Abstract
Background
Brucea javanica (L.) Merr. is a plant from the genus Brucea, which is used in local traditional medicine to treat various diseases. Recent studies revealed an impressive anticancer efficiency of B. javanica extract in different types of cancer cells.
Purpose
In this study, we have investigated the cytotoxic effects of the B. javanica hexane, ethanolic extracts against colon cancer cells. HT29 colon cells were selected as an in vitro cancer model to evaluate the anticancer activity of B. javanica ethanolic extract (BJEE) and the possible mechanisms of action that induced apoptosis.
Methods
3-(4,5-dimethylthiazol-2-yl)-2, 5,-diphenyltetrazolium bromide (MTT), lactate dehydrogenase, acridine orange/propidium iodide, and annexin-V-fluorescein isothiocyanate assays were performed to determine the antiproliferative and apoptosis validation of BJEE on cancer cells. Measurement of reactive oxygen species (ROS) production, caspase activities, nucleus factor-κB activity, and gene expression experiments was done to investigate the potential mechanisms of action in the apoptotic process.
Results
The results obtained from this study illustrated the significant antiproliferative effect of BJEE on colorectal cancer cells, with a concentration value that inhibits 50% of the cell growth of 25±3.1 µg/mL after 72 h of treatment. MTT assay demonstrated that the BJEE is selectively toxic to cancer cells, and BJEE induced cell apoptosis via activation of caspase-8 along with modulation of apoptosis-related proteins such as Fas, CD40, tumor necrosis factor-related apoptosis-inducing ligands, and tumor necrosis factor receptors, which confirmed the contribution of extrinsic pathway. Meanwhile, increased ROS production in treated cells subsequently activated caspase-9 production, which triggered the intrinsic pathways. In addition, overexpression of cytochrome-c, Bax, and Bad proteins along with suppression of Bcl-2 illustrated that mitochondrial-dependent pathway also contributed to BJEE-induced cell death. Consistent with the findings from this study, BJEE-induced cancer cell death proceeds via extrinsic and intrinsic mitochondrial-dependent and -independent events.
Conclusion
From the evidence obtained from this study, it is concluded that the BJEE is a promising natural extract to combat colorectal cancer cells (HT29 cells) via induction of apoptosis through activation of extrinsic and intrinsic pathways.
Keywords: Brucea javanica, apoptosis, cancer, HT29, mitochondrial pathway, apoptosis protein array
Introduction
Cancer is a debilitating disease that is known as one of the major health problems of global concern, and a substantial portion of the world population is currently affected by different types of cancers irrespective of age.1 Among the various types of cancer, colon cancer is the second and third leading cause of cancer deaths among men and women, respectively, in the USA. Surgery and chemotherapy are the main modes of medical treatments used in treating diagnosed colorectal cancer patients; nevertheless, there are innumerable failure cases during treatment because of delay of diagnosis or drug resistance.2–4
Natural products and extracts derived from plants have been used traditionally from thousands of years for the survival and treatment of various types of ailments and diseases.5,6 The usage of medicinal plants has been practiced among the people from China, India, and South-East Asian countries from the early civilization.7 There is a similarity between body composition and plant ingredients as per the traditional Chinese medicine (TCM), believed for more than 2,500 years, which has increased the interest in plant-derived medicines.8 TCM is recognized as an effective complementary and alternative medicine modality by the National Institutes of Health (USA), while TCM is expanding in the USA and global use in the world is also rising.9 Recently, the effects of medicinal plants derived from TCM herbs on cancer treatment has increased the attention of researchers to do experiments on the cytotoxic effects of the medicinal plants on cancer cells.10 These medicinal plant products induce cell death by various types of mechanisms. The most common mechanism is programmed cell death, which includes apoptosis and necrosis.11
Apoptosis is a mode of cellular suicide, which is a programmed process of cellular self-destruction. This process involves individual cells and it does not have any inflammatory effects on the neighboring cells.12 Thus, the characterization of apoptosis includes a series of biochemical and morphologic changes in cells, which are cell shrinkage, nuclear fragmentation, chromatin condensation, membrane blebbing, chromosomal DNA fragmentation, and externalization of phosphatidylserine (PS).13 Two different pathways, namely, extrinsic and intrinsic can trigger the apoptotic process. In the intrinsic pathway, mitochondrial dysfunction events and cell stress signals make the cells to be ready for suicide, while in the extrinsic pathway cell kills itself via outside signals, where in both the pathways cell death will be induced by activation of caspases or antiapoptotic gene degradation. In addition, overproduction of reactive oxygen species (ROS) leads to increased oxidative stress and decrease of the glutathione levels in cells, which triggers apoptosis signaling.14,15
Brucea (Family: Simaroubaceae) is a widely distributed genus occurring in tropical Africa and tropical Asia. Certain species of Brucea genus have been used traditionally to combat various types of illnesses like amoebiasis, malaria, diabetes, and cancer.16 Brucea javanica is one of the species derived from Brucea genus that has a long history of medical use for treatment of numerous diseases in China. Different parts of this plant contain a variety of active compounds, and the corresponding extracts were reported for anti-inflammatory, antidiabetic, anticancer, and antimalarial activities.17,18 To confirm these traditional usages, extracts of these plants were tested against 1210 lymphoid leukemia, solid murine tumors, lung carcinoma cells, and B-16 melanocarcinoma, which showed the potent cytotoxic effects against these cancer cells.19
Several efforts have been made to identify the bioactive chemical compounds from B. javanica extracts, and some of these chemical components include quassinoids, alkaloids, triterpenoids, flavonoids, steroids, and fatty acids. Quassinoids are the major components from B. javanica extracts that are represented for the antitumor, anticancer, and antimalarial properties. The quassinoids could be found in methanol, chloroform, and aqueous extracts from B. javanica plant. Quassinoids are most abundant in the seeds and fruits of the plant. Quassinoid glycosides and Bruceoside C have shown to exhibit cytotoxic activities against melanoma, ovarian cancers, and KB (a human epidermoid carcinoma of the nasopharynx) cell lines.20
According to previous studies, this plant has shown promising anticancer properties; hence, this study was designed to investigate the anticancer activities of B. javanica dried fruit extracts on the HT29 colorectal carcinoma cell line.
Materials and methods
Plant materials
The fruits of B. javanica plant were collected from Rimba Ilmu Botanical Garden, University Malaya. A herbarium (KLU) sample of the plant with the number KLU.48132 was deposited in the garden. The B. javanica fruits were air dried and crushed, and the plant material (300 g) was extracted by the method described by Kim et al with some modifications.21 The crushed fruits were defatted with 1 L hexane (merckmillipore) by soaking for 3 days, and then the mixture was exhausted and the hexane extract was clarified with filter paper. The resulted hexane extract was concentrated via rotary evaporator and then dry residues of crushed fruits from hexane extract were soaked in 1 L absolute ethanol (99.5% purity) at room temperature for 3 days, and the mixture was filtered. Finally, the crude extract was concentrated in a rotary evaporator (Buchi rotavapor R-124) at 40°C to produce B. javanica ethanolic extract (BJEE). The extracts were stored in the refrigerator at 4°C for further experiments.
Cell culture and 3-(4,5-dimethylthiazol-2-yl)-2, 5,-diphenyltetrazolium bromide (MTT) assay
Human colon cancer cells HT29 (American Type Culture Collection [ATCC®] HTB-38™), human breast cancer cell MDA-MB-231 (ATCC HTB-26™), human cervical cancer cell HeLa 229 (ATCC CCL-2.1™), normal human liver cell WRL-68 (ATCC CL-48™), human fibroblast cells BJ-5ta (ATCC CRL-4001™), and normal human colon epithelial cell CCD-841 CoN (ATCC CRL-1790™) were purchased from the ATCC (Manassas, VA, USA). Colon cancer and normal cells were cultured in McCoy’s 5A (Sigma, St Louis, MO, USA) medium, and rest of the cells were maintained in Dulbecco’s Modified Eagle’s Medium (Sigma, St Louis, MO, USA), supplemented with 10% fetal bovine serum (Biowest, MO, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco, Thermo Fisher Scientific) and incubated in a humidified atmosphere incubator with 5% CO2 at 37°C. Untreated medium with vehicle dimethyl sulfoxide (DMSO) (0.1%) was used as negative control for all the assays.22
The MTT assay was used to identify the effects of B. javanica extracts on cell viability of normal and cancer cells and the following method previously described by Hajiaghaalipour et al was used. In brief, normal and colon cells (5×103 cells/well) were treated with hexane and BJEE at different concentrations in a 96-well plate. Cells were incubated at 37°C for 24, 48, and 72 h. MTT solution was prepared in phosphate-buffered saline and 20 µL/well was added at a concentration of 5 mg/mL to stain the cells and incubated the plates for 4 h. After incubation time, the cells’ media were replaced by DMSO to dissolve the formazan crystals. Then the absorbance of the solution was read by using a microplate reader (Tecan Infinite 200 Pro) at 570 nm wavelength. 5-Fluorouracil (5-FU, Sigma, St Louis, MO, USA) was used as a positive control, and untreated media that contained DMSO (0.1%) was used as a vehicle control in this study.23 The potency of cell growth inhibition was expressed as IC50 (concentration that inhibits 50% of the cell growth) value, which was calculated by the following formula:
Antioxidant activity
2,2 diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay
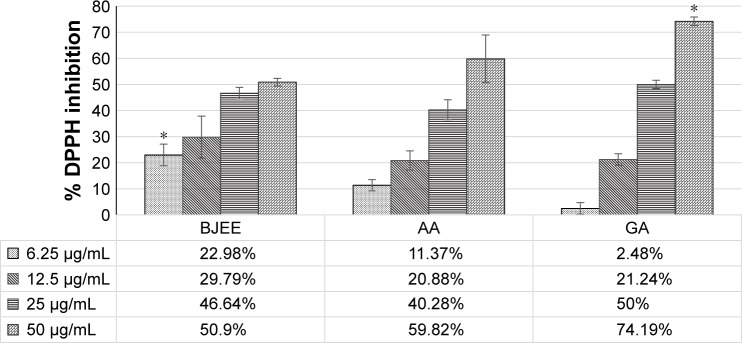
DPPH assay was employed to measure the free radical- scavenging activity of the extract, and the following method was previously reported by Ablat et al with slight modifications.24 Briefly, 10 µL of sample, which is the BJEE, and standards (ascorbic acid and gallic acid) with different concentrations (6–50 µg/mL) were mixed with 190 µL of 50 µM DPPH solution in ethanol. The mixture was immediately shaken and incubated for 20 min in the dark at room temperature. Absorbance was read at 515 nm wavelength after incubation with a microplate reader (Tecan Infinite 200 Pro). The plate was kept incubated for 1.5 h and the absorbance was measured again. The percentage of inhibition of DPPH was calculated according to the following formula (Figure 1):
Figure 1.
DPPH radical-scavenging activity (%) versus concentration (µg/mL).
Notes: *p<0.05; Indicated the significant differences of DPPH inhibition compared to other groups for the same dosage.
Abbreviations: AA, ascorbic acid; BJEE, Brucea javanica ethanolic extract; DPPH, 2,2 diphenyl-1-picrylhydrazyl; GA, gallic acid.
Ferric reducing antioxidant power (FRAP) assay
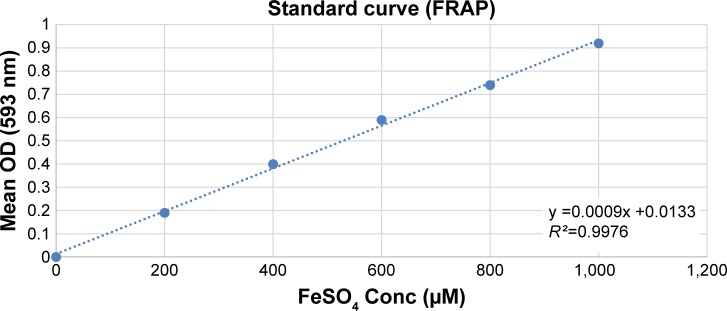
The antioxidant capacity of BJEE was measured as previously described by Lu et al with modifications.25 Ten microliters of sample was loaded with 300 µL of FRAP reagent, which contained 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) in 40 mM HCl, 2.5 mL of 20 mM FeCl3, and 24 mL of 300 mM acetate buffer (pH 3.6) in a 96-well plate. FeSO4 solution was used as standard to calibrate standard curve. The plates were incubated at 37°C for 1 h before absorbance was recorded at 593 nm. Gallic acid and ascorbic acid were used as positive controls. The FRAP values were calculated according to the standard curve equation (Figure 2) and the measurement unit was mmol Fe2+/g of extract from triplicate tests.
Figure 2.
FeSO4 standard curve plotted against concentration versus mean OD of the samples; standard equation: y =0.0009x +0.0133; R2=0.9976.
Abbreviations: Conc, concentration; FRAP, ferric reducing antioxidant power; OD, optical density.
Lactate dehydrogenase (LDH) measurement
Commercial LDH kit (Promega, USA) was used to measure LDH release of cell medium. LDH release assay is used to identify the cytotoxicity of extract or compounds. The method was used following the manufacturer’s protocol. Briefly, HT29 cells were seeded at a density of 5×104 cells/well in the 96-well plates and treated with BJEE at different concentrations for 24 h. One hundred microliters of treated cells medium was transferred to a new 96-well plate, then 100 µL of LDH reaction solution (CytoTox-ONE™) was added to each well, and the plates incubated at room temperature for 10 min. The LDH release activity was measured by recording the absorbance at an excitation wavelength of 560 nm and emission wavelength of 590 nm using a Tecan Infinite 200 Pro (Tecan, Männedorf, Switzerland) microplate reader. Cells were treated with Triton X-100, which was used as a positive control.26
Acridine orange (AO) and propidium iodide (PI) double staining
A PI and AO double staining assay was performed to detect morphologic features of apoptosis in the treated cells with BJEE by using a fluorescent microscope (Olympus BX51). HT29 cells were plated at a density of 5×104 cells/mL in a T-25 culture flask. Cells were treated with BJEE at different concentrations for 24 h. Then, cells were harvested and stained with equal volume of AO and PI (10 µg/mL) dyes. The morphology of the cells was observed under a UV-fluorescent microscope within 30 min.27
Annexin-V assay
Annexin-V (AV) assay was performed to quantify early and late apoptosis in HT29 cells treated with BJEE. This assay was carried out by using cytometry analysis using the commercial kit of BD Pharmingen Annexin V-FITC Apoptosis Detection Kit (APOAlert® Annexin V; Clontech, Mountain View, CA, USA). The method was followed as per the manufacturer’s instructions. Briefly, the HT29 cells were seeded at a density of 1×105 cells/mL at culture flasks, and then the BJEE was added to the cells at an IC50 concentration for 24 h after incubation time. The cells were washed and stained with AV-fluorescein isothiocyanate (FITC) and PI. BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA) instrument was used for fellow cytometric analysis and quadrant statistics was used to identify early and late apoptotic cells and necrotic cells.28
Measurement of ROS production
Production of ROS levels in HT29 cells treated with the BJEE at different concentrations were carried out by using a commercial kit, Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, Cambridge, UK; ab113851). The method used was based on the manufacturer’s protocols. Briefly, HT29 cells were seeded into 96-well plates at a density of 1×104 per well. The cells incubated overnight in a 37°C incubator, then the cells were treated with different concentrations of BJEE (25, 50, and 100 µg/mL) for 12 h. Dihydroethidium (DHE) probe was oxidized to ethidium in the presence of superoxides, and the fluorescence intensity was read using a fluorescent plate reader (Tecan, Männedorf, Switzerland) at an excitation wavelength of 500 nm and emission wavelength of 580 nm. Formation of ROS was quantified by using DHE staining. The following method was previously described by Rouhollahi et al. Briefly, 5×104 cells were seeded on cover slips and incubated overnight. The cells were treated at an IC50 concentration of BJEE and incubated for 12 h. After incubation, the cells were stained by DHE dye for 30 min and washed with washing buffer. ROS formation was identified under fluorescence microscope (Olympus BX51).29
Measurement of caspase-3/7, -8, and -9 activities
Caspase-3/7, -8, and -9 were measured using the commercial Caspase-Glo-3/7, -8, and -9 assay kits (Promega, USA). The activation of caspases was determined according to the manufacturer’s protocol. Briefly, HT29 cells were seeded at white-walled 96-well plates and then treated with BJEE for 24 h in a concentration-dependent manner (25, 50, and 100 µg/mL). After incubation time, the treated cells were supplemented with caspase-Glo reagent (100 µL) and then the sealed plates were incubated for 30 min at room temperature. Finally, the caspase activities were analyzed by a laminator plate reader (GloMax microplateluminescence reader, Promega Company, USA).30
Measurement of nucleus factor-κB (NF-κB) activity
NF-κB translocation assay was previously described by Hajrezaie et al. In brief, HT29 cells were seeded at six-well plates on top of cover slips, and then was exposed to a BJEE IC50 concentration for 3 h. One group of cells was stimulated with TNF-α (5 ng/mL) for 30 min. The staining of cells was done according to the manufacturer’s instructions. Cellomics NF-κB Activation Kit was used. The translocation of NF-κB was determined by florescence microscope (Olympus BX51).22
Gene expression by real-time quantita tive polymerase chain reaction (RT-PCR)
The mRNA expressions of six proteins that play key roles in the apoptosis process were quantified by RT-PCR. The experimental method was used as described by Mohan et al. In brief, HT29 cells were seeded in a T25 cm2 flask with a density of 1×105 µg/mL, which were incubated overnight. Then the cells were treated with an IC50 concentration of BJEE for 24 h. The total RNA of treated and untreated cells was extracted using the commercial available kit, which is RNAeasy Mini kit (Qiagen, Germany), followed by quality identification of RNA using the NanoDrop 2000c and gel electrophoresis. Complementary DNA (cDNA) synthesis and cDNA conversion were performed using high-capacity RNA-to-cDNA kit and TaqMan® Gene Expression Master Mix kit, respectively, accordingly to the manufacturer’s instructions. Two endogenous housekeeping genes GAPDH (Hs03929097_g1) and ACTB (Hs99999903_m1) were used as positive references and were applied to normalize the target mRNA. Specific primers include BCL2-associated X (BAX, Hs00180269_m1), BCL2 (BCL2, Hs00608023_m1), caspase-3 (CASP3, Hs0,02,34,387_m1), caspase-8 (CASP8, Hs01018151_m1), caspase-9 (CASP9, Hs00609647_m1), p53 (DM02154335-g1), and NF-κB (NFKBIL1, Hs00428211-m1) and were bought from TaqMan (MGB probes, FAM™ dye-labeled) for the gene expression analysis. RT-PCR was performed on the StepOne PLUS real-time PCR machine (Applied Biosystems, Carlsbad, CA, USA) and results were analyzed by ΔΔCt method to calculate the relative changes in gene expression specified from RT-PCR.31
Apoptosis proteomic profile array
To identify the potential apoptotic pathways induced by BJEE, we performed an assay to determine involvement of apoptosis-related proteins using the Proteome Profiler Array (RayBio® Human Apoptosis Antibody Array C1 Kit, Raybiotech, Inc., Norcross, GA, USA), according to the manufacturer’s instructions. In brief, the HT29 cells here treated with BJEE at an IC50 dose (65 µg/mL) for 24 h. Around 300 µg of extracted protein from each sample was incubated with the human apoptosis antibody array overnight. The membranes were used to quantify the apoptosis array data via scanning on a Biospectrum AC ChemiHR 40 (UVP, Upland, CA, USA). The membranes’ image file was analyzed using ImageJ analysis software and the signal fold expression was determined according to the manufacturer’s instruction.32
Statistical analysis
All experimental data were presented as mean ± SD of three independent experiments, and also for in vitro studies. The statistical analysis was performed using Excel 2010, SPSS (version 18.0) statistical software, one-way analysis of variance with Tukey’s multiple comparisons, and the Student’s t-test for the statistical and graphical evaluation. All p-values <0.05 were considered as statistically significant.
Results and discussion
Cancer cell growth inhibition by B. javanica extracts
Carcinogenesis is a multistage process that includes unregulated cell proliferation and reduction in apoptosis. One of the most basic requirements of a potential anticancer product is to induce apoptosis in carcinoma cells. Apoptosis is recognized as a programmed cell death that plays a crucial role in the treatment of cancer.33 Medicinal plants were traditionally used to combat various types of diseases as these plants contain extensive and diverse chemical compositions, of which some of them have a potent activity for cancer treatment.34 Plants in the genus of Brucea are included in this category as they are potential producers of anticancer compounds; however, only two species of Brucea genus, B. javanica and B. antidysenterica, have been extensively used in traditional medicine and researched. The chemical constituents of these plants have shown extensive biologic activities, which include antimalarial, antidiabetic, anticancer, antitumor, antiviral, and anti-inflammatory activities.19 The first step to confirm cytotoxicity of anticancer agents on cancer cells is to evaluate the IC50 concentration of the drug.35 In this study, MTT assay was performed to determine the effects of hexane and BJEE on human cancer cells and normal cells. The cell viability of three different cancer cell lines after 48 h treatment with extracts showed extensive range of cytotoxicity on cancer cells upon treating with hexane and ethanolic extracts, and IC50 ranged from 48±2.5 to 112±4.3 µg/mL (Table 1). The results from MTT assay presented that B. javanica extract is selectively toxic to cancer cells and BJEE has shown the highest cytotoxicity effects on HT29 colon cancer cells at an IC50 value of 48±2.5 µg/mL after 48 h treatment. HT29 cells have recently been characterized as a suitable model for colon cancer studies. As shown in Table 2, IC50 value of BJEE at different times (24–72 h) was between 65±4.2 and 25±3.1 µg/mL. Relatively, there was a higher IC50 value of BJEE (>1,000 µg/mL) on normal colon cells CCD-841 CoN compared to the colon cancer cells of HT29, which suggested that this extract is nontoxic toward normal human cells. Furthermore, the results of this assay revealed that BJEE has an antiproliferative effect on human colon cancer cells (HT29) in a time- and dose-dependent manner. As BJEE had shown strong cytotoxic effects on HT29 cells, we carried out additional assays on colon cancer cell line for validation of mechanism of action of the extract.
Table 1.
IC50 values of B. javanica extracts on six different cell lines after 48 h of treatment
| Extract | Cell lines, mean ± SD
|
|||||
|---|---|---|---|---|---|---|
| HT29 | MDA-MB-231 | HeLa 229 | CCD-841 CoN | BJ-5ta | WRL-68 | |
| Hexane, IC50 (µg/mL) | 65±4.3 | 89±3.0 | 112±4.3 | >1,000 | 200 | 670 |
| Ethanol, IC50 (µg/mL) | 48±2.5 | 76±0.8 | 98±4.1 | >1,000 | 167 | 500 |
Notes: Cells were treated with different concentrations of hexane and ethanolic extract of B. javanica for 48 h. The IC50 values represent the concentration of the BJEE that could inhibit 50% of the cell growth. The data represent the mean ± SD of three independent experiments.
Abbreviations: BJEE, Brucea javanica ethanolic extract; IC50, concentration that inhibits 50% of the cell growth.
Table 2.
Cytotoxic effect of BJEE on colorectal cancer cell lines (HT29) after 24, 48, and 72 h
| Sample | HT29 treatment time periods; BJEE IC50 (μg/mL) for each period | ||
|---|---|---|---|
| HT29 | 24 h | 48 h | 72 h |
| BJEE | 65±4.2 | 48±2.5 | 25±3.1 |
Notes: HT29 cells were treated with different concentration of BJEE at different time periods (24, 48, and 72 h). The IC50 values of BJEE were detected for different times of treatment. The data represent mean ± SD.
Abbreviations: BJEE, Brucea javanica ethanolic extract; IC50, concentration that inhibits 50% of the cell growth.
Antioxidant activity of BJEE
Most of the medicinal plants are rich sources of natural antioxidants.24 DPPH radical-scavenging assay (1,1-diphenyl-2-picryl hydrazyl radical reducing power methods) and FRAP assay were the two bioassays that were used to identify the antioxidant activity of the extract.25
DPPH assay
In the DPPH radical-scavenging assay, antioxidants react with DPPH, followed by its conversion to the yellow-colored a,a-diphenyl-β-picryl hydrazine (DPPH-H). The degree of discoloration identified the radical-scavenging potential of the sample.36 The results from Figure 1 showed the percentage inhibition of DPPH by BJEE and standards at different concentrations. Table 3 revealed that IC50 of BJEE is 41.83 µg/mL, which is almost similar to that of ascorbic acid (38.9 µg/mL), while the highest antioxidant activity belongs to gallic acid with an IC50 of 25 µg/mL followed by ascorbic acid and BJEE at the end (Table 3).
Table 3.
Concentration that inhibits 50% of the DPPH radical values for DPPH inhibition
| Samples | Concentration that inhibits 50% of the DPPH radical (μg/mL) |
|---|---|
| BJEE | 41.83±3.1 |
| AA | 38.90±2.8 |
| GA | 25.00±1.5 |
Note: Measuring the concentration that inhibits 50% of the DPPH radical values of extracts and standards for inhibition of free radicals (DPPH). The data represent the mean ± SD of three independent experiments.
Abbreviations: BJEE, Brucea javanica ethanolic extract; AA, ascorbic acid; GA, gallic acid; DPPH, 2,2 diphenyl-1-picrylhydrazyl.
FRAP assay
The FRAP assay was performed to evaluate the antioxidant capacity of samples based on the reaction between antioxidant potential and Fe3+-TPTZ complex to produce Fe2+-TPTZ, which is blue in color.25 Figure 2 showed the standard curve of ferrous sulfate within the range of 0.2–1.0 mM with a good linearity (R2=0.9976). The results of FRAP assay have been tabulated in Table 4, which revealed the highest antioxidant activity for ascorbic acid (3.98±0.9 mmol Fe2+/g), gallic acid (2.09±0.7 mmol Fe2+/g), and BJEE (0.23±0.003 mmol Fe2+/g) (Table 4). The observed results from antioxidant assays illustrated that the BJEE has less antioxidant effect to inhibit the formation of oxygen-derived free radicals compared to standards, which is in the line with previous study that has been done on the extract of B. javanica.24
Table 4.
Ferric reducing antioxidant capacity of ethanolic extract and standards
| Sample | Concentration (mg/mL) |
Antioxidant capacity (mmol/Fe2+/g) |
|---|---|---|
| BJEE | 1.0 | 0.23±0.003 |
| GA | 1.0 | 2.09±0.7 |
| AA | 1.0 | 3.98±0.9 |
Note: The data represent the mean ± SD of three independent experiments.
Abbreviations: AA, ascorbic acid; BJEE, Brucea javanica ethanolic extract; GA, gallic acid.
BJEE elevated LDH activity
LDH assay is a test to identify the cytotoxicity of an anticancer agent on cancer cells by measuring the level of LDH release from the treated cells into the medium. LDH release in the medium is an indicator that determines cell membrane damages, apoptosis, or necrosis.37 The loss of membrane integrity was reflected in LDH elevation in the cell medium treated with BJEE for 24 h (Figure 3). The LDH levels were increased by 3.2%, 11.7%, and 13% following exposure to 50, 100, and 200 µg/mL of BJEE, respectively. There is a linear correlation between LDH activity and cell viability (Figure 3). Our results from this assay indicated BJEE cytotoxicity on HT29 by significant elevation of LDH release into medium via the loss of membrane integrity, through the activation of apoptosis or necrosis pathways.
Figure 3.
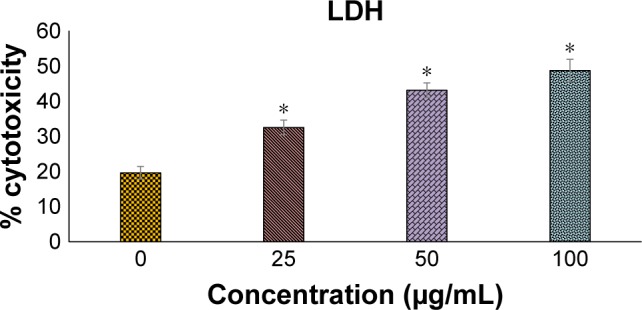
LDH assay confirmed the cytotoxicity of BJEE against HT29 cells in a dose-dependent manner. The data represent the means ± SD of three independent experiments. *p<0.05 compared with the untreated group.
Abbreviations: BJEE, Brucea javanica ethanolic extract; LDH, lactate dehydrogenase.
Quantified assessment of apoptosis using AO/PI double staining
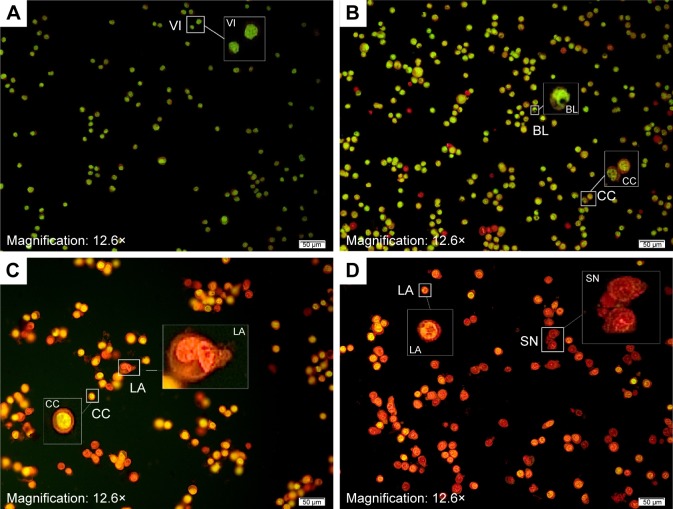
The mechanism of apoptosis is complex and many morphologic changes such as membrane permeability, chromatin condensation, and cell shrinkage are a few of the contributing factors in occurrence of apoptosis.13 There are two distinct phases in apoptotic process, named early and late apoptosis, which could be distinguished with intracellular staining assay. AO/PI double staining is one of the in vitro staining assays that can quantitatively distinguish viable cells from dead cells by analyzing cell morphologic features by using fluorescence microscope. AO stain with special characterization emits different colors of fluorescence accordingly when bound with different cellular organelles. AO emits green fluorescence when binding to dsDNA, while orange fluorescence color of AO binds to ssDNA. AO is able to penetrate plasma membrane of viable cells or early apoptotic cells with fragmented DNA. However, PI stain cannot cross the membrane of viable cells and can be observed only if there are dead cells. It can help in the detection of necrotic or late apoptotic cells via red fluorescence emission.38 In this study, the morphologic changes of treated HT29 cells with different concentrations (25, 50, and 100 µg/mL) of BJEE were observed under fluorescent microscope. Figure 4 showed that the untreated cells have healthy intact nuclei with green color, while membrane blebbing and chromatin condensation were detected after 24 h in cells treated with 25 µg/mL BJEE, which are the signs of early apoptosis. Further, presence of reddish-orange color was detected in cells treated with a higher concentration of BJEE (50 and 100 µg/mL), which is represented as late stage of apoptosis or necrosis. The AO/PI results illustrated morphologic features of apoptosis in a concentration-dependent manner.
Figure 4.
Microscopic detection of apoptosis using AO/PI double staining in untreated and treated HT29 cells with BJEE.
Notes: HT29 cells treated with BJEE at different concentrations (25, 50, and 100 µg/mL) during 24 h. Untreated cells are shown normal structure of cell without significant apoptosis or necrosis (A). After treatment with 25 µg/mL of BJEE, EA features including blebbing and chromatin condensation were observed (B). In 50 µg/mL treatment, LA significantly increased with orange color cells (C). After cell treatment with high dose of BJEE, 100 µg/mL, SN cells were noticed by red color (D), magnification: 20×.
Abbreviations: AO, acridine orange; BJEE, Brucea javanica ethanolic extract; BL, blebbing cell; CC, chromatin condensation; EA, early apoptosis; LA, late apoptosis; PI, propidium iodide; SN, secondary necrosis; VI, viable cell.
Externalization of PS induced by BJEE
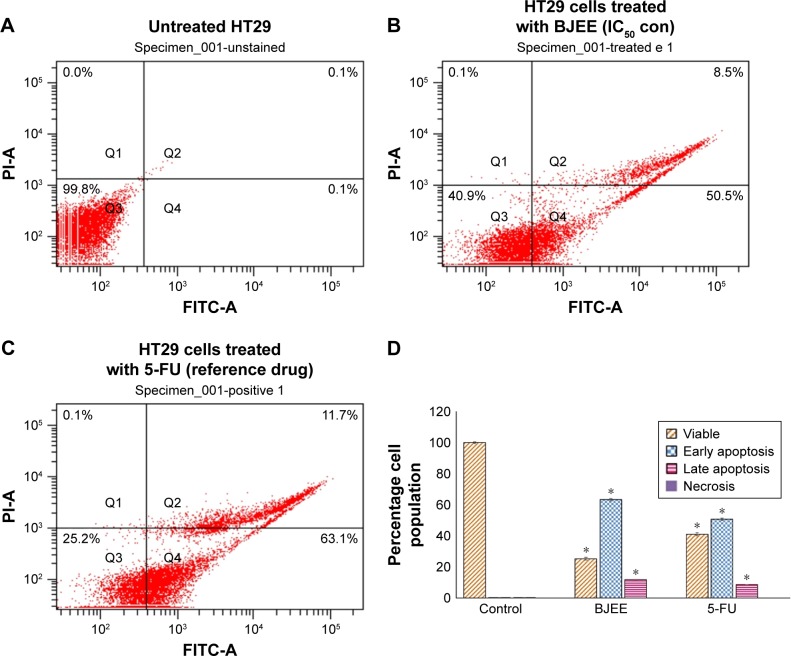
PS externalization is one of the biomarkers of apoptosis, which could be identified by AV flow cytometry assay. AV-FITC is designed for early detection of apoptosis by a probe with high affinity for PS, because exposure of PS is a prevalent event during apoptosis process, which occurs during DNA damages and membrane leakage.39 Therefore, staining with FITC in conjunction with PI dye helps to identify cells in early and late apoptosis stage. Viable cells with intact membranes exclude PI, whereas the membranes of dead and damaged cells are permeable to PI. Thus, cells that are considered viable are both AV and PI negative. In contrast, the cells observed both stain AV and PI positive marked the cells in the late apoptosis stage. Cells at early stages of apoptosis are AV positive and PI negative because of the affinity strength between AV-FITC and PS, which leads to transportation from inner leaflet to the outer surface of the plasma membrane. In addition, AV negative and PI positive staining represent the necrotic cells.40 The characteristic dot plots of Figure 5A–C indicated the flow cytometric valuation of apoptosis compared to the control (untreated cells), and the percentage of early apoptosis significantly increased (p<0.05) in the cells treated with BJEE and the reference drug (5-FU). Moreover, the percentage of cells at late apoptosis stage remarkably increased (p<0.05) to 8.5% and 11.7% in the cells treated with BJEE and 5-FU, respectively, while there are no significant necrotic cells after 24 h of treatment (Figure 5D). Overall, the results demonstrated that the BJEE treatment has an antiproliferative effect on HT29 cells by activating the induction of apoptosis cells.
Figure 5.
Flow cytometric analysis of early and late apoptosis in treated HT29 cells with BJEE.
Notes: HT29 cells were treated with BJEE and reference drug (5-FU) at IC50 concentrations, and then maintained for 24 h at 37°C in a CO2 incubator. The cells were analyzed after staining with FITC-conjugated AV and PI by flow cytometer. The dot plot represents the untreated HT29 cells as a control group (A), treated HT29 cells with IC50 concentration of BJEE (B), and treated HT29 cells with reference drug (5-FU) (C). The early apoptosis events (AV+/PI−) shown in lower right quadrant (Q4). The late stage of apoptosis/dead cells (AV+/PI+) is shown in quadrant Q2. Bar chart represents the percentage of viable, early apoptosis, late apoptosis, and necrotic cells in treatment with BJEE on HT29 cells (D). The results are shown as mean ± SD of three independent experiments with *p<0.05.
Abbreviations: 5-FU, 5-fluorouracil; AV, annexin-V; BJEE, Brucea javanica ethanolic extract; FITC, fluorescein isothiocyanate; IC50, concentration that inhibits 50% of the cell growth; PI, propidium iodide.
Effect of BJEE on ROS generation
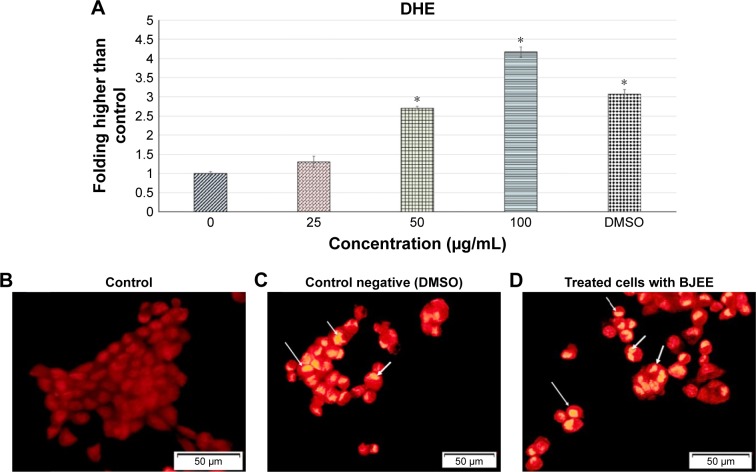
ROS generation plays a crucial role in the cell signaling and cell apoptosis pathways via activation of mitochondrial-intrinsic events or lipid peroxidation leading to cell death. Overproduction of ROS in cytoplasm involves different factors of non-receptor-mediated stimuli, which include lipid oxidation, DNA and protein damage, and p53 expression that induced tumor genesis or cell apoptosis.41 DHE is the most specific fluorescent probe used for superoxide detection. Cytosolic superoxide production could be detected by the DHE dye.42 In this study, ROS production levels have been identified using DHE staining, which exhibited the influence of BJEE on ROS production at HT29 cells as shown in Figure 6. Our results revealed a significant elevation in the ROS production at HT29 cells after 12 h of treatment with different concentrations (0, 25, 50, and 100 µg/mL) of BJEE (Figure 6A). Intracellular superoxide generation was evaluated by DHE staining. In comparison with the control group, the production of ROS was significantly increased at HT29 cells’ exposure to BJEE during 12 h (Figure 6).
Figure 6.
ROS production in the presence of BJEE in HT29 cells.
Notes: Bar chart represents quantification of DHE fluorescence intensity at different treatment concentrations (0, 25, 50, and 100 µg/mL) (A). DHE fluorescence folding increased at higher concentrations of BJEE treatment. Untreated HT29 cells stained with DHE (B). The yellow staining of nuclei reflects the intracellular generation of ROS. A large number of HT29 cells showed intense staining for ROS (arrows) after dimethyl sulfoxide treatment (control negative) (C), and this reaction was developed in HT29 cells treated with BJEE for 12 h (D). A significant formation of ROS observed in treated HT29 cells with BJEE. Data are expressed as the mean ± SD of triplicate measurements. *p<0.05 compared with the no-treatment group, magnification: 200×.
Abbreviations: BJEE, Brucea javanica ethanolic extract; DHE, dihydroethidium; ROS, reactive oxygen species.
Evaluation of caspase activity
Apoptosis is a complex mechanism that has many pathways that may be involved in the cell death processes such as mitochondrial membrane dysfunction, mitochondrial pathway, and extrinsic pathway. Caspase cascade events are an important factor in the induction of apoptosis via extrinsic or intrinsic pathways. Activity of caspase enzymes such as caspase-9, -3/7, and -8 in treated cells is closely linked to the apoptosis signaling through the intrinsic and extrinsic pathways. In many cases, caspase-9 activation is a downstream production from ROS elevation, which is linked to mitochondrial intrinsic pathway. Activation of caspase-3/7 triggered by caspase-9 and -8 activations is the last stage leading the cell to apoptosis. Moreover, the significant increase of caspase-8 in treated cells closely related to the extrinsic pathway, and in many cases, caspase-8 could be linked to the mitochondrial pathway by upregulation of Bid protein from Bcl-2 family members.43
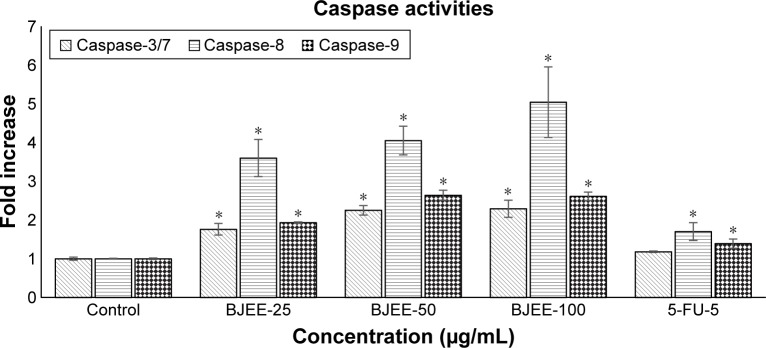
In this study, the results from bioluminescent intensities showed caspase-3/7, -8, and -9 activities in HT29 cells treated with different concentrations of BJEE for 24 h. Figure 7 demonstrated that caspase-8 remarkably has higher activation in comparison to caspase-3/7 and -9 during treatment with BJEE. Caspase-3/7 and caspase-9 also revealed a significant increase in activation during 24 h of treatment. The elevation of caspase-3/7 activity after 24 h of treatment confirmed induction of apoptosis in colon cancer HT29 cells. Furthermore, the expression level of caspase-9 activity elevated in treated cells is considered as the intrinsic pathway contribution during apoptotic process. In addition, ROS assay results showed that elevation of ROS production may lead to the activation of the caspase cascade and the results are in accordance with the caspase activity assay. The significant increase in caspase-8 level could confirm occurrence of extrinsic pathway in apoptosis of HT29 cell.
Figure 7.
Effect of different concentrations of BJEE (25, 50, and 100 µg/mL) and reference drug (5-FU) on caspase-3/7, -8, and -9 activations in HT29 cells after 24 h of treatment.
Notes: The results showed significant activation of caspase-3/7, -8, and -9 compared to nontreated control group. Data are expressed as the means ± SD of triplicate individual experiments. *p<0.05.
Abbreviations: BJEE, Brucea javanica ethanolic extract; 5-FU, 5-fluorouracil.
Inhibition of NF-κB translocation by BJEE
NF-κB activation and translocation from cytoplasm to nucleus play a critical role in inhibition of apoptosis in cells. This translocation will be induced by incubation of cells in the presence of TNF-α. NF-κB has a critical role as apoptosis inhibitor by the regulation of various genes in nucleus, and then the suppressive activity from translocation of NF-κB closely associated with the activation of the extrinsic apoptotic pathway in cancer cells.44
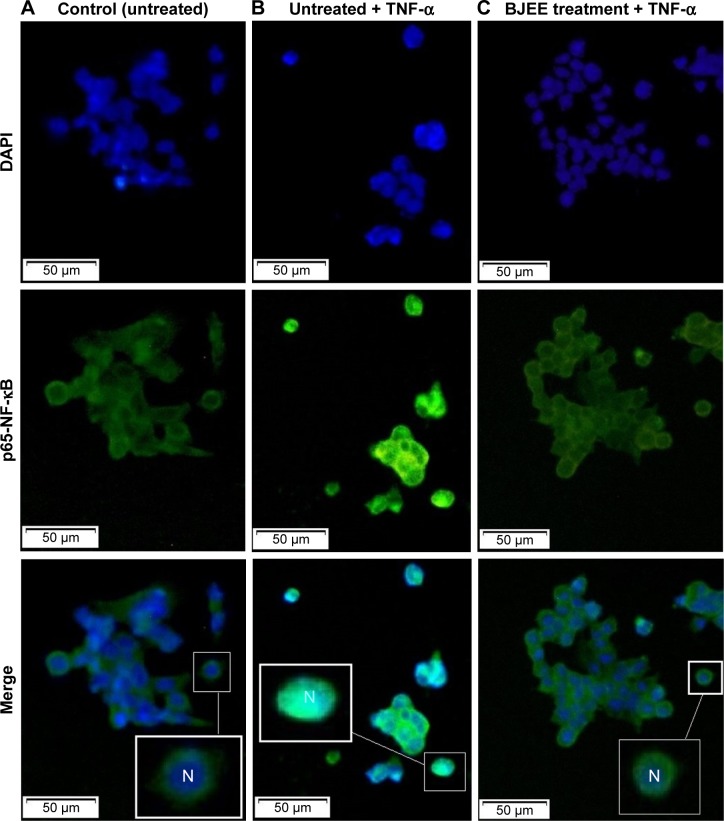
As shown in Figure 8, HT29 cells incubated with TNF-α remarkably induced NF-κB translocation compared to control group (untreated cells). However, the treatment of cells with BJEE at an IC50 concentration during 3 h showed inhibitory effect on NF-κB translocation in the presence of TNF-α (Figure 8). Since treatment with BJEE, HT29 cancer cells showed inhibitory effect against NF-κB translocation from cytoplasm to nucleus; therefore, we could assume induction of apoptosis via suppression of NF-κB as well.
Figure 8.
Inhibition effects of BJEE on translocation of TNF-α-induced NF-κB in HT29 cells.
Notes: Microscopic tracking of NF-κB translocation by staining of DAPI (nucleus) and NF-κB in HT29 cells. Cells were pretreated with 50 µg/mL of BJEE for 3 h, followed by TNF-α (5 ng/mL) stimulation for 30 min. Nonstimulated cells with no treatments are represented in group (A), the untreated cells stimulated for 30 min with TNF-α (NF-κB activation) is represented in group (B), and treated cells with BJEE reduced the fluorescent intensity of nuclear NF-κB (C), which confirmed that BJEE inhibited TNF-α-induced translocation of NF-κB from the cytoplasm to the nucleus (magnification: 200×).
Abbreviations: BJEE, Brucea javanica ethanolic extract; N, nucleus; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor alpha.
Gene expression analysis
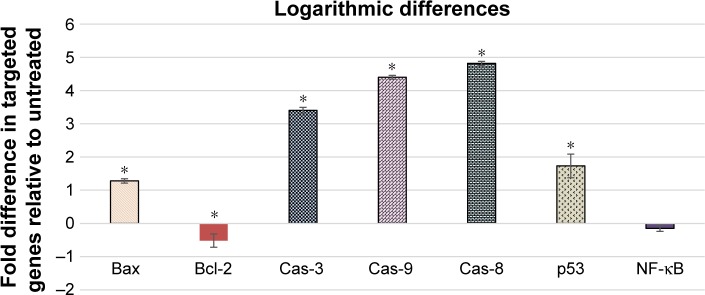
Due to the important role of caspase activity in stimuli of apoptosis, we had investigated the expression of these markers at the gene level, using the RT-PCR analyses. The mRNA expression from this study has revealed a significant expression of the Bax, caspase-3, caspase-8, caspase-9, and p53 after 24-h treatment of HT29 cells with BJEE. Furthermore, the gene expressions of antiapoptotic proteins such as Bcl-2 and NF-κB were inhibited after treatment (Figure 9). The results showed that the caspase-8, -9, and -3 have the higher upregulation of genes with approximately log based 2 values of 3.4-, 4.4-, and 4.8-fold higher than control, respectively. mRNA expressions of p53 and Bax proteins were significantly upregulated but log base 2 of folding values were 1.7 and 1.3, respectively, after 24 h of treatment. In addition, antiapoptotic genes such as Bcl-2 showed the significant downregulation after treatment, while the downregulation of NF-κB gene was not statistically significant but its expression was slightly inhibited during treatment. This suppression of NF-κB expression confirmed findings from NF-κB trans-location inhibition in treated (BJEE) HT29 cells.
Figure 9.
Gene expression analysis using RT-PCR in HT29 cells after 24 h of treatment with BJEE.
Notes: Quantitative analysis of gene expression results illustrated significant elevation in the expression of Bcl-2, Bax, p53, Cas-3, -8, and -9 genes, compared to the untreated control group. Meanwhile, gene expression of NF-κB was slightly inhibited but it has not revealed statistical significant changes. The gene expressions were shown in 2 log fold changes of mean ± SD. *p<0.05 versus control group.
Abbreviations: BJEE, Brucea javanica ethanolic extract; RT-PCR, real-time quantitative polymerase chain reaction.
Upregulation of caspase genes is in line with caspase activity and ROS findings. In addition, obtained results from overexpression of caspase and p53 genes are also corresponding with the caspase activity and ROS findings.
According to the earlier studies, the excessive production of ROS activated downstream production of p53, which is a mitochondrial apoptotic-stimuli mediator to activate caspase molecules and consequently lead to apoptotic cell death.45,46 In this experiment, a significant amount of ROS production in HT29 cells after 12-h treatment with BJEE was noticed along with the activation and upregulation of p53 and caspases according to the results from gene expression.
Effect of BJEE on apoptotic proteins
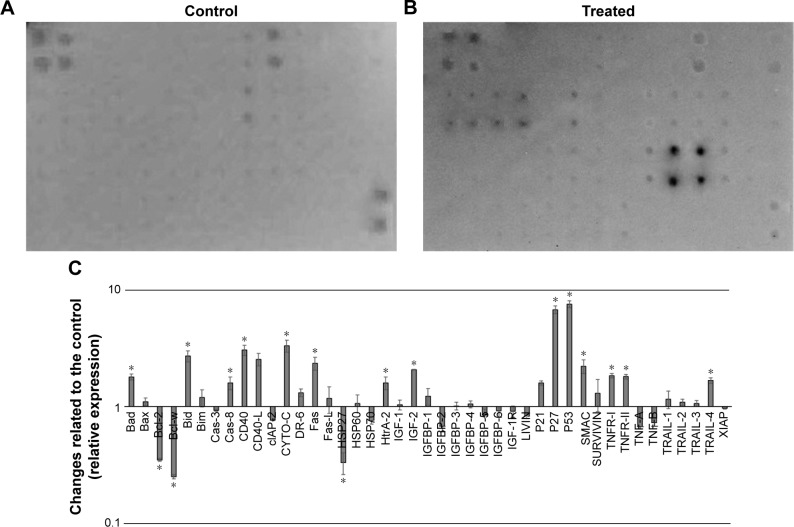
After exposure of HT29 cells to BJEE for 24 h, the critical proteins were implicated with cell death and apoptotic pathways were observed via human apoptosis protein array. In Figure 10A–C, the images showed the changes of apoptotic proteins in treated and untreated cells. Many proteins were observed to be up- or downregulated according to their role in apoptotic pathways. As shown in Figure 10C, the important proteins that play a critical role in the intrinsic apoptosis such as Bax, Bim, cytochrome c, SMAC (second mitochondria-derived activator of caspases), and HtrA-2 were significantly upregulated after 24 h of treatment, while expressions of antiapoptotic proteins including Bcl-2, Bcl-w, cIAP-2, XIAP, and LIVIN were inhibited. There are evidences that show that the members of the Bcl-2 family proteins such as Bax, Bim, Bad, Bcl-2, and Bcl-w play a key role in mediating mitochondrial pathway via releasing the cytochrome c from mitochondria into cytosol, which triggers the formation of apoptosome and activation of downstream caspase-9, which leads to activation of caspase-3, and -7, and overall these intermittent cellular activities were called the intrinsic apoptosis pathway.47 Moreover, SMAC/DIABLO and HtrA2/Omi markers inhibit the activity of apoptosis protein suppressor, such as cIAP-2 and XIAP,48 whose expressions were reduced in our experiment.
Figure 10.
Quantitative analysis of the human apoptosis proteome profiler array in treated and untreated HT29 cells.
Notes: Cells were treated with IC50 concentration of BJEE for 24 h, then cells were lysed and protein arrays were performed. Equal amounts (300 µg) of protein from each control and treated sample were used for the assay. Representative images of the apoptotic protein array are shown for the control (A) and treatment (B), and a bar graph shows the difference in the apoptotic markers between treated cells as well as untreated control cells (C). The data are expressed as the mean ± SD for three independent experiments. *Indicates a significant difference from control (*p<0.05).
Abbreviations: BJEE, Brucea javanica ethanolic extract; IC50, concentration that inhibits 50% of the cell growth.
In addition, earlier studies had revealed that the tumor suppressor protein p53 plays a critical role in leading the cancer cells into apoptotic death through dependent or independent pathways. This p53 protein may be interlinked with the mitochondrial pathway via regulation of Bcl2 protein family or could be involved in the cell cycle arrest through p21 protein overexpression.49 p53 also was induced expression of p27, which is associated with apoptotic stimuli by correlation with Bax expression.50 The obtained results from this study showed that overexpression of p53, p27, and p21 is supportive of the results from gene expression analysis. Further, HSP70 and HSP27 were significantly downregulated in the induction of apoptosis. In addition, highly overexpressed p53, along with p27, are cell proliferation suppressor markers, which induced apoptosis in HT29 cells by activation of proapoptotic Bcl2 family proteins and cell cycle arrest (Figure 10).
Additionally, apoptotic markers including Bad, Bid, caspase-8, Fas, CD40, DR6, TNF receptors (TNFR-I, TNFR-II), and the TNF-related apoptosis-inducing ligand TRAIL-4 proteins were overexpressed during treatment with BJEE, which showed the contribution of the extrinsic pathway in apoptosis of HT29 cells. Earlier studies had indicated that the activation of death receptors such as Fas, CD40, TRAILs, TNFR-I, and TNFR-II mediated extrinsic pathway of apoptosis, which in turn increased the caspase-8 expression to activate downstream effector caspases (caspase-3/7), or may be interlinked with mitochondrial pathway through cleavage of Bid to tBid.51 In agreement with previous studies, the obtained results from apoptosis proteome array revealed involvement of both intrinsic (mitochondrial) and extrinsic (death receptors Fas, TNFR, and TRAIL) pathways to induce apoptosis in HT29 cells after treatment with BJEE.
Conclusion
Overall, we had provided evidence that the BJEE has caused cytotoxic effects toward HT29 colorectal cancer cells. The obtained results from this study also revealed that the extract induced apoptosis in HT29 cells through the caspase activation via the ROS production, and p53, Bax, and NF-κB involvement. In addition, contributions of both extrinsic and intrinsic pathways could be concerned in the induction of apoptosis via elevation of caspase-8 enzyme level and inhibition of NF-κB translocation from cytoplasm to the nucleus. The association of mitochondrial-intrinsic events guided by mRNA and upregulation of p53, Bax, and cytochrome c might have triggered mitochondrial dysfunction via increased level of caspase-9 activity. These results of the study indicated that B. javanica is a promising plant in the fight against cancer. However, further in vitro and in vivo studies on the probable active compounds of this plant responsible for the above activities are still required to be investigated.
Acknowledgments
The authors would like to express their utmost gratitude and appreciation to the University of Malaya for supporting this project through the IPPP grant (PG053–2013A), and the UMRG research grant (RP021A-14AFR) to conduct this study. The funding sponsors had no role in the design of the study, data collection and analyses, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Author contributions
EB executed in vitro experiments, analyzed the data, and wrote the manuscript. FH helped in data analysis and rewriting some parts of the paper. SN is the principal investigator, contributed to the design of work, critical review of study protocol, and editing of manuscript and review of the paper before submission to the journal. NS is the co-investigator of the project, conceived the idea, and edited the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.David AR, Zimmerman MR. Cancer: an old disease, a new disease or something in between? Nat Rev Cancer. 2010;10(10):728–733. doi: 10.1038/nrc2914. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49(1):33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 3.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 4.Mishra J, Drummond J, Quazi SH, et al. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit Rev Oncol Hematol. 2013;86(3):232–250. doi: 10.1016/j.critrevonc.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palombo EA. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res. 2006;20(9):717–724. doi: 10.1002/ptr.1907. [DOI] [PubMed] [Google Scholar]
- 7.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Sucher NJ. The application of Chinese medicine to novel drug discovery. Expert Opin Drug Discov. 2013;8(1):21–34. doi: 10.1517/17460441.2013.739602. [DOI] [PubMed] [Google Scholar]
- 9.Schuster TL, Dobson M, Jauregui M, Blanks RH. Wellness lifestyles I: a theoretical framework linking wellness, health lifestyles, and complementary and alternative medicine. J Altern Complement Med. 2004;10(2):349–356. doi: 10.1089/107555304323062347. [DOI] [PubMed] [Google Scholar]
- 10.Sałaga M, Zatorski H, Sobczak M, Chen C, Fichna J. Chinese herbal medicines in the treatment of IBD and colorectal cancer: a review. Curr Treat Options Oncol. 2014;15(3):405–420. doi: 10.1007/s11864-014-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullah MF, Bhat SH, Husain E, et al. Cancer chemopreventive pharmacology of phytochemicals derived from plants of dietary and non-dietary origin: implication for alternative and complementary approaches. Phytochem Rev. 2014;13(4):811–833. [Google Scholar]
- 12.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 15.Nair P, Lu M, Petersen S, Ashkenazi A. Apoptosis initiation through the cell-extrinsic pathway. Methods Enzymol. 2014;544:99–128. doi: 10.1016/B978-0-12-417158-9.00005-4. [DOI] [PubMed] [Google Scholar]
- 16.Abebe W. An overview of Ethiopian traditional medicinal plants used for cancer treatment. Eur J Med Plants. 2016;14(4):1–16. [Google Scholar]
- 17.Houël E, Stien D, Bourdy G, Deharo E. Quassinoids: anticancer and antimalarial activities. Nat Products. 2013:3775–3802. [Google Scholar]
- 18.Monteiro LDS, Bastos KX, Barbosa-Filho JM, de Athayde-Filho PF, Diniz MDFFM, Sobral MV. Medicinal plants and other living organisms with antitumor potential against lung cancer. Evid Based Complement Altern Med. 2014;2014 doi: 10.1155/2014/604152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Li C, Zhang Y, Wen Q, Ren D. Phytochemical and biological activities of an anticancer plant medicine: Brucea javanica. Anticancer Agents Med Chem. 2014;14(3):440–458. doi: 10.2174/18715206113136660336. [DOI] [PubMed] [Google Scholar]
- 20.Alves IA, Miranda HM, Soares LA, Randau KP. Simaroubaceae family: botany, chemical composition and biological activities. Rev Bras Farmacogn. 2014;24(4):481–501. [Google Scholar]
- 21.Kim SH, Liu CY, Fan PW, et al. The aqueous extract of Brucea javanica suppresses cell growth and alleviates tumorigenesis of human lung cancer cells by targeting mutated epidermal growth factor receptor. Drug Des Devel Ther. 2016;10:3599–3609. doi: 10.2147/DDDT.S117443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajrezaie M, Paydar M, Zorofchian Moghadamtousi S, et al. A Schiff base-derived copper (II) complex is a potent inducer of apoptosis in colon cancer cells by activating the intrinsic pathway. ScientificWorldJournal. 2014;2014:540463. doi: 10.1155/2014/540463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajiaghaalipour F, Kanthimathi M, Sanusi J, Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015;169:401–410. doi: 10.1016/j.foodchem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Ablat A, Mohamad J, Awang K, Shilpi JA, Arya A. Evaluation of antidiabetic and antioxidant properties of Brucea javanica seed. ScientificWorld Journal. 2014;2014:786130. doi: 10.1155/2014/786130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y, Khoo T, Wiart C. Antioxidant activity determination of Citronellal and crude extracts of Cymbopogon citratus by 3 different methods. Pharmacol Pharm. 2014;5(4):395–400. [Google Scholar]
- 26.Zorofchian Moghadamtousi S, Rouhollahi E, Karimian H, et al. The chemopotential effect of Annona muricata leaves against azoxymethane-induced colonic aberrant crypt foci in rats and the apoptotic effect of acetogenin annomuricin E in HT-29 cells: a bioassay-guided approach. PLoS One. 2015;10(4):e0122288. doi: 10.1371/journal.pone.0122288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajiaghaalipour F, Faraj F, Bagheri E, et al. Synthesis and characterization of a new benzo indole derivative with apoptotic activity against colon cancer cells. Curr Pharm Des. 2017 Mar 20; doi: 10.2174/1381612823666170321093345. Epub. [DOI] [PubMed] [Google Scholar]
- 28.Hajiaghaalipour F, Bagheri E, Faraj FL, Abdulla MA, Majid NA. Underlying mechanism for the modulation of apoptosis induced by a new benzoindole derivative on HT-29 colon cancer cells. RSC Adv. 2017;7(61):38257–38263. [Google Scholar]
- 29.Rouhollahi E, Moghadamtousi SZ, Paydar M, et al. Inhibitory effect of Curcuma purpurascens BI. rhizome on HT-29 colon cancer cells through mitochondrial-dependent apoptosis pathway. BMC Complement Altern Med. 2015;15(1):15. doi: 10.1186/s12906-015-0534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajrezaie M, Paydar M, Looi CY, et al. Apoptotic effect of novel Schiff Based CdCl2 (C14H21N3O2) complex is mediated via activation of the mitochondrial pathway in colon cancer cells. Sci Rep. 2015;5:9097. doi: 10.1038/srep09097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan S, Abdelwahab SI, Kamalidehghan B, et al. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine. 2012;19(11):1007–1015. doi: 10.1016/j.phymed.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadipour F, Noordin MI, Mohan S, et al. Koenimbin, a natural dietary compound of Murraya koenigii (L) Spreng: inhibition of MCF7 breast cancer cells and targeting of derived MCF7 breast cancer stem cells (CD44+/CD24-/low): an in vitro study. Drug Des Devel Ther. 2015;9:1193–1208. doi: 10.2147/DDDT.S72127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14(1):48. doi: 10.1186/s12943-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngarivhume T, van’t Klooster CI, de Jong JT, Van der Westhuizen JH. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J Ethnopharmacol. 2015;159:224–237. doi: 10.1016/j.jep.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Florento L, Matias R, Tuaño E, Santiago K, dela Cruz F, Tuazon A. Comparison of cytotoxic activity of anticancer drugs against various human tumor cell lines using in vitro cell-based approach. Int J Biomed Sci. 2012;8(1):76–80. [PMC free article] [PubMed] [Google Scholar]
- 36.Hajiaghaalipour F, Sanusi J, Kanthimathi M. Temperature and time of steeping affect the antioxidant properties of white, green, and black tea infusions. J Food Sci. 2016;81(1):H246–H254. doi: 10.1111/1750-3841.13149. [DOI] [PubMed] [Google Scholar]
- 37.Cummings BS, Wills LP, Schnellmann RG. Measurement of cell death in Mammalian cells. Curr Protoc Pharmacol. 2012:12–18. doi: 10.1002/0471141755.ph1208s56. chapter 12 (unit 12.8) [DOI] [PubMed] [Google Scholar]
- 38.Tischlerova V, Kello M, Budovska M, Mojzis J. Indole phytoalexin derivatives induce mitochondrial-mediated apoptosis in human colorectal carcinoma cells. World J Gastroenterol. 2017;23(24):4341–4353. doi: 10.3748/wjg.v23.i24.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen ME, Jansen ES, Sanchez W, Waterhouse NJ. Flow cytometry based assays for the measurement of apoptosis-associated mitochondrial membrane depolarisation and cytochrome c release. Methods. 2013;61(2):138–145. doi: 10.1016/j.ymeth.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Abbaspour Babaei MA, Huri HZ, Kamalidehghan B, Yeap SK, Ahmadipour F. Apoptotic induction and inhibition of nF-κB signaling pathway in human prostatic cancer Pc3 cells by natural compound 2, 2′-oxybis (4-allyl-1-methoxybenzene), biseugenol B, from Litsea costalis: an in vitro study. Onco Targets Ther. 2017;10:277–294. doi: 10.2147/OTT.S102894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojtala A, Bonora M, Malinska D, Pinton P, Duszynski J, Wieckowski MR. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 2014;542:243–262. doi: 10.1016/B978-0-12-416618-9.00013-3. [DOI] [PubMed] [Google Scholar]
- 43.Fulda S, Debatin K. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 44.Turner DJ, Alaish SM, Zou T, Rao JN, Wang JY, Strauch ED. Bile salts induce resistance to apoptosis through NF-κB-mediated XIAP expression. Ann Surg. 2007;245(3):415–425. doi: 10.1097/01.sla.0000236631.72698.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Chen Y, Clair DKS. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44(8):1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Youle RJ. The role of mitochondria in apoptosis. Ann Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang QH, Church-Hajduk R, Ren J, Newton ML, Du C. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev. 2003;17(12):1487–1496. doi: 10.1101/gad.1097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen A, Huang X, Xue Z, et al. The role of p21 in apoptosis, proliferation, cell cycle arrest, and antioxidant activity in UVB-irradiated human HaCaT keratinocytes. Med Sci Monit Basic Res. 2015;21:86–95. doi: 10.12659/MSMBR.893608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujieda S, Inuzuka M, Tanaka N, et al. Expression of p27 is associated with Bax expression and spontaneous apoptosis in oral and oropharyngeal carcinoma. Int J Cancer. 1999;84(3):315–320. doi: 10.1002/(sici)1097-0215(19990621)84:3<315::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 51.Feltham R, Vince JE, Lawlor KE. Caspase-8: not so silently deadly. Clin Transl Immunol. 2017;6(1):e124. doi: 10.1038/cti.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]