Abstract
Introduction
Viral load (VL) monitoring for people on antiretroviral therapy (ART) is extremely challenging in resource-limited settings. We assessed the VL testing scale-up in six Médecins Sans Frontières supported health centres in Maputo, Mozambique, during 2014–15.
Methods
In a retrospective cohort study, routine programme data were used to describe VL testing uptake and results, and multi-variate logistical regression to estimate predictors of VL testing uptake and suppression.
Results
Uptake of a first VL test was 40% (17 236/43 579). Uptake of a follow-up VL test for patients with a high first VL result was 35% (1095/3100). Factors associated with a higher uptake included: age below 15 years, longer time on ART and attending tailored service delivery platforms. Virological suppression was higher in pregnant/breastfeeding women and in community ART Group members. Patients with a high first VL result (18%; 3100/17 236) were mostly younger, had been on ART longer or had tuberculosis. Out of 1095 attending for a follow-up VL test, 678 (62%) had virological failure. Of those, less than one-third had started second line ART.
Conclusion
This was the first study describing the uptake and results of VL testing scale-up in Mozambique. Identified gaps show patient and programmatic challenges. Where service delivery was customized to patient needs, VL monitoring was more successful.
Keywords: Antiretroviral therapy, HIV, Mozambique, Operational research, Sub-Saharan Africa, Viral load monitoring.
Introduction
To control the HIV epidemic by 2020, the 90-90-90 targets were set by UNAIDS in 2014. These targets require that 90% of the population know their HIV status, 90% of all people with diagnosed HIV infection are receiving sustained antiretroviral therapy (ART) and 90% of all people on ART are virologically suppressed. This implies that every person on ART should have access to viral load (VL) testing.1 From 2013, WHO has recommended yearly routine VL monitoring for all patients who have been on ART for more than 6 months. In most high- and middle-resourced settings, VL measurement every 3–6 months has been the standard of care for over a decade.2 In resource-limited settings the combination of financial, logistic and human resource-related constraints make widespread VL testing implementation extremely challenging.3
Médecins sans Frontières (MSF) has initiated and run various VL programmes funded by UNITAID in seven sub-Saharan African (sSA) countries with high HIV prevalence and large ART cohorts, including Mozambique.4 Routine VL testing in Mozambique has been rolled-out in MSF-supported health programmes in Maputo, the capital city, since October 2013.
Most studies on VL monitoring in sSA countries have assessed determinants of virological outcomes and the initiation of second-line therapy.5,6 However, the feasibility of large-scale implementation of VL monitoring in high prevalence, resource-limited settings has not been extensively studied. A study from Malawi demonstrated that good quality VL monitoring is feasible and affordable in their setting.7 Another study from Swaziland showed the same results, but stressed the needs for close monitoring of gaps in the ‘VL cascade’. The VL monitoring cascade is a management process for VL testing. If a first VL result is high, enhanced adherence counselling (EAC) is offered and then, if a follow-up VL result remains high, the patient should be considered for second line ART.8 At all steps of this cascade, from initial VL testing to switching to second line ART, patients are ‘lost’.
We know that implementation of VL monitoring in resource-limited settings is challenging in many ways, but there are no studies that examine which patient and programme level factors are associated with the uptake of first and follow-up VL testing. Identification of such factors is important if successful implementation of the WHO and UNAIDS’ recommendation of universal VL testing is to be achieved.
In this Mozambican study, we describe the VL cascade in six MSF-supported health centres (HCs) in Maputo city, including the uptake of first and follow-up VL testing and VL results. In addition, we study the patient and programme factors influencing the uptake of VL testing and VL results, with an emphasis on the performance of different service delivery platforms.
Methods
Design
This took the form of a retrospective cohort study using routine program data.
General setting
Mozambique is a large country situated in South-east Africa, with a population of over 26 million. Despite its’ economic growth, Mozambique is still one of the poorest countries in the world. Mozambique scores among the lowest in the world when it comes to trained nurses and doctors with only 0.04 physicians and 0.412 nurses/midwives per 1000 people reported in 2012.9 However, numbers and densities of health workers are improving, showing the impact of the human resources development strategy in the country.10
The country has a high HIV prevalence, with about 1.5 million people living with HIV/AIDS (PLWH). Approximately 10.5% of the population between 15 and 49 years of age are HIV positive. The country ranks third in the world for new paediatric infections and almost 110 000 teenagers are living with HIV.11 Maputo, the capital, has a population of 1.2 million, and an HIV prevalence of 16.8%. Although over 300 000 PLWH take first line ART in Maputo, only a few hundred people are on second line ART.12
VL testing roll-out in Mozambique
In the Mozambican National Strategic Plan for the HIV/AIDS response from 2010 to 2014 (NSP III), a phased roll-out of VL testing was foreseen. Initially, testing was to be implemented for early infant diagnosis, as well as targeted VL testing, when therapeutic failure was suspected (clinically or immunologically).13 The next NSP aims to further scale-up routine VL testing for all children between 2 and 5 years of age, and pregnant/breastfeeding women on ART, with a goal to reach routine VL monitoring for all by 2017.11
The guidelines for routine VL monitoring in Mozambique at the time of the study were as follows: a patient was eligible for a first VL test if they had spent more than 6 months on ART or at 3 months if pregnant/breast feeding. In case of a high VL (at the time of the study, the cut-off was set if more than or equal to 3000 copies/ml) the VL test should be repeated 6 months later, after one or more session of EAC. All patients with repeated high VLs (two times more than or equal to 3000 copies/ml, taken a minimum of 2 months apart) had to be presented to and approved by the ART Committee responsible for deciding if they should switch to second line ART. The actual starting of patients on second-line ART, and subsequent follow-up was performed at the referral hospital.
Implementation of routine VL testing in MSF-supported health centres in Maputo
Since 2001, MSF and the Direction of Health in Maputo City have collaborated in supporting HIV/AIDS activities in the health areas of Chamanculo and Mavalane. The seven HCs scaling-up routine VL monitoring at the time of the study were Chamanculo, Xipamanine, Alto-Mae, Maxaquene and Porto in the area of Chamanculo, and Primeiro de Maio and Albasine in the area of Mavalane. HIV care, including VL testing, is integrated into primary healthcare delivery.
In October 2013, a VL machine (type bioMérieux, NucliSENS EasyQ® HIV-1 V2.0, Paris, France) was fully installed at the laboratory in Jose Macamo Hospital and became functional. Henceforth, the MSF-supported HCs started sending dried blood spots (DBS) VL samples for routine testing.14
Medical staff included VL monitoring in their daily work, including prescribing and explaining the test, taking the venous blood samples, preparing the DBS samples for transport, receiving and classifying the VL results in the patient files, informing the patients about their results during the next consultation and making clinical decisions accordingly.15
Study site, period and population
The study included patients from six MSF-supported HCs who did roll-out routine VL monitoring in Maputo city, Mozambique: Chamanculo, Xipamanine, Alto Maé, Maxaquene, Porto and Primeiro de Maio. All patients who had spent more than 6 months on first line ART (eligible for routine VL testing) with at least one consultation between 2014 and 2015 were included. Patients who started ART after March 2015 were excluded. VL results were retrieved if sampled in the study period. Each HC offered a mix of different service delivery platforms (Box 1).
Box 1.
Service delivery platforms in six MSF supported health facilities in Maputo city, Mozambique, between 2014 and 2015
Conventional care:
|
Conventional care with iDART (intelligent dispensing of ART):16
|
|
Urban community ART groups (CAG):17
|
One-Stop models: service delivery platforms where all aspects of HIV care, including testing, ART and counseling, are provided in an integrated way. Service for adolescent care (SAAJ: Serviços de Amigos dos Adolescentes e Jovens):
|
Antenatal care (ANC) and postnatal care (PNC):
|
TB service:
|
Source of data
Study variables were extracted from the database used by MSF at all six HCs studied for HIV programme monitoring, called the Three Information Register Net Worked (Tier.net), and from the MSF second line referral and switch register.
Variables included sociodemographic patient characteristics, service delivery platforms (conventional, conventional with intelligent dispensing of ART (iDART),16 community ART groups (CAG),17 Serviços de Amigos dos Adolescentes e Jovens (SAAJ), antenatal care (ANC)/postnatal care (PNC), TB; see Box 1), year of ART initiation, VL results and dates, treatment outcomes (active in treatment, transferred out, dead, lost to follow-up) and dates, ART Committee referral and approval dates, and second line ART starting date.
Data analyses
Proportions were calculated for categorical variables, median and interquartile ranges (IQR) for numeric variables to report on demographic and clinical characteristics of patients, service delivery platforms (Box 1), uptake of first and follow-up VL testing, virological outcomes, second line ART referral, and switching to second line ART. Patients were attributed to a specific service delivery platform if they had attended at least four consultations during the study period in the specific platform.
‘Lost to follow-up’ was defined as being more than 3 months late for the last appointment. VL results were categorized as: first VL, virological rebound, follow-up VL, virological failure and low follow-up VL (Box 2).
Box 2.
Definition of viral load (VL) variables
| First VL: A first VL taken from a patient more than 6 months on first-line antiretroviral therapy, between 2014 and 2015. |
| Low first VL: A first VL<3000 copies/ml. |
| Virological rebound or high first VL: A first VL≥3000 copies/ml. |
| Follow-up VL: A VL taken from a patient with a first VL≥3000 copies/ml, more than 60 days after the first VL, between 2014 and 2015. |
| Virological failure or high follow-up VL: A follow-up VL≥3000 copies/ml. |
| Low follow-up VL: A follow-up VL<3000 copies/ml. |
We employed univariate and multivariate logistic regression to estimate the association between patient and programme level factors, and coverage and results of first and follow-up VL tests. P-values of <0.05 were considered as significant. Analyses were performed with Excel and Stata (version 11.2).
Ethical approval
The study was approved by the Maputo City Health Directorate and met the MSFs’ Ethics Review Board-approved criteria for the analysis of routinely-collected program data. Since the data were anonymized and routinely-collected, patient consent was not required.
Results
Baseline characteristics of study population
During the study period, 43 579 patients were eligible to do a first VL test, of which 98% never had a previous VL. Table 1 shows the baseline characteristics of eligible patients. Two-thirds were women and older than 35 years, while only 4% were younger than 15 years. It can be seen that the majority of patients were attended in conventional care, with or without iDART, while other service delivery platforms were attended as shown in Table 1. Patients had a median of 42 (IQR: 21–71) months on ART.
Table 1.
Characteristics of patients eligible for first viral load testing in six MSF-supported health centers in Maputo, Mozambique, between 2014 and 2015
| Characteristics | Number of eligible patientsa | |
|---|---|---|
| N | (%) | |
| Total | 43 579 | (100) |
| Sex, female | 29 657 | (68) |
| Age category (years) | ||
| <5 | 488 | (1) |
| 5≤|15 | 1510 | (3) |
| 15≤|25 | 2220 | (5) |
| 25≤|35 | 12 117 | (28) |
| ≥35 | 27 244 | (63) |
| Year ART start | ||
| <2010 | 11 241 | (26) |
| 2010−2013 | 21 989 | (50) |
| 2014−2015 | 10 349 | (24) |
| Facility | ||
| Primeiro de Maio | 8691 | (20) |
| Alto Mae | 10 211 | (23) |
| Chamanculo | 10 079 | (23) |
| Maxaquene | 2946 | (7) |
| Porto | 4382 | (10) |
| Xipamanine | 7270 | (17) |
| Service delivery | ||
| Conventionalb | 30 989 | (71) |
| Conventionalb with iDART | 6687 | (15) |
| CAG | 1202 | (3) |
| SAAJ | 451 | (1) |
| ANC/PNC | 3723 | (9) |
| TB | 527 | (1) |
a Eligible for first viral load testing included all patients more than 6 months on first line ART.
b Conventional care is the care provided to all patients not attending the other mentioned service delivery platforms.
ART: antiretroviral therapy; ANC: Antenatal Care; CAG: community ART groups; PNC: Postnatal Care; SAAJ: Servicio Apoio Amigos Jovens (Adolescent Friendly service); iDART: intelligent dispensing of ART-pharmacy software to dispense and monitor ART.
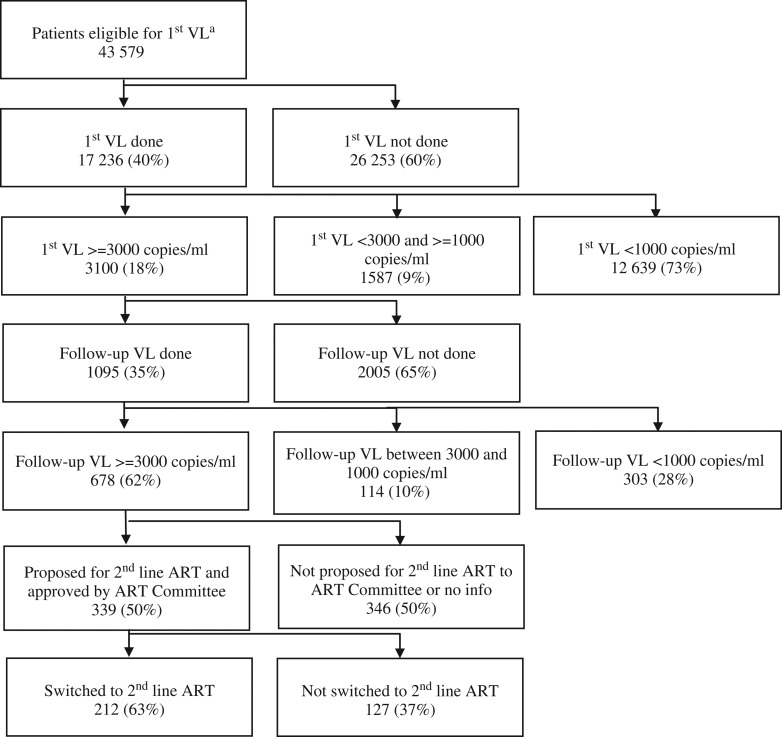
Serious gaps were observed throughout the VL cascade. Low levels of uptake of first (40%, 17 236/43 579) and follow-up (35%, 1095/3100) VL was observed. Only one in three patients identified with virological failure was switched to second line ART (Fig. 1).
Figure 1.
Viral load cascade of eligible patients in MSF-supported health centers in Maputo, Mozambique, between 2014 and 2015.
ART: antiretroviral therapy; VL: viral load. a Eligible for first VL testing included all patients more than 6 months on first line ART.
Uptake and results of first VL testing
Among eligible patients, 40% (17 236) had a first VL test during the 2-year study period, with a higher uptake in men, children younger than 15 years and patients who had been on ART longer, all statistically significant. Certain service delivery platforms performed significantly better than conventional care. The uptake of first VL test was highest in the more heavily MSF-supported services (SAAJ, CAG, TB; Table 2). In SAAJ, uptake was the highest. Among patients who never had a first VL, 8006 (31%) were lost to follow-up (LTFU), 164 (0.6%) were reported dead and 895 (3.4%) were transferred out.
Table 2.
Patient and programmatic factors influencing first viral load uptake and result in six MSF-supported health centers in Maputo, Mozambique, between 2014 and 2015
| First viral load uptake | First viral load result | |||||||
|---|---|---|---|---|---|---|---|---|
| Eligible first VLa | With first VL (%) | aOR (95% CI) | p value | With first VL | First VL≥3000 (%) | aOR (95% CI) | p value | |
| Total | 43 579 | 17 326 (40) | NA (NA) | NA | 17 326 | 3100 (18) | NA (NA) | NA |
| Gender | 0.001 | NS | ||||||
| Female | 29 657 | 11 501 (39) | 1 (NA) | 11 501 | 1978 (17) | 1 (NA) | ||
| Male | 13 922 | 5825 (42) | 1.08 (1.03–1.13) | 5825 | 1122 (19) | 1.04 (0.95–1.13) | ||
| Age category (years) | <0.001 | <0.001 | ||||||
| <5 | 488 | 254 (52) | 2.4 (1.99–2.91) | 254 | 102 (40) | 3.71 (2.87–4.82) | ||
| 5–15 | 1510 | 1066 (71) | 3.1 (2.75–3.49) | 1066 | 347 (33) | 2.52 (2.19–2.91) | ||
| 15–25 | 2220 | 757 (34) | 0.75 (0.67–0.83) | 757 | 203 (27) | 2.13 (1.76–2.58) | ||
| 25–35 | 12 117 | 3893 (32) | 0.75 (0.71–0.79) | 3893 | 687 (18) | 1.31 (1.18–1.45) | ||
| >35 | 27 244 | 11 356 (42) | 1 (NA) | 11 356 | 1761 (16) | 1 (NA) | ||
| Year ART start | <0.001 | NS | ||||||
| <2010 | 11 241 | 5 658 (50) | 4.14 (3.87–4.42) | 5658 | 1009 (18) | 1.21 (1.05–1.40) | ||
| 2010–13 | 21 989 | 9 568 (44) | 3.23 (3.05–3.43) | 9568 | 1751 (18) | 1.18 (1.04–1.35) | ||
| 2014–15 | 10 349 | 2 100 (20) | 1 (NA) | 2100 | 340 (16) | 1 (NA) | ||
| Service delivery | <0.001 | <0.001 | ||||||
| Conventionalb | 30 989 | 10 845 (35) | 1 (NA) | 10 845 | 1930 (18) | 1 (NA) | ||
| Conventionalb & iDART | 6687 | 3486 (52) | 2.07 (1.96–2.19) | 3486 | 705 (20) | 1.23 (1.12–1.36) | ||
| CAG | 1202 | 761 (63) | 2.6 (2.30–2.93) | 761 | 95 (12) | 0.75 (0.60–0.94) | ||
| SAAJ | 451 | 372 (82) | 9.61 (7.32–12.61) | 372 | 120 (32) | 1.18 (0.92–1.52) | ||
| ANC/PNC | 3723 | 1604 (43) | 2.44 (2.26–2.64) | 1604 | 192 (12) | 0.63 (0.53–0.74) | ||
| TB | 527 | 258 (50) | 3.58 (2.98–4.30) | 258 | 58 (22) | 1.43 (1.06–0.94) | ||
a Eligible for first VL testing included all patients more than six months on first line ART.
b Conventional care is the care provided to all patients not attending the other mentioned service delivery platforms.
aOR: adjusted odds ratio; ART: antiretroviral therapy; CAG: community ART groups; iDART: intelligent Dispensing of ART - pharmacy software to dispense and monitor ART; NA: not applicable; NS: not significant; SAAJ: Servicio Apoio Amigos Jovens (Adolescent Friendly service); VL: viral load;
First VL results were significantly higher across all age categories below 35 years. The younger the patients were and the longer they had been on ART, the higher was the probability of virological rebound. Patients in CAG and pregnant/breastfeeding women were more likely to have a low first VL.
Uptake and results of follow-up VL testing
The median time between first and follow-up VL testing was 214 days (IQR: 152–323). Of patients with virological rebound, 35% (1095) had a follow-up VL test, with a higher uptake in women, children between 5 and 15 years old, and patients who had been on ART longer, all of which were statistically significant (Table 3). Adults between 25 and 35 years old had the lowest uptake. Specific service delivery platforms performed significantly better than conventional care. Follow-up VL uptake was higher in conventional care with iDART in place. Among patients who never had a follow-up VL test, 351 (18%) were LTFU, 25 (1%) were reported dead and 124 (6%) were transferred out. Patients in CAG and pregnant/breastfeeding women were more likely to have a low follow-up VL test rate, indicating effective viral suppression.
Table 3.
Patient and programmatic factors influencing second viral load uptake and result in six MSF-supported health centers in Maputo. Mozambique, between 2014 and 2015
| Follow-up viral load uptake | Patients with 2nd viral load done | |||||||
|---|---|---|---|---|---|---|---|---|
| Eligible follow-up VLa | With follow-up VL (%) | aOR (95% CI) | p aOR: adjusted Odds Ratio p value | With follow-up VL | Follow-up VL≥3000 (%) | aOR (95% CI) | p value | |
| Total | 3100 | 1095 (35) | NA (NA) | NA | 1095 | 678 (62) | NA (NA) | NA |
| Gender | NS | NS | ||||||
| Female | 1978 | 709 (36) | 1 (NA) | 709 | 428 (60) | 1 (NA) | ||
| Male | 1122 | 386 (34) | 0.82 (0.70–0.96) | 386 | 250 (65) | 1.15 (0.88–1.51) | ||
| Age category (years) | <0.001 | NS | ||||||
| < 5 | 102 | 32 (31) | 1.06 (0.68–1.65) | 32 | 17 (53) | 0.62 (0.30–1.29) | ||
| 5≤15 | 347 | 160 (46) | 1.46 (1.14–1.87) | 160 | 103 (64) | 0.95 (0.64–1.39) | ||
| 15≤25 | 203 | 85 (42) | 1.11 (0.78–1.59) | 85 | 59 (70) | 1.13 (0.63–2.00) | ||
| 25≤35 | 687 | 183 (27) | 0.70 (0.57–0.86) | 183 | 119 (65) | 1.38 (0.95–1.98) | ||
| >35 | 1761 | 635 (36) | 1 (NA) | 635 | 380 (60) | 1 (NA) | ||
| Year ART start | <0.001 | NS | ||||||
| < 2010 | 1009 | 406 (40) | 3.84 (2.71–5.42) | 406 | 247 (61) | 1.13 (0.60–2.11) | ||
| 2010–2013 | 1751 | 641 (37) | 3.59 (2.57–5.00) | 641 | 403 (63) | 1.22 (0.66–2.25) | ||
| 2014-2015 | 340 | 48 (14) | 1 (NA) | 48 | 28 (58) | 1 (NA) | ||
| Service delivery | <0.001 | <0.001 | ||||||
| Conventionalb | 1930 | 622 (32) | 1 (NA) | 622 | 398 (64) | 1 (NA) | ||
| Conventionalb & iDART | 705 | 271 (38) | 1.32 (1.10–1.59) | 271 | 169 (62) | 0.92 (0.68–1.24) | ||
| CAG | 95 | 54 (57) | 2.67 (1.75–4.07) | 54 | 19 (35) | 0.30 (0.17–0.55) | ||
| SAAJ | 120 | 71 (59) | 2.66 (1.71–4.13) | 71 | 54 (76) | 1.74 (0.90–3.34) | ||
| ANC/PNC | 192 | 52 (27) | 1.05 (0.73–1.50) | 52 | 25 (48) | 0.46 (0.25–0.84) | ||
| TB | 58 | 25 (43) | 2.67 (1.50–4.73) | 25 | 13 (52) | 0.59 (0.26–1.34) | ||
a Eligible for a follow-up VL testing included all patients on ART with a first VL above 3000 copies/ml, and who had done their first VL more than 2 months ago.
b Conventional care is the care provided to all patients not attending the other mentioned service delivery platforms.
aOR: adjusted odds ratio; ANC: antenatal care; ART: antiretroviral therapy; CAG: community ART Groups; iDART: intelligent dispensing of ART, pharmacy software to dispense and monitor ART; NA: not applicable; NS: not significant; PNC: postnatal care; SAAJ: Servicio Apoio Amigos Jovens (Adolescent Friendly service); VL: viral load.
Discussion
This is the first study describing the uptake of VL testing during the scale-up of routine VL monitoring in Maputo, Mozambique. Serious gaps were observed throughout the VL cascade with low levels of first and follow-up VL uptake. Understanding the cascade and the reason for patients dropping out is an essential step in bridging those gaps. Patient and programme factors contributed to these findings. The benefits of routine VL monitoring in this programme were seriously undermined. Few patients who required second line ART gained access to it. These findings are important as they point to areas of care that need to be strengthened if the goal of universal VL monitoring is to be reached.1,2 A recent study reported the outcomes of national scale-up of routine VL monitoring in seven sSA countries (Côte d'Ivoire, Kenya, Malawi, Namibia, South Africa, Tanzania and Uganda) and showed big differences in uptake of first VL test, ranging from 3% to 95%.18 A study from a heavily MSF-supported VL programme in Swaziland reported better VL uptakes, with 73% of 16,349 eligible patients who undertook a first VL test. Of patients with a high first VL result, 60% had a follow-up VL test done within 6 months.6
We found that the proportion of patients with virological rebound was 18%, similar to that reported by other studies. The Swaziland study showed 16% high first VL results.6 The study from seven sSA countries showed that virological rebound ranged between 6% in Uganda and 22% in South Africa.18 Another study also described a heterogeneity of virological outcomes in patients on ART in sSA and Asia.19 In Mozambique, thresholds for virological rebound recently changed from 3000 copies/ml to 1000 copies/ml in October 2015. This will lead to increased second line ART needs. Applying this new threshold to our data, an additional 9% of patients would have had virological rebound in Maputo (Fig. 1).
Our findings supported the WHO recommendation that a patient with virological rebound should be addressed by EAC, as well as repeat VL measurement before considering ART switch.2
Patient and programme factors influencing VL monitoring
Patient factors associated with VL uptake included time on ART and age. The longer patients were on ART, the higher the uptake of first and follow-up VL testing. This finding is important, especially in programmes with ageing ART cohorts, since the risk of virological failure increases with time on ART, mainly due to drug resistance.20 Another reason why early VL testing should be systematized, is the bigger potential yield of EAC for failing patients when introduced early.21
Uptake of first VL test was high among children of different age categories, reflecting the priority given to this vulnerable subgroup in the scale-up of routine VL monitoring in the Maputo HCs. The younger the children, the more likely they were to have a high first VL result. This finding confirms what has been published elsewhere and requires programmatic responses to prevent and address high VL results in children.22
Adolescents had a lower uptake of first VL testing if they attended conventional care, as opposed to those who attended SAAJ, a specialized adolescent service, where uptake was excellent. Overall, adolescents were seen to have statistically significant higher levels of virological rebound, when compared to adults. This is in line with previous research, describing worse suppression rates in adolescents.23 Internationally, there is a consensus that adolescents living with HIV need to be addressed in a specific way, and innovative ideas are needed to further improve retention in care and treatment adherence.24 SAAJ can be one such innovation, where experience can guide similar roll-outs elsewhere.
Not only SAAJ, but also the service delivery platforms ANC/PNC and TB, showed an improved uptake of first and follow-up VL testing in vulnerable groups, when compared with conventional care. The benefits of these one-stop models of HIV care have already been highlighted in other studies,24,25 and have been recognized by the Mozambican government.25,26
ANC/PNC services were prioritized in the scale-up of VL monitoring by the Ministry of Health in Mozambique, which has opted for the option B+ approach since 2013.13 Although some studies show worrying retention in care and adherence levels among pregnant/breastfeeding women,27 our results show better first VL testing uptake and outcomes when compared with conventional care. Given the importance of having a low VL result at delivery and during breast feeding, extra support for these services is needed to reach the goal of elimination of mother to child transmission.
When addressing the community models of care in previous studies on CAG, an excellent retention in care of patients on ART was shown. However, VL testing uptake and virological outcomes were not studied.17 Our results show that both VL testing uptake and virological suppression was better in CAG members when compared with conventional care.
The pharmacy software program iDART was piloted in one MSF-supported HC in Maputo at the time of the study.16 It was not only useful for dispensing ART, but also to recall patients for EAC, which positively influenced the uptake of follow-up VL testing. However, the influence of iDART alone was difficult to measure, since other task forces were similarly organized at a pharmacy level in that HC to improve follow-up VL uptake. Future studies will need to show the effect of monitoring and communication technology on adherence.
Feasibility of routine VL monitoring
In well-resourced settings, HIV care needs to be tailored to the individual patient, including the choice of ART regimen and VL monitoring. However, the reality is different in low-resource, high HIV prevalence settings, such as Mozambique. VL monitoring is complex and resource-intensive, and includes many tasks: venous blood sampling, preparation and transport of samples, archiving of VL results inside the medical files of patients, and clinical decision-making based on virological outcomes. In Maputo, the bulk of this additional workload is currently dependent on scarce clinicians. Even though supported by a non-governmental organization, whose activities were integrated into the framework of the Mozambican Ministry of Health, the implementation of routine VL monitoring remained a big challenge at all levels in Maputo's HCs.
How to maximize access to routine VL
First, awareness about the importance of VL testing has to be raised, in patients as well as providers. Second, in a context of scarce resources, policy makers have to make hard choices. For example, VL monitoring could replace CD4 monitoring.28 In addition, national surveillance systems of resistance patterns should further orientate the periodic revision of the first line ART regimen. Third, decentralization of HIV care is a must.2,29 Not only first line ART delivery and care, but also the decision-making about when to switch to second line ART, as well as the follow-up of patients on second line ART, should be integrated in primary healthcare services. Community ART delivery and spaced clinical appointments for stable patients may decompress already overburdened HCs and ease the medical follow-up of people living with a chronic lifelong condition.17 Fourth, new tools and procedures for VL collection and analysis, such as DBS, finger prick sampling, pooled blood sampling and new VL platforms, including point-of-care technology,14 may bring VL testing closer to the people. Finally, besides a simplification of VL monitoring, further task shifting holds great potential.30 Lay counsellors are key to the good functioning of HIV care.31 Drawing on the literature of chronic disease care, also PLWH can be trained and made partially responsible in the follow-up of their own health status, once the appropriate tools are available.32
In the meantime, HIV programmes scaling-up routine VL monitoring should continue to prioritize the most vulnerable patients—sick patients (including co-infected patients with TB), children and adolescents, and pregnant/breastfeeding women.
There are a number of strengths in our study. The size of the sample cohort was large, permitting the evaluation of patient and programme factors. Relying on routinely-collected patient-level programme data also permitted these findings to reflect the reality of the ART programme in Maputo city. Additionally, data handling was carefully managed: they were retrieved from the programme database from different HCs with well-established and supervised data cleaning procedures. Data from the VL laboratory were double encoded during most of the study period. Hence, we believe the results are based on robust data.
However, this study has some important limitations. The study findings may have been affected by bias inherent to the observational design. Unmeasured factors related to the organization of care may have contributed to our findings. Since data on adherence counselling was not systematically encoded during the whole study period, this important step of the VL cascade was not reported in this study. Moreover, high patient attrition and programmatic gaps throughout the VL cascade hampered an accurate estimation of the real need for second line ART. Some groups were under-represented in our study. For example, children under 5 years old represented only 1% of our study population. Some delivery platforms had relatively few patients attending, but these numbers reflect the reality of the programme.
Conclusion
This study of 2 years’ outcomes of routine VL monitoring in Maputo city showed many gaps throughout the VL cascade, which reflect the challenges linked to large VL monitoring scale-up. One-stop services for vulnerable patients, CAGs, and patient recall systems at the pharmacy, showed additional benefits in the uptake of VL testing. Continuous efforts to further simplify and decentralize VL monitoring are needed. This study has pointed the way forward to achieve universal VL testing in Mozambique. The mantra of 90% virological suppressed patients on ART, as the ultimate objective of the VL cascade, is even a step further away.
Acknowledgments
Authors’ contributions: SS, TD and TR conceived the study and designed the study protocol. SS, TD, AWT, WM collected, analysed and interpreted the data. SS wrote the first draft of the paper. All co-authors contributed to the subsequent draft and approved the final version. TR is the guarantor of the study.
Acknowledgements: Special thanks are extended to all the hardworking Mozambican staff involved in the roll-out of VL monitoring in Maputo.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf [accessed 6 October 2015].
- 2. World Health Organization (WHO) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Second edition. Geneva: WHO; 2016. Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1 [accessed 25 September 2016]. [PubMed]
- 3. Roberts T, Cohn J, Bonner K et al. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis 2016;62:1043–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNITAID. Implementation of CD4 and viral load testing in decentralized, remote and resource-limited settings (2012–2016). Available from: https://www.unitaid.eu/project/implementation-cd4-viral-load-testing-decentralized-remote-resource-limited-settings/ [accessed 6 July 2017].
- 5. Johnston V, Fielding K, Charalambous S et al. outcomes following virological failure and predictors of switching to second-line antiretroviral therapy in a South African treatment programme. J Acquir Immune Defic Syndr 2012;61:370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jobanputra K, Parker LA, Azih C et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One 2015;10:e0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rutstein S, Hosseinipour MC, Kamwendo D et al. Dried blood spots for viral load monitoring in Malawi: feasible and effective. PLoS One 2015;10:e0124748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jobanputra K, Parker LA, Azih C et al. Impact and programmatic implications of routine viral load monitoring in Swaziland. J Acquir Immune Defic Syndr 2014;67:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO Global Health Observatory Data Repository: health workforce aggregated data by country, density per 1000. Geneva: WHO; 2014. Available from: http://apps.who.int/gho/data/node.main.A1444?lang=en&showonly=HWF [accessed 8 April 2016].
- 10. MOH, Mozambique Inquérito Nacional de Prevalência, Riscos Comportamentais e Informação sobre o HIV e SIDA em Moçambique (INSIDA). Maputo: MOH, Mozambique; 2009. Available from: http://dhsprogramme.com/pubs/pdf/HF33/HF33p.pdf [accessed 8 April 2016].
- 11. UNAIDS Mozambique. HIV and AIDS estimates (2015). Available from: http://www.unaids.org/en/regionscountries/countries/mozambique [accessed 2016 April 8].
- 12. Bila DCA, Boullosa LT, Vubil AS et al. Trends in prevalence of HIV-1 drug resistance in a public clinic in Maputo, Mozambique. PLoS One 2015;10(7):e0130580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MOH, Mozambique National HIV/AIDS Strategic Plan 2010–2014. Maputo: MOH, Mozambique; 2010. Available from: http://www.nationalplanningcycles.org/sites/default/files/country_docs/Mozambique/national_strategic_hiv_and_aids_response_plan_2010-2014.pdf [accessed 6 Oct 2015].
- 14. MSF Putting HIV treatment to the test. A product guide for viral load and point-of-care CD4 diagnostic tools. Paris: MSF; 2013. Available from: http://www.msf.fr/actualite/publications/putting-hiv-treatment-test-product-guide-viral-load-and-point-care-cd4-diagno [accessed 8 April 2016].
- 15. WHO Technical and operational considerations for implementing HIV viral load testing. Geneva: WHO; 2014. Available from: http://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/ [accessed 6 October 2015].
- 16. Rivett U, Tapson J. The Cell-Life Project: Converging technologies in the context of HIV/AIDS. Gateways: International Journal of Community Research and Engagement 2009;2:82–97. [Google Scholar]
- 17. Decroo T, Koole O, Remartinez D et al. Four-year retention and risk factors for attrition among members of community ART groups in Tete, Mozambique. Trop Med Int Health 2014;19:514–21. [DOI] [PubMed] [Google Scholar]
- 18. Lecher S, Ellenberger D, Kim AA et al. Scale-up of HIV viral load monitoring in seven sub-Saharan African countries. MMWR Morb Mortal Wkly Rep 2015;64:1287–90. [DOI] [PubMed] [Google Scholar]
- 19. Aghokeng AF, Monleau M, Eymard-Duvernay S et al. Extraordinary heterogeneity of virological outcomes in patients receiving highly antiretroviral therapy and monitored with the World Health Organization Public Health Approach in Sub-Saharan Africa and Southeast Asia. Clin Infect Dis 2014;58:99–109. [DOI] [PubMed] [Google Scholar]
- 20. Stadeli KM, Richman DD. Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antivir Ther 2013;18:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kerschberger B, Boulle AM, Kranzer K et al. Superior virologic and treatment outcomes when viral load is measured at 3 months compared to 6 months on antiretroviral therapy. J Int AIDS Soc 2015;18:20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernheimer JM, Patten G, Makeleni T et al. Paediatric HIV treatment failure: a silent epidemic. J Int AIDS Soc 2015;18:20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee S, Hazra R.. Achieving 90-90-90 in paediatric HIV: adolescence as the touchstone for transition success. J Int AIDS Soc 2015;18:20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. UNAIDS All In, to end the AIDS epidemic, start focusing on adolescents. Geneva: UNAIDS; 2015. Available from: http://allintoendadolescentaids.org [accessed 8 April 2016].
- 25. Kerschberger B, Hildebrand K, Boulle AM et al. The effect of complete integration of HIV and TB services on time to initiation of antiretroviral therapy: a before-after study. Plos One 2012;7:e46988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sweeney S, Dayo Obure C, Maier CB et al. Costs and efficiency of integrating HIV/AIDS services with other health services: a systematic review of evidence and experience. Sex Transm Infect 2012;88:85–99. [DOI] [PubMed] [Google Scholar]
- 27. Ngarina M, Kilewo C, Karlsson K et al. Virologic and immunologic failure, drug resistance and mortality during the first 24 months postpartum among HIV-infected women initiated on antiretroviral therapy for life in the Mitra plus Study, Dar es Salaam, Tanzania. BMC Infect Dis 2015;15:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ford N, Stinson K, Davies MA et al. Is it safe to drop CD4+ monitoring among virologically suppressed patients: a cohort evaluation from Khayelitsha, South Africa. AIDS 2014;28:2003–5. [DOI] [PubMed] [Google Scholar]
- 29. Kredo T, Ford N, Adeniyi FB et al. Decentralising HIV treatment in lower- and middle-income countries. Cochrane Database Syst Rev 2013;6:CD009987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Callaghan M, Ford N, Schneider H.. A systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MSF HIV/TBcounselling: who is doing the job? Time for recognition of lay counsellors. South Africa: MSF; 2015. Available from: http://www.samumsf.org/ [accessed 8 April 2016].
- 32. Kuo CC, Lin CC, Tsai FM.. Effectiveness of empowerment-based self-management interventions on patients with chronic metabolic diseases: a systematic review and meta-analysis. Worldviews Evid Based Nurs 2014;11:301–15. [DOI] [PubMed] [Google Scholar]