Abstract
Arsenic, a naturally occurring environmental contaminant, is harmful to humans at elevated concentrations. Increased levels of arsenic in the environment occur as a result of human activities and from natural geologically sourced leaching into ground and surface water. These sources pose an exposure risk above the USEPA standard to individuals whose food and water sources become contaminated. Arsenic exposure negatively impacts organ function and increases the risk for developing pathologies, including cancer. Some of the effects of arsenic on cancer translate to normal cell function in wound healing. To evaluate whether arsenic influences wound healing, an in vitro scratch assay was employed to study the effects of arsenic on cellular migration, which is a key component in the normal wound-healing process. In this study, skin cells were exposed to environmentally relevant concentrations of arsenic, and wound closure was evaluated. Results indicated that arsenic significantly decreased the rate of cellular migration in the scratch assay when compared with controls. In addition, estradiol, which has been shown to positively influence cellular and tissue processes involved in wound healing, reversed the slowing effects of arsenic on wound closure. These results suggest that arsenic contamination may inhibit, and estrogen may provide a therapeutic benefit for individuals with arsenic-contaminated wounds.
Keywords: : arsenic, estrogen, in vitro, scratch assay, wound healing
Introduction
Environmental contaminants influence numerous physiological processes. Tissue surrounding skin wounds may be particularly susceptible to effects from exposure to environmental chemical contaminants due to the skin's direct exposure to the external environment. This route of contamination directly influences the skin and wound sites, or it indirectly affects health through systemic toxicity and comorbidities of patients who are exposed to contaminated water and who are part of at-risk populations for chronic wounds (e.g., elderly, Native Americans, especially in the Southwest United States, and diabetics).
Inorganic arsenic is a ubiquitous environmental toxicant found throughout the world in ground and surface water resources.1–4 In the Southwest United States, numerous groundwater, well water, and springs have been documented to contain concerning levels of arsenic.5 For example, in Verde Valley, AZ, Montezuma Well contains 210 μg/L of arsenic, groundwater around the Verde River, AZ contains 16 μg/L of arsenic on average with peak values reaching 1.3 mg/L.6,7 Notably, many wells in the Verde River water shed exceed 50 μg/L.8 These environmental arsenic levels are not limited to Arizona in the United States. In Rio Puerco, NM, and Rio Salado, NM, surface river water samples have been measured to contain between 111 and 346 μg/L arsenic.9
Chronic environmental arsenic exposure through consumption of geologically contaminated water is correlated with increased incidences of several cancers, autoimmune disease, diabetes, and cardiovascular diseases along with integumentary complications.10–20 Most relevant to this study, Sherwood et al. found that 10 μg/L arsenic exposure of human bronchial epithelial cells inhibited wound repair efficiency through influencing Ca2+ signaling.21 Gentry et al. reviewed the literature up to 2010 regarding arsenic-induced shifts in gene expression in numerous tissues and in vivo studies.22 They found that even at low ppb exposure levels (below current drinking water levels), arsenic exposure caused shifts in expression of genes that are responsible for numerous cellular processes, including oxidative stress, inflammation, proteotoxicity, proliferation, DNA repair, cell cycle control, and apoptosis, all processes that influence wound healing.23 Further, patients exposed to environmentally relevant levels of arsenic in the Philippines demonstrate several dermatological outcomes, suggesting that arsenic influences skin health.24 Although the mechanism of action through which arsenic affects these disease processes is not fully understood, environmental arsenic exposure is demonstrated to have long-term health consequences in human populations above or even below the current USEPA regulatory limit of 10 ppb.25 Collectively, the aforementioned studies indicate that arsenic negatively impacts many different organs, which leads us to believe that arsenic may also negatively impact skin functions in wound healing and, more specifically, cellular processes that govern wound healing.
Steroid hormones have strong influences on wound healing. In particular, the steroid hormone, estradiol, promotes wound healing, and treatment with aromatase inhibitors that block estradiol synthesis leads to adverse outcomes in wounds.26–30 Further, both the nuclear ER-α and ER-β play a role in activating components of the wound-healing response.31,32 These effects of estradiol involve processes involved in fibroblast function, Erk/Akt signaling, matrix metalloproteinase (MMP) activity, and monocyte attraction.33–35 Collectively, these studies demonstrate that steroid hormone signaling is important to wound healing, and a more comprehensive understanding of how compounds that interact with hormones may influence wound repair may help lead to targeted treatments.
Little is understood about the implications of arsenic contamination on skin cell migration and wound healing. Many investigators have eluded to arsenic-fibroblast interactions such as MMP production and activity, reactive oxygen species activity, and fibroblast proliferation.36–39 These studies indicate that arsenic may impact many levels of wound healing; however, arsenic effects on fibroblast migration, a pivotal step in wound healing, are not well described in literature. In this study, the effects of environmentally relevant arsenic exposure on dermal fibroblast migration in an in vitro mock-wound model (scratch assay) were evaluated. These initial proof-of-concept experiments were employed to first understand the effects that arsenic may have on skin cell migration. In addition, estrogen–arsenic interactions have been shown to mediate various pathways in wound healing and cancer; thus, the ability for the estrogen hormone to positively influence cellular migration in arsenic-contaminated cells was evaluated.40
The purpose of this study was to determine whether arsenic alone negatively influences cellular migration and whether estradiol reverses the potential effects of arsenic on wound closure.
Materials and Methods
Cell culture
Human neo-natal dermal fibroblasts (third passage; Life Technologies, Carlsbad, CA) were grown as a monolayer into a tissue-culture-treated T75 culture flask (Corning, Inc., Corning, NY) at a density of 5000 cells/cm2. The cells were grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (FBS; Life Technologies). Cells were incubated at 37°C, 5% CO2. Once the cells reached optimal density (∼70% confluence), they were sub-cultured into a tissue-culture-treated 12-well plate (Corning, Inc.) and incubated for 2 days or until the cells reached 100% confluence. Upon reaching confluence, the cells were scratched by using a P200 pipette tip (Gilson, Inc., Middleton, WI) across the monolayer with the initial scratch width measuring 1.5 ± 0.5 mm.41 Next, the media was removed, the cells were rinsed with 1 × Hanks balanced salt solution (Life Technologies), and the respective treatments were added.
Arsenic and estradiol preparations
Arsenic (Sigma-Aldrich) was weighed out to 2.2 mg and diluted into 22 mL of DMEM supplemented with 10% FBS to make a 1 μg/10 μL stock arsenic solution. The 1.0 (75 μg/L) and 0.1 μM (7.5 μg/L) treatments were made from the stock arsenic solution and added to the specified wells. These treatment concentrations were selected from published literature values as environmentally relevant doses as previously described.6–8 A stock 17-β estradiol (E2; Sigma-Aldrich) solution of 1 μg/μL 95% ethanol was diluted for a final treatment concentration of 1 nM E2 in 0.000026% EtOH and added to specified wells. Ethanol was added to all treatments at the same concentration, and a nonethanol treatment group was used for comparison to ensure that there was no ethanol-related effect. Sample sizes (n) for all treatment groups was n = 10.
Image analysis
Images were captured every 4 hours over a 24-hour period to analyze wound closure by using a Leica inverted microscope (Leica DMi1, Wetzlar, Germany). Photos were analyzed by using Image J (V1.48) analysis software (National Institute of Health, Bethesda, MD). Wound widths in each well were measured at 10 unique locations along the length of the scratch, and an average was reported.
Wound width and area under the curve calculation
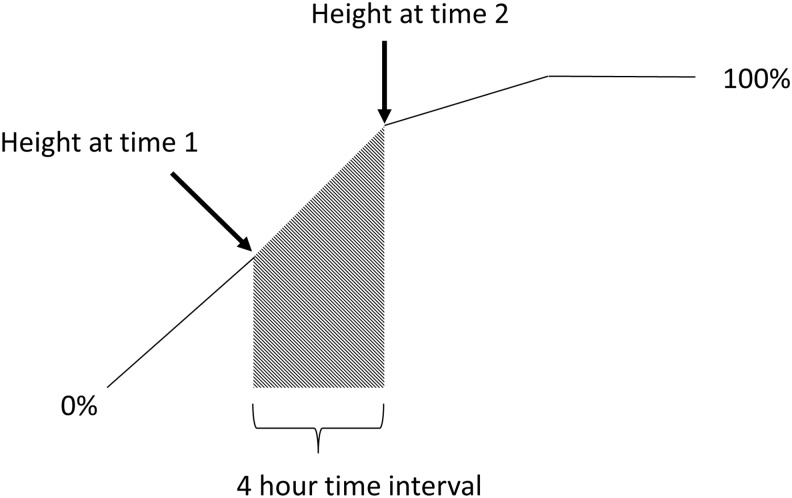
The measured distance between the leading edges of the cells (wound width) after the cell monolayer was scratched was 0.5 ± 1.0 mm. The raw wound width measurements were used to calculate percent wound closure. Percent wound closure was calculated as: (initial width − final width)/initial width × 100. Percent wound closure was graphed across time from which the area under the curve (AUC) could be calculated following the trapezoidal rule to access a single measure of wound closure across all time points for each sample (Fig. 1).
FIG. 1.
Schematic representation of the method used to calculate AUC from percent wound closure. The shaded area represents a 4-hour time interval of wound closure. This area was calculated by using a trapezoid area formula. This was repeated from 0 hour through complete wound closure (∼24 hour) for all controls and treatments. Areas were summed and represented in Figure 3. AUC, area under the curve.
Statistical analysis
JMP Pro (version 12) was used for statistical analysis. A two-way analysis of variance (ANOVA) interaction with a Tukey adjustment was used to identify statistical differences between arsenic-contaminated cells and controls (p < 0.01).
Results
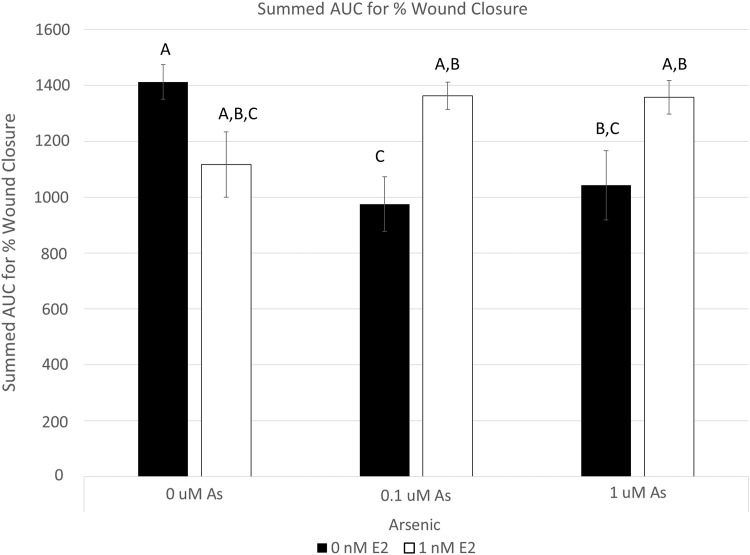
Sample sizes (n) for all treatment groups was n = 10. Uniform scratches were observed across all treatment groups and controls. All scratch widths measured between 0.5 and 1.5 mm (Fig. 2). There was no statistical difference between EtOH and non-EtOH media (data not shown); thus, only results from EtOH control are reported (Fig. 3). Arsenic and estrogen effects were represented as summed AUC from an initial percent wound closure calculation. Control wounds had a summed AUC of 1412.40, which was the fastest closure time. Estrogen control wounds had a summed AUC of 1116.67, which was not statistically different from the control wounds. Both arsenic 0.1 and 1 μM treatments statistically decreased the summed AUC values (migration times) at 975.17 and 1042.60, respectively, compared with control wounds. Arsenic 0.1 and 1 μM supplemented with 1 nM estrogen reversed the negative effects seen in arsenic alone, and the summed AUC values were 1362.85 and 1357.70, respectively (Fig. 3). The two-way ANOVA resulted in an interaction effect between arsenic and estradiol exposure (F = 9.03; p < 0.001). Post hoc tests revealed that both arsenic exposure concentrations led to lower AUC values, indicating inhibited scratch closure (inhibited wound healing), and estradiol rescued the effect of arsenic (p < 0.05 for all significant tests).
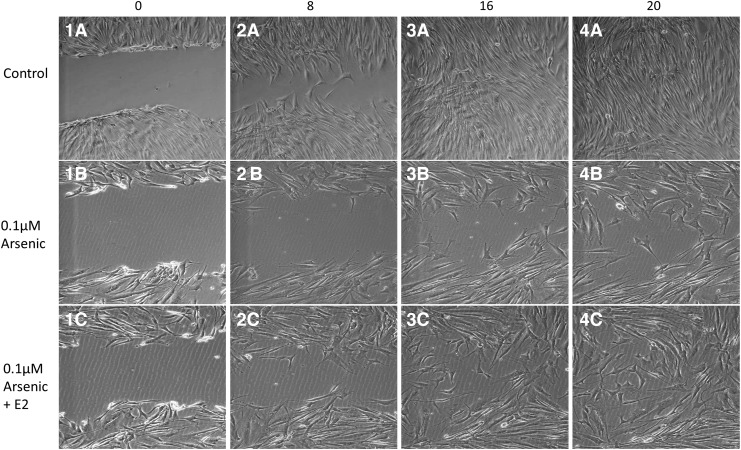
FIG. 2.
Representative cellular migration images of the in vitro “scratch” wound-healing model over a 20-hour period. Images (1A–4A) show the control scratch wound closing over a 20-hour period. Images (1B–4B) show the 0.1 μM arsenic scratch wound closing over a 20-hour period. Images (1C–4C) show the 0.1 μM arsenic+estradiol (E2) scratch wound closing over a 20-hour period. Arsenic alone (1B–4B) deleteriously affected wound closure compared with control wounds. Arsenic with the addition of estradiol (E2) (1C–4C) showed a reverse effect, indicating that E2 may have a therapeutic benefit to arsenic-contaminated cells.
FIG. 3.
Arsenic inhibits wound healing in an in vitro model, and estrogen treatment counteracts arsenic's effects. hDFn were grown in 12-well plates and incubated until the cells reached 100% confluence. The cells were scratched by using a P200 pipette tip, and the wells were imaged every 4 hours for 24 hours to determine the degree to which the cells filled in the scratch space. The % closure was determined at each time point, and the AUC was determined for summed % closure across all time points as a measure of wound healing. A significant two-way ANOVA interaction effect was found (p < 0.001). Statistical significance is shown by using the “compact letter display” notation, commonly used in statistical analysis with multiple comparisons. Treatments that do not share a common letter are statistically significant from each other (p < 0.05). Treatments distinguished by different letters are significantly different by a Tukey's post hoc analysis (p < 0.05). ANOVA, analysis of variance.
Discussion
The bench-top wound-healing assay in this study was successfully used to investigate the hypothesized effects of arsenic on in vitro cellular migration, as well as the hypothesized therapeutic benefit of estrogen hormone as a wound-healing stimulant. The results suggest that environmentally relevant concentrations of arsenic may slow wound healing, and that low-dose estradiol may provide a therapeutic method for reversing the effects of arsenic exposure. This assay has been well described in literature as a simplistic, high-throughput, and timely method of achieving early cellular migration pilot data that can be used to develop a strategic study design before in vivo testing.41,42 Dermal fibroblast cells were used in this assay to best represent the cellular environment predominantly affected by both skin wounds and contamination through arsenic exposure to the skin. This assay was also used as a means to target a dose range that would most accurately represent levels of arsenic exposure seen in the environment. The literature describes broad-ranging levels of arsenic in the environment that bracket the values tested in this study.6–8 These levels have been shown to also contribute to various pathologies, cancers, and diseases.10–20 Further, levels of arsenic that best represent environmental exposure, at 0.1 and 1 μM, were used in this study.20
The results indicate that arsenic directly influences cellular migration in vitro. A decrease in cellular migration could have downstream effects that negatively impact wound-healing outcomes in both acute and chronic wounds. Initial recruitment of cells to the site of tissue damage is a key first step in many processes involved in healing.43 For example, inflammatory cells quickly invade the damaged tissue and release various molecules (cytokines, growth factors, pro-angiogenic factors, etc.) that stimulate the release and migration of additional cells to the wound site to initiate tissue repair.43,44 More specifically, dermal fibroblasts migrate and close the open wound to prevent further exposure to foreign pathogens, begin to lay down new extracellular matrix to provide structural support, and secrete additional growth factors for continued healing, all of which demonstrate the importance of fibroblast migration to the wound site. If arsenic is present during a wounding injury or is present in trace amounts in the skin from prior exposure and bioaccumulation, initial cellular migration may be slowed, which would delay the entire wound-healing process and prevent fibroblast functions, such as secretion of fibrous proteins needed for remodeling the extracellular matrix and release of cytokines and growth factors for wound repair. Further, there are specific populations of individuals who may be at higher risk for cellular toxicity that may cause delayed wound healing and arsenic contamination, namely diabetic patients. Diabetic individuals who have pre-existing chronic non-healing wounds and who live in high arsenic exposure locations may be further affected by the presence of arsenic in the wound site.
In the United States, Native American populations are at the highest risk for arsenic exposure through increased levels of naturally occurring arsenic on Tribal Lands.45 The Native American population also has the highest diabetic rate of any ethnicity at ∼15%, and it is increasing every year.46 The increased diabetic incidence rate among Native Americans raises the likelihood of forming non-healing chronic diabetic ulcers that could likely become contaminated with arsenic.47 The results from this study indicate that arsenic negatively impacts in vitro wound healing and may possibly have health implications in Native American individuals who are both diabetic and exposed to elevated environmental arsenic levels. With this knowledge, potential therapeutic remedies must be evaluated to rescue damaged, contaminated wounds from further insult. This was a substantial motivation in this study for investigating the estrogen hormone's ability to reverse the negative effects of arsenic on wound closure.
Estradiol was used to treat arsenic-contaminated cells to evaluate whether estrogen would reverse the negative effects of arsenic on cellular migration. Estrogen replacement therapy has been shown to support skin homeostasis seen in postmenopausal women, which includes thinning of the skin and decreased collagen production.31 In addition, both human and animal experiments have demonstrated decreases in wound-healing rates associated with estrogen deficiencies.48 Estrogen replacement has also been shown to accelerate healing in both aged humans and estrogen-depleted animal models.31 Therefore, we used estrogen as a wound-healing stimulant in this in vitro simulation of wound healing to promote fibroblast migration after arsenic contamination. Results suggest that estrogen does, in fact, rescue arsenic-contaminated cells and promotes cellular migration that is more representative of control wounds. This finding may indicate that estrogen acts through an independent pathway associated with arsenic contamination that specifically influences wound healing that is separate from normal physiologic estrogen pathways. If estrogen can promote positive wound healing in a contaminated scenario, estrogen may have the potential to be used as a therapeutic remedy in areas where arsenic levels of exposure are at dangerously high levels. Ongoing research will address the molecular mechanisms through which arsenic inhibits wound healing, and it will determine whether estradiol interferes either directly or indirectly with the deleterious actions of arsenic on wound healing.
Conclusions
We concluded that arsenic-contaminated fibroblasts had a decreased cellular migration rate compared with control wounds in an in vitro scratch assay. This indicates that arsenic may play a role in wound healing in exposed individuals. Estradiol, however, was able to rescue arsenic-contaminated cells and increase cellular migration rates. This result supports other findings that estradiol plays a role in wound healing and may provide a pathway toward therapeutic treatment of arsenic-contaminated wounds. Further mechanistic research will be conducted to address specific roles of signaling pathways associated with arsenic-impacted wound healing.
Acknowledgments
1. Technology and Research Initiative Fund (TRIF).
2. Northern Arizona University Initiative for Maximizing Student Diversity in the Biomedical Sciences, National Institute of Health (NIH #R25GM056931-14).
3. Southwest Health Equity Research Collaborative (NIH RCMI, 1 U54 MD012388-01).
Author Disclosure Statement
No competing financial interests exist in this study.
References
- 1.Agusa T, Trang PT, Lan VM, et al. . Human exposure to arsenic from drinking water in Vietnam. Sci Total Environ 2014:488–489;562–569 [DOI] [PubMed] [Google Scholar]
- 2.Ayotte JD, Belaval M, Olson SA, et al. . Factors affecting temporal variability of arsenic in groundwater used for drinking water supply in the United States. Sci Total Environ 2015:505;1370–1379 [DOI] [PubMed] [Google Scholar]
- 3.Dummer TJ, Yu ZM, Nauta L, et al. . Geostatistical modeling of arsenic in drinking water wells and related toenail arsenic concentrations across Nova Scotia, Canada. Sci Total Environ 2015:505;1248–1258 [DOI] [PubMed] [Google Scholar]
- 4.Sorg TJ, Chen AS, Wang L. Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Res 2014:48;156–169 [DOI] [PubMed] [Google Scholar]
- 5.Beamer PI, Klimecki WT, Loh M, et al. . Association of children's urinary CC16 levels with arsenic concentrations in multiple environmental media. Int J Environ Res Public Health 2016:13;pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foust RD, Mohapatra P, Compton-O'Brian AM, et al. . Groundwater arsenic in the Verde Valley in central Arizona, USA. Appl Geochem 2004:19;251–255 [Google Scholar]
- 7.Robertson FN. Arsenic in ground-water under oxidizing conditions, southwest United States. Environ Geochem Health 1989:11;3–4 [DOI] [PubMed] [Google Scholar]
- 8.Uhlman K. Arsenic in Arizona Ground Water: Source and Transport Characteristics. Arizona Cooperative Extension, AZ 1453. The University of Arizona, Tucson, AZ; 2008 [Google Scholar]
- 9.Branvold LA, Branvold DK. A Compilation of Trace Metal Values in Water and Sediments Collected Along the Rio Grande and its Tributaries in New Mexico. Data from Selected Published and Unpublished Sources. Open File Report 359. Socorro New Mexico: New Mexico Bureau of Mines and Mineral Resources; 1990 [Google Scholar]
- 10.Garcia-Esquinas E, Pollan M, Umans JG, et al. . Arsenic exposure and cancer mortality in a US-based prospective cohort: The strong heart study. Cancer Epidemiol Biomarkers Prev 2013:22;1944–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saint-Jacques N, Parker L, Brown P, et al. . Arsenic in drinking water and urinary tract cancers: A systematic review of 30 years of epidemiological evidence. Environ Health 2014:13;44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong J, Erdei E, Rubin RL, et al. . Mercury, autoimmunity, and environmental factors on cheyenne river sioux tribal lands. Autoimmune Dis 2014:2014;325461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettinger AS, Zota AR, Amarasiriwardena CJ, et al. . Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect 2009:117;1059–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CF, Chen YW, Yang CY, et al. . Arsenic and diabetes: Current perspectives. Kaohsiung J Med Sci 2011:27;402–410 [DOI] [PubMed] [Google Scholar]
- 15.James KA, Marshall JA, Hokanson JE, et al. . A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ Res 2013:123;33–38 [DOI] [PubMed] [Google Scholar]
- 16.Maull EA, Ahsan H, Edwards J, et al. . Evaluation of the association between arsenic and diabetes: A national toxicology program workshop review. Environ Health Perspect 2012:120;1658–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abhyankar LN, Jones MR, Guallar E, et al. . Arsenic exposure and hypertension: A systematic review. Environ Health Perspect 2012:120;494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balakumar P, Kaur J. Arsenic exposure and cardiovascular disorders: An overview. Cardiovasc Toxicol 2009:9;169–176 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Karagas MR. Arsenic and cardiovascular disease: New evidence from the United States. Ann Intern Med 2013:159;713–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdul KS, Jayasinghe SS, Chandana EP, et al. . Arsenic and human health effects: A review. Environ Toxicol Pharmacol 2015:40;828–846 [DOI] [PubMed] [Google Scholar]
- 21.Sherwood CL, Lantz RC, Boitano S. Chronic arsenic exposure in nanomolar concentrations compromises wound response and intercellular signaling in airway epithelial cells. Toxicol Sci 2013:132;222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentry PR, McDonald TB, Sullivan DE, et al. . Analysis of genomic dose-response information on arsenic to inform key events in a mode of action for carcinogenicity. Environ Mol Mutagen 2010:51;1–14 [DOI] [PubMed] [Google Scholar]
- 23.Anderson B, Clark RAF, Henson PM. Life Sciences: The Molecular and Cellular Biology of Wound Repair, vol. 78 Research Triangle Park, NC: The Scientific Research Society; 1990, pp. 273–274 [Google Scholar]
- 24.Sheena S, Salud C, Yap-Silva C, et al. . A retrospective review of the dermatologic manifestations of chronic arsenic poisoning in the Philippines. Int J Dermatol 2017:56;721–725 [DOI] [PubMed] [Google Scholar]
- 25.Gentry PR, Clewell HJ, III, Greene TB, et al. . The impact of recent advances in research on arsenic cancer risk assessment. Regul Toxicol Pharmacol 2014: 69;91–104 [DOI] [PubMed] [Google Scholar]
- 26.Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric 2005:8;110–123 [DOI] [PubMed] [Google Scholar]
- 27.Calvin M. Oestrogens and wound healing. Maturitas 2000:34;195–210 [DOI] [PubMed] [Google Scholar]
- 28.Emmerson E, Hardman MJ. The role of estrogen deficiency in skin ageing and wound healing. Biogerontology 2012:13;3–20 [DOI] [PubMed] [Google Scholar]
- 29.Hall G, Phillips TJ. Estrogen and skin: The effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol 2005:53;555–568 [DOI] [PubMed] [Google Scholar]
- 30.Howgate DJ, Gamie Z, Panteliadis P, et al. . The potential adverse effects of aromatase inhibitors on wound healing: In vitro and in vivo evidence. Expert Opin Drug Saf 2009:8;523–535 [DOI] [PubMed] [Google Scholar]
- 31.Campbell L, Emmerson E, Davies F, et al. . Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J Exp Med 2010:207;1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emmerson E, Rando G, Meda C, et al. . Estrogen receptor-mediated signalling in female mice is locally activated in response to wounding. Mol Cell Endocrinol 2013:375;149–156 [DOI] [PubMed] [Google Scholar]
- 33.Midgley AC, Morris G, Phillips AO, et al. . 17 Beta-estradiol ameliorates age-associated loss of fibroblast function by attenuating IFN gamma/STAT1-dependent miR-7 upregulation. Aging Cell 2016:15;531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pincus DJ, Kassira N, Gombosh M, et al. . 17 Beta-estradiol modifies diabetic wound healing by decreasing matrix metalloproteinase activity. Wounds 2010:22;171–178 [PubMed] [Google Scholar]
- 35.Plackett TP, Gregory MS, Kovacs EJ. Effects of high estrogen levels on monocyte chemoattractant protein-1 and wound healing. Adv Wound Care 2015:4;92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Y, Li P, Zhao J, et al. . Arsenic trioxide regulates the production and activities of matrix metalloproteinases-1, −2 and −9 in fibroblast THP-1. Chine Med J 2012:125;4481–4487 [PubMed] [Google Scholar]
- 37.Olsen C, Liguori A, Zong Y, et al. . Arsenic upregulates MMP-9 and inhibits wound repair in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2008:295;L293–L303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chayapong J, Madhyastha H, Madyastha R, et al. . Arsenic trioxide induces ROS activity and DNA damagae, leading to G0/G1 extension in skin fibroblasts through the ATM-ATR-associated Chk pathway. Environ Sci Pollut Res Int 2017:24;5316–5325 [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi Y, Madhyastha H, Madhyastha R, et al. . Arsenic acid inhibits proliferation of skin fibroblasts and increases cellular senescence through ROS mediated MST1-FOXO signaling pathway. J Toxicol Sci 2016:41;105–113 [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Mukherjee TK, Guptasarma P. Arsenic 17-β-estradiol bind to each other and neutralize each other's signaling effects. Biochem Biophys Res Commun 2016:477;575–580 [DOI] [PubMed] [Google Scholar]
- 41.Pinto B, Tabor A, Stearns D, et al. . A bench-top in-vitro wound assay to evaluate wound closure. Appl In Vitro Toxicol 2016:2;151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang C, Park A, Guan J. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007:2;329–333 [DOI] [PubMed] [Google Scholar]
- 43.Tabor A, Robinson A, Pinto B, et al. . Platelet rich plasma combined with an electrospun collagen scaffold: In-vivo and in-vitro wound healing effects. Clin Res Dermatol 2016:3;1–8 [Google Scholar]
- 44.Martin P. Wound healing-aiming for perfect skin regeneration. Science 1997:276;75–81 [DOI] [PubMed] [Google Scholar]
- 45.Gribble M, Howard B, Umans J, et al. . Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol 2012:176;865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. 2011. Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention [Google Scholar]
- 47.Young BA, Maynard C, Reiber G, et al. . Effects of ethnicity and nephropathy on lower-extremity amputation risk among diabetic veterans. Diabetes Care 2003:26;495–501 [DOI] [PubMed] [Google Scholar]
- 48.Stevenson S, Taylor AH, Meskiri A, et al. . Differing responses of human follicular and nonfollicular scalp cells in an in vitro wound healing assay: Effects of estrogen on vascular endothelial growth factor secretion. Wound Repair Regen 2008:16;243–253 [DOI] [PubMed] [Google Scholar]