Abstract
Expansins are a family of extracellular proteins proposed to play a key role in wall stress relaxation and, thus, in cell and tissue growth. To test the possible function of expansins in morphogenesis, we have developed a technique that allows transient local microinduction of gene expression in transgenic plants. We have used this system to manipulate expansin gene expression in various tissues. Our results indicate that local expansin expression within the meristem induces a developmental program that recapitulates the entire process of leaf formation. Moreover, local transient induction of expansin expression on the flank of developing primordia leads to the induction of ectopic lamina tissue and thus modulation of leaf shape. These data describe an approach for the local manipulation of gene expression and indicate a role for expansin in the control of both leaf initiation and shape. These results are consistent with the action of cell division-independent mechanisms in plant morphogenesis.
It has been proposed that modulation of cell wall extensibility could play a key role in plant morphogenesis (1). In addition to theoretical considerations supporting this hypothesis, data have accumulated indicating that a family of extracellular proteins (expansins) functions in vivo to modulate cell wall extensibility and thereby regulates organ growth and morphogenesis (2, 3). Expansins were first identified as cell wall-associated proteins that could function in vitro to increase cell wall extensibility (4). It has subsequently become apparent that expansins are widespread in the plant kingdom, and that they tend to be encoded by relatively large gene families whose patterns of transcript accumulation and activity frequently correlate with specific processes of growth, morphogenesis, and differentiation (2). Moreover, data have accumulated supporting a functional role for expansins in these processes. For example, experiments in which expansin expression was suppressed in differentiating vascular tissue in transgenic Arabidopsis plants led to a marked phenotype of dwarfed leaves and altered morphology (5), supplying expansin protein to BY2 suspension cultures led to an increase in average cell size (6), and local application of expansin to tomato meristems induced morphogenesis (3).
Although expansin-induced morphogenesis was sufficient to induce a program that recapitulated at least some aspects of normal leaf development, the expansin-induced structures were never capable of forming phenotypically normal leaves, most noticeable in the absence of vasculature and limited lamina formation. Later experiments (7) indicated that exogenously supplied expansin was likely to affect only the outermost epidermal cell wall, and that this pattern was very different from the endogenous accumulation of expansin transcripts, which defined a zone encompassing many cell layers of the meristem in the presumptive region of leaf initiation (3, 8). We therefore hypothesized that the formation of incomplete leaf structures after exogenous expansin supply might reflect a technical limitation leading to a failure of the exogenous protein to mimic the endogenous pattern of expansin expression observed during leaf initiation.
To test this hypothesis, we have generated transgenic tobacco plants in which expansin gene expression can be chemically induced. At the same time, we have developed a microinduction system that allows us to induce gene expression in very small tissue parts (fractions of a meristem). Using this system, we demonstrate that local induction of ectopic expansin expression in the meristem induces morphogenesis leading to the formation of leaves that are phenotypically similar to normally generated leaves. Moreover, we show that local manipulation of expansin expression during the earliest stages of leaf development is sufficient to alter local lamina growth leading to modification of leaf shape.
Materials and Methods
Plant Material and Transformation.
R7 Nicotiana tabacum seedlings (a gift of C. Gatz, University of Goettingen, Goettingen, Germany) were transformed (9). Regenerants were grown in a greenhouse and F1 seeds collected for analysis. For microinduction experiments, plants were grown in soil in a growth chamber (16 h light at 24°C/8 h dark at 20°C) or on half-strength Murashige and Skoog medium (pH 5.6), 1% (wt/vol) agar (16/8 h light/dark cycle, 24°C, 100 μmol m−2⋅s−1). For RNA blot and expansin activity analyses, ≈100 4-week-old seedlings were grown with gentle shaking (60 rpm) in 2-liter Erlenmeyer flasks containing 200 ml of liquid MS medium (pH 5.6), with or without anhydrotetracycline (Ahtet), at the concentrations and for the times given in Results.
DNA Manipulation.
The CsExp1 coding sequence (10) was inserted as a transcriptional fusion into the KpnI/SalI sites of the pBinHyg-Tx vector (a gift of C. Gatz). The resultant clone, pBinHyg-Tx- CsExp1, was introduced into R7 tobacco plants, as was a pBinHyg-Tx-β glucoronidase (GUS) construct in a parallel experiment. All DNA manipulations were by standard techniques (11).
Microinduction.
Ahtet dissolved in DMSO was mixed with melted lanolin/3% paraffin at 60°C and rapidly cooled to generate a paste. Ahtet concentrations used ranged from 0.1 to 100 μg/ml. Portions of paste were applied by using stretched plastic tips to the surfaces of dissected meristems and primordia under a Leica MZ12 (Deerfield, IL) microscope. Controls were performed by using DMSO/lanolin/paraffin paste without Ahtet. At the time of apex dissection, plants had 10–15 leaves, 2–3 of which were left intact on the meristem before microinduction experiments. For lamina experiments, primordia at stage P2 or P3 were microinduced along approximately one-third of the flank. After manipulation, apices were grown on half-strength MS medium (pH 5.6), 1% (wt/vol) agar in a growth chamber (16/8 h light/dark cycle, 24°C, 100 μmol m−2⋅s−1) for 2 weeks before transfer to multiwell plates with water. After growth and root regeneration under the same growth conditions, plantlets were transferred to soil for further growth and analysis.
RNA Analysis.
For blots, total RNA was extracted from 4-week-old seedlings by using RNeasy columns (Qiagen, Chatsworth, CA). Gel electrophoresis, blotting, and hybridization with a radiolabeled probe for CsExp1 was by standard methods (11). In situ hybridization was as previously described (12), by using digoxygenin-labeled sense and antisense riboprobes for CsExp1.
Expansin and GUS Activity Assays.
Expansin activity assays were performed as previously described (13). Briefly, equivalent amounts of cell wall protein from Ahtet- and control-treated plantlets were added to a cellulose/xyloglucan matrix in the presence of 50 mM sodium acetate (pH 4.5). Expansin activity was calculated as the rate of extension of the material in the first 10 min after protein addition minus the prior rate of tissue extension. GUS activity was visualized in intact apices (14) and either hand sections taken or tissue embedded in Technovit resin (Haska, Bern, Switzerland) for thin-section analysis (according to the manufacturer's instructions).
Electron Microscopy.
Cryoscanning electron microscopy was as previously described (7) of apices 3 days after manipulation.
Results
Generation of Transgenic Plants with Inducible Expansin Activity.
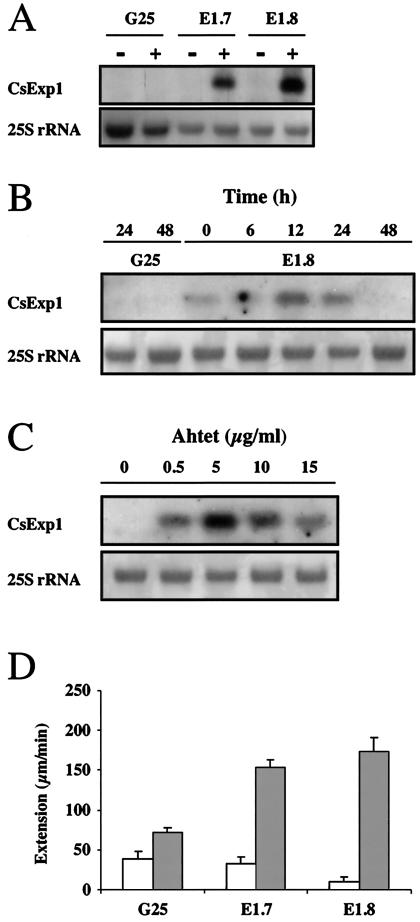
Plasmid pBinHyg-Tx-CsExp1 containing a cDNA encoding the expansin CsEx29 protein originally identified in cucumber hypocotyls (4) was introduced into tobacco R7 plants engineered to constitutively overexpress the tetracycline (Tet) repressor protein (15). The repressor protein binds to the TetO sequence in the pBinHyg-Tx vector to repress transcription of downstream sequence. Ahtet binds to the repressor protein to alleviate this transcriptional repression. Seeds were collected from 11 independent primary transformants and each line tested for the accumulation of CsExp1 transcript with or without prior induction with Ahtet. This experiment led to the identification of six lines showing accumulation of CsExp1 transcripts after induction with undetectable expression under noninducing conditions. The results for two of these lines (E1.7 and E1.8) are shown in Fig. 1A. These lines were used for further analysis. A time course of CsExp1 induction by using 15 μg/ml of Ahtet revealed a transient transcript accumulation with a maximum after 12 h (Fig. 1B). Incubation with various concentrations of Ahtet indicated that relatively low levels were sufficient to induce transcript accumulation with a maximum at 5 μg/ml; at higher concentrations, induction was reduced (Fig. 1C). Controls with plants engineered to express the GUS reporter gene under Ahtet-inducible transcription (Tet∷GUS) revealed no cross hybridization of the CsExp1 probe with endogenous transcripts under the conditions used (Fig. 1 A and B). To test whether the observed changes in CsExp1 transcript level had an influence on endogenous expansin activity, we performed extensibility tests by using an in vitro system (13). The results (Fig. 1D) indicate that in both lines E1.7 and E1.8, Ahtet induction led to an increased level of endogenous expansin activity (5-fold over noninduced levels for line E1.7, 17-fold over noninduced levels for line E1.8).
Figure 1.
Tet-inducible expansin expression. (A) RNA blot analysis of total RNA (10 μg/lane) from transgenic tobacco plants containing either the GUS reporter gene (G25) or CsExp1 sequence (lines E1.7 and E1.8) under Tet-inducible transcriptional regulation. Plants were treated with 15 μg/ml Ahtet (+) or buffer (−) for 24 h before RNA extraction. Blots were hybridized with a radiolabeled probe for CsExp1. Methylene blue staining of 25S rRNA is shown as a loading control. (B) Time course of CsExp1 transcript accumulation by RNA gel blot analysis of E1.8 and G25 plants after treatment with 15 μg/ml Ahtet for the times indicated. Hybridization was as in A with 10 μg of RNA/lane. (C) Ahtet concentration dependence of CsExp1 transcript accumulation in line E1.8 by RNA gel blot analysis. Plants were treated for 24 h with the concentration of Ahtet indicated before hybridization as in A with 10 μg of RNA/lane. (D) Expansin activity measurements in plants from lines E1.7, E1.8, or G25 treated with Ahtet (2.5 μg/ml) (shaded columns) or buffer (open columns) for 24 h. Bars represent SE (n = 12).
Microinduction of Gene Expression.
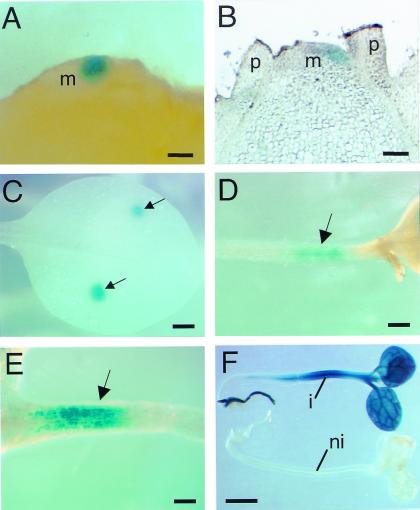
To locally induce gene expression, we took the following strategy. Small portions of lanolin were impregnated with Ahtet and then manipulated onto the surface of dissected apical meristems, the aim being to generate a local source of Ahtet and, thus, a localized area of induction (Fig. 2). To validate this method, we first performed a series of experiments with Tet∷GUS plants. Local induction of GUS expression was obtained to a resolution of a fraction of a meristem (Fig. 3A). Analysis of thin sections (Fig. 3B) revealed that GUS expression was induced in several cell layers in a restricted area within the meristem. By using this approach, local GUS induction could be achieved (to varying resolution) in various organs and tissues, including leaves (Fig. 3C), hypocotyls (Fig. 3D), and roots (Fig. 3E). By varying the Ahtet concentration, the amount of lanolin, and the time for which the lanolin was left on the tissue, the target tissue area and signal intensity could be controlled (data not shown). That virtually all tissues could respond to the inducer is shown in Fig. 3F by the induction of a seedling after immersion in Ahtet.
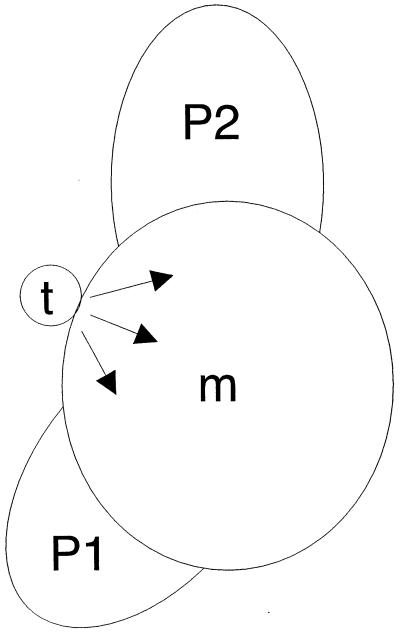
Figure 2.
Strategy to locally induce gene expression in the shoot apex. The meristem (m) is surrounded by primordia (P2, P1). Ahtet-loaded lanolin (t) placed on the meristem acts as a local source of inducer (arrows), leading to the localized induction of transgene transcription.
Figure 3.
Microinduction of gene expression. (A) Hand section of a tobacco apex showing localization of GUS expression (blue) to a portion of the apical meristem after manipulation of Ahtet-impregnated lanolin onto the meristem surface. (B) Section of an apex treated as in A showing localized GUS expression in several cell layers on one flank of the meristem. (C) Localized GUS expression on the leaf lamina in two spots corresponding to areas of lanolin–Ahtet treatment (arrows). (D) Localized GUS expression (arrow) in the hypocotyl after treatment, as in C. (E) Localized GUS expression (arrow) in the root after treatment, as in C. (F) Whole seedling induction of GUS expression after induction (i) or noninduction (ni) with Ahtet. m, meristem; p, primordium. (Bar: A and B = 50 μm; C = 500 μm; D = 500 μm; E = 500 μm; F = 1 mm.)
Local Induction of Expansin Leads to Leaf Initiation and Reversal of Phyllotaxis.
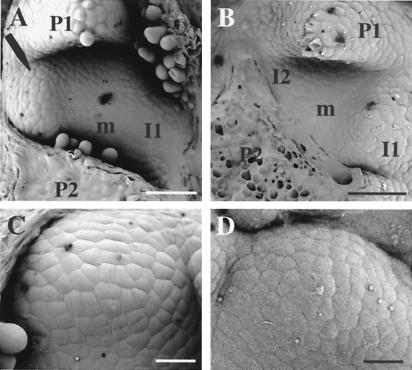
Having established a method for the local induction of gene expression, we proceeded to analyze the outcome of localized expansin induction in various plant organs. In this analysis, we concentrated on the apical meristem, because our previous data indicated that local alteration in expansin activity was likely to lead to altered morphogenesis in this tissue (3). Ahtet-impregnated lanolin was manipulated onto the I2 position of the apical meristem. This is an area that will not normally generate a leaf primordium until after the I1 position (138° distant) has undergone organogenesis. When Ahtet had been locally applied to the I2 position of a Tet∷E1.7 meristem, morphogenesis occurred at the I2 position to generate a bulge between the P2 and P1 primordia (Fig. 4A). In control experiments in which either Ahtet was manipulated onto the I2 position of Tet∷GUS meristems or buffer was manipulated onto the I2 position of Tet∷E1.7 plants, no altered morphogenesis was observed (Fig. 4B). In these cases, a primordium bulge arose at the expected time at the I1 position. Comparison of the expansin-induced bulges (Fig. 4C), and normal leaf primordia at a similar stage of development (Fig. 4D) revealed no overt differences in terms of morphology, epidermal cell size, or structure.
Figure 4.
Expansin-induced morphogenesis. (A) Scanning electron micrograph (SEM) of an apex from an E1.7 plant in which Ahtet-impregnated lanolin was manipulated onto the I2 position of the meristem (m) between primordia P1 and P2. After 72 h, a bulge has formed (arrow) at this position opposite the expected I1. (B) SEM of an E1.7 apex treated with buffer at the I2 position. No morphogenesis has occurred. (C) SEM of expansin-induced primordium. (D) SEM of normally formed primordium. (Bar: A and B = 150 μm; C and D = 25 μm.)
Initiation of leaf structures was observed after localized expansin induction in 19% (Tet∷E1.7) and 18% (Tet∷E1.8) of cases for the two independent transgenic lines analyzed, with no such altered morphogenesis observed in mock-treated Tet∷E1.7 lines (28 plants tested) or Tet∷E1.8 lines (26 plants tested), or in an Ahtet-treated Tet∷GUS line (49 plants treated) (Table 1).
Table 1.
Induction of expansin expression leads to leaf initiation and reversed phyllotaxis
| Plant line | Treatment | Apices analyzed | Leaf initiation | Reverse phyllotaxy |
|---|---|---|---|---|
| E1.7 | + | 73 | 14 | 14 |
| − | 26 | 0 | 0 | |
| E1.8 | + | 55 | 10 | 10 |
| − | 28 | 0 | 0 | |
| G25 | + | 49 | 0 | 1 |
| − | ND | ND | ND |
Meristems were induced on I2 position with Ahtet (+) or treated with buffer (−). The number showing novel leaf initiation and reversed phyllotaxis was counted. ND, not determined.
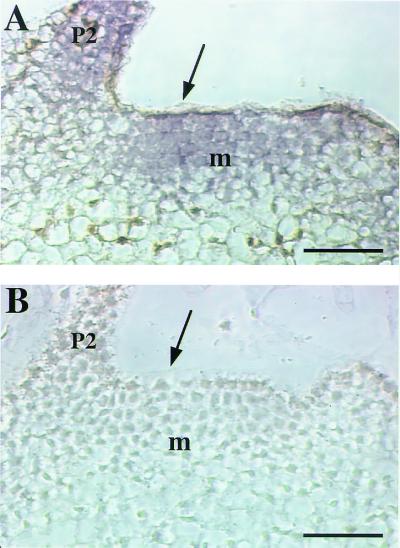
To confirm that the manipulations of the meristem did lead to a local accumulation of CsExp1 transcripts, we performed in situ hybridizations of induced and noninduced meristems. Local application of Ahtet led to accumulation of the CsExp1 transcript principally in the meristem, although occasionally signal was observed in adjacent primordia (Fig. 5A). The CsExp1 probe used did not cross hybridize with endogenous expansin transcripts, shown by hybridizations with buffer-treated control tissue (Fig. 5B).
Figure 5.
Localized accumulation of CsExp1 transcripts in the meristem. (A) Longitudinal section of an apex from an E1.8 plant locally induced (arrow) with Ahtet and hybridized with an antisense probe for CsExp1. A high signal (blue/violet) is seen in the meristem. (B) As in A, except the meristem was treated with buffer alone. m, meristem; p, primordium. (Bar = 50 μm.)
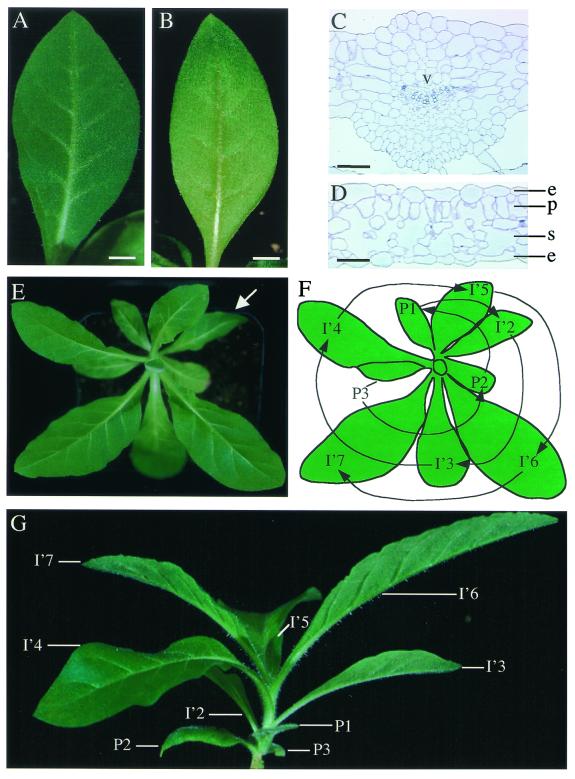
After initiation, the expansin-induced primordia grew to form leaf structures that were indistinguishable from normally formed leaves. The expansin-induced leaves (Fig. 6A) were ovate and had a system of venation and lamina growth comparable to that observed in normally formed leaves (Fig. 6B). Histological analysis confirmed the presence of all of the major expected cell types in the appropriate position within the induced leaves, both in the region of the vascular tissue (Fig. 6C) and the lamina (Fig. 6D).
Figure 6.
Expansin-induced primordia develop into normal leaves and lead to reversed phyllotaxy. (A) Expansin-induced leaf. (B) Normally formed leaf. (C) Cross section of the midrib of an expansin-induced leaf. (D) Cross section of the lamina of an expansin-induced leaf. (E and F) Reversal of phyllotaxy after the initiation of a leaf (I′2) (arrow in E) by localized induction of expansin expression. As shown in diagram (F), initially leaves (P3, P2, P1) were formed in an anticlockwise direction. Leaves formed subsequent to I′2 (I′3- I′7) have been formed in a clockwise direction. (G) Side view of plant shown in E to demonstrate leaf insertion points along the stem. e, epidermis; p, palisade; s, spongy mesophyl. (Bar: A and B= 5 mm; C and D = 125 μm.)
After the formation of an Ahtet-induced leaf, the apical meristem continued to generate phenotypically normal leaves but with a reversed phyllotaxis (Table 1). For example, a plant in which the original phyllotaxis was anticlockwise generated leaves I′3–I′7 in a clockwise fashion subsequent to the formation of an expansin-induced leaf at the I2 position (Fig. 6 E and F). Observation of the order of leaf initiation for the plant shown in Fig. 6E is facilitated by the side view of the plant, where the insertion points of the leaves along the stem can be seen (Fig. 6G).
Manipulation of Leaf Shape by Local Induction of Expansin.
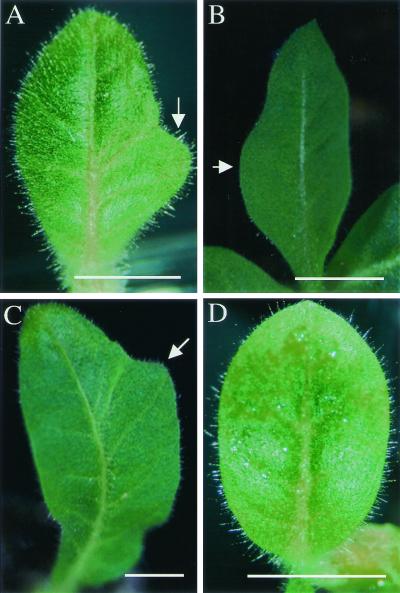
In addition to local induction of expansin expression within the meristem, inductions were also performed on the flanks of young leaf primordia (P2-P3 stage). Local induction of expansin on the primordium flank led to increased local growth of the lamina and to altered leaf shape in 11 cases of 20 (Fig. 7). Three examples are shown in Fig. 7 A–C. In each case, the outgrowth of the lamina has occurred only on the side of the leaf where the induction was performed, the opposite side of the lamina showing normal morphology. Manipulations in which Tet∷E1.7 primordia were mock-treated, or Tet∷GUS primordia were induced with Ahtet, did not lead to any change in leaf morphology (Fig. 7D).
Figure 7.
Manipulation of leaf shape by local induction of expansin expression. (A–C) Primordia (P2 stage) of E1.7 plants were induced on one flank with Ahtet then allowed to grow for 2–4 weeks. Ectopic lamina is formed on the induced flank (arrows). (D) Normally formed leaf primordium. (Bar = 1 cm.)
Discussion
Microinduction of Gene Expression.
To test the molecular basis of morphogenesis, it is necessary to develop techniques that allow the expression of specific genes in specific cells and to observe the effect of altered gene expression on the process under investigation. Constitutive overexpression of morphogenically important gene products may be lethal, may tend to highlight early acting pathways in development, or may even induce compensatory mechanisms so that any phenotype is either obscured or variable. One way to circumvent this problem is to use promoter elements that direct gene expression to specific tissues at particular time points (16). However, the number of elements at present characterized is rather limited, and there may be patterns of gene expression that are impossible to reconstruct by using such an approach. The results reported in this paper describe an alternative strategy. This strategy involves the adaptation of the well-established Tet-inducible promoter system (15) by locally applying the inducer to various plant tissues. This approach allowed the local induction of gene expression in all tissues tested. By varying the concentration and time of application, a variety of patterns of gene induction could be achieved. At the extreme, we were able to transiently induce transgene expression down to a resolution of less than 50 μm. This manipulation would not have been possible by using any of the promoter elements presently available. Our microinduction approach thus represents a powerful adjunct to methods already established for the manipulation of gene expression.
Local Expansin Expression Is Sufficient to Initiate the Entire Program of Leaf Development.
By using the microinduction technique, we were able to locally induce expansin gene expression within the meristem. This manipulation led to the initiation of leaf development. These results corroborate earlier data indicating that local ectopic application of expansin protein to meristems induced morphogenesis (3, 7). The data also extend these observations by demonstrating that induction of expansin endogenously in several cell layers of the meristem initiates a program of development generating leaves that, at the level of overall morphology and histology, are indistinguishable from normally formed leaves. Expansin-induced leaves also influence the subsequent phyllotaxis of the plant, consistent with previous observations and theories on the function of newly generated leaves in generating signals that influence meristem activity (3, 17). Taken together with data showing a specific accumulation of expansin transcripts at the presumptive site of leaf initiation (8, 18), our data fit with a model according to which local increase in growth (via modulation of cell wall extensibility) is a key event in leaf initiation. These data support the concept that alterations in the biophysical context of a tissue can influence development (1, 19).
Local Expansin Expression Modulates Leaf Shape.
That local increase in cell wall extensibility can influence morphogenesis was also demonstrated by experiments in which local induction of expansin expression on the flank of leaf primordia led to local altered growth and eventual modification of leaf shape (increased lamina formation). The histology of the ectopic lamina was very similar to that of normally formed leaves (data not shown), indicating the close interaction between cell growth, division, and differentiation. In this context, cell division is not driving morphogenesis; rather, there is a programmed pattern of cell division/differentiation that appears to fill the available space within the organ. This is further evidence for the existence of cell-division-independent mechanisms controlling morphogenenesis (20, 21).
Whether local modulation of expansin expression plays a role in the endogenous mechanism controlling leaf morphology remains to be determined. Our analysis shows that expansin genes are expressed in young tobacco leaf tissue (data not shown), and our future research will be focused on identifying expansin genes that might play a role in the mechanism of leaf morphogenesis.
Acknowledgments
We thank Dr. Didier Reinhardt (University of Bern, Bern, Switzerland) for discussions on microinduction, Dr. Martin Müller [Swiss Federal Institute of Technology (ETH)-Zurich] for access to the cryo-scanning electron microscopy, and Prof. Nikolaus Amrhein (ETH-Zurich) for providing lab space and encouragement throughout the project. A.J.F. is supported by a START Fellowship from the Swiss National Science Foundation, which provided funding for the project under Grant 31–49337.
Abbreviations
- Ahtet
anhydrotetracycline
- GUS
β glucoronidase
- Tet
tetracycline
Footnotes
See commentary on page 10981.
References
- 1.Green P B. Trends Plant Sci. 1997;2:365–366. [Google Scholar]
- 2.Cosgrove D J. Nature (London) 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 3.Fleming A J, McQueen-Mason S, Mandel T, Kuhlemeier C. Science. 1997;276:1415–1418. [Google Scholar]
- 4.McQueen-Mason S, Durachko D M, Cosgrove D J. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho H-T, Cosgrove D J. Proc Natl Acad Sci USA. 2000;97:9783–9788. doi: 10.1073/pnas.160276997. . (First Published August 8, 2000; 10.1073/pnas.160276997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Link B M, Cosgrove D J. Plant Physiol. 1998;118:907–916. doi: 10.1104/pp.118.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming A J, Caderas D, Wehrli E, McQueen-Mason S, Kuhlemeier C. Planta. 1999;208:166–174. [Google Scholar]
- 8.Reinhardt D, Wittwer F, Mandel T, Kuhlemeier C. Plant Cell. 1998;10:1427–1437. doi: 10.1105/tpc.10.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi L, Escudero J, Hohn B, Tinland B. Plant Mol Biol Rep. 1993;11:220–229. [Google Scholar]
- 10.Shcherban T Y, Shi J, Durachko D M, Guiltinan M J, McQueen-Mason S J, Shieh M, Cosgrove D J. Proc Natl Acad Sci USA. 1995;92:9245–9249. doi: 10.1073/pnas.92.20.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. [Google Scholar]
- 12.Pien S, Wyrzykowska J, Fleming A J. Plant J. 2001;25:663–674. doi: 10.1046/j.1365-313x.2001.01002.x. [DOI] [PubMed] [Google Scholar]
- 13.Whitney S E C, Gidley M J, McQueen-Mason S J. Plant J. 2000;22:327–334. doi: 10.1046/j.1365-313x.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 14.Jefferson R A, Kavanagh T A, Bevan M W. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatz C, Frohbergand C, Wendenburg R. Plant J. 1992;2:397–404. doi: 10.1111/j.1365-313x.1992.00397.x. [DOI] [PubMed] [Google Scholar]
- 16.Schoof H, Lenhard M, Haecker A, Mayer C F X, Jürgens G, Laux T. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 17.Snow M, Snow R. Philos Trans R Soc London Ser B. 1931;221:1–31. [Google Scholar]
- 18.Cho H-T, Kende H. Plant J. 1998;15:805–812. doi: 10.1046/j.1365-313x.1998.00258.x. [DOI] [PubMed] [Google Scholar]
- 19.Ingber D. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 20.Day S J, Lawrence P A. Development (Cambridge, UK) 2000;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- 21.Smith L G, Hake S, Sylvester A W. Development (Cambridge, UK) 1996;122:481–489. doi: 10.1242/dev.122.2.481. [DOI] [PubMed] [Google Scholar]