Abstract
Background and Aims:
Propofol injection pain is an unresolved problem in children. Although medium and long chain triglyceride (MCT-LCT) propofol has shown promising results in adults, its efficacy in children is not proven. In a prospective observational study the incidence and severity of pain with MCT-LCT and LCT propofol in children was compared.
Methods:
After obtaining approval from the Institutional Ethics Committee, 170 children (age group 6 months to 8 years) scheduled for various surgeries were included in this study. Following standard pre-medication, propofol 1% either LCT or MCT-LCT in a dose of 2–4 mg/kg along with preservative-free lignocaine (2% lignocaine 1 mg in propofol 10 mg) was administered. The primary objective was to study injection pain on scale of 0–6. For children ≤2 years doubling of motor event score (0–3) and for children >2 years, addition of motor (0–3) and verbalisation scores (0–3) were considered. Mann–Whitney U test was used for statistical analysis.
Results:
MCT-LCT group had lower incidence of pain (17 patients (20%) versus 35 patients (35.3%), P = 0.026) and severe pain (zero patients (0%) versus six patients (7.1%), P = 0.029) as compared to LCT group. MCT-LCT group had significantly lower mean rank of motor (79.65 versus 91.35), verbal (77.29 versus 90.79) and total score (77.76 versus 93.24) as compared to LCT group (P = 0.037, 0.002, and 0.009, respectively).
Conclusion:
MCT-LCT propofol is associated with significantly lower injection pain as compared to LCT propofol in children, when both are combined with lignocaine.
Key Words: Children, injection, lignocaine, pain, propofol, triglyceride
INTRODUCTION
Propofol, 2,6-di-isopropylphenol, has become the most popular intravenous anaesthetic drug for induction and sedation. However, one troublesome issue which limits its use is the propofol-induced injection pain, the exact mechanism of which is unknown. The concentration of free propofol in the aqueous phase is claimed to be associated with higher intensity of pain on injection.[1]
A novel lipid-formula, which contains a mixture of medium and long chain triglyceride (MCT-LCT) propofol has less free propofol concentration in aqueous phase. It has shown lesser injection pain as compared to long chain triglyceride (LCT) propofol in adults. However, results in paediatric population remain inconclusive. Major limitations with previous studies are inclusion of only older children,[2,3] omission of lignocaine[4] or addition of lignocaine in only LCT group.[5]
This prospective observational study was conducted to overcome the lacunae of previous studies and evaluate the incidence and severity of injection pain with LCT and MCT-LCT propofol in children when lignocaine was added to both the groups.
METHODS
After obtaining the Institutional Ethics Committee (IEC) approval and written informed consent from parents of participating children, 170 children (aged 6 months to 8 years) belonging to the American Society of Anaesthesiologists' physical status I or II, scheduled for various procedures requiring propofol as the induction agent were enrolled in this study. Patients with known allergy to eggs or with bradycardia and hypotension were excluded from the study. All children were thoroughly evaluated regarding fitness for general anaesthesia and were fasted for 6 h for solids, 4 h for breast milk and 2 h for clear fluids. Inside the operation theatre, electrocardiograph, peripheral oxygen saturation (SpO2), heart rate, systolic and diastolic blood pressures were monitored and baseline values were noted. Intravenous (IV) cannula (24G) was inserted in the ward over the dorsum of the hand.
IV access of all children was checked for pain-free injection of saline in the pre-operative room in the presence of parent when the child was calm. Glycopyrrolate 0.004 mg/kg, midazolam 0.02 mg/kg and fentanyl 2 μg/kg were administered intravenously to all children 5 min before the induction of anaesthesia. Children who continued to cry or did not calm down after 5 min of the above mentioned medication were not considered for the study. Thereafter, induction of anaesthesia was carried out with freshly prepared (only taken out from the refrigerator) propofol 1%, either LCT propofol (Vital Healthcare Ltd, India) or MCT-LCT (Fresenius Kabi, India Pvt. Ltd, Mfg. Fresenius Kabi, Austria), at a dose of 2–4 mg/kg administered over a period of 20 s along with preservative-free lignocaine (Loxicard, of Neon Laboratories, India) in the proportion of 2% lignocaine 1 mg in propofol 10 mg, until the loss of consciousness. The preparation of injection was done as follows: in the syringe 4 mg/kg either of the propofol preparation (LCT/MCT-LCT) was aspirated and then, 0.4 mg/kg of preservative-free lignocaine was added using 1 ml syringe. Patients received either of the formulation of propofol at the attending anaesthesiologist's discretion. All patients receiving LCT propofol were labelled as Group LCT and patients receiving MCT-LCT propofol were labelled as Group MCT-LCT.
Injection pain following propofol was assessed in all patients until the loss of consciousness, by an anaesthesiologist who was unaware of the type of propofol being used. Pain scale[6] used is depicted in Table 1. In children ≤2 years, only motor events were observed and in children >2 years, both motor as well as verbalisation scale was used. Thus, in children ≤2 years, pain score evaluated on the basis of motor events was doubled to obtain a maximum score of 6. Pain score of ≥3 was considered as severe pain and inhalation of sevoflurane in oxygen at fresh gas flow rate of 6 L/min was started at 1% and increasing at every 3 breaths by 1% until the child had pain score <3 or loss of eyelash reflex, whichever occurred earlier. After the induction, anaesthesia was subsequently carried on as decided by the attending anaesthesiologist.
Table 1.
Pain scale for evaluation of propofol-induced injection pain

The primary outcomes of the study were incidence of pain and severe pain on injection and pain scores. The secondary outcomes studied were change in heart rate and blood pressure and adverse events (if any). Adverse events such as rash, urticaria or myoclonic movements were noted. The sample size estimation was based on the assumptions from a previous study where pain in control group was observed to be in 22% of patients while that in experimental group, it was found to be in 7% of patients.[6] Considering 80% power and 5% significance, it was estimated that 85 patients would be required to be evaluated in each group. The statistical software SPSS version 16.0 was used for the analysis of data. Continuous data such as age and weight were analysed using the Student's t-test. Categorical data such as gender, incidence of pain and severe pain were analysed using Chi square test. Pain score in both the groups was analysed using Mann–Whitney U test. A value of P < 0.05 was considered statistically significant.
RESULTS
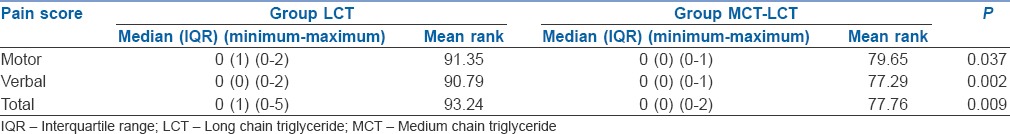
The study included 170 children [Figure 1]. There were no significant differences between the groups with respect to demographic parameters [Table 2]. MCT-LCT group had significantly lower incidence of pain (20% vs. 35.3%, P = 0.026) and severe pain (0% vs. 7.1%, P = 0.029) as compared to LCT group [Table 3]. The pain scores were not normally distributed and hence are depicted as median, inter-quartile range and minimum to maximum score [Table 4]. MCT-LCT group had significantly lower mean rank of motor (79.65 vs. 91.35), verbal (77.29 vs. 90.79) and total score (77.76 vs. 93.24) as compared to LCT group (P = 0.037, 0.002 and 0.009, respectively).
Figure 1.
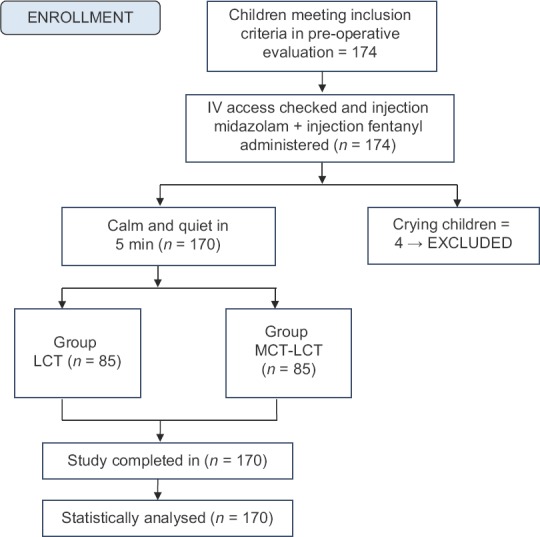
Patient Flow Diagram
Table 2.
Demographic characteristics of patients
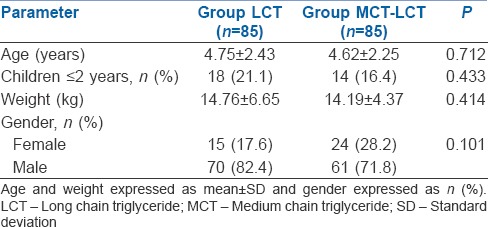
Table 3.
Incidence of pain and severe pain on injection

Table 4.
Pain score
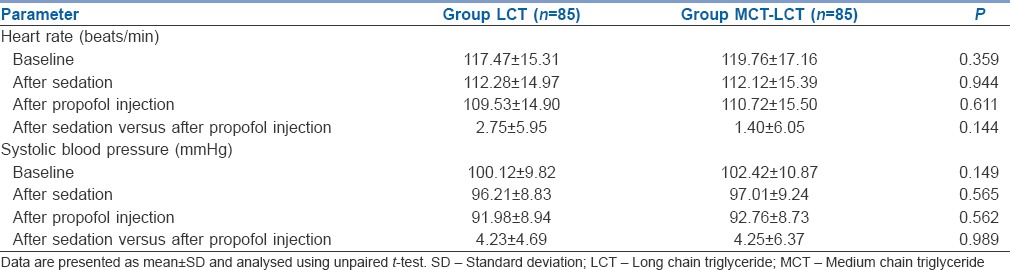
Heart rate and blood pressure remained stable, and there was no significant change in heart rate and blood pressure following propofol injection [Table 5]. No adverse events were seen in any of the patients.
Table 5.
Comparison of haemodynamic values in two groups of patients studied
DISCUSSION
Due to its desirable properties, propofol (2,-6-di-isopropylphenol) is now becoming the dominating IV anaesthetic agent. Since the introduction of propofol, numerous attempts have been made in an effort to alleviate propofol-induced injection pain, a troublesome issue which still persists, especially in children.
The exact mechanism of propofol-induced injection pain is not fully understood. It has been postulated that the intensity of this pain correlates with the concentration of free propofol in the aqueous phase.[1,7,8] Both bradykinin generation and complement activation were similarly higher with LCT and MCT-LCT propofol as compared with saline when blood obtained from 13 volunteers was mixed with one of these agents.[1] Another study found the significantly lower incidence of injection pain with MCT-LCT propofol compared to LCT propofol in 200 adult patients. The authors of this study proposed reduced concentration of propofol in the aqueous phase to be the most likely contributor to lesser injection pain with MCT-LCT propofol. The ability of new lipid formula, MCT-LCT propofol in reducing this pain at the injection site has been effectively revealed in adults.[9,10,11] However, the medical literature is quite limited and inconclusive with regard to its efficacy in children.
So far, of the six paediatric studies[2,3,4,5,6,12] conducted to compare injection pain by MCT-LCT and LCT propofol, only one study included children ranging from 0 to 7 years of age.[6] Thus, the evaluation of injection pain in children <2 years age is not very evident.[2,3,4,5,12] The large sample size (n = 170) and the inclusion of younger age group (6 months to 8 year) helped making our results easy to generalise for paediatric anaesthesia practice. To avoid other factors, such as site and size of IV cannula, which could contribute to injection pain, we used 24G cannula for all children secured over the dorsum of the hand. IV access was checked for pain-free injection of saline in all children. Of all the interventions used so far, local anaesthetic lignocaine-either as a pre-treatment with venous occlusion or as an admixture has proven to be the most effective method for reducing this discomfort.[13,14] Thus, we added lignocaine in both the groups.
Inadequate expression of the degree of pain by children makes it a difficult task to evaluate pain during the induction of anaesthesia. Thus, like various other paediatric studies, pain evaluation in our study was also an investigator-based pain assessment. We used a pain score similar to the one used in a previous study which included both motor as well as verbal scale to appropriately evaluate pain in all the age groups.[6] To further make it as objective as possible, the verbal scale was not included for children ≤2 years of age and in such children motor score calculated was doubled to obtain the total pain score.
A new environment and the pre-existing anxiety or restlessness in a child makes it difficult to assess the injection pain by propofol. Of the six studies,[2,3,4,5,6,12] three have not used any pre-medication.[3,4,12] Of the remaining three, one used oral diazepam[2] and another[6] used rectal midazolam, which is not the routine practice in our set-up. Since it is a normal protocol to administer IV midazolam and fentanyl, we used the same and waited for the children to be calmed down. Any child still crying or not calmed down after 5 min of sedation was excluded from the study.
Our results concur with previous two studies finding lesser injection pain with MCT-LCT propofol. The study included 85 patients in MCT-LCT group compared to 20 and 42 patients in those two studies.[2,6] The incidence of pain with MCT-LCT propofol was 7.5% in a previously conducted study compared to 20% in this study.[6] Perhaps, it could be due to the use of nitrous oxide in 55 patients in their study to secure venous cannula.
In contrast with the above studies, no significant difference in the pain scores between MCT-LCT and LCT propofol was observed in one study.[3] None of the 84 children enrolled in this study were administered any pre-medication or sedation. The varying distraction techniques used by them to calm the children before injection of the drug could be the reason for this differing result. Propofol LCT with or without lignocaine and propofol MCT-LCT with or without lignocaine were studied with regard to pain on injection. The highest pain scores were found in propofol MCT-LCT group without lignocaine and the lowest pain scores were found in propofol MCT-LCT group with lignocaine.[12] The use of tourniquet in their study could itself be a potential source of discomfort as tourniquets are not as easily tolerated by the children as the adults. Much higher pain incidence was observed with MCT-LCT propofol when used as a plain solution compared to LCT propofol with added lignocaine.[5] The use of lignocaine with only LCT propofol probably explains the confounding variable in their study and the reason behind increased pain with MCT-LCT propofol. In another study where lignocaine was not added to any groups, MCT-LCT propofol was associated with higher pain incidence than LCT propofol.[4] Since the use of lignocaine has reduced propofol injection pain in most of the studies, it is our routine practice to add lignocaine to propofol irrespective of the type of formulation used.
In our study, the incidence of severe pain, requiring supplementation of sevoflurane, was 0% in MCT-LCT group and 7.1% in LCT group. This could be attributed to the use of fentanyl 5 min before injection of propofol and addition of lignocaine to both the groups. In a study comparing four different groups, the pain incidence was 5% with group fentanyl lignocaine (fentanyl 0.1 mg 3 min before propofol mixed with lidocaine 40 mg; 40% in group fentanyl (fentanyl 0.1 mg 3 min before propofol); 35% in group lidocaine (lidocaine 40 mg added to 200 mg propofol); and 80% in control group.[15]
This study suffers from a few limitations. Since only 18.82% of children were belonging to ≤2 years, subgroup analysis could not be performed. Further, this was not a randomised controlled trial. An appropriately powered study on the incidence of pain and severity of pain in the younger age group is suggested. Although the incidence of severe pain is 0% in MCT-LCT group, some amount of pain is present in 20% of children inspite of premixing with lignocaine and premedication with fentanyl. Therefore, there is a need to invent either different formulation or additive measures to reduce the pain.
CONCLUSION
The novel lipid formula MCT-LCT propofol along with lignocaine significantly reduces the incidence as well as the severity of injection pain in the paediatric population. Thus, MCT-LCT propofol can be considered as a preferable alternative to the traditional LCT propofol when used in children for IV induction of anaesthesia or for sedation purpose.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ohmizo H, Obara S, Iwama H. Mechanism of injection pain with long and long-medium chain triglyceride emulsive propofol. Can J Anaesth. 2005;52:595–9. doi: 10.1007/BF03015768. [DOI] [PubMed] [Google Scholar]
- 2.Larsen R, Beerhalter U, Erdkönig R, Larsen B. Injection pain from propofol-MCT-LCT in children. A comparison with propofol-LCT. Anaesthesist. 2001;50:676–8. doi: 10.1007/s001010100213. [DOI] [PubMed] [Google Scholar]
- 3.Varghese E, Krishna HM, Nittala A. Does the newer preparation of propofol, an emulsion of medium/long chain triglycerides cause less injection pain in children when premixed with lignocaine? Paediatr Anaesth. 2010;20:338–42. doi: 10.1111/j.1460-9592.2010.03272.x. [DOI] [PubMed] [Google Scholar]
- 4.Beyaz SG, Eman A. Injection pain of propofol in children: A comparison of two formulations without added lidocaine. J Anaesthesiol Clin Pharmacol. 2012;28:314–7. doi: 10.4103/0970-9185.98322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyman Y, von Hofsten K, Georgiadi A, Eksborg S, Lönnqvist PA. Propofol injection pain in children: A prospective randomized double-blind trial of a new propofol formulation versus propofol with added lidocaine. Br J Anaesth. 2005;95:222–5. doi: 10.1093/bja/aei156. [DOI] [PubMed] [Google Scholar]
- 6.Rochette A, Hocquet AF, Dadure C, Boufroukh D, Raux O, Lubrano JF, et al. Avoiding propofol injection pain in children: A prospective, randomized, double-blinded, placebo-controlled study. Br J Anaesth. 2008;101:390–4. doi: 10.1093/bja/aen169. [DOI] [PubMed] [Google Scholar]
- 7.Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J. Reducing pain during propofol injection: The role of the solvent. Anesth Analg. 1996;82:472–4. doi: 10.1097/00000539-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Klement W, Arndt JO. Pain on injection of propofol: Effects of concentration and diluent. Br J Anaesth. 1991;67:281–4. doi: 10.1093/bja/67.3.281. [DOI] [PubMed] [Google Scholar]
- 9.Rau J, Roizen MF, Doenicke AW, O'Connor MF, Strohschneider U. Propofol in an emulsion of long- and medium-chain triglycerides: The effect on pain. Anesth Analg. 2001;93:382–4. doi: 10.1097/00000539-200108000-00029. [DOI] [PubMed] [Google Scholar]
- 10.Sun NC, Wong AY, Irwin MG. A comparison of pain on intravenous injection between two preparations of propofol. Anesth Analg. 2005;101:675–8. doi: 10.1213/01.ANE.0000157564.91910.04. [DOI] [PubMed] [Google Scholar]
- 11.Doenicke AW, Roizen MF, Rau J, O'Connor M, Kugler J, Klotz U, et al. Pharmacokinetics and pharmacodynamics of propofol in a new solvent. Anesth Analg. 1997;85:1399–403. doi: 10.1097/00000539-199712000-00040. [DOI] [PubMed] [Google Scholar]
- 12.Beyaz SG, T Fek A, Tokgöz O. The effect of propofol lipuro with and without lidocaine on injection pain in children. Niger J Clin Pract. 2011;14:60–4. doi: 10.4103/1119-3077.79252. [DOI] [PubMed] [Google Scholar]
- 13.Picard P, Tramèr MR. Prevention of pain on injection with propofol: A quantitative systematic review. Anesth Analg. 2000;90:963–9. doi: 10.1097/00000539-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Cameron E, Johnston G, Crofts S, Morton NS. The minimum effective dose of lignocaine to prevent injection pain due to propofol in children. Anaesthesia. 1992;47:604–6. doi: 10.1111/j.1365-2044.1992.tb02335.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi Y, Naganuma R, Seki S, Aketa K, Ichimiya T, Namiki A, et al. Reduction of pain on injection of propofol: A comparison of fentanyl with lidocaine. Masui. 1998;47:963–7. [PubMed] [Google Scholar]


