Highlights
-
•
Phytocompounds detection from the aqueous and methanolic extract of herbal remedy triphala and its constituting plants.
-
•
Antioxidant potential of triphala and its constituting plants.
-
•
Triphala and its constituting plants as potential antibacterial agents.
Keywords: Phytochemicals, Antioxidant, Triphala, Ayurveda, Antibacterial
Abstract
Triphala, an Indian ayurvedic triherbal formulation, is an equiproportional mixture of fruits of three herbs, amalaki (Emblica officinalis), haritaki (Terminalia chebula) and bibhitaki (Terminalia bellerica). The present study focused on phytocompounds detection and comparative analysis of various biochemical activities in the aqueous and methanolic extracts of triphala and its constituting herbs. Antioxidant activity was determined by 1, 1-diphenyl-2-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), super oxide dismutase (SOD), catalase assay. Antibacterial potential was determined by broth dilution and agar well diffusion assays.
Results revealed the presence of valuable bioactive compounds such as flavonoids, alkaloids, phenols, etc which might be responsible for biochemical activities. Extracts exhibited satisfactory radical-scavenging activity comparable with ascorbic acid. Methanolic extracts demonstrated higher antioxidant activity compared to aqueous extract. Extracts showed promising antibacterial potential against tested strain comparable to ampicillin. Hence, it can be concluded that triphala may be a promising candidate in pharmaceuticals and future medicine.
1. Introduction
With the advancement in human livings effected by technology and such innovations, they say our disease-causing organism have also developed themselves. This is currently the most focused aspect of humans, the new or improved ways of defence and cure from the life-threatening disease. The present chemical based pharmaceutical is looking new hopes in traditional or natural origin medicines which would be efficiently effective against disease causing microorganism which are now said to immune themselves from the chemical based medicine available in the market.
Nature has all the solutions to mankind problems; such is the natural or ayurvedic medicine derived from the plants, animals or marine. Ayurveda is one known traditional Indian medicinal system being practiced for thousands of years. Many natural products such as plants, animals and minerals have been used for the treatment of human diseases [32]. Therapeutic materials have been isolated form variety of plants of therapeutic use. The modern pharmaceutical industry has also realised the importance of Ayurveda and hence now number of drugs is being introduced using these natural products for making effective drugs against many diseases [37,35]. History of medicine dates back practically to the existence of human civilization. The current accepted modern medicine or allopathy has gradually developed over the years by scientific and observational efforts of scientists [9].
Nowadays, the use of natural formulations as medicine is gaining more popularity. Infact, several natural formulations which make use of herbal extracts have been found to be safer medicines with minimum side effects when compared to chemical drugs. According to the Bulletin of the World Health Organization (WHO), around 65% of the world’s population relied on medicinal plants as their primary healthcare source [8]. Also, it has been estimated that about 50% of the medicines developed since1980 have been natural products, their derivatives, or their analogs [30,46]. Further, it has been predicted that approximately 25% of the currently used modern medicines are derived from plants [46]. Amongst them, analgesics (morphine), cardiotonics (digoxin), anticancer drugs (paclitaxel and the vinca alkaloids) and the antimalarials (quinine and artemisinin) are note worthy [34].
Triphala is a familiar ayurvedic formulation, commonly prescribed by most healthcare practitioners in India. It is an equiproportional mixture of fruits of three medicinal herbs, amalaki (Emblica officinalis), haritaki (Terminalia chebula) and bibhitaki (Terminalia bellerica), also known as the ‘three myrobalans’ [49,50]. It exhibits anti-viral, anti-bacterial, anti-fungal and anti-allergic properties. Triphala and its constituents act as cardio-tonic, control blood pressure, improve blood circulation and reduce cholesterol levels. Triphala shows immunomodulatory properties and helps in improving the body’s defence system [17,22,23,25,26]. Triphala formulation is rich in antioxidants, is a frequently used ayurvedic medicine to treat many diseases such as anaemia, jaundice, constipation, asthma, fever and chronic ulcers [40,[42], [43], [44]. Triphala is traditionally been used as laxative in chronic constipation, colon cleansing, digestion problems and poor food assimilation [43,44]. It has also been used in cardiovascular disease, high blood pressure disease, serum cholesterol reduction, poor liver function, large intestine inflammation, and ulcerative colitis [42]. In recent years there are also several reports in the literature which suggest that triphala possesses antimutagentic, radioprotecting and antioxidant activity [3,[13], [14], [15],19,24,29,45].
With these multiple medicinal properties of triphala known, it has been contemplated to concentrate our studies on phytochemical studies of this rarely explored formulation and to investigate its medicinal properties. Accordingly, in the present study, the phytochemicals of herbal plant triphala and its constituents was extracted with various solvents (water and methanol) and these extracts were studied for their in vitro antimicrobial and antioxidant properties. Their antimicrobial activities were determined using broth dilution assay and agar well diffusion assay and the antioxidant activity was evaluated by 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, Ferric Reducing Antioxidant power (FRAP), Super Oxide Dismutase (SOD), and catalase assays.
2. Material and methodology
2.1. Plant materials
Fruits of Terminalia chebula Retz (Combretaceae), Terminalia belerica Linn. (Combretaceae) and Emblica officinalis Gaertn. (Euphorbiaceae), were purchased from ayurvedic clinic. Seeds from individual fruits were removed and the dried fruit pulp was crushed to powder using an electric blender. Triphala was prepared from these powders by mixing them in equal proportions (1:1:1) based on formula of Ayurvedic Formulary of India, to give in-house sample. These powders were stored in a closed vessel for future use.
2.2. Chemicals and reagents
The chemicals used for the study were all analytical grade. All solvents and chemicals (analytical grade) used for phytochemical screening, antioxidant, anti-inflammatory and antibacterial assay were purchased from Merck, SRL, Himedia, India. DPPH and TPTZ were purchased from Sigma-Aldrich, India.
2.3. Test microorganisms
The tested bacterial strains were identified and obtained from NCCS, Pune, India. These included Staphylococcus aureus (MTCC 902), Escherichia coli (MTCC 443), Bacillus subtilis (MTCC 736) and Pseudomonas aeruginosa (MTCC 2453). The bacterial stock was maintained at 4 °C on nutrient agar slants.
2.4. Preparation of triphala extracts
The fruits of triphala (equiproportional mixture of three herbs) and its constituent plants namely amalaki (Emblica officinalis), bibhitaki (Terminalia bellerica) and haritaki (Terminalia chebula) were taken and ground to get their powder form. The powdered triphala and its constituents were dissolved in the solvents i.e. distilled water and methanol to get aqueous extracts and methanolic extracts respectively. The plant extracts were soaked overnight and then were centrifuged at 8000 rpm for 15 min. The supernatant was collected, filtered with 0.45 μm membrane filter and used for the further activities. The aqueous extract for triphala and its constituents were termed as TAE (triphala aqueous extract), BAE (bibhitaki aqueous extract), HAE (haritaki aqueous extract), AAE (amalaki aqueous extract) and the methanolic extract were termed as TME (triphala methanolic extract), BME (bibhitaki methanolic extract), HME (haritaki methanolic extract), AME (amalaki methanolic extract extract).
2.5. Phytochemical screening
Freshly prepared extracts of triphala and its constituents were subjected to the standard methods of phytochemical analysis [12,21] to detect the presence of various valuable phytoconstituents, viz. flavanoids, alkaloids, xanthoproteins, carboxylic acid, carbohydrates, glycosides, saponins, tannins, proteins etc.
2.6. Determination of antibacterial activity
2.6.1. Broth dilution method
Antimicrobial activity of the aqueous and methanolic extract was tested against two gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and two gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) by the method of Shamsi et al. [38]. Overnight cultures were prepared in Luria broth (LB) media by inoculation with a single colony from agar plates and incubated at 37 °C for 12 h. Overnight cultures were diluted with fresh LB media to approximately 104 colony forming units (CFU) and incubated at 37 °C for 12–14 h in the presence of triphala and its constituting plants extract compared to the growth of the control culture where only media and bacterial inoculum was taken. The experiment was repeated twice for the confirmation. The percentage mean growth inhibition (%MGI) was calculated by using the formula:
| % MGI = [(dc − dt)/dc] x 100, |
2.6.2. Agar well diffusion method
In vitro antibacterial screening of aqueous and methanolic extracts of triphala and its constituents against four bacterial strains was performed by Agar well-diffusion method by the method of Singh et al. [39]. The log phase bacterial cultures (secondary culture) was spread on potato dextrose agar medium plates using a sterile spreader in order to get a uniform bacterial growth on test plates. Wells were punched over the agar plates using sterile cork borer. About 10–20 μl of each extract was added using sterile syringe into the wells and allowed to diffuse at room temperature for 2 h. Ampicillin (50 mg/ml) was used as standard antibacterial drug. The plates were then incubated at 37 °C for 18–24 h. The diameter of the inhibition zone (mm) was measured. The results (zone of inhibition) were compared with the activity of the standards, ampicillin (50 mg/ml).
2.7. Determination of anti-inflammatory activity
2.7.1. Inhibition of albumin denaturation
The anti-inflammatory assay was done using method of Mizushima et al. with minor changes [27]. A range of diluted working solutions of the triphala and its constituents were prepared and mixed with 1% aqueous solution of bovine serum albumin (BSA) fraction. The samples were incubated at 37 °C for 20 min and then heated at 57 °C for 20 min. 1% BSA was taken as control and Tris buffer was taken as blank. Aspirin (100 μg/ml) was taken as standard drug. After cooling the samples, the turbidity was measured spectrophotometrically at 660 nm. Percent inhibition of protein denaturation was calculated using formula:
| Percentage inhibition = (Abscontrol − Abssample) X 100/Abscontrol |
2.7.2. Proteinase inhibitory action
The test was performed according to the modified method of Oyedepo et al. [31]. The reaction mixture (2 ml) was containing 0.06 mg trypsin, 1 ml 20 mM Tris HCl buffer (pH 7.4) and 1 ml test sample of different concentrations. The mixture was incubated at 37 °C for 5 min and then 1 ml of 0.8% (w/v) casein was added. The mixture was incubated for an additional 20 min. 2 ml of 70% perchloric acid was added to terminate the reaction. Cloudy suspension was centrifuged and the absorbance of the supernatant was read at 210 nm against buffer as blank. The experiment was performed in triplicate. The percentage inhibition of proteinase inhibitory activity was calculated.
| Percentage proteinase inhibition = (Abscontrol − Abssample) X 100/Abscontrol |
2.8. Determination of antioxidant activity
2.8.1. Catalase (CAT) assay
This assay was performed to assess the antioxidant potential of extracts. The assay is based on the reaction of CAT with methanol in the presence of hydrogen peroxide, producing formaldehyde which is measured colorimetrically using 4-amino-3-hydrazino-5- mercapto-1,2,4-triazole (purpald) as the chromogen. Purpald forms a bicyclic heterocycle with aldehydes, which upon oxidation changes from colorless to a purple color. CAT activity in each sample was expressed in nmol/min/ml. One unit is defined as the amount of enzyme that caused the formation of 1.0 nmol of formaldehyde per minute at 25 °C [16].
2.8.2. DPPH assay
The antioxidant activity of triphala, its constituents and the standard (ascorbic acid) were checked on the basis of the free radical scavenging effect of the stable DPPH by the method of Braca et al. with minor modifications [5]. A range of diluted working solutions of the Triphala and its constituent plants were prepared in distilled water and methanol respectively. Ascorbic acid (1 mg/ml) in distilled water was used as standard. 0.1 mM DPPH was prepared in 80% methanol and 500 μl of this solution was mixed with 500 μl of working sample solutions and standard solution separately. These solution mixtures were kept in dark for 30 min and optical density was measured at 517 nm using spectrophotometer. 0.1 mM DPPH solution was used as control. The range of diluted aqueous and methanolic extracts was taken as blank. The optical density was recorded and DPPH scavenging was calculated using the formula given below:
| DPPH scavenging Activity (%) = [(dc − dt)/dc] x 100, |
Where dc and dt represent the absorbance at 517 nm of control and test sample respectively.
2.8.3. FRAP assay
Antioxidant activity assay was also done following the ferric-reducing antioxidant power (FRAP) method described by Benzie & Strain with minor modifications [4]. FRAP reagents was freshly prepared by mixing 10 ml acetate buffer (300 mM, pH 3.6), 1 ml 2,4,6-tris (2-pyridyl)-S-triazine (TPTZ) solution (10 mM TPTZ in 40 mM/L HCl) and 1 ml FeCl3 (20 mM) water solution. A range of diluted working solutions of the Triphala and its constituent plants were prepared in distilled water and methanol. Each sample (200 μl) was added in 1.5 ml of freshly prepared FRAP reagent and mixed and after 5 min, absorbance was measured at 593 nm, using FRAP working solution as blank. Ascorbic acid was used as standard. The results were expressed in mM Fe2+/ml of aqueous and methanolic extract. Higher absorbance indicates higher reducing power.
2.8.4. Superoxide dismutase (SOD) assay
SOD assay was performed according to the method of Kakkar et al. with some modifications [18]. The assay mixture contained sodium pyrophosphate buffer, phenazine methosulfate (PMS), nitro blue tetrazolium chloride (NBT), aqueous and methanolic extracts of triphala and its constituents and distilled water. The reaction was initiated by the addition of nicotinamide adenine dinucleotide (NADH). The mixture was incubated at 30 °C for 90 s and arrested by the addition of glacial acetic acid. The reaction mixture was then shaken with n-butanol, allowed to stand for 10 min and centrifuged. The assay of SOD is based on the inhibition of the formation of NADH-phenazine methosulphate-nitroblue tetrazolium formazon. The colour formed at the end of the reaction can be extracted into butanol and measured at 560 nm in a spectrophotometer. One unit of enzyme activity is defined as the amount of extract that gave 50% inhibition of NBT reduction in one minute.
2.9. Determination of antifungal activity
The minimum inhibitory concentration of Triphala aqueous and methanolic extract against Candida cells (ATCC 10261 and ATCC 90028) was determined by broth dilution by the method as described by the Clinical and Laboratory Standards Institute (CLSI). Cultures were grown with or without test compounds in the media. Two fold dilutions of the test compound were carried out as an initial step. Also, antifungal activity of aqueous and methanolic extract in solid media was determined by the Disc Diffusion Method. Candida cells (105cells/ml) were inoculated in molten yeast extract peptone dextrose (YEPD) agar (∼40 °C) and poured into a petri plates. Filter discs were placed on solid agar and different concentrations of test compounds were applied on the disc in 10 μl volume. The average diameter of the zone of inhibition was recorded in millimeters. The experiment was performed in triplicates.
2.10. Statistical analysis
The experiments were done in triplicates. Results were expressed as graphs representing Mean ± SEM (Standard Error of Mean) using the software Graph Pad Prism 5.0.
3. Results
3.1. Phytochemical analysis
The presence of various phytochemicals was analysed qualitatively using various standard methods based on chromogenic reactions. Both the aqueous and methanolic extract showed the presence of various important phytocompounds viz. alkaloids, flavonoids, phenols, sterols, resins, quinines, xanthoproteins and terpenoids. However, both the extracts depicted absence of steroids, saponins, tannins, glycoside and carboxylic acid. The methanolic extract gave highly intense chromogenic reaction as compared to the aqueous extract which suggested that methanol solvent extracted more phytochemicals in the extract than the water solvent. The various results obtained after phytochemical analysis of aqueous and methanolic extracts of triphala and its constituting plants were shown in Table 1.
Table 1.
Tabular representation of the phytocompounds detected in the aqueous and methanolic extracts of triphala and its constituting plants.
| Plants | Triphala | Emblica officinalis | Terminalia bellerica | Terminalia chebula | ||||
|---|---|---|---|---|---|---|---|---|
| Tests | TAE | TME | AAE | AME | BAE | BME | HAE | HME |
| Test For Alkaloids | + | ++ | + | ++ | + | ++ | + | ++ |
| Test For Flavonoids | + | ++ | + | ++ | + | ++ | + | ++ |
| Test For Steroids | − | − | − | − | − | − | − | − |
| Test For Saponins | − | − | − | − | − | − | − | − |
| Test for Phenols | + | ++ | + | ++ | + | ++ | + | ++ |
| Test for Tannins | − | − | − | − | − | − | − | − |
| Test for Glycoside | − | − | − | − | − | − | − | − |
| Test for Carboxylic Acid | − | − | − | − | − | − | − | − |
| Test for Sterols | + | ++ | + | ++ | + | ++ | + | ++ |
| Test for Resins | + | ++ | + | ++ | + | ++ | + | ++ |
| Test for Quinines | + | ++ | + | ++ | + | ++ | + | ++ |
| Test-for Xanthoproteins | + | ++ | + | ++ | + | ++ | + | ++ |
| Test for Terpenoids | + | ++ | + | ++ | + | ++ | + | ++ |
3.2. Antibacterial assay
3.2.1. Broth dilution method
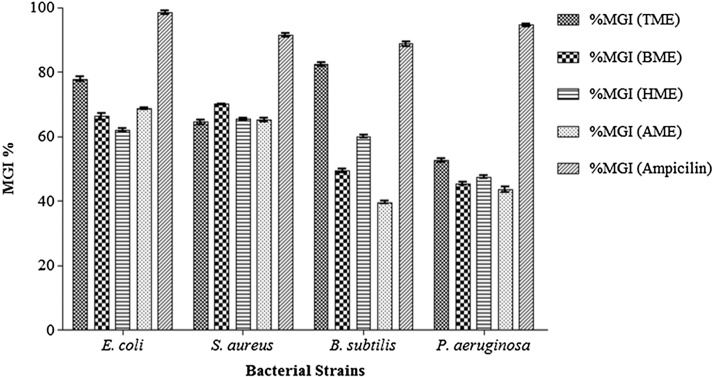
Antibacterial assay of the aqueous and methanolic extract was examined against various bacterial strains by accessing the%MGI in presence of triphala and its constituting herbs. Results obtained were compared to the ampicillin (standard antibiotic). Positive control was taken where only media and bacterial cultures were added. The results suggested that triphala exhibits bactericidal property in-vitro i.e. the growth of microorganisms was inhibited in the presence of both the extracts as shown in Fig 1, Fig. 2.
Fig 1.
Bar diagrammatic representations of in vitro antibacterial activity by broth dilution assay. The bars represent the percentage mean growth inhibition (%MGI) assessed by aqueous extract of triphala and its constituting plants when tested against 4 bacterial strains. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
Fig. 2.
Bar diagrammatic representations of in vitro antibacterial activity by broth dilution assay. The bars represent the percentage mean growth inhibition (%MGI) by methanolic extract of triphala and its constituting plants when tested against 4 bacterial strains. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
It was found that TAE had strongest inhibitory activity against B. subtilis i.e. 94.931 ± 0.342% whereas; it showed least inhibition against P. aeruginosa i.e. 36.780 ± 0.279% as shown in Fig. 1. In the case of BAE, S. aureus was the least sensitive with 66.438 ± 0.172% while B. subtilis was the most sensitive with 80.721 ± 0.194%, for HAE B. subtilis was least sensitive with 31.884 ± 0.132% and E. coli was most sensitive with 48.254 ± 0.387%. Whereas, AAE depicted least sensitivity against S. aureus with 53.875 ± 0.134% and most sensitivity against E. coli strain with 82.183 ± 0.160 inhibition. Ampicillin has shown maximum inhibition against E. coli (MGI% 98.37 ± 0.572) and minimum inhibition against B. subtilis (MGI% 88.67 ± 0.643). Above analysis and observations revealed that TME possessed the maximum inhibitory activity against all the four bacterial strains which serves it as the most potential antibacterial agent as compared to other constituents of triphala.
In case of methanolic extracts, it was found that TME had the highest inhibitory activity against B. subtilis with 82.493 ± 0.570% while least inhibition was showed against P. aeruginosa with 52.537 ± 0.569%. BME showed the strongest inhibition against S. aureus with 70.037 ± 0.142% and least inhibition was exhibited against P. aeruginosa with 45.43 ± 0.449%. HME showed maximum inhibition against S. aureus with 65.41 ± 0.411% and lowest against P. aeruginosa with 47.411 ± 0.420%. In case of AME, highest inhibition was exhibited against E. coli with 68.64 ± 0.38% and lowest was showed against B. subtilis with 39.63 ± 0.354%. Fig. 2 represents percent inhibition of TME, BME, HME and AME against all four bacterial strains. The above observations and analysis also proved that TME acted as the most potential antibacterial agent as it showed the maximum percent inhibition against all the four bacterial strains compared to other methanolic extracts of constituents of triphala.
3.2.2. Well diffusion assay
The aqueous and methanolic extracts of triphala and its constituting plants were immediately tested for respective antibacterial activity towards both gram positive and gram negative bacterial strains using well diffusion assay showing the zones of inhibition. Based on the zone of inhibition produced, triphala and its constituents prove to exhibit good antibacterial activity against bacterial strains. On the other hand, control did not exhibit any antibacterial activity. The results depicted that both type of extracts showed the fair antibacterial activities which was reflected through greater inhibition zone. The results of antibacterial activities of extracts evaluated from the well diffusion assay were given in Table 2 and Fig. 3.
Table 2.
Tabular representation of zone of inhibition of aqueous extracts of triphala and its constituents and standard ampicillin. The growth of E. coli and S. aureus on potato dextrose agar plate was inhibited by the aqueous extracts viz. TAE, HAE, AAE, BAE and the ampicillin. The results obtained in well diffusion assay showing fair antibacterial activity when compared with standard antibiotic.
| Components | Zone of inhibition (mm) |
|
|---|---|---|
| E.coli | S.aureus | |
| Control | No zone of inhibition | No zone of inhibition |
| Ampicillin | 25 | 24 |
| TAE | 17 | 15 |
| AAE | 8 | 15 |
| BAE | 11 | 16 |
| HAE | 9 | 25 |
Fig. 3.
Well diffusion assay of the aqueous and methanolic extract of triphala and its constituents. Plates of potato dextrose agar showing zone of inhibition compared with standard antibiotic ampicillin. (A) is showing the zone of inhibition in the growth of E. coli in the presence of TAE, BAE, HAE, AAE and ampicillin. (B) is showing zone of inhibition in the growth of S. aureus in the presence of TAE, BAE, HAE, AAE and ampicillin. (C) is showing zone of inhibition in the growth of P. aeruginosa in the presence of TME, BME, HME, AME and ampicillin.
3.3. Antioxidant assay
3.3.1. DPPH assay
Both the aqueous and methanolic extracts were checked for DPPH radical scavenging activity and both of the extracts showed significant amount of DPPH scavenging activity. The scavenging activities of all the extracts were compared with the scavenging activity of ascorbic acid taken as standard control for all the extracts (Table 3).
Table 3.
Tabular representation of zone of inhibition of methanolic extracts of triphala and its constituents. The methanolic extracts (TME, HME, AME, BME) also showed an appreciable zone of inhibition in the plate of P. aeruginosa which was compared with ampicillin. The results obtained in well diffusion assay showing fair antibacterial activity when compared with standard antibiotic.
| Components | Zone of inhibition (mm) in P. aeroginosa |
|---|---|
| Control | No zone of inhibition |
| Ampicillin | 20 |
| TME | 16 |
| AME | 18 |
| BME | 5 |
| HME | 4 |
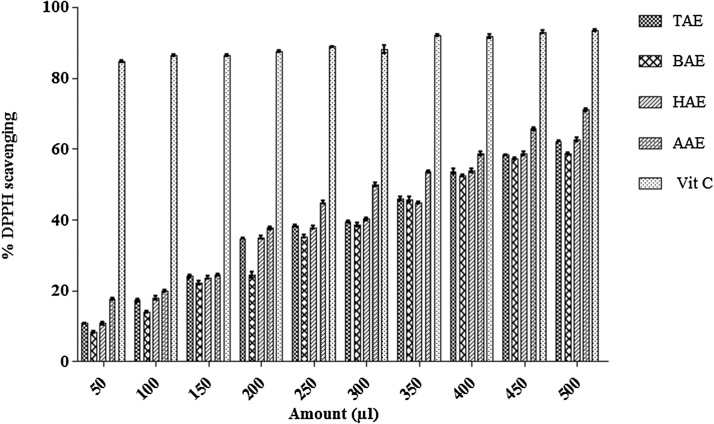
DPPH free radical scavenging activity of aqueous extracts of triphala and its constituting plants was calculated and compared to ascorbic acid. Extract showed radical scavenging activity in a concentration–dependent manner with the highest and lowest scavenging at amount 500 μl and 50 μl of the extracts. TAE showed maximum DPPH radical scavenging activity with 62.185 ± 0.247% and lowest with 10.749 ± 0.256%. BAE showed maximum activity with 58.55 ± 0.288% and lowest with 8.369 ± 0.311%. In case of HAE, maximum DPPH radical scavenging activity was 62.729 ± 0.53% and lowest was 10.755 ± 0.429%. AAE showed maximum activity with 71.024 ± 0.467% and lowest with 17.677 ± 0.319%. The graph represented that aqueous extract of triphala and its constituting plants showed significant amount of free radical scavenging activity when compared to ascorbic acid (vitamin C) where its maximum DPPH radical scavenging activity was 93.351 ± 0.292% and lowest was 84.798 ± 0.307%. AAE showed the high DPPH scavenging activity followed by HAE, TAE and then the least could be seen in BAE as shown in Fig. 4.
Fig. 4.
% DPPH free radical scavenging activity of aqueous extract triphala and its constituting plants was calculated. The results were compared to ascorbic acid. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
DPPH free radical scavenging activity of methanolic extracts of triphala and its constituting plants was also calculated and compared to ascorbic acid. TME showed maximum DPPH radical scavenging activity with 96.139 ± 0.258% and lowest with 19.311 ± 0.309%. BME showed maximum activity with 94.406 ± 0.275% and lowest with 16.577 ± 0.305%. In case of HME, maximum DPPH radical scavenging activity was 94.482 ± 0.492% and lowest was 22.814 ± 0.554%. AME showed maximum activity with 94.747 ± 0.251% and lowest with 15.907 ± 0.52%. The results revealed that methanolic extracts of triphala and its constituting plants represents significant amount of free radical scavenging activity when compared to ascorbic acid (Vitamin C) where its maximum DPPH radical scavenging activity was 93.351 ± 0.292% and lowest was 84.798 ± 0.307%. The TME showed the highest DPPH scavenging followed by BME, AME and then the least could be seen in HME as shown in Fig. 5.
Fig. 5.
% DPPH free radical scavenging activity of methanolic extract of triphala and its constituting plants was calculated and compared to ascorbic acid. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
3.3.2. Catalase assay
Hydrogen peroxide is a non-radical reactive oxygen species with weak oxidizing activity. It diffuses through cell membranes rapidly and interacts with Fe2+ and possibly Cu2+ ions to form hydroxyl radicals and other free radicals. It is therefore biologically advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate. The hydrogen peroxide scavenging ability of aqueous and methanolic extract of triphala and its constituting plants were shown in Fig. 6.
Fig. 6.
Hydrogen peroxide degradation per minute was assessed in the presence of aqueous and methanolic extracts of triphala and its constituents. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
The results show that TAE exhibited significant hydrogen peroxide scavenging activity i.e. 0.03 ± 0.0005 unit per minute H2O2 degradation and the TME showed 0.016 ± 0.0003 unit per minute. AAE showed 0.013 ± 0.0003 unit per minute degradation of H2O2, whereas the AME 0.014 ± 0.0003 unit per minute degradation. The hydrogen peroxide scavenging activity of BAE was noted 0.014 ± 0.0003 unit per minute and BME was 0.034 ± 0.0005 unit/min H2O2 degradation. HAE showed 0.042 ± 0.0003 while HME showed 0.024 ± 0.0005 unit/min. Results revealed that in aqueous extracts, HAE showed highest H2O2 degradation/min followed by TAE, BAE and AAE while in methanolic extract, BME showed the highest H2O2 degradation followed by HME, TME, and AME.
3.3.3. SOD assay
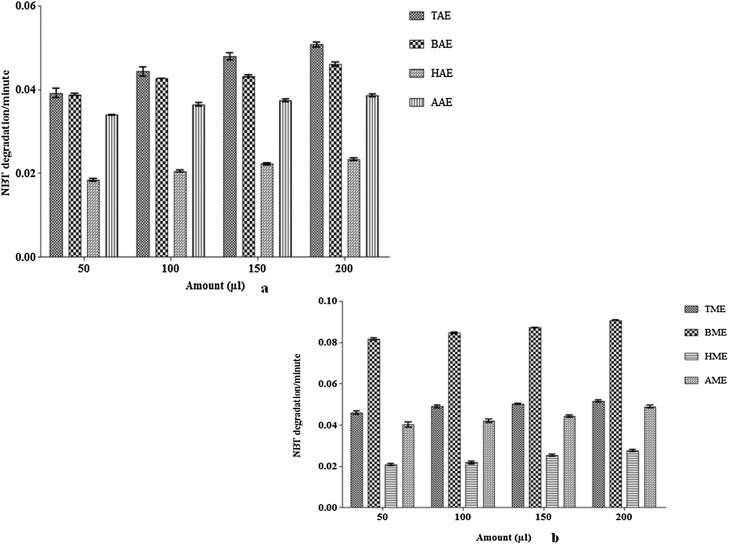
Superoxide anion reduces NBT into formazan at pH 7.8 at room temperature and formazan generation can be measured by spectrophotometry at 560 nm. The decrease of absorbance at 560 nm with antioxidants thus indicates the consumption of superoxide anion in the reaction mixture. In this assay, a significant amount of free radical scavenging activity was noted in triphala plant extract. The results revealed that, TAE showed NBT degradation by 0.039 ± 0.001 unit/min and 0.051 ± 0.001 unit/min at 50 μl (minimum degradation) and 200 μl (maximum degradation) extract volume respectively. While, BAE showed 0.039 ± 0.0003 and 0.046 ± 0.0005 unit/minute, HAE showed 0.018 ± 0.0004 and 0.023 ± 0.0004 unit/min and AAE showed 0.034 ± 0.00005 and 0.039 ± 0.00035 unit/min NBT degradation at 50 μl and 200 μl extract volume respectively. The results confirmed that highest NBT degradation/min was observed by TAE followed by BAE, AAE and HAE at 200 μl extract (Fig. 7a).
Fig. 7.
(a) Bar graph showing the NBT degradation/min in the presence of aqueous extracts of triphala and its constituting plant at different extract concentrations. The results were expressed in degradation/min. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments. (b) Bar graph showing the NBT degradation/min in the presence of methanolic extracts of triphala and its constituting plant at different extract concentrations. The results were expressed in degradation/min. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
In methanolic extracts, TME showed NBT degradation by 0.046 ± 0.0007 unit/min and 0.052 ± 0.005 unit/min at 50 μl (minimum degradation) and 200 μl (maximum degradation) extract volume respectively. While, BME showed 0.082 ± 0.0005 and 0.091 ± 0.0001 unit/minute, HME showed 0.021 ± 0.001 and 0.028 ± 0.001 unit/min and AME showed 0.040 ± 0.001 and 0.049 ± 0.001 unit/min NBT degradation at 50 μl and 200 μl extract volume respectively. The results confirmed that highest NBT degradation/min was observed by BME followed by TME, AME and HME at 200 μl extract (Fig. 7b).
3.3.4. FRAP assay
The ability of the plants extracts to reduce ferric ions was determined using the FRAP assay. An antioxidant capable of donating a single electron to the ferric-TPTZ (Fe (III)-TPTZ) complex would cause the reduction of this complex into the blue ferrous-TPTZ (Fe (II)-TPTZ) complex which absorbs strongly at 593 nm. Triphala and its constituting plants namely showed FRAP antioxidant activity in a concentration–dependent manner. Both the aqueous and methanolic extracts were checked for FRAP assay. Results were compared with ascorbic acid taken as standard.
Extracts and ascorbic acid showed FRAP activity in a concentration–dependent manner with the highest and lowest scavenging at amount 500 μl and 50 μl of the extracts. TAE showed a maximum of 1.007 ± 0.018 mM and a lowest of 0.192 ± 0.003 mM. BAE showed a maximum of 0.927 ± 0.007 mM and lowest of 0.128 ± 0.002 mM. HAE showed a maximum activity of 0.976 ± 0.009 mM and lowest 0.162 ± 0.002 mM. AAE showed maximum 0.992 ± 0.005 mM and lowest of 0.147 ± 0.008 mM (Fig. 8). From observations, it is inferred that triphala and its constituents showed satisfactory results in comparison with ascorbic acid which showed highest FRAP value of 1.151 ± 0.028 mM and a lowest of 0.318 ± 0.007 mM. The highest FRAP value was observed in TAE followed by AAE, HAE and BAE at 500 μl extract.
Fig. 8.
Ferric reducing antioxidant power of aqueous extracts of triphala and its constituting plant were estimated and compared to the ascorbic acid as standard. The results were expressed in mM of FRAP value. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
TME showed a maximum of 1.018 ± 0.005 mM while lowest being 0.232 ± 0.005 mM. BME showed a maximum of 0.942 ± 0.005 mM and lowest 0.150 ± 0.002 mM. HME showed a maximum of 0.988 ± 0.001 mM and a lowest of 0.194 ± 0.002 mM. AME displayed a maximum of 0.951 ± 0.005 mM and a lowest of 0.190 ± 0.003 (Fig. 9). The results were compared with ascorbic acid which showed highest FRAP value of 1.134 ± 0.011 mM and a lowest of 0.319 ± 0.008 mM. Results confirmed that TME has highest FRAP value followed by HME, AME and BME at 500 μl extract.
Fig. 9.
Ferric reducing antioxidant power of methanolic extract of triphala and its constituting plants. Results obtained were estimated and compared to the ascorbic acid as standard. The results were expressed in mM of FRAP value. Results were described in the means ± SEM (standard error of mean) value from at least three separate experiments.
3.4. Anti-inflammatory assay
3.4.1. Inhibition of albumin denaturation
Protein denaturation is the main cause observed in the process of inflammation. Various anti-inflammotory drugs such as salicylic acid, aspirin, phenylbutazone, flufenamic acid are known to prevent heat induced protein denaturation in dose-dependent manner [27]. The anti-inflammatory activity of the aqueous and methanolic extracts of triphala and its constituting plant to inhibit protein denaturation was studied. Both type of extracts induced the BSA denaturation which resulted into the formation of dense protein precipitates and aggregates, which otherwise was supposed to be inhibited in the presence of extracts to establish the anti-inflammatory property of the extracts. Hence, the results confirmed that the triphala is not an anti-inflammatory agent, lower concentration might result in the anti-inflammatory property.
3.4.2. Inhibition of proteinase action
Inflammation is a complex process which is linked with pain, swelling and results into the increase of protein denaturation, vascular permeability, and membrane alteration. Tissue damage is caused by stress, heat burning, radiation, bacterial or viral invasion which is characterized by redness, pain, heat, and swelling and loss of function in the injured area. Serine proteinases are found in abundant amount in the neutrophils and are localized at lysosomes. Previous study revealed that during inflammatory process, leukocytes proteinase play a key role in the development of tissue damage and protection can be provided by various proteinase inhibitors which could result in the prevention of inflammation [16]. Here, in the current study, the inhibition of proteinase action was also performed to confirm the anti-inflammatory nature of triphala and its constituting plants. The results again depicted no inhibition of proteinase action. Hence, this confirmed that triphala and its constituting plants is non-anti-inflammatory agent.
3.5. Determination of antifungal assay
Antifungal assay of the triphala and its constituents extract was performed against Candida cells (ATCC 10261 and ATCC 90028). The extract showed no zone of inhibition up to 1.5 mg/ml as well as there is no inhibition in growth of Candida cells up to 1 mg/ml in broth as well. Our results showed that triphala and its constituents extract showed no potent anticandidal activity up to 1 mg/ml, increased concentration of extracts might lead the anticandidal effect.
4. Discussion
Triphala is considered as the most potential ayurvedic formulation by the practitioner but as far as our knowledge on study of triphala goes, it is exploited for its potential use as an immunomodulatory [6], anti-diabetic [10] anti-mutagenic and anticancer activity [42]. It is interesting to note that the extracts of triphala and its constituents showed the presence of flavonoids and alkaloids in abundant quantity. Several flavonoid derivatives were reported to be effective antimicrobial substances against different microorganisms. Their mode of action may be due to their ability to form complexes with extracellular and soluble proteins and with bacterial cell wall as well. The flavonoids being more lipophilic may also disrupt microbial membranes. In addition to being effective against bacteria, these compounds exhibit inhibitory effects against viruses and parasites [7]. It has been well established that flavonoids in nature are the potential antioxidants. Quercetinm, a flavonoids that exists in numerous plants, possesses very good antioxidant activity and hence it is currently being used in health food stores [36]. Another class of natural products, viz. alkaloids, are complex heterocyclic nitrogenous compounds commonly found to possess antimicrobial properties. They are quite useful against viral and protozoan infections. In case of highly aromatic planar quaternary alkaloids, their mechanism of action is due to their ability to intercalate with DNA [7]. Saponins, which are amphipathic glycosides, maybe mono-or poly desmodic, depending on the number of attached sugar moieties.
These bio-active ingredients are commonly present in licorice root (Glycyrrhiza glabra), and possess expectorant, bacteriostatic and antiviral activities [7]. The ginsenosides are a class of natural saponins and are believed to be responsible for immunostimulant and antinociceptive (pain-relieving) properties [28]. Similarly, tannins are well-known for their antimicrobial and antioxidant activities [36]. According to some reports, certain tannins are considered to be potential cytotoxic and antineoplastic agents [1]. Against this background, our work on Triphala and its constituents prove to be quite interesting due to the presence of all the above-mentioned important classes of bioactive phytochemicals in the plant. Further, it provides scientific validation for usage of the plant extracts in folk medicine in our region. The present work on preliminary phytochemical screening of triphala extracts certainly encourages future advanced research activities on chromatographic isolation of these compounds in their pure state. In recent years, multiple drug resistance has been developed due to indiscriminate use of existing drugs in the treatment of infectious diseases. The major thrust is to establish alternative antimicrobial agent in order to treat microbial infections with less or no toxicity and less or negligible side effects. The herbal medicines have shown potential to overcome the limitation associated with conventional drugs [39]. At present, there is a lot of scope and importance for development of new antimicrobials in treatment of microbial infections. The latest trend shows that the plant-based antimicrobial agents have an enormous therapeutic potential since they do not show any major side effects on human beings [25].
In our work, the extracts of triphala and its constituents showed a good antimicrobial activity against all the bacterial strains tested. The results for antibacterial assay of aqueous extract is described from the highest to lowest inhibition against 4 bacterial strains i.e. TAE: B. subtilis > E. coli > S. aureus > P. aeruginosa; BAE: B. subtilis > P. aeruginosa > E. coli > S. aureus; HAE: E. coli > P. aeruginosa > S. aureus > B. subtilis; AAE: E. coli > P. aeruginosa > B. subtilis > S. aureus. The methanolic extract showed the pattern of inhibition against bacterial strains as follows: TME: B. subtilis > E. coli > S. aureus > P. aeruginosa BME: S. aureus > E. coli > B. subtilis > P. aeruginosa; HME: S. aureus > E. coli > B. subtilis > P. aeruginosa; AME: E. coli > S. aureus > P. aeruginosa > B. subtilis. Hence, it can be expected that the plant possesses various phytochemicals which might be responsible for inhibition of bacterial metabolism.
The triphala is known to possess different variety of phytosignatures drawn from the mixture of seeds of three plants. Antioxidants are important, as they are free radical neutralizers. They are essential as excess of free radical causes diseases like Alzheimer's, Parkinson’s, various types of cancers, ophthalmic disorders etc. Further, antioxidant study of extracts which evaluates the free radical scavenging property of triphala and its constituents using various assays confirmed the antioxidant potential of extracts [20]. The result of antioxidant assay confirms the methanolic extract to be more powerful free radical scavenger than aqueous extract. Antioxidant studies indicated that triphala should possess the ability of either inhibiting free radical formation, or itself is a free radical scavenger. During oxidative stress, excessive free radicals are produced, which are known to cause damage to biomolecules [47,11]. Most of the free radicals are reactive and short-lived and in order to monitor their reactions, one has to employ fast kinetic methods [11]. Free radicals have one or more unpaired electrons; therefore, they react with substrates either by electron transfer or by transfer of hydrogen atom causing damage to them [41]. The results obtained suggested that the triphala and its constituents may scavenge these free radicals from the cellular system and can prevent oxidative cell damage. In the present study, we have investigated the antimicrobial, antioxidant activity of triphala that formed the basis for evaluation as potential antibacterial and antioxidant drug for prevention of microbial infections and oxidative stress based disorders. In our study, we have explored triphala and its constituents as an effective drug for use in prevention of diseases caused by microbes and due to excess of free radical production [33]. The results proved that each extract had a variety of bio-signatures with different properties including bactericidal, antioxidant potentials. The MGI% and zone of inhibition value showed aqueous and methanolic extract to possess antimicrobial activity at par with each other.
Triphala, a well-known ayurvedic formulation, has been found to be an excellent scavenger of superoxide radicals, whose excessive formation is implicated in oxidative stress. Employing other non-biological and stable free radicals such as DPPH, the% free radical scavenging ability of triphala and its constituents was estimated and the results were compared with ascorbic acid. The aqueous and methanolic extracts of triphala and its constituents were evaluated for the DPPH scavenging activity and the results were compared to each other. The highest to lowest activity in aqueous and methanolic extract was as follows AAE > HAE > TAE > BAE and TME > BME > AME > HME respectively. The antioxidant activity of the aqueous and methanolic extracts of triphala and its constituting plants were also evaluated by SOD assay, catalase assay and FRAP assay and the results were compared and described in the Table 4. The results in SOD assay showed dose-dependent scavenging of NBT (degradation/min) radicals by both the extracts of triphala and its constituting plants. Catalase assay showed the fair hydrogen peroxide degradation/min in the presence of extracts. FRAP value was the highest in the triphala aqueous and methanolic extract than the other constituting plant extracts which confirmed that triphala possess highest reducing power.The triphala and its constituents didn’t show any anti-inflammatory and antifungal activity.
Table 4.
Comparative analysis of biological activities (from highest to lowest) of aqueous and methanolic extract of triphala and its constituting plants.
| Activities/phytochemicals | Aqueous extract | Methanolic extract |
|---|---|---|
| Phytochemicals | lower | higher |
| Antibacterial assay | TAE: B. subtilis > E. coli > S. aureus > P. aeruginosa | TME: B. subtilis > E. coli > S. aureus > P. aeruginosa |
| BAE: B. subtilis > P. aeruginosa > E. coli > S. aureus | BME: S. aureus > E. coli > B. subtilis > P. aeruginosa | |
| HAE: E. coli > P. aeruginosa > S. aureus > B. subtilis | HME: S. aureus > E. coli > B. subtilis > P. aeruginosa | |
| AAE: E. coli > P. aeruginosa > B. subtilis > S. aureus | AME: E. coli > S. aureus > P. aeruginosa > B. subtilis | |
| DPPH scavenging activity | AAE > HAE > TAE > BAE | TME > BME > AME > HME |
| Catalase assay | HAE > TAE > BAE > AAE | BME > HME > TME > AME |
| SOD assay | TAE > BAE > AAE > HAE | BME > TME > AME > HME |
| FRAP assay | TAE > AAE > HAE > BAE | TME > HME > AME > BME |
The studies are of great significance as the demand for herbal products as natural antioxidants and antimicrobial agents is increasing constantly [2,48]. The observed values of free radical scavenging of these extracts support the significance of fruits of triphala extracts as promising natural source of antioxidants and hence they can be used in nutritional or pharmaceutical areas for the prevention of free-radical-mediated diseases. Thus, this ancient ayurvedic plant showed scientific evidences of its therapeutic properties. The various experimental setups and assays showed the presence of various phytochemical constituents which could be significantly important for its medicinal properties such as antibacterial, antifungal, antioxidant and anti-inflammatory.
5. Conclusion
The present study disclosed that the aqueous and methanolic extract of triphala and its constituents exhibited presence of various secondary metabolites viz. flavonoids, phenols, alkaloids etc. The performed study on Triphala and its constituents also proved to have strong antimicrobial and antioxidant properties. Therefore, it may be used as a potential source of natural antimicrobial and antioxidant agents. Triphala may be use as an easily accessible source of natural antioxidants and as a possible food supplement or in pharmaceutical industry. It can be inferred from the present study that the plant could be investigated for some new compounds of plant origin that may be more potent antimicrobials and antioxidants. Further exploration of triphala and its constituents is required to isolate and identify active molecules for detailed evaluation of in vivo biological activities of such isolated compounds.
Conflicts of interests
The authors declare that they have no conflict of interest. It has not been published elsewhere. That it has not been simultaneously submitted for publication elsewhere. All authors agree to the submission to the journal.
Acknowledgement
We are very thankful to Department of Biotechnology, Jamia Millia Islamia for providing us the infrastructure to carry out the proposed research work.
References
- 1.Aguinaldo A.M., Espeso E.L., Guovara B.Q. A guide book to plants screening: phytochemical and biological. In: Guevara B.Q., editor. Phytochemistry. University of Santo Tomas; Manila, Philippines: 2005. pp. 121–125. [Google Scholar]
- 2.Arora R., Gupta D., Chawla R., Sagar R., Sharma A., Kumar R., Prasad J., Singh S., Samanta N., Sharma R.K. Radioprotection by plant products: present status and future prospects (review) Phytother. Res. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 3.Arora S., Kaur K., Kaur S. Indian medicinal plants as a reservoir of protective phytochemicals. Teratog. Carcinog. Mutagen. 2003;1:295–300. doi: 10.1002/tcm.10055. [DOI] [PubMed] [Google Scholar]
- 4.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 5.Braca A., Sortino C., Politi M., Morelli I., Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002;79(3):379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 6.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cseke L.J., Kirakosyan A., Kaufman P.B., Warber S., Duke J.A., Brielmann H.L. second ed. CRC Press; BocaRaton, USA: 2006. Natural Products from Plants. p. 17. [Google Scholar]
- 8.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109(1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnsworth N.R., Akerele O., Bingle A.S., Sojarto D.D., Guo Z. Medicinal plant in therapy. Bull. WHO. 1985;63:965–981. [PMC free article] [PubMed] [Google Scholar]
- 10.Graefe E.U., Derendorf H., Veit M. Pharmacokinetics and bioavail-ability of the flavonol quercetin in humans. Int. J. Clin. Pharmacol. Ther. 1999;37:219–233. [PubMed] [Google Scholar]
- 11.Halliwell B., Gutteridge J.M.C. Clarendon Press; Oxford: 1993. Free Radicals in Biology and Medicine; pp. 22–81. [Google Scholar]
- 12.Harborne J.B. second ed. Chapman A. & Hall; London, UK: 1998. Photochemical Methods: A Guide to Modern Techniques of Plant Analysis; pp. 4–84. [Google Scholar]
- 13.Jagetia G.C., Baliga M.S., Malagi K.J., Sethukumar K.M. The evaluation of the radio protective effect of triphala (an ayurvedic rejuvenating drug) in the mice exposed to gamma radiation. Phytomedicine. 2002;9:99–108. doi: 10.1078/0944-7113-00095. [DOI] [PubMed] [Google Scholar]
- 14.Jagetia G.C., Malagi K.J., Baliga M.S., Venkatesh P., Veruva R.R. Triphala, an ayurvedic rasayana drug, protects mice against radiation-induced lethality by free-radical scavenging. J. Altern. Complement. Med. 2004;10:971–978. doi: 10.1089/acm.2004.10.971. [DOI] [PubMed] [Google Scholar]
- 15.Jagetia G.C., Rao S.K., Baliga M.S., Babu K. The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: a preliminary study. Phytother. Res. 2004;18:561–565. doi: 10.1002/ptr.1494. [DOI] [PubMed] [Google Scholar]
- 16.Jambunathan N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 2010;639:292–829. doi: 10.1007/978-1-60761-702-0_18. [DOI] [PubMed] [Google Scholar]
- 17.Juss S.S. Triphala–the wonder drug. Ind. Med. Gaz. 1997;131:194–196. [Google Scholar]
- 18.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 19.Kaur S., Arora S., Kaur K., Kumar S. The in vitro antimutagenic activity of triphala an Indian herbal drug. Food Chem. Toxicol. 2002;40:527–534. doi: 10.1016/s0278-6915(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 20.Kaur S., Michael H., Arora S., Harkonen P.L., Kumar S. The in vitro cytotoxic and apoptotic activity of triphala–an Indian herbal drug. J. Ethnopharmacol. 2005;97:15–20. doi: 10.1016/j.jep.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 21.Kokate C.K. Vallabh Prakashan; Delhi, India: 2000. Practical Pharmacognosy. p. 218. [Google Scholar]
- 22.Kolonel L.N., Altshuler D., Henderson B.E. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat. Rev. Cancer. 2004;4:519. doi: 10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni P.H. Clinical assessment of the effect of sookshma triphala in lipoma. J. Ayurvedic Res. Pap. 1995;1995:66–71. [Google Scholar]
- 24.Kumar P.V., Kuttan R., Kuttan G. Radioprotective effects of rasayanas. Ind. J. Exp. Biol. 1996;34:848–850. [PubMed] [Google Scholar]
- 25.Lwu M.W., Duncan A.R., Okunji C.O. New antimicrobials of plant origin. In: Janick J., editor. Perspectives on New Crops and New Uses. ASHS Press; Alexandria, USA: 1999. pp. 457–462. [Google Scholar]
- 26.Mehta B.K., Shitut S., Wankhade H. In vitro antimicrobial efficacy of triphala. Fitoterapia. 1999;64:371–372. [Google Scholar]
- 27.Mizushima Y., Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968;20:169–173. doi: 10.1111/j.2042-7158.1968.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 28.Nah J.J., Hahn J.H., Chung S. Effect of ginsenosides, active Components of ginseng, on capsaicin-induced pain-related behaviour. Neuropharmacology. 2000;39:2180–2184. doi: 10.1016/s0028-3908(00)00048-4. [DOI] [PubMed] [Google Scholar]
- 29.Naik G.H., Priyadarsini K.I., Bhagirathi R.G., Mishra B., Mishra K.P., Banavalikar M.M., Mohan H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother. Res. 2005;19:582–586. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- 30.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 31.Oyedepo O.O., Femurewa A.J. Anti-protease and membrane stabilizing activities of extracts of Fagra zanthoxiloides, Olax subscorpioides and Tetrapleura tetraptera. Int. J. Pharm. 1995;33:65–69. [Google Scholar]
- 32.Patwardhan B., Hooper M. Ayurveda and future drug development. Int. J. Altern. Complement. Med. 1992;10:9–11. [Google Scholar]
- 33.Ramasundaram S., Parthasarathy N.J., Rathinasamy S. Immunomodulatory activity of triphala on neutrophil functions. Biol. Pharm. Bull. 2005;28(8):1398–1403. doi: 10.1248/bpb.28.1398. [DOI] [PubMed] [Google Scholar]
- 34.Ramawa K.G., Das S., Mathur M. The chemical diversity of bioactive molecules and therapeutic potential of medicinal plant. In: Ramawa K.G., editor. Herbal Drugs: Ethnomedicine to Modern Medicine. Springer; Berlin Heidelberg, Germany: 2009. pp. 7–32. [Google Scholar]
- 35.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother. Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Rivière C., Hong V.N.T., Pieters L. et al.. Polyphenols isolated from Antiradical extracts of Mallotus metcalfianus. Phytochemistry. 2009;70:86–94. doi: 10.1016/j.phytochem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Sane R.T. Standardization, quality control and GMP for herbal drug. Indian Drugs. 2002;39(3):184190. [Google Scholar]
- 38.Shamsi T.N., Parveen R., Rehsawla R., Afreen S., Azam M., Fatma T., Haque Q.M.R., Fatima S. In-vitro biological characterization of indian honey. Int. J. Pharm. Res. 2016;8(1):33–38. [Google Scholar]
- 39.Singh D., Chauhan N., Sawhney S.S., Painuli R.M. Biochemical characterization of triphala extracts for developing potential herbal drug formulation for ocular diseases. Int. J. Pharm. Pharm. Sci. 2011;3(5):516–523. [Google Scholar]
- 40.Singh P.K. Mycotoxin elaboration in triphala and its constituents. Indian Phytopathol. 2003;56:380–383. [Google Scholar]
- 41.Spinks J.W.T., Woods R.J. John Wiley; New York: 1991. Introduction to Radiation Chemistry; p. 243. [Google Scholar]
- 42.Srikumar R., Parthasarathy N.J., Devi R.S. Immunomodulatory activity of triphala on neutrophil functions. Biol. Pharm. Bull. 2005;28:1398–1403. doi: 10.1248/bpb.28.1398. [DOI] [PubMed] [Google Scholar]
- 43.Tariq M., Hussain S.J., Asif M., Jahan M. Protective effects of fruits of extracts of Emblica officinalis Gaertn. and Terminalia bellerica Roxb. in experimental myocardial necrosis in rats. Ind. J. Exp. Biol. 1977;15:485–486. [PubMed] [Google Scholar]
- 44.Thakur C.P., Thakur B., Singh S., Sinha P.K., Sinha S.K. The Ayurvedic medicines, haritaki, amla and bahira reduce cholesterol- induced atherosclerosis in rabbits. Int. J. Cardiol. 1988;21:167–175. doi: 10.1016/0167-5273(88)90219-7. [DOI] [PubMed] [Google Scholar]
- 45.Vani T., Rajani M., Sarkar S., Shishoo C.J. Antioxidant properties of the ayurvedic formulation triphala and its constituents. Int. J. Pharmacogn. 1997;35:313–317. [Google Scholar]
- 46.Verpoorte R. Overview and introduction. In: Mander L., Lui H.W., editors. Comprehensive Natural Products-II—Chemistry and Biology. Elsevier Science; Oxford, UK: 2010. pp. 1–4. [Google Scholar]
- 47.Von Sonntag C. Taylor and Francis; London: 1987. The Chemical Basis of Radiation Biology; pp. 65–84. [Google Scholar]
- 48.Weiss J.F., Landauer M.R. Protection against ionising radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 49.Wong A.H., Gottesman I.I., Petronis A. Phenotypic differences in genetically identical organisms: the epigenetic perspective. Hum. Mol. Genet. 2004;14(1):11–18. doi: 10.1093/hmg/ddi116. [DOI] [PubMed] [Google Scholar]
- 50.Das S.N., Chatterjee S. Long term toxicity study of ART-400. Indian Indg. Med. 1995;16(2):117–123. [Google Scholar]