Abstract
Background and Aims:
Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) during apnoea has shown to delay desaturation. The primary objective was to compare time to desaturate to <90% during apnoea with THRIVE versus traditional preoxygenation followed by apnoeic oxygenation.
Methods:
This prospective, randomised, single-blinded study was conducted in 10 adult patients presenting for direct laryngoscopy under general anaesthesia without endotracheal intubation. Group P patients were preoxygenated with 100% oxygen, and in Group H, high-flow humidified oxygen was delivered using nasal cannula for 3 min. After induction and neuromuscular blockade, time to desaturate to 90%, while receiving apnoeic oxygenation, was noted. Chi-square test and Mann–Whitney tests were used.
Results:
Group H had a significantly longer apnoea time as compared to Group P (796.00 ± 43.36 vs. 444.00 ± 52.56 s). All patients in Group H continued to have nearly 100% saturation even at 12 min of apnoea. However, in Group P, 80% of patients desaturated to <90% after 6 min of apnoea. Baseline blood gases, that following preoxygenation and at 3 min of apnoea time were comparable in both groups. At 6 min, Group H had a significantly higher PaO2 (295.20 ± 122.26 vs. 135.00 ± 116.78) and PaCO2 (69.46 ± 7.15 vs. 59.00 ± 4.64). Group H continued to have a PaO2 of >200 mmHg even at 12 min of apnoea with a significant rise in PaCO2 along with fall in pH after 6 min.
Conclusion:
During apnoeic periods time to desaturate to <90% was significantly prolonged with use of THRIVE.
Key Words: Apnoea, blood, gases, insufflation, oxygen, PaO2
INTRODUCTION
Surgeries like biopsy with direct laryngoscopy sometimes require immobile vocal cords during the procedure with adequate space at the glottic opening for surgical manipulations intraoperatively. Sometimes, these demands could only be met by providing an apnoeic anaesthetised patient who is not intubated. Usually, effective preoxygenation followed by apnoeic oxygenation enables anaesthesiologists to safely prolong the apnoea time. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) using high-flow oxygen therapy for preoxygenation and oxygen supplementation during apnoea has shown promising results in this regard.[1,2,3,4]
The primary objective of our study was to compare time to desaturate to <90% during apnoea following THRIVE versus traditional preoxygenation using tidal volume breathing (TVB) followed by apnoeic oxygenation. Secondary objectives of this study included assessment of changes in arterial partial pressures of oxygen, carbon dioxide, bicarbonate and lactate levels along with pH and haemodynamic changes during these techniques.
METHODS
This prospective, randomised, single-blinded study was conducted from January 2017 to November 2017 after obtaining Institutional Ethical Committee clearance and patients' consent. The study was performed in 10 adult American Society of Anesthesiologists (ASA) physical Status I and II patients coming for direct laryngoscopy under general anaesthesia without endotracheal intubation. Those with room air saturation of <98%, anticipated difficult airway, chronic obstructive pulmonary disease, obesity, pregnancy, thyrotoxicosis, pheochromocytoma, hyperkalaemia, significant cardiac illness with pulmonary hypertension and raised intracranial pressure were excluded from the study.
Patients were randomly allotted to either Group P or Group H based on computer-generated random sequence of numbers, and allocation concealment was done using sequentially numbered opaque sealed envelopes. After obtaining an intravenous line and attaching pulse oximeter, non-invasive blood pressure monitor and electrocardiograph, glycopyrrolate 0.2 mg and midazolam 1 mg were given and patients were nebulised with 4 ml of 4% lignocaine. Following topicalization of the airway, a quick and gentle laryngoscopy was performed, and only those with Cormack Lehane Grade 1 and 2 were recruited into the study. An arterial cannula was then placed in the radial artery under local anaesthesia. The Group P patients were preoxygenated with 100% oxygen using a tight-fitting face mask at a rate of 6 L/min for 3 min with endtidal gas monitoring. In Group H, high-flow humidified oxygen warmed to 37°C was delivered through nasal cannula using AIRVO™ 2 (Fisher and Paykel Healthcare Ltd., New Zealand) at the rate of 30 L/min for 3 min. AIRVO™ 2 is a humidifier with integrated flow generator that delivers high flow of warmed and humidified oxygen up to 60L/min [Figure 1]. In both groups, patients were asked to perform TVB for a minimum period of 3 min targeting an endtidal oxygen of >0.9 and all received general anaesthesia as per a standardised protocol. Anaesthesia was induced with propofol 2 mg/kg body weight after administering fentanyl 2 μg/kg and an additional midazolam 1 mg intravenously. After induction of anaesthesia atracurium was given at a dose of 0.5 mg/kg.
Figure 1.
Patient receiving transnasal humidified rapid-insufflation ventilatory exchange
The apnoea time began from the onset of cessation of breathing as evidenced by a flat line in the capnogram with the absence of chest movements. This was either after induction of anaesthesia, confirmed with loss of response to verbal commands, or after administration of atracurium. During apnoea, all the patients received apnoeic oxygenation. In Group P, this was through a nasopharyngeal catheter with oxygen flow at the rate of 12 L/min and in Group H, using a nasal cannula with oxygen flow of 60 L/min.
After a time period of 3 min of giving atracurium, to ensure adequate relaxation and immobility, the surgical procedure was commenced. The surgical procedure was allowed to continue till the pulse oximeter saturation fell (SpO2) to 90%. The study was terminated if SpO2 fell <90%, at appearance of spontaneous respiratory efforts, end of surgery, development of arrhythmias, hypotension (mean arterial pressures [MAP] <20% from baseline), bradycardia or to a maximum time frame of 12 min. Ventilation was reinstituted with any of these events. The hypotension and arrhythmias were treated according to existing guidelines.
Duration of safe apnoea was defined as the time taken by an apnoeic patient to desaturate to 88%–90%.[5] At that point, either trachea was intubated, if glottic opening was directly visible and accessible during the procedure, and ventilated with 100% oxygen or mask ventilation was resumed. Once SpO2 was 100%, the procedure was resumed, if not already completed.
Blood sample for arterial blood gas (ABG) analysis was taken before preoxygenation (baseline), end of preoxygenation, at the time patient became apnoeic, then every 3 min till SpO2 dropped to 90%, or at 12 min of apnoea.
Heart rate (HR) and MAP were documented at the same time points when arterial gases were sampled. Arterial partial pressures of oxygen, carbon dioxide, bicarbonate, base excess, pH and the time to desaturate to 90% were noted.
Intravenous propofol 20–30 mg bolus was given every 3 min during apnoea or if the HR or MAP increased to >20% of the baseline to maintain the depth of anaesthesia. At the end of the procedure, patients were mask ventilated till adequate tidal volume, respiratory rate and saturation of 98%–100% were regained. Once awake and protective airway reflexes had returned, patients were shifted to Intensive Care Unit.
As there was no similar study published, we conducted a pilot study in 20 patients to calculate the sample size taking into account the time taken to desaturate to 90% as primary objective (Group H 839 ± 17.0 s and Group P 298.7 ± 165.7 s). Anticipating a mean difference of 540 s with alpha error of 0.05 and power of 99% the estimated sample size was three per group. However, we were able to recruit another 10 cases during the study period who were equally allocated to two groups. Using the observed mean difference of 352.0 s in the time taken to desaturate to 90% among the groups, with standard deviations of 52.56 in Group P, and 43.36 in Group H, the calculated power of the present study comes to 100%.
Chi-square test was used to compare gender and ASA physical status and Mann–Whitney test was used to compare all the continuous variables among the groups. Statistical analyses were conducted using SPSS Version 20.0 for Windows (IBM Corporation ARMONK, NY, USA).
RESULTS
The study was performed in 10 patients recruited into two equal groups [Figure 2]. Distribution of gender, ASA physical status as well as mean age, height and weight of patients in both groups were comparable [Table 1]. Group H had a significantly higher apnoea time as compared to Group P (796.00 ± 43.36 vs. 444.00 ± 52.56 s, P = 0.008) as well as a higher value of lowest saturation recorded (99.40 ± 1.34 vs. 85.80 ± 9.31) [Table 1]. In Group H, at 12 min of apnoea, in one patient, SpO2 dropped to 97% while others continued to have 100% SpO2. However, in Group P, 4 patients out of 5 desaturated to <90% by 6 min of apnoea. No patient required intubation as SpO2 improved with mask ventilation. Only one patient in this group continued to have SpO2>90% at 9 and 12 min. Hence, the study duration for calculating mean blood gas variables was taken as 12 min for Group H and 6 min for Group P.
Figure 2.
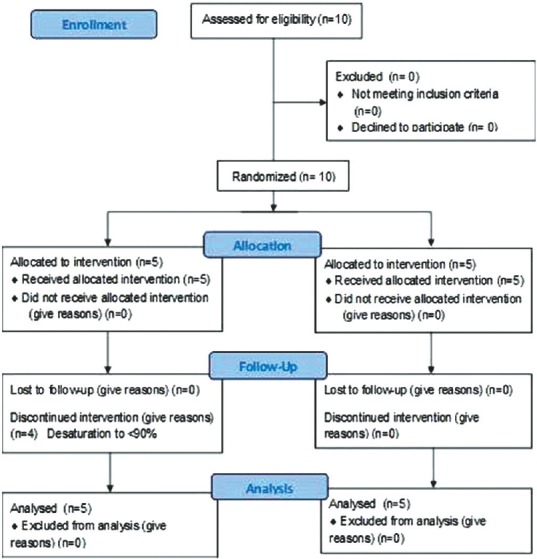
CONSORT flowchart
Table 1.
Comparison of demographics, American Society of Anesthesiologists physical status, apnoea time, arterial blood gas variables and saturation
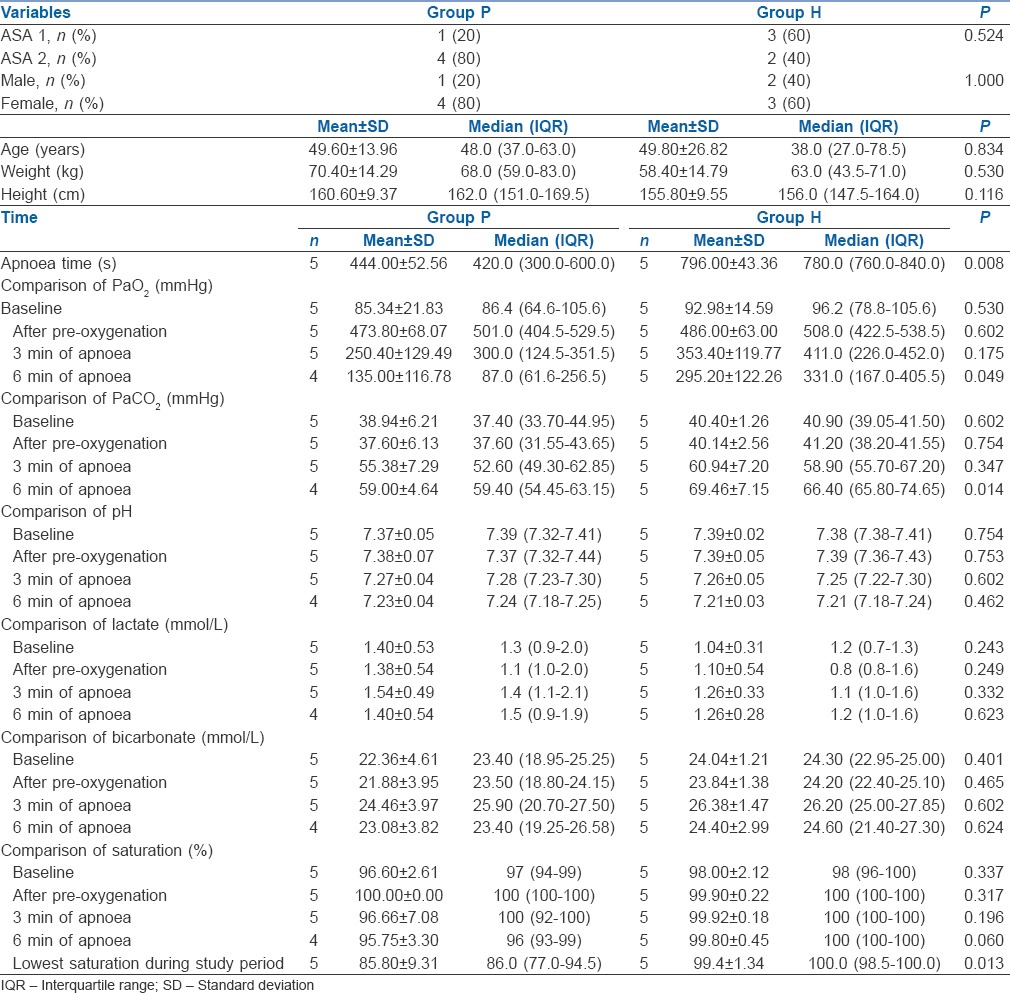
Baseline PO2, that following preoxygenation and at 3 min of apnoea time did not show any statistically significant difference between the groups. However, at 6 min, Group H had a significantly higher PO2(295.20 ± 122.26 vs. 135.00 ± 116.78, P = 0.049). Similarly, PCO2 was also comparable in both groups till 3 min, but at 6 min Group H had significantly higher PCO2(69.46 ± 7.15 vs. 59.00 ± 4.64). Comparison of pH, lactate, bicarbonate and base excess up to 6 min of apnoea did not show any significant difference among the groups [Table 1].
Baseline haemodynamic variables were comparable in both groups. Although there was an increase in HR from baseline in both groups during apnoea, only at 3 min, there was a significant difference between the groups with Group P having a significantly higher HR. MAP was comparable in both groups till 6 min of apnoea. Changes in PaO2 and PaCO2 in both groups during the study period as well as the haemodynamic changes in both groups are depicted in Figures 3–5, respectively.
Figure 3.
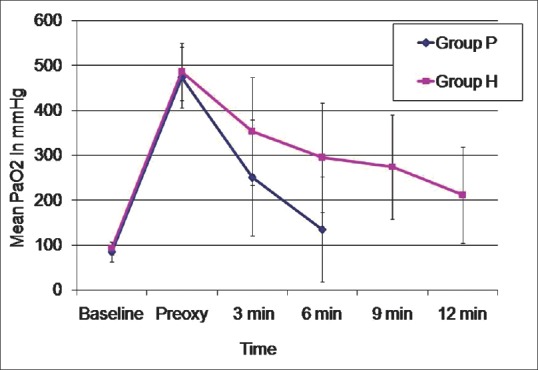
Changes in partial pressures of arterial oxygen
Figure 5.
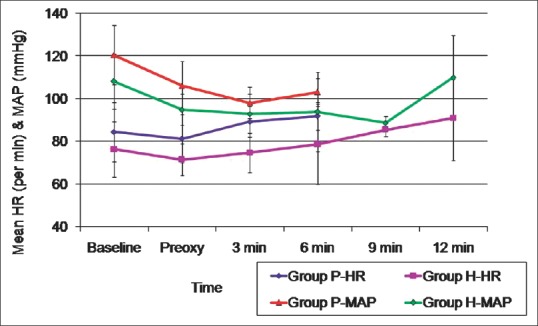
Changes in haemodynamics
Figure 4.
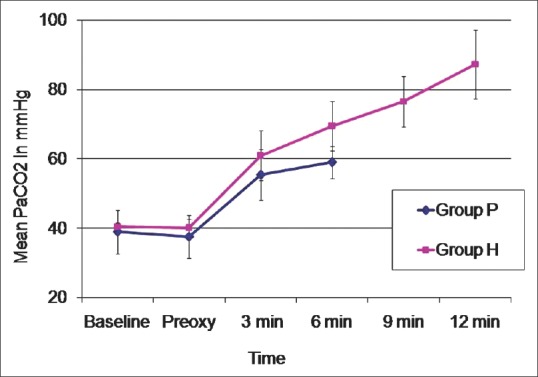
Changes in partial pressures of arterial carbon dioxide
DISCUSSION
Desaturation to SpO2< 90% was significantly prolonged with use of THRIVE as compared to traditional preoxygenation with TVB and apnoeic oxygenation. Most patients following traditional preoxygenation desaturated to <90% by 6 min of apnoea whereas all patients receiving THRIVE continued to have nearly 100% saturation even at 12 min of apnoea. Although in Group H, mean PO2 remained >200 mmHg even at 12 min of apnoea, after 6 min, there was a significant rise in PCO2 along with fall in pH in those patients.
Traditional methods of preoxygenation include TVB for 3 min or eight vital capacity breaths (VCBs) over a minute. Eight VCB had shown to result in significantly higher PaO2 with an almost doubled apnoea-induced desaturation time as compared to TVB for 3 min.[6] Four VCBs were found to be inadequate for this purpose.[7] The indices of effective preoxygenation are increased alveolar oxygen fraction, decreased alveolar nitrogen fraction with an improved PaO2. End-tidal oxygen concentration of 90% or end-tidal nitrogen concentration of 5% is considered as endpoint of maximal preoxygenation.[8]
Desaturation following preoxygenation, while keeping the patient apnoeic, can be delayed with apnoeic diffusion oxygenation, continuous positive airway pressure (CPAP) and/or positive end-expiratory pressure (PEEP), bi-level positive airway pressure and THRIVE.[8] Although proper preoxygenation provides improved margin of safety during apnoeic periods, it could result in delayed detection of oesophageal intubation and absorption atelectasis. Adoption of CPAP, PEEP and/or vital capacity maneuvers reduce the risk of atelectasis. Although reduction of inspired fraction of oxygen to 0.8 reduces this risk, the efficiency of preoxygenation will be diminished.[8]
Because of conflicting results from recent trials, the efficacy of apnoeic oxygenation remains unclear. Apnoeic oxygenation using high-flow nasal cannula at 15 L/min has not shown to result in any significant difference in severe hypoxemia during rapid sequence intubation.[9] Whereas three systematic reviews and meta-analysis have shown its clear benefit in prolonging safe apnoeic time and thereby reducing the incidence of desaturation during intubation.[10,11,12]
THRIVE is associated with a flow-dependent dead space flushing resulting in gas exchange. It combines benefits of CPAP and apnoeic oxygenation.[2] It has shown to maintain oxygen saturation up to 40 min[13] and has been successfully used to preoxygenate patients at high risk of hypoxia during intubation.[4,14] A patent upper airway is a prerequisite whenever THRIVE is used, and in the obese or patients with upper airway obstruction, its effectiveness may be reduced.[15] Most of the studies advocating the utility of THRIVE in prolonging safe apnoeic period had not assessed its effects on arterial carbon dioxide levels or acid-base balance. Serial ABG analysis has revealed progressive hypercarbia with acute respiratory acidosis in our patients, even though partial pressure of oxygen remained in safe and acceptable limits with no arterial desaturation. The haemodynamic changes observed could be secondary to sympathetic stimulation resulting in increased plasma catecholamine levels.
Acute respiratory acidosis results in more prompt myocardial depression as compared to that due to metabolic causes because of rapid entry of carbon dioxide into the cardiac cells. Though there is stimulation of the sympathetic-adrenal axis, this effect is blunted by reduced responsiveness of adrenergic receptors to circulating catecholamines. Acidosis decreases the threshold for ventricular fibrillation and the decrease in cardiac contractility seen with acidaemia is directly proportional to the degree of fall in pH. Acute respiratory acidaemia causes marked increases in cerebral blood flow and thereby increases intracranial tension as well.[16]
Although sample size calculation based on a pilot study necessitated recruitment of only three patients per group, we included five patients in each group. The data of the 20 patients of the pilot study were omitted. As results of any study need to be extrapolated to a larger population, ours with a small sample size could only be considered as a preliminary study, which was a major drawback. We tried to prevent the development of awareness by premedicating with midazolam and intermittent boluses of propofol intraoperatively, but depth of anaesthesia was not assessed. The availability of BIS monitoring would have provided more reliable information regarding the same. Because of the intermittent propofol bolus administration during our study, the HR and MAP responses cannot be considered as a true reflection of changes in haemodynamics that occur during apnoea.
Based on the observation of development of progressive acute respiratory acidosis during the study, intermittent positive pressure ventilation for short periods after 6 min of apnoea while using THRIVE to aid carbon dioxide elimination might increase the safety of its use.
CONCLUSION
During apnoeic periods, the time to desaturate to <90% was significantly prolonged with the use of THRIVE as compared to traditional preoxygenation followed by apnoeic oxygenation. However, in view of the development of significant hypercarbia and acute respiratory acidosis, continued use of THRIVE beyond 6 min of apnoea could not be recommended even though there was no arterial hypoxemia or desaturation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.English J, Norris A, Bedforth N. Anaesthesia for airway surgery. Contin Educ Anaesth Crit Care Pain. 2006;6:28–31. [Google Scholar]
- 2.Patel A, Nouraei SA. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE): A physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–9. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59:165–750. doi: 10.1016/j.annemergmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Doyle AJ, Stolady D, Mariyaselvam M, Wijewardena G, Gent E, Blunt M, et al. Preoxygenation and apneic oxygenation using transnasal humidified rapid-insufflation ventilatory exchange for emergency intubation. J Crit Care. 2016;36:8–12. doi: 10.1016/j.jcrc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 5.De Jong A, Futier E, Millot A, Coisel Y, Jung B, Chanques G, et al. How to preoxygenate in operative room: Healthy subjects and situations “at risk”. Ann Fr Anesth Reanim. 2014;33:457–61. doi: 10.1016/j.annfar.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Rajan S, Mohan P, Paul J, Cherian A. Comparison of margin of safety following two different techniques of preoxygenation. J Anaesthesiol Clin Pharmacol. 2015;31:165–8. doi: 10.4103/0970-9185.155142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Can J Anaesth. 2009;56:449–66. doi: 10.1007/s12630-009-9084-z. [DOI] [PubMed] [Google Scholar]
- 8.Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: Physiologic basis, benefits, and potential risks. Anesth Analg. 2017;124:507–17. doi: 10.1213/ANE.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 9.Riyapan S, Lubin J. Apneic oxygenation may not prevent severe hypoxemia during rapid sequence intubation: A Retrospective helicopter emergency medical service study. Air Med J. 2016;35:365–8. doi: 10.1016/j.amj.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Binks MJ, Holyoak RS, Melhuish TM, Vlok R, Bond E, White LD, et al. Apneic oxygenation during intubation in the emergency department and during retrieval: A systematic review and meta-analysis. Am J Emerg Med. 2017;35:1542–6. doi: 10.1016/j.ajem.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Pavlov I, Medrano S, Weingart S. Apneic oxygenation reduces the incidence of hypoxemia during emergency intubation: A systematic review and meta-analysis. Am J Emerg Med. 2017;35:1184–9. doi: 10.1016/j.ajem.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Wong DT, Yee AJ, Leong SM, Chung F. The effectiveness of apneic oxygenation during tracheal intubation in various clinical settings: A narrative review. Can J Anaesth. 2017;64:416–27. doi: 10.1007/s12630-016-0802-z. [DOI] [PubMed] [Google Scholar]
- 13.Desai N, Fowler A. Use of transnasal humidified rapid-insufflation ventilatory exchange for emergent surgical tracheostomy: A Case report. A A Case Rep. 2017;9:268–70. doi: 10.1213/XAA.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 14.Hengen M, Willemain R, Meyer A, Langer B, Joshi GP, Diemunsch P, et al. Transnasal humidified rapid-insufflation ventilatory exchange for preoxygenation before cesarean delivery under general anesthesia: A Case report. A A Case Rep. 2017;9:216–8. doi: 10.1213/XAA.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad I, Keane O, Muldoon S. Enhancing airway assessment of patients with head and neck pathology using virtual endoscopy. Indian J Anaesth. 2017;61:782–6. doi: 10.4103/ija.IJA_588_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das B. Acid base disorders. Indian J Anaesth. 2003;47:373–9. [Google Scholar]


