Abstract
Background and Aims:
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is becoming the standard treatment option for peritoneal carcinomatosis but is associated with high rates of morbidity and mortality. Our aim was to retrospectively analyse and evaluate intra-operative factors associated with morbidity and mortality of CRS and HIPEC.
Methods:
Intra-operative data were collected for cases done over 1 year (24 cases) and analysed for the primary outcome of post-operative ventilation >24 h, and secondary outcome of length of the Intensive Care Unit (ICU) stay >5 days. Statistical analysis was carried out in STATA 11 software.
Results:
Higher peritoneal carcinoma index (PCI), (P = 0.0047), longer duration of surgery (P = 0.0016), higher delta temperatures (P = 0.0119), increased estimated blood loss (EBL) (P = 0.0054), high intraoperative fluid requirement (P = 0.0038), lower mean arterial pressure (MAP) (P = 0.0021) and higher blood products requirement were associated with >24 h ventilation. These factors were also associated with longer ICU stay. All these factors associated with >24 h ventilation and prolonged ICU stay are related to the PCI which is an indicator of the extent of surgery.
Conclusion:
Higher PCI, longer duration of surgery, higher delta temperatures, increased EBL, high intraoperative fluid requirement, lower mean arterial pressure and higher blood products requirement were associated with >24 h postoperative ventilation as well as ICU stay >5 days. All these factors are related to the PCI, which is a major predictor of post-operative morbidity.
Key Words: Anaesthesia, cytoreductive surgery, hyperthermic intraperitoneal chemotherapy, intensive care, ventilation
INTRODUCTION
Cytoreductive surgery (CRS) combined with perioperative hyperthermic intraperitoneal chemotherapy (HIPEC) is becoming the standard treatment option for peritoneal carcinomatosis that was deemed inoperable. Peritoneal carcinomatosis had a rather dismal prognosis with poor median survival of 6[1] months without treatment till the introduction of HIPEC by Sugarbaker in the 1990s as an aggressive form of locoregional therapy.[2]
We have been anaesthetising CRS-HIPEC since 2014.[3] CRS-HIPEC presents the anaesthetist with numerous challenges perioperatively. Optimal management of these patients needs an understanding of the details of surgery and chemoperfusion. CRS targets macroscopic disease and consists of stripping off of all the affected visceral and parietal peritoneum and resection of involved organs (often multiple) and reconstruction. HIPEC follows CRS and consists of circulating the peritoneal cavity with heated chemotherapeutic fluid for 60–90 min at 42°C–43°C. HIPEC achieves high peritoneal concentrations of chemotherapy with limited systemic absorption.[4]
An observed increase in survival rates with CRS and HIPEC has brought normally inoperable cases under the purview of surgery, so the anaesthesiologist requires knowledge of physiology and temperature changes during CRS-HIPEC, the tremendous volume loss during CRS, abdominal hypertension, electrolyte abnormalities, coagulopathies, increased cardiac index, oxygen consumption and decreased systemic vascular resistance during the HIPEC phase, apart from knowledge of the toxicity of chemotherapy being used.[5] With the aim of finding the patient-specific and intra-operative factors associated with morbidity and mortality of CRS and HIPEC we decided to retrospectively analyse the patient data of CRS-HIPEC done in the previous year.
METHODS
We retrospectively reviewed information on all patients with primary or recurrent peritoneal carcinomatosis, who were consecutively treated with CRS and HIPEC at tertiary cancer centre between January 2016 and January 2017. Data for this retrospective analysis were retrieved from the tumour registry and our hospital electronic database, there was no exclusion criteria. Missing data were excluded from the analysis for that particular variable.
All patients had pre-anaesthesia check and were optimised with supplementary vitamins and minerals, high protein diet and incentive spirometry. Patients were counselled on the day before surgery on their expected course during operative and post-operative period. Pre-medication was with oral ranitidine 150 mg on the day before and on the morning of surgery, No sedative pre-medication was given. All patients received thoracic epidural analgesia with bupivacaine 0.125% to achieve analgesia from T4 to L1 with morphine 40 μg/kg eighth hourly. Anaesthesia was induced with fentanyl 1–1.5 μg/kg, propofol 1.5–2 mg/kg and vecuronium 0.1 mg/kg and was maintained with O2 and N2O with desflurane or sevoflurane to minimum alveolar concentration of 0.8–0.9. Monitoring included Electrocardiogram, non-invasive blood pressure, pulse oximetry, nasopharyngeal temperature, end-tidal carbon dioxide and agent concentration along with invasive arterial and central venous pressures. Non-invasive continuous cardiac output monitoring with FloTrac (EV1000 Edwards Lifesciences Corp, Irvine, CA, USA), was used whenever the peritoneal carcinoma index (PCI) was over 15 or if the patient had persistent haemodynamic perturbations.
Laparotomy was performed with an extended midline incision and the PCI was assessed to find the extent of peritoneal cancer by dividing the peritoneal cavity into 13 well-defined regions [Figure 1]. In each region, the size of the largest tumour nodule was measured and scored as follows: if no tumour - '0', if nodule <0.5 cm, '1', if 0.5 cm - 5 cm, '2', lesions >5 cm, '3'. The PCI is addition of all 13 regions with a maximum score of 39 (13 × 3). At this juncture, we connected the FloTrac to the arterial line if PCI was >15. Surgery proceeded with total peritonectomy and removal of involved viscera and all macroscopic peritoneal disease, as described by Sugarbaker in 1995.[2]
Figure 1.
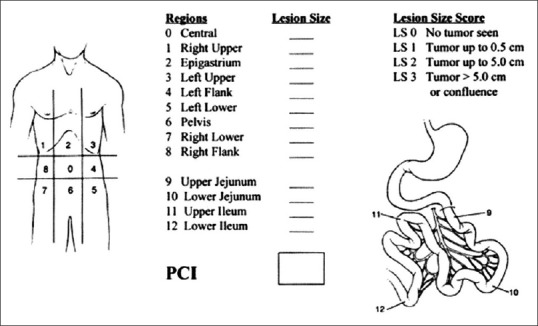
Peritoneal carcinoma index scoring
Intraoperative crystalloid, colloid and blood product administration was at the discretion of the anaesthesiology team based on maintenance of blood pressure within 20% of the baseline and urine output >0.5 mL/kg/h and the stroke volume variations and cardiac index trends. Our vasopressor of choice was dopamine followed by noradrenaline and adrenaline. Our colloid of choice was gelatine based. Blood products were replaced based on estimated blood loss (EBL) and laboratory haemoglobin values and the transfusion trigger was kept at 9 gm%. Maintenance of normothermia was by convective warming blankets and blood/fluid warmers. Cooling with ice packs was done as needed during HIPEC if the core temperatures went over 39°C.
HIPEC, which targets microscopic metastasis in the peritoneum[6] was by a closed technique, wherein the abdominal cavity was filled with heated (42°C) peritoneal dialysis fluid (1.5% dextrose) about 3–5l. After reaching the desired temperature in the abdomen, selected chemotherapeutic drug was added to the circulating fluid (mitomycin C for peritoneal malignancy, cisplatin for ovarian cancer or oxaliplatin for colonic cancer), with a roller pump keeping the drug circulated. The duration of HIPEC ranged from 30 to 90 min depending on the histology. During HIPEC, urine output was maintained at 0.5 ml/kg/15 min with fluid boluses and intravenous (IV) furosemide. After HIPEC, the abdomen was washed, any anastomosis needed was done and abdomen was closed.
Following surgery, all patients were transferred to the Intensive Care Unit (ICU). Decision of on table or interval extubation was made depending on patient having pain score of <3/10 on numerical scale and generating tidal volumes of at least 5 ml/kg with normal haemodynamics on one vasopressor. Post-operative analgesia was achieved with epidural local anaesthetic infusion and epidural morphine bolus at 40 μg/kg 8th hourly over 72 h. Opioid boluses were continued for a further 48 h before epidural catheter removal if prothrombin time/international normalised ratio (INR), platelet counts were normal. Intensive care management was done according to the patient's cardiorespiratory status.
Data collection included patient demographics, operative time, intra-operatively administered fluids, blood and blood products, fluid losses, urine volume, intra-operative haemodynamics and respiratory monitoring parameters as well as temperature and arterial blood gas (ABG) values and the need for more than two inotropes. ABG were determined at six-time points, namely, after surgical incision (baseline value B), at the end of cytoreductive phase around 30 min before the start of HIPEC (H0), first quarter (H1), second quarter (H2), end of HIPEC (H3) and the end of surgery (E). Post-operative data collected were the length of ICU stay, INR on day 1, and hours of ventilation (> or <24 h). The primary outcome was post-operative ventilation of >24 h and the secondary outcome was length of ICU stay >5 days.
Descriptive statistics are presented as median and ranges for continuous variables (demographic and haemodynamic parameters) and as counts for categorical variables (hypoalbuminemia, ascites, etc.). Continuous variables were compared by Student's t-test and categorical variable using Chi-square test. Fisher's exact test was used wherever applicable. ANOVA was used to compare the effects of more than two continuous variables. All tests were two-sided at significance level of ≤0.05. Statistical analysis was carried out in STATA 11 software. (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP.).
RESULTS
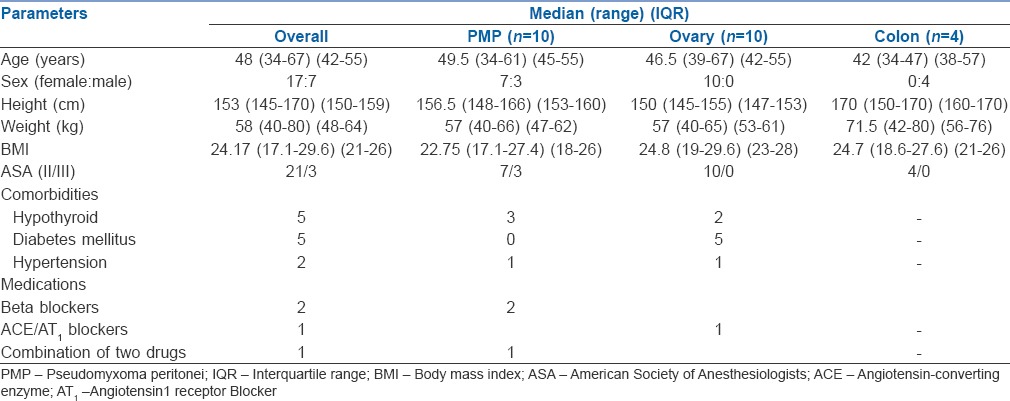
Of the data collected for 24 cases, ten were pseudomyxoma peritonei (PMP), ten were carcinoma ovary (O) and four were colonic malignancy (C). The demographics of the patients are in Table 1. Of the 24 patients, three were American Society of Anesthesiology (ASA) physical status 3 and they all belonged to the PMP group. Higher ASA did not correlate with longer ventilation or ICU stay in our patients.
Table 1.
Demography of patients undergoing cytoreductive surgery-hyperthermic intraperitoneal chemotherapy (n=24)
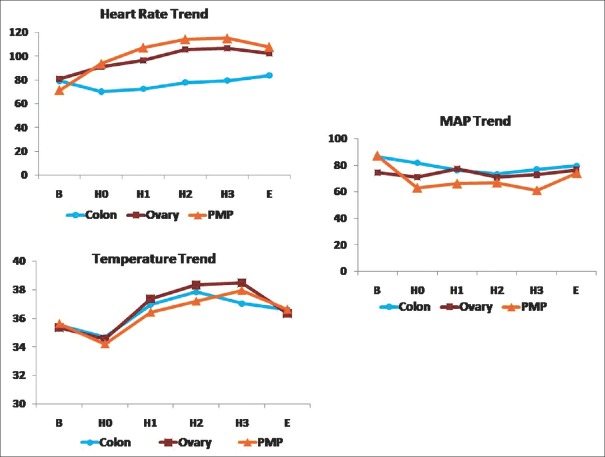
Calculating the difference of the least temperature during surgery and highest temperatures during HIPEC [Figure 2] measured with nasopharyngeal probe showed that the mean delta temperature was 3.97°C ± 1.98°C. Higher Delta temperatures [Table 2] were statistically associated with longer ventilation (P = 0.0119) and ICU stay (P = 0.036).
Figure 2.
Intra-operative mean arterial pressure, heart rate and temperature trend for pseudomyxoma peritonei, ovary and colon groups
Table 2.
Perioperative variables associated with ventilation >24 h and Intensive Care Unit stay >5 days with P values
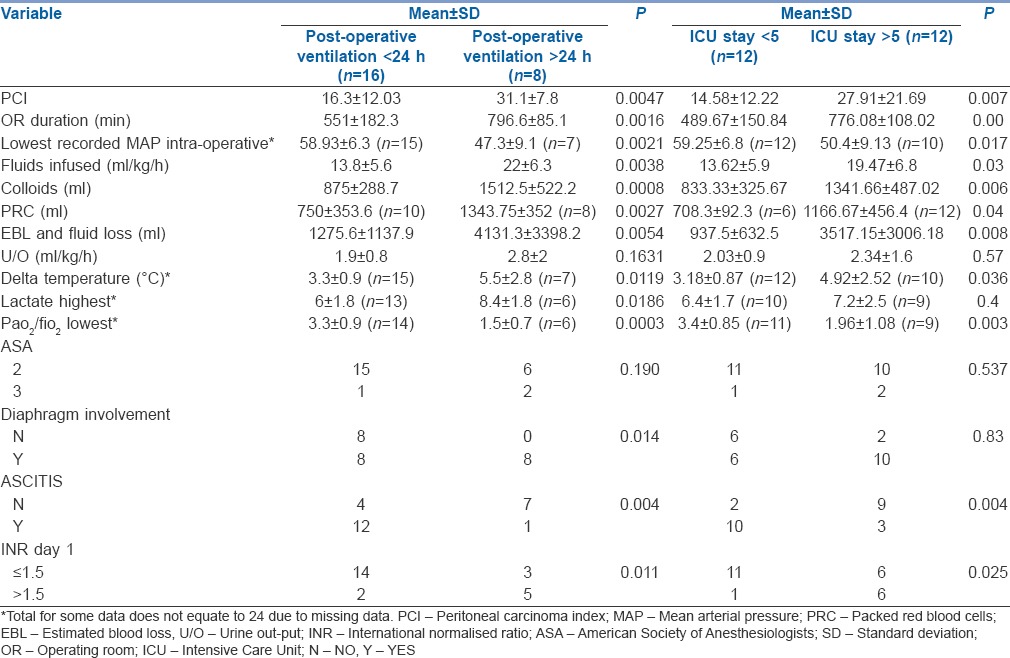
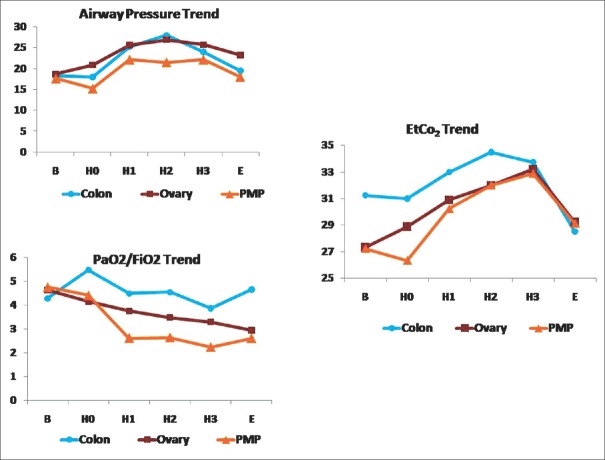
Peak mean airway pressures increased during HIPEC [Figure 3] in comparison to the values at the end of CRS due to the chemotherapeutic fluid in the abdomen (3–5 L). There was a similar increase in mean end-tidal carbon dioxide values. Mean oxygenation ratio (PaO2/FiO2) showed a falling trend and was associated with longer ventilation (P = 0.0003) and longer ICU stay (P = 0.003). The mean highest documented lactate during HIPEC for PMP was 7.1, and was associated with longer ventilation (P = 0.018) but not ICU stay. Intra-operative blood glucose rose, especially during HIPEC to levels from 250 to even 400 in one patient, but this did not predict increased ventilation or ICU stay.
Figure 3.
Respiratory variables – airway pressure, end-tidal carbon dioxide and PaO2/FiO2 trend during the set points. B – Baseline; H0– 30 min before start of hyperthermic intraperitoneal chemotherapy; H1– First quarter; H2– Second quarter; H3– Third quarter; E – End of surgery
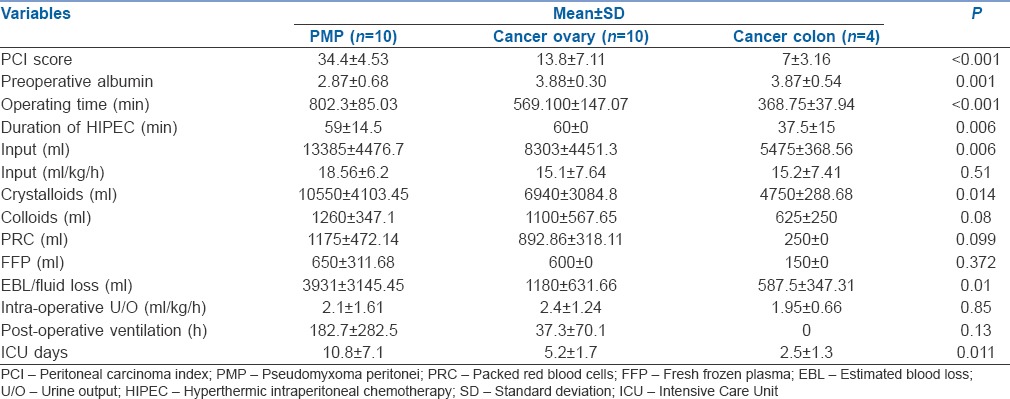
Twenty-two of the 24 patients needed inotropes during surgery, 13 patients needed two inotropes intraoperatively and 4 needed three inotropes. The patients needing 3 inotropes had also been infused over 20 ml/kg/h of IV fluids, out of these two patients died of intractable shock post-operatively. Sixteen (66%) patients were extubated within 24 h. Mean length of ICU stay [Table 3] was 10.8 ± 7.07 for PMP, 5.2 ± 1.7 for ovary and 2.5 ± 1.3 for colorectal group.
Table 3.
Perioperative variables for pseudomyxoma peritonei, ovary and colon subgroups with P values
On examining the intraoperative variables associated with ventilation of >24 h [Table 2], it was found that higher PCI (p = 0.0047), longer duration of surgery (p = 0.0016), increased estimated blood and fluid loss (0.0054), increased intraoperative fluid requirement (0.0038), lower mean arterial pressure (MAP) (p = 0.0021) and higher blood products requirement (0.0027) were statistically significant. These factors while interrelated were statistically significant with p < 0.05 for longer ICU stay too. Categorical variables such as diaphragmatic involvement (p = 0.014), ascites (p = 0.004) and INR. greater than 1.5 on day 1 (p = 0.011), were associated with longer ventilation. Ascites and raised INR on day 1 were also associated with longer ICU stay (p > 0.05).
DISCUSSION
Among the 24 consecutive patients for CRS and HIPEC in our study, our primary endpoints of post-operative ventilation >24 h and ICU stay >5 days were both associated with the longer operative time, higher PCI, greater blood loss and higher requirements of crystalloids, colloids and blood products and lower PaO2/FiO2 ratio with statistical significance of P < 0.05. PCI, which is related to the extent of resection and consequently various associated sequelae was not comparable in the PMP, ovarian and colonic groups. In colorectal cancer, as PCI is closely correlated with the prognosis after HIPEC,[7] with higher PCI, the prognosis becomes worse, hence this subset had a lower mean PCI of 7 ± 3.2 and had a relatively smooth course. In the 10 ovarian cancer patients, the mean PCI was 13.8 ± 7.1. In primary peritoneal malignancy such as low-grade PMP the approach is more aggressive; these patients have excellent long-term outcomes despite having a very high PCI as the disease-free survival in such patients does not depend on PCI but on R0 resection (R0 resection denotes the completeness of cytoreduction (CCR) as defined by the Sugarbaker criteria as: CCR-0 = no residual tumour).[8] Even in PMP perioperative mortality was significantly associated with a PCI >24.[9] Our PMP patient's PCI ranged between 27 and 39 pre-operatively with mean of 34.4 ± 4.5 – in effect all our PMP patients had PCI >24. There are reports stating morbidity becomes severe with PCI >30.[10] We feel that it is essential to compare the morbidity and mortality associated of CRS and HIPEC by subgroup analysis of patients depending on PCI.
In a recent review article,[11] patient-specific factors such as hypoalbuminemia, performance status, age and the operative factors such as PCI, multiorgan resection, diaphragmatic involvement and surgical experience have been found to have a strong association with morbidity. The mean pre-operative albumin levels in our patients was 2.87 ± 0.7 g for PMP group depicting their disease burden and associated ascites in this cohort (P = 0.001). As discussed by Raspe et al.,[12] all our PMP patients needed post-operative albumin at least for 5 days as their pre-operative albumin was low and resection was extensive with lot of fluid losses. Due to the aggressive nature of the surgery in the PMP group, multivisceral resections were common (9 of the ten patients had more the 2 organs removed with associated anastomosis). Diaphragmatic involvement was present in all of our PMP patients and was associated with longer ventilation but not ICU stay. Diaphragmatic involvement has been associated with increased morbidity[13] and mortality[14] in various reports.
The perioperative coagulopathy, hemodynamic instability and infectious complications that may occur during CRS-HIPEC have raised concerns about the safety of epidural analgesia. Bell et al.[15] caution the use of epidural analgesia in this group of patients citing the incidence of epidural abscess in the survey conducted by them but others have endorsed epidural use[16] and claim decreased blood loss as an advantage, we placed epidural catheter in all our patients and did not have any complications.
Haemodynamic parameters such as HR and MAP (p = 0.017) correlated with the extent of resection and were more labile for the PMP group. Liberal fluid administration has been emphasised in these patients.[17] Kajdi et al.[18] have used liberal fluid management and transfused 10 ml/kg/h, but the mean fluid requirement in our cohort was 16.5 ml/kg/h and was highest in the PMP group (18.6 ml/kg/h) and was related to the PCI. The mean PCI in their group was 21.5. Fluids administered in our patients who stayed in ICU <5 days was 13.62 ml/kg/h (PCI − 14.58) compared to 19.47 ml/kg/h (PCI 27.9) for patients who stayed >5 days. Colantonio et al.[19] advocate goal-directed fluid therapy and say it decreases morbidity and hospital length of stay and mortality. Our patients required fluid of over 18.6 ml/kg/h despite using goal-directed fluid management with EV 1000 monitor in the PMP group, we feel this is due to the high PCI in our cohort. As CVP is no longer accepted as predictor of intravascular volume status,[20] we did not use it to guide fluid therapy. As discussed by Shiralkar et al.,[21] various IV fluid strategies did not influence the incidence of post-operative acute renal failure in our patients. None of our patients developed immediate post-operative kidney injury, based on the RIFLE criteria.[22] One of the reasons could be that we used only gelatin-based colloids and did not use any of the Starches.[18]
In the PMP group, two patients received fluids of more than 20 ml/kg/h, one of these patients needed three inotropes and was on ventilatory support till death (day 10 due to multiple organ dysfunction syndrome). Of the 10 ovarian cases, two had received fluids >20 ml/kg/h, one of these patients had a cardiac arrest on day 2 due to progressive hypotension.
The numbers studied are small to clearly say if fluids >20 ml should be avoided but non-invasive cardiac output measurements with goal-directed fluid management intraoperatively can be routinely used in such massive resections. The mean blood loss and blood product replacements too depended on the extent of resection and by implication, the PCI and this is similar to other studies.[17] Average blood transfusion was between 500 and 2000 ml in our patients. Exposure to blood transfusions is associated with an increased morbidity and mortality in surgical oncology[23] increasing the chances of coagulopathy. The cause of coagulopathy in CRS-HIPEC is ill understood, 7 of our 24 patients had raised INR on post-operative day 1 which normalised over the next 2–4 days; all of them belonged to the PMP group, which correlates with high blood and fluid transfusion with dilution of coagulation factors.
Base excess and serum lactate at the end of CRS-HIPEC and the delta base excess at 48 h is quoted as a predictor of complications in CRS-HIPEC,[24] but in our cohort, the base excess or the lactate levels did not predict the length of ICU stay. Lactate levels during HIPEC were associated with prolonged ventilation (P = 0.0186).
Delta temperature defined as the difference between least and highest temperatures during CRS-HIPEC was found to be a significant predictor of ICU stay >5 days [Figure 2]. Decreasing temperature during long resection phase between B and H0 was the cause for higher delta temperature. This was highest in patients with high PCI necessitating longer, aggressive resection. Hypothermia is associated with cardiac morbidity, decreased humoral and cell-mediated immunity and affects acid-base balance thus reflecting the higher ICU stay.[25]
We were able to extubate 37.5% of patient on table and 66% were extubated by 24 h, this was similar to the recent report from Singapore.[26] It is proposed that routine intensive care admission is not mandatory in such patients,[27] however we admitted all our patients to the ICU as we are still a young unit in terms of CRS-HIPEC. Cooksley and Haji-Michael[28] extubated all their patients on the table, but their mean PCI score was much less at 10.5.
In a recent review on intensive care outcomes after CRS-HIPEC,[29] the authors state that pre-operative medical co-morbidities, extent of surgical debulking (PCI), intraoperative blood losses, amount of intra-operative blood products required and total operative time are the factors affecting ICU length of stay. These were the factors that were statistically significant in predicting longer ICU stay in our cohort too. The mean PCI in their group was 21.5 in the patients transferred to ICU, in our patients, it was 27.91 in the patients staying for >5 days in ICU. As we found PCI to be the main predictor of morbidity in CRS-HIPEC, subgroup analysis according to the site of primary tumour [Table 3] showed that PCI (p = 0.0.000), operative time (p = 0.000), duration of HIPEC (p = 0.006), EBL (p = 0.01), total amount of fluids infused, (p = 0.006) crystalloids infused (P = 0.014) and duration of ICU stay (p = 0.011) were higher in the PMP group which had a PCI of 34.4 ± 4.53.
An important limitation intrinsic to our study is that it is retrospective, and the recruited numbers were too small to conclude on the association of fluid infused with morbidity and thus the duration of ventilation and ICU stay. The event rate and sample size was insufficient to undertake a multivariate analysis to determine factors independently associated with the primary and secondary outcomes. In our cohort, post-operative ICU stay and morbidity was significantly associated with PCI.
CONCLUSION
PCI is a major predictor of post-operative morbidity. Higher PCI was associated with longer of duration of surgery, increased estimated blood and fluid loss, increased intraoperative fluid requirement, lower MAP and increased Delta temperature with statistical significance of P < 0.05 for ventilation of >24 h and ICU stay of >5 days. Diaphragmatic involvement and pre-operative ascites were associated with ventilation >24 h Goal-directed fluid management and avoidance of hypothermia during resection phase are associated with decreased ICU stay. PCI should be the basis for comparison of various data in CRS-HIPEC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We wish to acknowledge our surgical oncologist's Dr. E. Hemant Raj and Dr. A. S. Ramakrishnan, but for whom we would not have had this data.
REFERENCES
- 1.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: New management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–28. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murughan K, Tiwari M, Balakrishnan K. Cytoreduction with hyperthermic intraperitoneal chemotherapy: An anaesthetic challenge. Indian J Anaesth. 2014;58:59–62. doi: 10.4103/0019-5049.126799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacquet P, Averbach A, Stephens AD, Stuart OA, Chang D, Sugarbaker PH, et al. Heated intraoperative intraperitoneal mitomycin C and early postoperative intraperitoneal 5-fluorouracil: Pharmacokinetic studies. Oncology. 1998;55:130–8. doi: 10.1159/000011847. [DOI] [PubMed] [Google Scholar]
- 5.Miao N, Pingpank JF, Alexander HR, Royal R, Steinberg SM, Quezado MM, et al. Cytoreductive surgery and continuous hyperthermic peritoneal perfusion in patients with mesothelioma and peritoneal carcinomatosis: Hemodynamic, metabolic, and anesthetic considerations. Ann Surg Oncol. 2009;16:334–44. doi: 10.1245/s10434-008-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27:365–74. doi: 10.1053/ctrv.2001.0232. [DOI] [PubMed] [Google Scholar]
- 7.Elias D, Mariani A, Cloutier AS, Blot F, Goéré D, Dumont F, et al. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur J Surg Oncol. 2014;40:1467–73. doi: 10.1016/j.ejso.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Vaira M, Cioppa T, DE Marco G, Bing C, D'Amico S, D'Alessandro M, et al. Management of pseudomyxoma peritonei by cytoreduction+HIPEC (hyperthermic intraperitoneal chemotherapy): Results analysis of a twelve-year experience. In Vivo. 2009;23:639–44. [PubMed] [Google Scholar]
- 9.Saxena A, Yan TD, Chua TC, Morris DL. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2010;17:1291–301. doi: 10.1245/s10434-009-0875-9. [DOI] [PubMed] [Google Scholar]
- 10.Baratti D, Kusamura S, Mingrone E, Balestra MR, Laterza B, Deraco M, et al. Identification of a subgroup of patients at highest risk for complications after surgical cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2012;256:334–41. doi: 10.1097/SLA.0b013e31825704e3. [DOI] [PubMed] [Google Scholar]
- 11.Newton AD, Bartlett EK, Karakousis GC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A review of factors contributing to morbidity and mortality. J Gastrointest Oncol. 2016;7:99–111. doi: 10.3978/j.issn.2078-6891.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raspe C, Piso P, Wiesenack C, Bucher M. Anesthetic management in patients undergoing hyperthermic chemotherapy. Curr Opin Anaesthesiol. 2012;25:348–55. doi: 10.1097/ACO.0b013e32835347b2. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed S, Levine EA, Randle RW, Swett KR, Shen P, Stewart JH, et al. Significance of diaphragmatic resections and thoracic chemoperfusion on outcomes of peritoneal surface disease treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2014;21:4226–31. doi: 10.1245/s10434-014-3891-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franssen B, Tabrizian P, Weinberg A, Romanoff A, Tuvin D, Labow D, et al. Outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on patients with diaphragmatic involvement. Ann Surg Oncol. 2015;22:1639–44. doi: 10.1245/s10434-014-4083-x. [DOI] [PubMed] [Google Scholar]
- 15.Bell JC, Rylah BG, Chambers RW, Peet H, Mohamed F, Moran BJ, et al. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: A multi-institutional experience. Ann Surg Oncol. 2012;19:4244–51. doi: 10.1245/s10434-012-2496-y. [DOI] [PubMed] [Google Scholar]
- 16.Owusu-Agyemang P, Soliz J, Hayes-Jordan A, Harun N, Gottumukkala V. Safety of epidural analgesia in the perioperative care of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2014;21:1487–93. doi: 10.1245/s10434-013-3221-1. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia. 2008;63:389–95. doi: 10.1111/j.1365-2044.2007.05380.x. [DOI] [PubMed] [Google Scholar]
- 18.Kajdi ME, Beck-Schimmer B, Held U, Kofmehl R, Lehmann K, Ganter MT, et al. Anaesthesia in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: Retrospective analysis of a single centre three-year experience. World J Surg Oncol. 2014;12:136. doi: 10.1186/1477-7819-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colantonio L, Claroni C, Fabrizi L, Marcelli ME, Sofra M, Giannarelli D, et al. Arandomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg. 2015;19:722–9. doi: 10.1007/s11605-015-2743-1. [DOI] [PubMed] [Google Scholar]
- 20.Esquivel J, Angulo F, Bland RK, Stephens AD, Sugarbaker PH. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open “coliseum technique”. Ann Surg Oncol. 2000;7:296–300. doi: 10.1007/s10434-000-0296-2. [DOI] [PubMed] [Google Scholar]
- 21.Shiralkar SP, Kerr P, Scott J, Sivalingam P. Anaesthetic management of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei: A retrospective audit. Anaesth Intensive Care. 2017;45:490–8. doi: 10.1177/0310057X1704500413. [DOI] [PubMed] [Google Scholar]
- 22.Acute Dialysis Quality Initiative Workgroup. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, et al. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon E, Datta I, Sutherland FR, Vauthey JN. Blood loss in surgical oncology: Neglected quality indicator? J Surg Oncol. 2009;99:508–12. doi: 10.1002/jso.21187. [DOI] [PubMed] [Google Scholar]
- 24.Eng OS, Dumitra S, O'Leary M, Wakabayashi M, Dellinger TH, Han ES, et al. Base excess as a predictor of complications in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2017;24:2707–11. doi: 10.1245/s10434-017-5869-4. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22:645–57. doi: 10.1016/j.bpa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Thong SY, Chia CS, Ng O, Tan G, Ong ET, Soo KC, et al. Areview of 111 anaesthetic patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Singapore Med J. 2017;58:488–96. doi: 10.11622/smedj.2016078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogal HD, Levine EA, Fino NF, Obiora C, Shen P, Stewart JH, et al. Routine admission to Intensive Care Unit after cytoreductive surgery and heated intraperitoneal chemotherapy: Not always a requirement. Ann Surg Oncol. 2016;23:1486–95. doi: 10.1245/s10434-015-4963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooksley TJ, Haji-Michael P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC) World J Surg Oncol. 2011;9:169. doi: 10.1186/1477-7819-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapoor S, Bassily-Marcus A, Alba Yunen R, Tabrizian P, Semoin S, Blankush J, et al. Critical care management and Intensive Care Unit outcomes following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. World J Crit Care Med. 2017;6:116–23. doi: 10.5492/wjccm.v6.i2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]