomy and function. BJU Int 2002;90:403-7. 10.1046/j.1464-410X.2002.02918.x [DOI] [PubMed] [Google Scholar]
- 40.Damaser MS, Whitbeck C, Chichester P, et al. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol 2005;98:1884-90. 10.1152/japplphysiol.01071.2004 [DOI] [PubMed] [Google Scholar]
- 41.Lin AS, Carrier S, Morgan DM, et al. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology 1998;52:143-51. 10.1016/S0090-4295(98)00136-8 [DOI] [PubMed] [Google Scholar]
- 42.Jiang HH, Damaser MS. Animal models of stress urinary incontinence. Handb Exp Pharmacol 2011;(202):45-67. 10.1007/978-3-642-16499-6_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badra S, Andersson KE, Dean A, et al. A nonhuman primate model of stable urinary sphincter deficiency. J Urol 2013;189:1967-74. 10.1016/j.juro.2012.09.103 [DOI] [PubMed] [Google Scholar]