Abstract
The importance of active, participant-centred monitoring of adverse events following immunisation (AEFI) is increasingly recognised as a valuable adjunct to traditional passive AEFI surveillance. The databases OVID Medline and OVID Embase were searched to identify all published articles referring to AEFI. Only studies which sought participant response after vaccination were included. A total of 6060 articles published since the year 2000 were identified. After the application of screening inclusion and exclusion criteria, 25 articles describing 23 post-marketing AEFI systems were identified. Most countries had a single system: Ghana, Japan, China, Korea, Netherlands, Singapore, Brazil, Cambodia, Sri Lanka, Turkey and Cameroon except the USA (2), Canada (4) and Australia (6). Data were collected from participants with and without AEFI in all studies reviewed with denominator data enabling AEFI rate calculations. All studies considered either a single vaccine or specified vaccines or were time limited except one Australian system, which provides continuous automated participant-centred active surveillance of all vaccines. Post-marketing surveillance systems using solicited patient feedback are emerging as a novel AEFI monitoring tool. A number of exploratory systems utilising e-technology have been developed and their potential for scaling up and application in low and middle income countries deserves further investigation.
Keywords: Adverse events, AEFI, Immunisation, Post-marketing surveillance, Technology, Vaccine safety
Introduction
Vaccination programmes contribute considerably to global health by providing protection against many important transmissible infections.1 Vaccines are a unique pharmaceutical product because they are recommended for nearly everyone in the community. As they are administered to people who are generally well, they are held to a higher level of safety than therapeutic drugs.2
For optimal disease control and community protection high immunisation rates are required. To achieve optimum coverage a high level of public confidence in vaccines and the vaccine program is required in all settings.3 Public confidence is tested during the introduction of new vaccines and as an immunisation programme matures. For example, concerns about new vaccines that do not yet have a known post-marketing safety profile may inhibit uptake, while as vaccine preventable disease (VPD) incidence decreases due to high sustained immunisation coverage, adverse events can become more common than the disease prevented, leading to paradoxically heightened vaccine safety concerns.
With accelerating introduction of underutilised vaccines in low and middle income countries (LMICs) and the development of novel vaccines for neglected diseases, such as malaria and dengue, the need for flexible low cost and integrated adverse events following immunisation (AEFI) surveillance systems in LMICs has emerged.4
Post-marketing AEFI surveillance is conducted by regulators and public health authorities to identify safety signals that require further investigation.5,6 Post-marketing surveillance has traditionally relied on passive (or spontaneous) reporting from consumers and health providers. Passive surveillance has certainly proven useful in the past. For example, in 1998, passive surveillance in the United States detected a fourfold increase in the number of intussusception cases expected after the administration of the Rotashield rotavirus vaccine and the manufacturer voluntarily withdrew the vaccine from the market.7 Passive reporting is the cornerstone of post-licensure AEFI surveillance because of ease of implementation, relatively low cost and ability to capture unexpected events.8 However, passive AEFI surveillance systems under report, have low sensitivity and do not allow risk estimate calculation.9 The 2015 Global Vaccine Safety Initiative meeting identified that the low rates of passive AEFI reporting are a significant barrier to detecting safety signals.10
Both LMICs and high income countries have invested in novel methods of enhancing AEFI surveillance. For example the roll out of conjugate meningococcal A vaccine (PsA-TT, MenAfriVac) in the sub-Saharan African meningococcal belt was supported by enhanced AEFI surveillance with reporting from sentinel health services in Burkina Faso and Mali.11,12 In Australia, the Paediatric Active Enhanced Disease Surveillance System has used sentinel surveillance at five participating paediatric hospitals to routinely screen for intussusception, seizures and acute flaccid paralysis as possible AEFIs.13 In New South Wales, Australia, word recognition algorithms search Emergency Department admission triage notes for mention of immunisation to detect possible adverse events.14
Sophisticated data linking systems in the United States are conducted at scale, linking vaccine histories and clinical presentations.15 Vietnam has established the first large linked database in a developing country, able to provide AEFI detection in real time and report rate ratios for observed medical events in the 60 days following measles vaccination in one province.16
Data linkage, however, has limitations because it requires considerable expertise and resources, access to data is often delayed, and privacy, legislative and ethical requirements are a barrier. Hence, this cannot be used for population level roll-out of new vaccines or older vaccines in potentially higher risk populations. Thus, attention has turned to systems that engage the vaccine recipient. We conducted a narrative review of the array of active AEFI surveillance systems from around the world published since 2000, which elicit data directly from the vaccinee or their parent or carer. We aimed to catalogue methods of active, participant-centred AEFI monitoring and describe how these approaches improve the understanding of vaccine safety.
Methods
Literature searches
The review aimed to include studies that described systems that had active contact with the participant after vaccination for AEFI surveillance. A set of focussed searches were conducted by an experienced medical librarian (author CK) to identify published manuscripts describing vaccine safety surveillance systems. The bibliographic databases OVID Medline (2000 to September Week 2 2016) and OVID Embase (2000 to Week 39 2016) were searched, with the final search completed on 25 September 2016. Database thesaurus terms used included ‘Immunization’, ‘Immunization programs’, ‘Vaccines’, ‘Safety’, ‘Adverse drug reaction reporting systems’, ‘Product surveillance, Post-marketing’, ‘Risk’ and ‘Drug evaluation’. Where possible, thesaurus term subheadings including ‘Adverse effects’ and ‘Complications’ were applied to further focus these terms. Matching textword terms, including ‘Vaccine safety’, ‘Adverse effect’, Adverse event’, ‘Adverse outcome’, ‘Post-marketing surveillance’, ‘Post-marketing monitoring’, ‘Postlicensure surveillance’, ‘Postlicensure monitoring’ and ‘AEFI’, were also used to maximise retrieval. Truncation was employed to ensure terms with variant endings were also identified. The results were limited to ‘Human’ and published since 2000 but no date or language limits were applied. A copy of the search strategy used is available upon application to the authors.
In addition, hand searching of references in selected articles was conducted to ensure that no relevant papers had been missed.
Inclusion and exclusion criteria
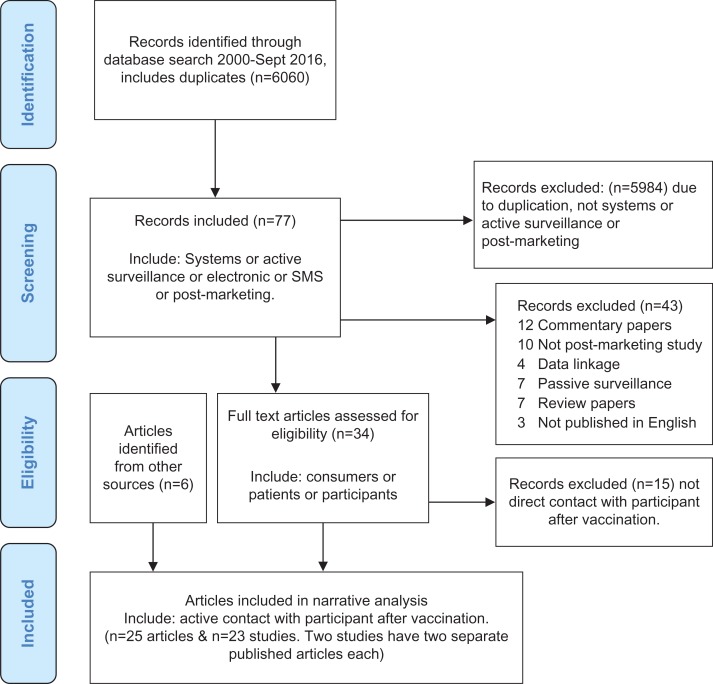
The abstracts of the articles retrieved from the database searches were screened against general inclusion criteria (Figure 1): 1. system or 2. active surveillance or 3. use of electronic or SMS, or 4. post-marketing surveillance AND general exclusion criteria 1. data linkage or 2. review papers or 3. not published in English or 4. sentinel active surveillance or 5. phase III trials.
Figure 1.
Inclusion and exclusion criteria study diagram of literature review for participant-centred active surveillance of adverse events following immunisation (AEFI).
Data extraction
The following data were extracted across the included studies: study population, setting, country, high versus LMICs, outcome measures and nature of surveillance system. Data extraction was consistently performed by the lead author (PC).
Search quality assessment
Quality assessment of the included studies was performed by critically appraising individual studies for risk of bias. The following aspects of individual studies were assessed by the lead author (PC); whether the research questions were well defined, sample representativeness, appropriateness of study design, clear and appropriate data analysis, and whether any confounders were accounted for.
Results
The focused database search located 6060 articles including duplicates. Application of inclusion criteria reduced the search to 77 articles and this decreased further to 34 articles after application of exclusion criteria. The 34 full text articles were reviewed to identify studies with active participant contact after vaccination for AEFI surveillance. Twenty-five articles describing 23 post-marketing AEFI systems were identified. Two systems had two published papers each describing different aspects of the project. Other papers were based on a common system but described discrete studies (Figure 1).
There were single studies from Ghana, Japan, China, Korea, Netherlands, Singapore, Brazil, Cambodia, Sri Lanka, Turkey, Cameroon, two from the USA, four from Canada and six from Australia (Table 1). Most of the active participant-centred AEFI surveillance in LMICs were by diary card, while in high income countries they were by SMS and/or web based. However, an SMS system was set up in the Cambodian17 study and the project in Brazil18 used email and telephone survey. The Cameroon19 study investigated the innovative low cost method of notifying an AEFI by an unanswered telephone call described as telephone ‘beep’ from participants. There were participant-centred active AEFI systems used in all WHO Regions except the Eastern Mediterranean Region; one project in the South-East Asia Region in Sri Lanka20; two in the African Region in Ghana21 and Cameroon19; two in the European Region in Turkey22 and The Netherlands23; seven in the Region of the Americas; and 11 in the Western Pacific Region.
Table 1.
The features of the papers identified from 2000 to September 2016 in literature review to have active contact with participant after vaccination for surveillance of AEFI (n=23)
| Year Published | n | Data on all vaccinated. Reactions and non-reactions | Cohort & age | Contact participant method | Response to surveillance rates | When surveillance occurred post- vaccination | Vaccine | Strengths | Weaknesses | Finding | Author | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | Two sites n=715 & n=822 | Yes | Active adult military personnel | Web or telephone | 66% & 86% | For 28 days after vaccination | Smallpox vaccine |
|
|
Electronic monitoring acceptable | Olmsted38,42 | US |
| 2007 | 406 | Yes | Infants at 4 clinics | Pink diary card & clinic interview & medical records | 91% completed the study | Diary card collected 4 weeks after final vaccination | DTPaHBVHiBx3 |
|
Small sample n=406. Descriptive | Agreement with other studies | Dodoo21 | Ghana |
| 2009 | n=8700 in study group. n=4130 control | Yes | Children <3 years in 6 cities | Postcards | 44% returned postcards (study group) 32% returned postcards(control group) | 2 weeks after vaccine | Oral polio vaccine | Comparison group | Unable to evaluate parental assessment | Mild diarrhoea in OPV group | Sugawara57 | Japan |
| 2010 | 95 244 | Yes | Children >4 years and adults from 245 schools | Diary cards and telephone interview | 31.2% by diary card. (20% Sample of persons not returned card telephoned) | Diary card complete days 1, 2, 3 & 7 after vaccination | 2009 Pandemic H1N1 |
|
Convenience sample limited to school students and family | Pandemic influenza vaccine had similar safety profile to seasonal vaccine | Wu25 | China |
| 2011 |
|
Yes | Children and adults | Computer assisted telephone interview | Not reported – CATI survey has data for 100% | Not reported | 2009 Pandemic influenza adjuvanted and non-adjuvanted |
|
|
Safety demonstrated | Choe26 | Korea |
| 2011 | 3569 | Yes | Adults >60 years at risk of influenza and staff | Email with web questionnaire | 5% lost to follow up for first email survey | 1 week after 1st vaccine and approximatly 1 week after 2nd vaccine. Third questionnaire 3 months after first questionnaire. | 2009 Pandemic vaccine |
|
|
One third reported AEFI | Harmark23 | Netherlands |
| 2011 | 2590 & 702 | Yes | Infants | Diary cards for 30 days after each dose and telephone call 6 mths after final dose | 96% completed study. Details of withdrawals provided | Diary card for 4 days after each dose AND telephone call 6 months after last study vaccine | DTPa-IPV/HiB × 4 plus Hep B × 3 and Rotarix Vs DTPa-HBV-IPV/HiB × 2 and DTPa-HPV-IPV/HiB × 1 plus Hep B × 2 and Rotarix |
|
|
Demonstrated safety of combination vaccines | Lim24 | Singapore |
| 2013 | 906 | Yes | Persons >60 years | Email and telephone | 84.7% interviewed | Interview 14 days after vaccine | Yellow fever vaccine |
|
Wide range of interval from vaccination to interview (6 to 155 days) | Pre-immunisation screening for YF vaccine in >60-year-olds | Miyaji18 | Brazil |
| 2013 | 184 | Yes | Adults >18 years | SMS | 71.9% replied. 54.9% immediate SMS reply & 16.8% SMS response after additional prompts | 48 hours after vaccine | All/any |
|
|
Proof of concept | Baron17 | Cambodia |
| 2014 | 9798 | Yes | Children | Self-administered questionnaire & diary card & MO visit and interview if report symptoms | 96.2% completed questionnaire & diary cards | Diary card for 2 weeks | Mouse-brain derived Japanese Encephalitis vaccine |
|
Authors report some incompleteness of self-assessment | AEFI incident rate several-fold higher than National passive surveillance rate | De Alwis20 | Sri Lanka |
| 2014 | 3281 & ongoing | Yes | All | Automated SMS tool | 72.6% responded by SMS. >80% responded within 24 h | Automated SMS 3 days after vaccination | All |
|
AEFI linked to vaccine by timing not causally linked | Complete & automated active AEFI surveillance system. Real time and rapid signal detection. | Leeb28 & second study same system by Westphall27 | Australia |
| 2014 | 477 | Yes | Children 6 months to <10 years | Automated email or SMS with link to web-survey | 57% & 61% response to online survey | Day 3 and day 42 after vaccination | Inactivated Influenza Vaccine |
|
|
Online system automated. Data quickly to public health authorities for rapid analysis. | Cashman30 | Australia |
| 2014 | 1230 | Yes | Children 6 months to 18 years | Email contact with online survey | 72% online plus 11% by phone | Day 8 after vaccination | Trivalent influenza vaccine and live attenuated intranasal vaccine |
|
Number of people approached but declined not recorded | AEFI rates lower than clinical trials and close to the rates for national passive surveillance | Bettinger37 | Canada |
| 2014 | 3,173 | Yes | Pregnant women | SMS with telephone survey for those with reporting symptoms | 83.6% replied to SMS | Day 7 after vaccination | Trivalent Influenza Vaccine |
|
May not be representative study group | Mobile phone enabled efficient timely surveillance | Regan29 | Australia |
| 2014 | 1422 | Yes | Infants | Diary card and telephone call 1 month after each dose | Not reported | Diary card for 3 days and telephone call 1 month after each dose | DTwP and DTaP |
|
Too small to detect rare events | DTaP less reactions than DTwP in infants | Korkmaz22 | Turkey |
| 2014 | 530 | Yes | Infants | SMS prompt for temperature SMS reply from parents for 7 days | 95.1% day 1 decreasing daily to 79.6% day 7 | Nightly for 7 days after vaccination | TIV and PCV 13 compared to TIV or PCV13 |
|
Single symptom | New finding of increased risk of fever with these concomitant vaccines TIV & PCV | Stockwell36 | USA |
| 2015 | 1086 pregnant & 314 non-pregnant | Yes | Pregnant women | SMS | 86% replied by SMS | Day 7 after vaccination | Influenza vaccine | Comparison group | Potential reporting bias | Influenza vaccine AEFI similar in pregnant and non-pregnant women | Regan34 | Australia |
| 2015 | 22 080 | Yes | Health care workers | Email with link to online survey | 68.7% | Day 8 after vaccination | Influenza vaccine |
|
HCW not representative of community for AEFI or Web survey completion | Rapid evaluation in light of safety signal | Bettinger32 | Canada |
| 2015 | 3340 | Yes | Children | SMS and email | 75% participation | Day 3 after vaccination | Trivalent Influenza Vaccine |
|
Interpret parental reports with care | National system. Rapid real time feedback to inform program rollout | Pillsbury31 | Australia |
| 2015 | 236 study group & 235 controls | No | Children | Telephone call from investigators. Response by ‘beep’ phone call not picked up | Unknown | Surveillance for 30 days after vaccination | Routine childhood EPI vaccines |
|
Mostly urban not rural participants | Telephone ‘beep’ increases community based AEFI reporting | Tsafack19 | Cameroon |
| 2016 | 76 | Yes | Adult hospital staff and family | App | 63% downloaded app. 50% completed all surveys | Day 8 and day 30 after vaccination | Influenza vaccine | App developed |
|
Proof of concept that app to demonstrate technology is functional | Wilson39 | Canada |
| 2016 | 5155 | Yes | Pregnant women | SMS | 84.3% replied by SMS | Day 7 after vaccination | TIV and dTpa |
|
AEFI data collection by SMS differs to other methods – further investigation required | Safety data supports antenatal vaccination | Regan35 | Australia |
| 2016 | 987 | Yes | University students and staff | Email with link to online survey | 33% | 8–10 days after each dose | Meningococcal B vaccine - 4CMenB | Support of emergency vaccine programme | Emergency response so full methodology previously developed unable to be employed | Medically attended events more frequent than in clinical trial data but local reactions consistent with previously reported | Langley33 | Canada |
Data collection methods used to contact the participant after vaccination included using diary cards (n=5), postcards (n=1), a computer assisted telephone survey (CATI) (n=1), an unanswered phone call signal (n=1), an online survey (n=8), SMS contact alone (n=6) and development of a mobile app (n=1). Many of the systems also used telephone calls for survey or for case follow-up if alerted by a SMS or web mechanism. The three studies investigating a change in the infant immunisation schedule from Ghana (n=406),21 Singapore (3292)24 and Turkey (n=1422)22 all used diary cards given to parents to record AEFI. Ten studies primarily investigated influenza vaccine alone, three of which investigated a 2009 H1N1 pandemic vaccine; i.e, China (n=95 244),25 Korea (n=9000)26 and the Netherlands (n=3569).23
All systems functioned to allow active post-marketing surveillance of a single vaccine or specific vaccine schedule change except the pilot study in Cambodia,17 which investigated all vaccines given to a cohort of adults, the study in Cameroon,19 which investigated Expanded Program on Immunization vaccines for infants and the SmartVax27,28 project in Australia which developed a tool to provide continuous automated patient-centred active surveillance of all vaccines administered.
Vaccine safety questions were addressed by surveying a specific risk cohort or by investigating a specific vaccine for known AEFI or concerns raised from passive surveillance. The yellow fever vaccine study in Brazil was conducted to examine viscerotropic events in a specific cohort of people over 60 years of age.18 The Fastmum29 system in Western Australia was established to provide influenza and pertussis containing vaccine safety data from and for pregnant women. The Japanese encephalitis vaccine study in Sri Lanka was conducted to investigate a rise in allergic reactions and seizures identified by the country's passive surveillance system and the attendant public vaccine safety concerns.20
Several of the systems utilised real-time active surveillance using rapid vaccinee responses to SMS or email surveys. The Smartvax Australian system reported that 80% of SMS replies were received within 2 hours.28 The web-based systems in the Netherlands23 and Vaxtracker30 in Australia were able to perform interim analysis in near real time enabling timely AEFI monitoring. The Australian AusVaxSafety project provided weekly analysis and reporting during the influenza season of real time safety data for Australian consumers and medical interests and in addition was able to publish seasonal vaccine experience in a timely way for authorities in the northern hemisphere in 2015 when the influenza vaccine strains in the ensuing influenza season were the same in both hemispheres.31 The Canadian health care worker influenza system was able to investigate a safety signal, detected in another country, in real time of the implicated product.32 The Canadian system was also able to adapt rapidly and be deployed for AEFI surveillance of meningococcal B vaccine (4CMenB, Bexsero) during a meningococcal B outbreak in university students.33
Response rates to surveys varied. Traditional diary card response rates varied from 31.2% in the Chinese study to 96% in the Sri Lankan study. The Chinese study was very large (n=95 244) with participants being performers in a civic parade immunised with the H1N1 pandemic vaccine. Although the return rate was only 31.2% it was still a large study with 29 710 returned diary cards and a further random sample of 20% of persons not returning the card contacted by telephone call adding another 11 603 interviews.25 The high return rate of the Sri Lankan study of 96% was achieved by the trained surveyors, who distributed and explained the questionnaire to parents/guardians, visiting the house of each participant to collect the questionnaires.20 Response rates for SMS contact had a narrower range from 72% to 91%. Regan et al. conducted three studies involving pregnant women in Western Australia with consistently high response rates of 83% to 86%.29,34,35 The small pilot study in Cambodia also sent SMS to vaccinated adults and achieved a response rate of 54.9% with another 16.8% responding after additional SMS prompts. The paper by Westphal et al. defines response rates for the SmartVax system in Australia as the proportion of patients who responded to the clinic's SMS with a reply SMS and reports a response rate of 74.2%, which were timely with 81.3% of replies received within 2 hours; 82.2% of people who responded ‘no’ to any reaction responded within 2 hours compared to 73.0% of people who reported ‘yes’ to any reaction.27 The study by Stockwell et al. in New York also used text message to prompt for participant temperatures on days 1 to 7 after vaccination; the response rate decreased over the 7 days from 95.1% on day 0 to 79.6% on day 7.36 Two studies used email to contact participants over 60 years of age; the Dutch23 study investigating H1N1 vaccine and had a response rate of 94.5% and the Brazilian18 yellow fever vaccine study using telephone and electronic mail did not a report response rate. The Canadian influenza vaccine online survey reported 72% responding online and an additional 11% were contacted by telephone.37 The online survey sent to university students for the follow up of meningococcal vaccine AEFIs at a university was the lowest with 33% responding to the online survey.33
The US programme of smallpox vaccination for military personnel published a separate analysis of patient experience of reporting vaccination-associated responses with an electronic (web-based) monitoring system. For smallpox vaccination, CDC guidelines instruct the vaccinee to maintain a written daily diary of symptoms for 28 days. These researchers replaced the written diary with a secure website or a call to an automated password protected telephone system to record their data daily. As well as the advantage of physician real time tracking, 84% of respondents reported a preference for electronic vaccine monitoring.38 Bettinger et al. in Canada surveyed respondents about the online AEFI survey after vaccination linked from an email; 98% rated the online survey easy to access and 99% easy to understand.37 Most (76%) online respondents used a computer to complete the survey. The Canadian proof of concept study for the mobile app included a usability survey, and although only 21 participants completed this survey, 86% preferred an app to online survey on a computer. The authors noted that only 63% of recruited participants successfully downloaded the app and logged in but also noted that the access to app technology is changing continually and that the sample of participants who completed the usability survey was more comfortable trying new technologies than the general public.39 A specific strength of all the systems reviewed was the collection of data from people reporting and not reporting AEFI, providing a denominator thus enabling rate calculation. There was, however, no consistent approach to keeping a count of all the people approached to participate in the active surveillance.
The authors of most the papers listed sample representativeness and selection bias as potential biasing weaknesses of their systems, with the Cambodian17 and Cameroon19 study indicating participants were more likely to be urban than rural. Certain authors cautioned about the interpretation of parental or vaccinee reporting of unverified symptoms. Three studies (New York,36 Singapore24 and Sri Lanka20) provided thermometers to participants to increase accuracy of temperature monitoring.
Discussion
Participant-centred active AEFI surveillance is an expanding method of post-marketing vaccine pharmacovigilance. Given the explosion in e-communication technology it is possibly surprising that there have only been 15 e-technology based attempts using 10 different systems at active AEFI surveillance found in this review. This appears to be an under-utilised opportunity for signal detection and deserves acceleration and scaling up based on the experience of the systems reviewed here, and also local context and resources.
Passive surveillance is integral to AEFI reporting but has the shortcomings of under-reporting, reporting bias and not being timely. Active surveillance by data linkage is established in the US and in one province in Vietnam. Bettinger et al. report that Vaccine Safety Datalink is not feasible in Canada as medical and immunisation records are not linked so they have developed systems for active AEFI surveillance by direct survey of participants now formalised as the Canadian National Vaccine Safety Network (CANVAS).37 This work is conducted annually with results succinctly communicated online. The high rates reported for ease of use is a strength of the study and encourages ongoing participation to support the system design of having the current year's influenza vaccine participants sent an online survey prior to the following season's influenza vaccine to act as controls.32,40
The emergence of systems directly approaching vaccinees or their parents/carers to address specific vaccine concerns relies on the relative simplicity of canvasing people directly for AEFI post-marketing surveillance. The established method of using diary cards supplemented with visits and telephone calls is useful but requires considerable resourcing with trained telephone survey operators. The systems identified in this review using diary cards were large scale coordinated studies. The use of e-technology has become more common with the initial attempts taking small scale study proof-of-concept approach with systematic and networked systems of AusVaxSafety31 and CANVAS32,40 more evident in the more recent papers reviewed here. The first SMS system identified in this review was used successfully with adult participants in Cambodia. SMS to health care workers in Cameroon has been deployed to stimulate and encourage MenAfriVac AEFI reporting.41 The oldest study from 2005 encapsulated the changes in soliciting participant involvement; the CDC recommended that daily participant monitoring after smallpox vaccine for 28 days by written diary record should be replaced by electronic monitoring and it enjoyed a high degree of acceptance.42
From this review it appears that building an information technology system to automate contact with vaccinees to solicit AEFI data has only occurred in Australia with the SmartVax27,28 and Vaxtracker30 systems. It is probable that there are other such systems in various stages of development around the world but they are currently unpublished. New work in this area by CANVAS in Canada has explored the use of an app instead of SMS or email prompts for participants to complete an AEFI surveillance survey. Participants are prompted on day 8 and day 30 by the app but can also report events spontaneously throughout the follow-up period.43
It is not surprising that there are more systems in Australia than any other country. The growth of active post-marketing surveillance activity in Australia is a response to the excess fever and febrile convulsions caused by the Australian manufactured trivalent influenza in 2010 followed by a forensic review by the Commonwealth government.44 This work is now coordinated nationally under the AusVAxSafety system.31 One of the Canadian papers referred to this experience in Australia and the 6 weeks taken to detect and investigate the safety signal was motivation to develop a Canadian real time vaccinee centred approach.29
Influenza vaccine safety is of particular concern to public health authorities. The short lead time to manufacture a new influenza vaccine to accommodate strain changes necessitates marketing with limited new annual safety data.45 We found pandemic and seasonal influenza vaccines the most commonly investigated in this review. Post-marketing surveillance by direct approach to people who have received influenza vaccines enables early safety signal detection.
Active surveillance is limited by the numbers of participants and does not have the power to detect very rare events in any of the included studies. However, the study of pandemic influenza vaccine from China25 vaccinated and followed up 95 244 recipients and separately conducted active surveillance for neurological conditions at all Beijing hospitals for 10 weeks after vaccination finding 27 cases of Guillain–Barré syndrome none of whom had the pandemic influenza vaccine and none of the study vaccine recipients followed through active surveillance identified a neurological condition.
Determining post-marketing AEFI rates by actively seeking participant input after vaccination is a methodology similar to phase III pre-licensure trials, which enables public health authorities and regulators to ensure a vaccine is performing in the community as anticipated. In a limited number of countries with good routine administrative systems, national immunisation programmes utilise vaccine distribution data or immunisation registers to provide a proxy denominator for AEFI rate calculation. This is not available for all countries. Post-marketing active AEFI surveillance by direct contact with participants can provide a timely denominator and the resultant calculation of AEFI rates as well as the detection of non-medically attended events allows comparison with pre-licensure trial data. Non-medically attended AEFI can be much more common and possibly provide an early vaccine safety signal. Equally importantly, collection of this data, particularly in the early stages of validating a specific active surveillance system, allows comparison with the rates of common vaccine-associated adverse events in clinical trials, validating the sensitivity of the system under investigation. Patient-centred AEFI surveillance improves reporting rates with active surveillance also eliciting more reports of minor events.46 The study from Sri Lanka followed up 9798 people for two weeks following Japanese encephalitis vaccine by self-reporting and found the incidence rate of AEFI was several-fold higher than through the national AEFI surveillance system.20 Bettinger et al.37 reported that post vaccination events such as fever were less common than reported in clinical trials and in line with Canadian passive surveillance whereas the Vaxtracker30 system in Australia found that the solicited nature of AEFI reporting in clinical trials and in participant-centred post-marketing active surveillance delivered similar rates for fever and local reactions. Further work needs to be done on the meaning and interpretation of the data generated on both expected vaccine reactions and AEFI from these systems approaching participants after vaccination and the appropriate comparison data.
Vaccine hesitancy and public concern about vaccine safety is a global issue. In Australia, consumer confidence in influenza vaccination diminished after the safety issues with the 2010 influenza vaccine in children.47 Actively seeking the input of consumers renders the gathering of AEFI data more transparent to the public and trustworthy. In the study identified from Ghana, the authors discuss the importance of public confidence in immunisation programme safety by having adequate reporting systems to support the Expanded Program on Immunisation.21 Participant-centred active AEFI surveillance can be small scale and able to be initiated where no AEFI monitoring was taking place. The authors of the project in Cambodia note that there was no functional pharmacovigilance programme in Cambodia at the time they conducted the SMS active surveillance pilot.17
Under reporting of AEFI by medical professionals may lead to doubts about vaccine safety reassurances by public health authorities. Consumers reporting directly to passive AEFI surveillance systems provide a different perspective to reports made by medical professionals. Consumer AEFI reporting has been shown to be reliable in a review in the Australian state of Victoria; consumers were 5% more likely to describe a serious AEFI that resulted in specialist clinic attendance than reports from health care professionals.48 Also patient reporting has been shown to concord with medical record review in the assessment of febrile seizures following vaccination in young children and the use of both sources was considered complementary.49 It is possible that safety data, which is actively sourced from consumers improves public perception that the data is trustworthy because the data collection process is more transparent and potentially less subject to health professional positive bias towards vaccination. The AusVaxSafety and the CANVAS programmes of participant-centred automated active surveillance make the data publically available on the web to close the feedback loop and further improve transparency aiming to bolster public trust in immunisation.40,50 The timely collection of AEFI data and potential signal detection occurring in the public gaze enable and ensure an appropriate and timely public health response.
A weakness mentioned by many of the authors in this review was selection bias. These were studies with a distinct vaccinated cohort and several authors were concerned that the study population may not be representative of the wider population such as canvasing input from urban rather than rural consumers. The same has been demonstrated in established systems which are open to consumer reporting; a CATI survey of consumers who reported AEFI to the passive system in South Australia found that awareness of the surveillance system did not increase reporting but was associated with demographic features such as being born in Australia.51 In designing future participant-centred active AEFI surveillance, selection bias is an important consideration. Both the cohort of vaccinated people and the people responding to the surveillance need to be representative of the target group for vaccination. For small focused vaccine safety projects addressing a particular concern randomisation of participants would be important. Another approach would be to embed the active surveillance into routine immunisation practice as has been achieved by the Smartvax system in a large and growing number of general practices throughout Australia, so that the range and number of consumers given the opportunity to participate would be vast thus improving representativeness and signal detection.
Advances in e-technology provides opportunity for both active and passive AEFI surveillance to gather input from the public. Consumer reporting to passive surveillance systems for adverse drug reactions including vaccines is being encouraged in Europe and technology is facilitating consumer involvement. For example, in Europe three smartphone apps have been developed by WEB-RADR to enable consumers and healthcare professionals to report adverse drug reactions to national passive systems and receive information and alerts. The Yellow Card system in the UK now has a Yellow Card app and two more apps have subsequently been developed for Netherlands (LAREB) and Croatia (HALMED).52
As this was a narrative, rather than systematic review, it did not search grey literature sources. The authors are aware of at least one active AEFI system that has not yet published its findings and thus there may be other start up or as yet unpublished projects gathering data that are not represented in this review.53 In a global survey of AEFI surveillance systems for pregnant women and their infants, six countries provided additional information on active systems five of which were unpublished.54 In addition, one published, short-term active surveillance study by nurse telephone call following trivalent influenza vaccine in Australia was not located in the very focussed search for this narrative review.55
Vaccine safety and pharmacovigilance is an essential element of immunisation programmes around the world. The changes in global vaccination with a shift towards vaccine development for health issues affecting LMIC and the shift towards vaccine manufacture in LMIC has also moved the vaccine safety focus to LMIC's. WHO has developed the Global Vaccine Safety Blueprint to help establish vaccine safety systems in all countries.4 Individual systems identified through this review demonstrated sustainability, flexibility, affordability and timeliness, which are the essential elements that have been identified for vaccine safety monitoring internationally.56 Participant-centred active surveillance offers a unique contribution and can potentially be embedded into post-licensure monitoring enabled by advances in technology.
Conclusions
Public health authorities require near real-time sensitive post-marketing AEFI surveillance systems to ensure public safety and public confidence in vaccines. Passive surveillance is the cornerstone of vaccine safety but has limitations of under reporting and imprecise risk estimates. Active surveillance can offer more sensitive surveillance, timely signal detection and provides phase IV (i.e., post-marketing safety) data for regulators and public health authorities. By having active surveillance, which directly surveys the consumers in near real time and makes the results publically available, active surveillance systems address transparency concerns and contributes to public confidence in the whole immunisation programme. A number of exploratory systems utilising e-technology have been developed and their potential for scaling up and application in developing settings deserves further investigation.
Acknowledgments
Authors’ contributions: PC: data extraction, analysis and draft manuscript. KM: advice on study design, analysis and manuscript review. GK: advice and manuscript review. CK: literature search, advice on study design and manuscript review. MG: advice and manuscript review. DD: advice on study design, analysis and manuscript review. All authors approved the final version submitted for review. PC is guarantor of the paper.
Funding: None.
Competing interests: KM, MG, DD and PC are involved in the Ausvaxsafety study described here. DD and PC are involved in the Vaxtracker study described here but have no financial interest or potential financial gains.
Ethical approval: Not required.
References
- 1. UN News on Millennium Development Goals. http://www.un.org/millenniumgoals/ [accessed 9 January 2017].
- 2. Offit PA, DeStefano F.. Vaccine Safety In: Plotkin S, Orenstein W, Offit P (editors), Vaccines 6th ed Elsevier: Saunders; 2013. [Google Scholar]
- 3. Ali M, Cahn DG, Clemens JD et al. The vaccine data link in Nha Trang, Vietnam: a progress report on the implementation of a database to detect adverse events related to vaccinations Vaccine 2003;21:1681–6. [DOI] [PubMed] [Google Scholar]
- 4. Amarasinghe A, Black S, Bonhoeffer J et al. Effective vaccine safety systems in all countries: a challenge for more equitable access to immunization. Vaccine 2013;31: B108–14. [DOI] [PubMed] [Google Scholar]
- 5. Chen RT, Rastogi SC, Mullen JR et al. The Vaccine Adverse Event Reporting System (VAERS). Vaccine 1994;12:542–50. [DOI] [PubMed] [Google Scholar]
- 6. Chen R, Shimabukuro T, Martin D et al. Enhancing vaccine safety capacity globally: A lifecycle perspective. Vaccine 2015;49:S364–76. [DOI] [PubMed] [Google Scholar]
- 7. Zanardi LR, Haber P, Mootrey GT et al. Intussusception among recipients of rotavirus vaccine: reports to the vaccine adverse event reporting system. Pediatrics 2001;107:E97. [DOI] [PubMed] [Google Scholar]
- 8. Mahajan D, Cook J, Dey A et al. Annual Report: Surveillance of adverse events following immunisation in Australia, 2011. Commun Dis Intell 2012;36: 315–32. [PubMed] [Google Scholar]
- 9. Crawford N, Clothier H, Hodgson K et al. Active surveillance for adverse events following immunization. Expert Rev Vaccines 2014;13:265–76. [DOI] [PubMed] [Google Scholar]
- 10.Olsson S. Achievements and challenges in global vaccine safety. Uppsala Reports 2016;72:25
- 11. Ouandraogo CR, Yameogo TM, Diomande FV et al. Adverse events following immunisation during mass vaccination campaigns at first introduction of a meningococcal A conjugate vaccine in Burkina Faso. Vaccine 2012;30(Suppl. 2):B46–51. [DOI] [PubMed] [Google Scholar]
- 12. Vannice KS, Keital M, Sow SO et al. Active surveillance for adverse events after a mass vaccination campaign with a group A meningococcal conjugate vaccine (PsA-TT) in Mali. Clin Infect Dis 2015;61(Suppl. 5):S493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The PAEDS Network. PAEDS Paediatric Active Enhanced Disease Surveillance http://www.paeds.edu.au/ [accessed 24 May 2017].
- 14. Epidemiology and Research Branch Public Health Real-time Emergency Dept Surveillance System (PDREDSS) Public health Unit Response. http://www1.health.nsw.gov.au/PDS/pages/doc.aspx?dn=GL2010_009 [accessed 24 May 2017].
- 15. Yih WK, Kulldorff M, Fireman BH et al. Active surveillance for adverse events: the experience of the vaccine safety datalink project. Pediatrics 2011;127(Suppl. 1)S54–64. [DOI] [PubMed] [Google Scholar]
- 16. Ali M, Rath B, Thiem VD. Vaccine safety monitoring systems in developing countries: An example of the Vietnam model. Current Drug Safety 2015;10:60–7. [DOI] [PubMed] [Google Scholar]
- 17. Baron S, Goutard F, Nguon K, Tarantola A. Use of a text message-based pharmacovigilance tool in Cambodia: pilot study. J Internet Res 2013;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyaji KT, Luiz AM, Lara AN et al. Active assessment of adverse events following yellow fever vaccination of persons aged 60 years and more. Hum Vacc Immunother 2013;9:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsafack M, Ateudjieu J. Improving community based AEFI (adverse events following immunisation) reporting rate through telephone “beep” in a Cameroon health district: a randomized field trial. Pan Afr Med J 2015;22:351–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Alwis KN, Abeysinghe MRN, Wickramesinghe AR, Wijesinghe PR. A cohort event monitoring to determine the adverse events following administration of mouse brain derived, inactivated Japanese Encephalitis vaccine in an endemic district in Sri Lanka. Vaccine 2014;32:924–30. [DOI] [PubMed] [Google Scholar]
- 21. Dodoo AN, Renner L, van Grootheest AC et al. Safety monitoring of a new pentavalent vaccine in the expanded program on immunisation in Ghana. Drug Safety 2007;30:347–56. [DOI] [PubMed] [Google Scholar]
- 22. Korkmaz HA, Aydin A, Unal B. Comparison of acellular pertussis-tetanus-diphtheria vaccines and whole-cell pertussis-tetanus-diphtheria vaccines in infancy. Paediatr Int Child Health 2014;34:198–202. [DOI] [PubMed] [Google Scholar]
- 23.Harmark L, van Hunsel F, Kak E, van Grootheest. Monitoring the safety of influenza A (H1N1) vaccine using web based intensive monitoring. Vaccine 2009;29:1941–7. [DOI] [PubMed] [Google Scholar]
- 24. Lim FS, Phua KB, Lee BW et al. Safety and reactogenicity of DTPa-HBV-IPV/HiB and DTPa-IPV/HiB vaccines in a port-marketing surveillance setting. Southeast Asian J Trop Med Public Health 2011;42:138–147. [PubMed] [Google Scholar]
- 25. Wu J, Xu F, Lu L et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med 2010;363:2416–23. [DOI] [PubMed] [Google Scholar]
- 26. Choe YJ, Cho H, Song KM et al. Active surveillance of adverse events following immunization against pandemic influenza A (H1N1) in Korea. Jpn J Infect Dis 2011:64;297–303. [PubMed] [Google Scholar]
- 27. Westphal D, Williams S, Leeb A, Effler P. Continuous active surveillance of adverse events following immunisation using SMS technology. Vaccine 2016;34:3350–5. [DOI] [PubMed] [Google Scholar]
- 28. Leeb A, Regan AK, Peters IJ et al. Using automated text messages to monitor adverse events following immunisation in general practice. Med J Aust 2014;200:416–18. [DOI] [PubMed] [Google Scholar]
- 29. Regan AK, Blyth CC, Mak DB et al. Using SMS to monitor adverse events following trivalent influenza vaccination in pregnant women. Aust N Z J Obstet Gynaecol 2014;54:522–8. [DOI] [PubMed] [Google Scholar]
- 30. Cashman P, Moberley S, Dalton C et al. Vaxtracker: Active on-line surveillance for adverse events following inactivated influenza vaccine in children. Vaccine 2014;32:5503–8. [DOI] [PubMed] [Google Scholar]
- 31. Pillsbury A, Cashman P, Leeb A et al. Real-time safety surveillance of seasonal influenza vaccines in children, Australia. Euro Surveill 2015;20:Article 30050. [DOI] [PubMed] [Google Scholar]
- 32. Bettinger JA, Rouleau I, Garlepy MC et al. Successful methodology for large-scale surveillance of severe events following influenza vaccination in Canada 2011 and 2012. Euro Surveill 2015;20:Article 21189. [DOI] [PubMed] [Google Scholar]
- 33. Langley JM, MacDougall DM, Halperin BA et al. Rapid surveillance for health events following a mass meningococcal B vaccine program in a university setting: A Canadian Immunisation Research Network Study. Vaccine 2016;34:4046–9. [DOI] [PubMed] [Google Scholar]
- 34. Regan AK, Tracey L, Blyth CC et al. A prospective cohort study comparing the reactogenicity of trivalent influenza vaccine in pregnant and non-pregnant women. BMC Pregnancy Childbirth 2015;15:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Regan AK, Tracey L, Blyth CC et al. A prospective cohort study assessing the reactogenicity of pertussis and influenza vaccines administered during pregnancy. Vaccine 2016;34:2299–304. [DOI] [PubMed] [Google Scholar]
- 36. Stockwell MS, Broder K, LaRussa P et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr 2014;168:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bettinger JA, Vanderkooi OG, MacDonald MD, Kellner JD. Rapid online identification of adverse events after immunization in children by PCIRN's National Ambulatory Network. Pediatr Infect Dis J 2014;33:1060–4. [DOI] [PubMed] [Google Scholar]
- 38. Olmsted SS, Grabenstein RP, Jain AK, Lurie N. Patient experience with, and use of, an electronic monitoring system to assess vaccination responses. Health Expect 2006;9:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willson K, Atkinson KM, Westeinde J et al. An evaluation of the feasibility and usability of a proof of concept mobile app for adverse event reporting post influenza vaccination. Hum Vacc Immunother 2016;12:1738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Canadian Vaccine Safety Network (CANVAS) Study results. http://cirnetwork.ca/network/national-ambulatory-network/ [accessed 24 May 2017].
- 41. Ateudjieu J, Stoll B, Nguefack-Tsague G et al. Vaccines safety; effect of supervision or SMS on rporting rates of adverse events following immunization (AEFI) with meningitis vaccine (MenAfriVacTM): A randomized controlled trial. Vaccine 2014;32:5662–8. [DOI] [PubMed] [Google Scholar]
- 42. Olmsted SS, Grabenstein JD, Jain AK et al. Use of an electronic monitoring system for self-reporting smallpox vaccine reactions. Biosecur Bioterror 2005;3:198–206. [DOI] [PubMed] [Google Scholar]
- 43. Willson K, Atkinson KM, Westeinde J et al. An evaluation of the feasibility and usability of a proof of concept mobile app for adverse event reporting post influenza vaccination. Hum Vaccin Immunother 2016;12:1738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horvath J. Review of the management of adverse events associated with Panvax and Fluvax. Commonwealth of Australia; Canberra, 2011.
- 45. Kelly HA. Safety and effectiveness of influenza vaccines. Med J Aust 2014;201:560–1. [DOI] [PubMed] [Google Scholar]
- 46. Isaacs D, Lawrence G, Boyd I et al. Reporting of adverse events following immunization in Australia. J Paediatr Child Health 2005;41:163–6. [DOI] [PubMed] [Google Scholar]
- 47. Blyth CC, Richmond PC, Jacoby P et al. The impact of pandemic (H1N1) pdm09 influenza and vaccine-associated adverse events on parental attitudes and influenza vaccine uptake in young children. Vaccine 2014;32:4075–81. [DOI] [PubMed] [Google Scholar]
- 48. Clothier HJ, Crawford NW, Kempe A, Buttery JP. Surveillance of adverse events following immunisation: the model of SAEFVIC Victoria. Commun Dis Intell 2011;35:294–8. [PubMed] [Google Scholar]
- 49. Ackerson BK, Sy LS, Yao JF et al. Agreement between medical record and parent report for evaluation of childhood febrile seizures. Vaccine 2013;31:2904–9. [DOI] [PubMed] [Google Scholar]
- 50. AusVaxSafety Current AusVaxSafety surveillance data. http://www.ncirs.edu.au/vaccine-safety/current-data/ [accessed 24 May 2017].
- 51. Parrella A, Gold M, Braunack-Mayer A. Consumer reporting of adverse events following immunization (AEFI): identifying predictors of reporting an AEFI. Hum Vaccin Immunother 2014;10:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. WEB-RADR Mobile Apps. https://web-radr.eu/mobile-applications-for-adr-submission/ [accessed 24 May 2017].
- 53. STARSS Team STARSS Vaccination Surveillance Study. http://www.adelaide.edu.au/trials/starss/ [accessed 15 December 2016].
- 54. Cassidy C, MacDonald N, Steeneek A et al. A global survey of adverse event following immunization surveillance systems for pregnant women and their infants. Hum Vaccin Immunother 2016;12:2010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wood N, Sheppeard V, Cashman P et al. Influenza vaccine safety in children less than 5 years old, the 2010 and 2011 experience in Australia. Pediatr Infect Dis J 2012:31:199–201. [DOI] [PubMed] [Google Scholar]
- 56. Izurieta HS, Zuber P, Bonhoeffer J et al. Roadmap for the international collaborative epidemiologic monitoring of safety and effectiveness of new high priority vaccines. Vaccine 2013;31:3623–7. [DOI] [PubMed] [Google Scholar]
- 57. Sugawara T, Ohkusa Y, Taya K et al. Diarrhea as a minor adverse effect due to oral polio vaccine. Jpn J Infect Dis 2009;62:51–3. [PubMed] [Google Scholar]