Abstract
Intersphincteric resection (ISR) has rapidly increased worldwide including laparoscopic surgery. However, there are some concerns for the definition of ISR, surgical technique, oncological outcome, anal function, and quality of life (QoL). The aim of the present study is to evaluate those issues. A review of this surgical technique was carried out by searching English language literature of the PubMed online database and appropriate articles were identified. With regard to open‐ISR, the morbidity rate ranged from 7.5% to 38.3%, with lower mortality rates. Local recurrence rates varied widely from 0% to 22.7%, with a mean follow‐up duration of 40–94 months. Disease‐free and overall 5‐year survival rates were 68–86% and 76–97%, respectively. Those outcomes were equivalent to laparoscopic‐ISR. Surgical and oncological outcomes of ISR were generally acceptable. However, accurate evaluation of anal function and QoL was difficult because of a lack of standard assessment of various patient‐related factors. The surgical and oncological outcomes after ISR seem to be acceptable. The ISR technique seems to be valid as an alternative to abdominoperineal resection in selected patients with a very low rectal cancer. However, both necessity for ISR and expectations of QoL impairment as a result of functional disorder should be fully discussed with patients before surgery.
Keywords: functional outcome, intersphincteric resection, local recurrence, oncological outcome, rectal cancer, survival
1. Introduction
Surgical treatment for very low rectal cancer is very difficult because of the higher rate of local recurrence (LR) and lower rate of survival. Abdominoperineal resection (APR) reported by Miles has been used for a long time as a standard surgical procedure for lower rectal cancer.1 However, APR characterized by a permanent colostomy has not been easily accepted by patients. In 1972, low anterior resection followed by hand‐sewn coloanal anastomosis (CAA) introduced by Parks became widely adopted around the world as an excellent procedure for lower rectal cancer to preserve the anus.2 However, anal preservation may have a higher risk of LR than non‐preservation. In the latter half of the 1900s, total mesorectal excision (TME),3 preoperative chemoradiotherapy (CRT), and optimal circumferential resection margin (CRM) suggested both good control of LR and survival benefit.4, 5 Also, CRT influenced down‐staging of the tumor, and allowed sphincter‐saving operation for some patients who may have required APR.6 In addition to those aspects, shorter distal resection margin proposed by clinicopathological studies has encouraged surgeons to preserve the anus.7, 8, 9, 10, 11, 12, 13 In 1994, Schiessel et al. introduced intersphincteric resection (ISR) followed by hand‐sewn CAA as an anal preservation procedure for very low rectal cancer closer to the anus.14 ISR is the ultimate anal preservation surgery by both abdominal and anal approaches which consists of TME and excision of the internal anal sphincter. The surgical technique changed the concept of anal preservation and, since 2000, has rapidly expanded not only in Europe, but also in Japan and other Asian countries.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 Also, laparoscopic‐ISR has come to be aggressively carried out.38, 39, 40, 41, 42 Many researchers have reported the surgical, oncological, and functional outcomes. However, some studies including conventional Parks’ CAA, or low anterior resection with stapled anastomosis have caused misunderstanding of ISR. Moreover, quality of life (QoL) impairment caused by fecal incontinence remains unclear.20, 46, 48, 54, 55, 56 The present review investigates and discusses the surgical, oncological and functional outcomes, as well as QoL, of ISR.
2. Methods
A literature search of PubMed online database in the English language was carried out and appropriate articles associated with ISR were identified including laparoscopic surgery. Some studies specializing in conventional Parks’ CAA and in stapler CAA (ultralow anterior resection with stapled anastomosis) were excluded. Multiple publications involving the same series of patients (or duplicate patient populations) were identified and grouped together with only the most recent or primary study to avoid double‐counting of patients.
3. Results
3.1. Indication
Available data were extracted from 22 articles21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 and are summarized in Table 1. The most common indication for ISR is a tumor with T1–3 categories and a tumor located at 10–50 mm from the anal verge. Contraindication is the presence of untreatable distant metastasis, poorly differentiated carcinoma, poor anal function, psychiatric disease, and a fixed tumor (T4 lesion) which invades the puborectal muscles and/or external anal sphincter.
Table 1.
Characteristics of patients and tumors
| Authora | Year | No. patients | Age (years) | Sex (M(%)/F) | Distance from AV (DL) (mm) | T category |
|---|---|---|---|---|---|---|
| Köhler et al.21 | 2000 | 31 | 60 | 17(55)/14 | 13 ± 9 (DL) | T1–T3 |
| Vorobiev et al.22 | 2004 | 27 | 55 (26–75) | 16(59)/11 | 10 (5–15) (DL) | T2–T3 |
| Schiessel et al.23 | 2005 | 121 | 65/62 (M/F) | 83(69)/38 | 30 (10–50) | T1–T3 |
| Rullier et al.24 | 2005 | 92 | 65 (25–86) | 57(62)/35 | 30 (15–45) | T1–T3 |
| Hohenberger et al.25 | 2006 | 65 | NR | NR | <20 (DL) | T1–T2 |
| Chin et al.26 | 2006 | 18 | 61 (42–79) | 7(39)/11 | 10–30 (DL) | T2–T3 (T4) |
| Saito et al.27 b | 2006 | 228 | 58 (27–77) | 168(74)/60 | 34 (20–50) | T1–T3 (T4) |
| Chamlou et al.28 | 2007 | 90 | 59 (27–82) | 59(66)/31 | 35 (22–52) | T1–T3 (T4) |
| Portier et al.29 | 2007 | 173 | 64 | 57(33)/116 | 41 ± 1.4 | T1–T3 (T4) |
| Krand et al.30 | 2009 | 47 | 57 (27–72) | 31(66)/16 | 33 (15–50) | T2–T3 |
| Han et al.31 | 2009 | 40 | 62 (34–73) | 24(60)/16 | 20–50 (DL) | T1–T2 |
| Weiser et al.32 | 2009 | 44 | 54 (28–78) | 25(57)/19 | 50 (30–60) | T3–T4 |
| Kuo et al.33 | 2011 | 26 | 51 (26–71) | 16(62)/10 | 35 (25–50) | T3–T4 |
| Gong et al.34 | 2012 | 43 | 53 | 27(63)/16 | <50 | T1–T2 |
| Akagi et al.35 | 2013 | 124 | 65 (32–81) | 77(62)/47 | 30 (10–40) | T1–T3 (T4) |
| Tokoro et al.36 | 2013 | 30 | 59 (31–75) | 16(53)/14 | 8.9 (–3–25) (DL) | Tis–T3 |
| Saito et al.37 | 2014 | 199 | 59 (27–80) | 144(72)/55 | 35 (10–55) | T1–T4 |
| Rullier et al.38 | 2003 | 32 | 64 (37–75) | 21(66)/11 | <50 | T1–T4 |
| Park et al.39 | 2011 | 210 | 61 | 141(67)/69 | 36–47 | T1–T4 |
| Laurent et al.40 | 2011 | 175 | 64 | 117(67)/58 | 35–40 | T1–T4 |
| Kuo et al.41 | 2013 | 58 | 53 | 36(62)/22 | 36 | T1–T4 |
| Kanso et al.42 | 2015 | 85 | 59 (32–82) | 62(73)/23 | 17 (0–35) (DL) | T0–T4 |
Available data were summarized.
Japanese experience, including our data.
AV, anal verge; DL, dentate line; F, female; M, male; NR, not reported.
3.2. Neoadjuvant chemoradiotherapy and surgical outcomes
Neoadjuvant chemoradiotherapy was commonly given, but its use varied widely, ranging from 0 to 100%,21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 as shown in Table 2.
Table 2.
Surgical procedures
| Author | No. patients | Pre‐op CRT (%) | Method of ISR P‐ST/T/ESR | J‐Pouch anastomosis (%) | Diverting stoma (%) |
|---|---|---|---|---|---|
| Köhler et al.21 | 31 | 0 | 31/0/0 | 0 | 100 |
| Vorobiev et al.22 | 27 | 7 | 0/27(100%)/0 | 100 (C‐pouch) | 100 |
| Schiessel et al.23 | 121 | 0 | P‐ST, T | 0 | 100 |
| Rullier et al.24 | 92 | 88 | P‐ST, T | 57 | 100 |
| Hohenberger et al.25 | 65 | 65 | P‐ST | Sometimes | 100 |
| Chin et al.26 | 18 | 33 | NR | 100 | 100 |
| Saito et al.27, a | 228 | 25 | 159/69 (T/ESR) | 22 | NR |
| Chamlou et al.28 | 90 | 41 | P‐ST | 100 | 100 |
| Portier et al.29 | 173 | 53 | P | NR | 100 |
| Krand et al.30 | 47 | 100 | 47/0/0 | 40 (coloplasty) | 100 |
| Han et al.31 | 40 | 2.5 | 35/5(13%)/0 | 18 | 28 |
| Weiser et al.32 | 44 | 100 | 44/0/0 | 48 | NR |
| Kuo et al.33 | 26 | 88 | 26/0/0 | 0 | 100 |
| Gong et al.34 | 43 | 0 | 43/0/0 | NR | 0 |
| Akagi et al.35 | 124 | 0 | T, ST | NR | 100 |
| Tokoro et al.36 | 30 | 0 | 14/12(40%)/4 | 87 | 100 |
| Saito et al.37 | 199 | 25 | 144/55 (/41) | NR | 100 |
| Rullier et al.38 | 32 | 91 | 32/0/0 | 100 (coloplasty) | 100 |
| Park et al.39 | 210 | 5.2 | NR | 0 | 9.5 |
| Laurent et al.40 | 175 | 90 | 119/56(32%)/0 | NR | 100 |
| Kuo et al.41 | 58 | 95 | NR | 0 | NR |
| Kanso et al.42 | 85 | 84 | 64/21/0 | 0 | 100 |
Japanese experience including our data.
ESR, external anal sphincter resection (ISR with combined resection of partial or extended external sphincter); ISR, intersphincteric resection; NR, not reported; P, partial; Pre‐op CRT, preoperative chemoradiotherapy; ST, subtotal; T, total.
3.3. Surgical technique
Based on the concept of TME,3 the rectum is mobilized down to the upper level of the levator ani muscle. Dissection of the intersphincteric space (ISS) between the internal anal sphincter (IAS) and external anal sphincter (EAS) is begun from the posterior side of the rectum by transecting the hiatal (anococcygeal) ligament. Then, circumferential dissection of the intersphincteric space in the anal canal is carried out from the bilateral lateral side to the anterior part. The dissection is advanced to a level lower than the dentate line (DL) in order to facilitate the transanal approach. Circular incision of the anal canal is started at the DL in partial‐ISR, between the dentate line and intersphincteric groove in subtotal‐ISR, and at the intersphincteric groove in total‐ISR.35 The IAS is dissected from the EAS, prostate, vagina, and puborectal muscle, and then the dissection is connected to the transabdominal dissection. After the rectum is completely separated from the anal canal structures, the specimen is taken out of the anus. Thereafter, hand‐sewn CAA is done using straight colon,21, 23, 33 J‐pouch,26, 28 coloplasty30 or C‐pouch.22 Smooth muscle plasty was devised as a neo‐sphincter to improve anal function.22, 30 Combined EAS resection (ESR) is sometimes carried out for tumors with suspected invasion into the intersphincteric space and/or EAS.17, 44, 53 Finally, protective diverting ileostomy or colostomy is commonly created. An example of open‐ISR technique is shown on the supplementary video (Video 1).17, 35, 49
3.4. Morbidity and mortality
Regarding open surgery, the rate of overall morbidity varied widely from 7.5% to 38.3% (Table 3).21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 Operative mortality was rare (0–1.7%). Morbidities included anastomotic leakage, pelvic abscess, colonic ischemia (or necrosis), ileus, ano‐vaginal fistula and others. Anastomotic leakage occurred in 4.3–48% of cases, and subsequent stenosis was observed in 8.4–23.3% of cases. These outcomes were almost equivalent to laparoscopic‐ISR.
Table 3.
Patient characteristics, surgical outcomes and postoperative complicationsa
| Item | Open‐ISR | Laparoscopic‐ISR |
|---|---|---|
| Age (years) | 51–65 | 55–64 |
| Gender: Male/Female (%) | 33–74/26–67 | 61–76/24–39 |
| Body mass index (kg/m2) | 25 | 21.4–24.3 |
| Distance from AV [DL] (mm) | 30–50 [10–50] | 33–55 [17] |
| T factor (T1/T2/T3/T4) (%) | 3/13/83/0 | 0–12/11–33/43–86/0–4 |
| Pre‐op CRT (%) | 0–100 | 26.9–100 |
| Type of ISR: P‐ST/T/ESR (%) | Almost 100/13–100/Few | 73–75/25–27/0 |
| J‐Pouch anastomosis (%) | Almost <50 | Almost <50 |
| Diverting stoma (%) | Almost 100 | 14–100 |
| Operating time (min) | 416 | 185–420 |
| Blood loss (mL) | 155–265 | 59–303 |
| Intraoperative transfusion (%) | 10 | 0–1.5 |
| Postoperative stay (days) | 16–18 | 9–15 |
| Operative mortality (%) | 0–1.7 | 0–1.1 |
| Leakage (%) | 4.3–48 | 3.8–24 |
| Vaginal fistula (%) | 0–19.4 | 1.5–2.8 |
| Vesical fistula (%) | 0–0.8 | 0 |
| Colonic ischemia (necrosis) (%) | 0–2.0 | 2.5–14.3 |
| Sepsis (%) | 0–8.7 | 0 |
| Pelvic abscess (%) | 0–5.6 | 0.8–8.1 |
| Pelvic hematoma (%) | 0–6.5 | 0 |
| Ileus (bowel obstruction) (%) | 0–8.5 | 1.5–15.4 |
| Stenosis (%) | 8.4–23.3 | 2.4–13 |
| Not closed (diverting stoma) (%) | 0–12.5 | NR |
| Additional surgeryb (%) | 0–12.9 | NR |
| Grade of morbidity (%) | ||
| Dindo I–II | 96 | 63‐95 |
| Dindo III–V | 3.8–27.7 | 5.4–37 |
| Overall morbidity (%) | 7.5–38.3 | 12.5–32.1 |
Available data from 22 articles were summarized.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42
Abdominoperineal resection, Hartmann's procedure, and/or re‐creation of stoma were required because of postoperative surgical and/or functional complications.
AV, anal verge; CRT, chemoradiotherapy; DL, dentate line; ESR, external anal sphincter resection (ISR with combined resection of partial or extended external sphincter); ISR, intersphincteric resection; P‐ST, partial‐subtotal ISR; T, total ISR.
3.5. Oncological outcomes
Oncological outcomes are summarized in Table 4. As to open‐ISR, the rate of radical surgery (R0 resection) was over 90%. The distal resection margin (DRM) was maintained from 5 to 25 mm. Frequency of a radial (circumferential) resection margin (CRM) ≤1 mm ranged from 4 to 19.6%. Rates of overall recurrence, distant metastasis, and local recurrence showed ranges of 13.3–20.0%, 0–19.0%, and 0–22.7%, respectively, within a mean follow‐up duration from 12 to 94 months. These outcomes were almost equivalent to laparoscopic‐ISR.
Table 4.
Oncological outcomesa
| Item | Open‐ISR | Laparoscopic‐ISR |
|---|---|---|
| TNM stage: I/II/III/IV (%) | 0–58/4–63/16–78/0–7 | 0–48/11–24/22–86/3–8 |
| R0 resection (%) | 90–100 | 95–96.4 |
| Distal resection margin (mm) | 5–25 | 12–30 |
| Radial resection margin ≤1 mm (%) | 4.0–19.6 | 5.0–15.5 |
| Retrieved lymph node (n) | 14.7 | 13.3–15.2 |
| Median follow up (months) | 12–94 | 31.5–53 |
| Overall recurrence (%) | 13.3–20.0 | 17.9–28.2 |
| Distant metastasis (%) | 0–19.0 | 8.5–24 |
| Local recurrence (%) | 0–22.7 | 2.6–8.2 |
| Disease‐free 3‐year survival (%) | 77.0 | 75.0–90.5 |
| Overall 3‐year survival (%) | 81.6 | 86.6–94.8 |
| Disease‐free 5‐year survival (%) | 68.4–86 | 70–82.8 |
| Overall 5‐year survival (%) | 76.5–97 | 85–88.4 |
Disease‐free and overall 5‐year survival rates were excellent, with ranges of 68–86% and 76–97%, respectively. Oncological outcomes after ISR were not markedly different from those after conventional Parks’ CAA or APR.29, 35 Only one study reported a significant difference in the overall and disease‐free survival rates between ISR and APR.33 Saito et al. reported a significant difference in overall survival rate between ISR and APR.52 Akagi et al. reported no significant difference in LR and recurrence‐free survival rates between ISR and APR which were carried out during the same time period.35 These outcomes were almost equivalent to laparoscopic‐ISR, but were not sufficiently evaluated because of the small number of patients and short‐term follow up.
3.6. Functional outcomes
Regarding open‐ISR, anal function was assessed at 1 year after stoma closure, and the available data were summarized from 14 articles,16, 18, 21, 22, 23, 24, 25, 26, 30, 31, 33, 45, 46, 47 as shown in Table 5. Stool frequency/24 h varied widely from 1.8 to 5.1. Rates of stool fragmentation, urgency, nocturnal soiling, daytime soiling, and pad wearing were as follows: 15–79%, 2–52%, 24–53%, 26–35%, and 19–57%, respectively. Wexner score and Kirwan grade showed a relatively good assessment with scores <12 and lower rates of grades IV (0–27%) and V (0–5.9%). Unexpectedly, anti‐diarrhea medication was not particularly necessary (0–33%). Patient satisfaction was approximately 70%. Functional outcomes of laparoscopic‐ISR were not sufficiently evaluated because of lack of data.
Table 5.
Functional outcomesa
| Assessment at ≥1 year after stoma closure | Open‐ISR | Laparoscopic‐ISR |
|---|---|---|
| Mean maximum resting pressure (cmH2O) | 42–75 | NR |
| Mean maximum squeeze pressure (cmH2O) | 186–259 | NR |
| Median stool frequency/24 h | 1.8–5.1 | 2–6 |
| 1–3 (%) | 50–85 | NR |
| 4–5 (%) | 12–57.1 | NR |
| >5 (%) | 0–36 | NR |
| Stool fragmentation (%) | 15–78.9 | 81 (NS) |
| Urgency (<15 min) (%) | 2–51.7 | 58‐83 |
| Incontinence for flatus (%) | 7.7–68.2 | 72.8 (NS) |
| Nocturnal soiling (%) | 23.8–52.9 | 92 (NS) |
| Daytime soiling (%) | 26–35 | 92 (NS) |
| Pad wearing (%) | 19–57 | NR |
| Feces and flatus discrimination (%) | 4–86 | NR |
| Anti‐diarrhea medication (%) | 0–33.3 | NR |
| Mean Wexner score (range) | 2.8–12 | 11–14 |
| Kirwan grade (%) | ||
| Grade I (perfect) | 13.9–84.6 | NR |
| Grade II (incontinence of flatus) | 7.7–36.6 | NR |
| Grade III (occasional minor soiling) | 3.8–38.6 | NR |
| Grade IV (frequent major soiling) | 0–27 | NR |
| Grade V (required colostomy) | 0–5.9 | 4.9 (NS) |
| Patient satisfaction (%) | ||
| Very low | 14–18 | |
| Medium | 11 | NR |
| Perfect (almost) | 71 | |
4. Discussion
4.1. Definition of intersphincteric resection
Schiessel et al. clearly defined the ISR technique, and classified the procedure into two types: subtotal ISR and total ISR.14 According to the clinical definition by a Japanese study group that included our institute, total‐ISR is defined as complete IAS removal at the intersphincteric groove (ISG); subtotal‐ISR is IAS removal between the DL and ISG, and partial‐ISR is defined as IAS removal at the DL (Fig. 1).57 ISR is a surgical procedure specializing in IAS removal followed by hand‐sewn CAA without mucosectomy. Partial‐ISR is defined as one‐third removal of the upper part of the IAS, subtotal‐ISR as two‐thirds removal of the IAS, and total‐ISR as complete removal of the IAS. ISR must be discriminated from conventional Parks’ CAA and stapler CAA.
Figure 1.
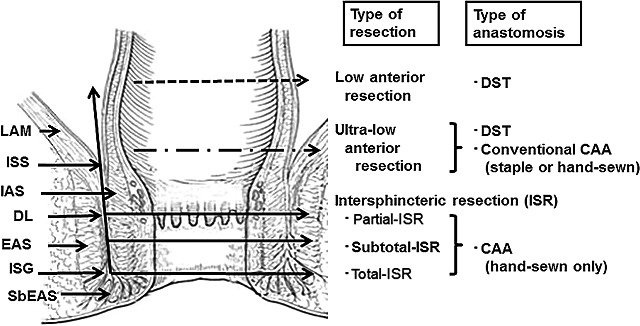
Definition of intersphincteric resection. The resection line of the rectum or anal canal varies depending on the location of the tumor from the anal verge. Total intersphincteric resection (total‐ISR) is defined as an internal sphincter resection at the intersphincteric groove (ISG), subtotal‐ISR is between the dentate line (DL) and ISG, and partial‐ISR is at the DL. CAA, coloanal anastomosis; DST, double stapling technique; EAS, external anal sphincter; IAS, internal anal sphincter; ISS, intersphincteric space; LAM, levator ani muscle; SbEAS, subcutaneous part of external anal sphincter.
4.2. Indication and preoperative evaluation
When planning treatment by ISR, careful patient selection is important. Indications for laparoscopic ISR do not differ from those for open surgery. Preoperative careful evaluation of patient and tumor should be carried out. Patients with severe preoperative complications including cardiac failure, liver cirrhosis, anal dysfunction, renal dysfunction, respiratory dysfunction, and psychiatric disease appear to not be suitable for ISR.
Many authors have reported that the oncological inclusion criteria are T1–T3 tumor showing well‐ to moderately differentiated adenocarcinoma. Oncological exclusion criteria include T4 tumor, fixed tumor, untreatable distant metastasis, and poorly differentiated adenocarcinoma. Digital examination is important for evaluating tumor mobility and for making a final surgical decision.24, 29, 31 Barium enema is shown in Figure 2. Anus preservation can be done by ISR or ESR technique for these rectal cancers.57 Also, estimating anal function by digital examination is useful,58 and comparable to manometry.59
Figure 2.
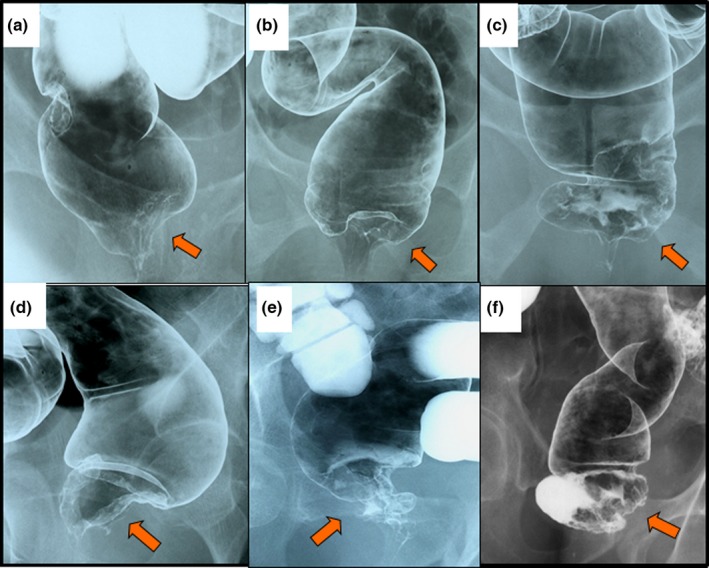
Barium enema of very low rectal cancers. Anus preservation can be carried out in patients with a very low rectal cancer by (a–c) intersphincteric resection or (d–f) external sphincter resection techniques. Arrow, location of rectal cancer.
4.3. Surgical margin
Correct evaluation of tumor invasion to the anal canal complex is essential to achieve both negative distal resection margin (DRM) and circumferential (radial) resection margin (CRM). In the 21st century, better understanding of the distal spread based on the pathological studies justified reduction of the DRM from 20 mm to 10 mm.7, 8 Neoadjuvant CRT enabled the DRM to be decreased to 5–10 mm.9, 10, 11 A DRM of 10 mm is thought to be safe and reasonable for anal preservation when ISR is applied for a very low rectal cancer closer to the anus.12
In addition, CRT is commonly used to avoid positive CRM and to decrease LR. The CRM is well known as a powerful indicator for LR,13 and the CRM around the anal canal is likely to represent a risk factor for LR when ISR is carried out. Computed tomography, magnetic resonance imaging (MRI), and digital examination are commonly used to evaluate tumor invasion to the anal canal complex. A MRI study has demonstrated no invasion to the EAS when the distance between the lower edge of the tumor and the DL is ≥2 cm.60 This study was supported by a histopathological investigation of whole‐mount sections.61 Moreover, Salerno et al. reported that MRI can predict invasion to the ISS.62 The utility of MRI has been emphasized for facilitating a successful operation with negative CRM.63, 64 In contrast, Dent et al. have reported that MRI cannot predict histological tumor involvement of CRM.65 The validity and reproducibility of the diagnosis require further investigation.65, 66
To avoid a risk of positive CRM, the ESR procedure may be suitable for a tumor with suspected invasion into the ISS and/or EAS.17, 49 The same strategy appears in Russian and Korean studies,44, 53 and the concept is supported by a histopathological investigation.61 However, the ESR showed a higher positive CRM rate (36.7%).37 Surgery alone seems to be difficult for achieving local control. Most authors agree that any tumor invading the EAS (T4 tumor) should be treated using chemoradiotherapy followed by APR.
4.4. Oncological outcomes
Local recurrence is a serious concern after ISR, and occurs in the pelvic cavity including at the anastomotic site. The rate of LR after ISR varies from 0% to 22.7%, lower than that after APR (10–57%) for mid or low rectal cancer.27, 31, 67 Neoadjuvant CRT affects the down‐sizing of tumor and down‐staging of disease, and is often used as a standard strategy to avoid a positive CRM and LR in rectal cancer patients.68, 69, 70, 71, 72 However, some questions remain as to whether neoadjuvant CRT should be more widely applied for patients who would undergo ISR. CRT is associated with higher surgical complications,68, 69 a negative impact on anal function,45, 70 and sexual disorder,71 and has no clear survival benefit.72 In Japan, preoperative neoadjuvant CRT has not been routinely carried out for resectable T1, T2 and T3 tumors regardless of the presence or absence of lymph node metastasis. Recently, Akagi et al. reported a low rate of LR (4.8%) without the use of neoadjuvant CRT.35 Disease‐free and overall 5‐year survival rates were excellent, with ranges of 68–86% and 76–97%, respectively.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 35 These results were consistent with those after APR or Parks’ CAA.29, 35, 58, 73 ISR seems to be oncologically acceptable, but ESR should be carefully selected because of worse survival compared to ISR.37
With regard to laparoscopic‐ISR, several surgeons reported that the surgical and oncological outcomes were equivalent to open surgery.38, 39, 40, 41, 42
However, the surgical techniques are not yet established, and regarded as more complex with difficulties in pelvic exposure, dissection, and sphincter preservation.
4.5. Functional outcomes
Anal dysfunction is one of the serious potential problems after ISR. However, data from laparoscopic‐ISR was not sufficient for estimation. Clinical assessment concerning stool frequency, fragmentation, urgency, soiling, and fecal incontinence varied widely in open‐ISR. Anal continence assessed by the Kirwan grade74 and the Wexner score75 appeared relatively good. Anorectal manometric examination may be useful for an objective assessment of anal function. Generally, maximum resting pressure (MRP) is mainly affected by the IAS and, in part, by the EAS.76 MRP gradually recovered over time after ISR,14, 15, 19 and anal function improved over time.23, 27 Some authors reported that colonic J‐pouch anastomosis offered superiority in bowel frequency, urgency control, tolerable volume, Wexner score, and fecal incontinence severity index (FISI)77 compared with the straight anastomosis.20, 43, 78 Moreover, the C‐pouch and smooth muscle plasty procedures improved anal function following ISR.30, 44 However, these procedures may be difficult in obese patients and/or in male patients with a narrow pelvis. Also, neoadjuvant CRT is an adverse factor for anal continence following ISR.28, 45 QoL such as physical, social and psychological aspects of a patient's life is likely to be affected by anal dysfunction.28, 79 QoL outcomes of ISR patients were relatively good based on the SF‐36, EORTC QLQ‐C30, and FIQL scales.20, 46, 48 However, further studies are required to evaluate the QoL.
5. Conclusion
Surgical and oncological outcomes after open‐ and laparoscopic‐ISR seem to be acceptable. The ISR technique seems to be a valid alternative to APR in selected patients with a very low rectal cancer. However, the necessity for ISR and expectations of QoL impairment as a result of functional disorder should be fully discussed with patients before surgery.
Conflicts of Interest
Authors declare no conflicts of interest for this article.
Supporting information
Video 1. Operative techniques of intersphincteric resection for lower rectal cancer, http://www.wiley.co.jp/journals/AGS1-1_Shirozu_Video1.html
Shirouzu K, Murakami N, Akagi Y. Intersphincteric resection for very low rectal cancer: A review of the updated literature. Ann Gastroenterol Surg. 2017;1:24–32. https://doi.org/10.1002/ags3.12003
Funding information
This study was not carried out under any commercial sponsorship or grant.
References
- 1. Miles WE. The present position of the radical abdomino‐perineal operation for cancer of the rectum in regard to mortality and post‐operative recurrence. Proc R Soc Med. 1931;24:989–91. [PMC free article] [PubMed] [Google Scholar]
- 2. Parks AG. Transanal technique in low rectal anastomosis. Proc R Soc Med. 1972;65:975–6. [PMC free article] [PubMed] [Google Scholar]
- 3. Heald RJ, Husband EM, Ryall RD. Mesorectum in rectal cancer surgery: the clue to pelvic recurrence. Br J Surg. 1982;69:613–6. [DOI] [PubMed] [Google Scholar]
- 4. Friedmann P, Park WC, Afonya II, et al. Adjuvant radiation therapy in colorectal carcinoma. Am J Surg. 1978;135:512–8. [DOI] [PubMed] [Google Scholar]
- 5. Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–11. [DOI] [PubMed] [Google Scholar]
- 6. Habr‐Gama A, de Souza PM, Ribeiro U Jr, et al. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41:1087–96. [DOI] [PubMed] [Google Scholar]
- 7. Shirouzu K, lsomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter‐preserving surgery. Cancer. 1995;76:388–92. [DOI] [PubMed] [Google Scholar]
- 8. Andreola S, Leo E, Belli F, et al. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40:25–9. [DOI] [PubMed] [Google Scholar]
- 9. Moore HG, Riedel E, Minsky BD, et al. Adequacy of 1‐cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined‐modality therapy. Ann Surg Oncol. 2003;10:80–5. [DOI] [PubMed] [Google Scholar]
- 10. Kuvshinoff B, Maghfoor I, Miedema B, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinoma: are < or = 1 cm distal margins sufficient? Ann Surg Oncol. 2001;8:163–9. [DOI] [PubMed] [Google Scholar]
- 11. Guillem JG, Chessin DB, Shia J, et al. A Prospective pathologic analysis using whole‐mount sections of rectal cancer following preoperative combined modality therapy: implications for sphincter preservation. Ann Surg. 2007;245:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1‐cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann Surg Oncol. 2012;19:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–12. [DOI] [PubMed] [Google Scholar]
- 14. Schiessel R, Karner‐Hanusch J, Herbst F, Teleky B, Wunderlich M. Intersphincteric resection for low rectal tumors. Br J Surg. 1994;81:1376–8. [DOI] [PubMed] [Google Scholar]
- 15. Rullier E, Zerbib F, Laurent C, et al. Intersphincteric resection with excision of internal anal sphincter for conservative treatment of very low rectal cancer. Dis Colon Rectum. 1999;42:1168–75. [DOI] [PubMed] [Google Scholar]
- 16. Renner K, Rosen H, Novi G, Hölbling N, Schiessel R. Quality of life after surgery for rectal cancer. Dis Colon Rectum. 1999;42:1160–7. [DOI] [PubMed] [Google Scholar]
- 17. Shirouzu K, Ogata Y, Araki Y, Kishimoto Y, Sato Y. A new ultimate anus‐preserving operation for extremely low rectal cancer and for anal canal cancer. Tech Coloproctol. 2003;7:203–6. [DOI] [PubMed] [Google Scholar]
- 18. Matzel KE, Bittorf B, Günther K, Stadelmaier U, Hohenberger W. Rectal resection with low anastomosis: functional outcome. Colorectal Dis. 2003;5:458–64. [DOI] [PubMed] [Google Scholar]
- 19. Saito N, Ono M, Sugito M, et al. Early results of intersphincteric resection for patients with very low rectal cancer: an active approach to avoid a permanent colostomy. Dis Colon Rectum. 2004;47:459–66. [DOI] [PubMed] [Google Scholar]
- 20. Bretagnol F, Rullier E, Laurent C, Zerbib F, Gontier R, Saric J. Comparison of functional results and quality of life between intersphincteric resection and conventional coloanal anastomosis for low rectal cancer. Dis Colon Rectum. 2004;47:832–8. [DOI] [PubMed] [Google Scholar]
- 21. Köhler A, Athanasiadis S, Ommer A, Psarakis E. Long‐term results of low anterior resection with intersphincteric anastomosis in carcinoma of the lower one‐third of the rectum. Dis Colon Rectum. 2000;43:843–50. [DOI] [PubMed] [Google Scholar]
- 22. Vorobiev GI, Odaryuk TS, Tsarkov PV, Talalakin AI, Rybakov EG. Resection of the rectum and total excision of the internal anal sphincter with smooth muscle plasty and colonic pouch for treatment of ultralow rectal carcinoma. Br J Surg. 2004;91:1506–12. [DOI] [PubMed] [Google Scholar]
- 23. Schiessel R, Novi G, Holzer B, et al. Technique and long‐term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum. 2005;48:1858–65. [DOI] [PubMed] [Google Scholar]
- 24. Rullier E, Laurent C, Bretagnol F, Rullier A, Vendrely V, Zerbib F. Sphincter‐saving resection for all rectal carcinomas: the end of the 2 cm distal rule. Ann Surg. 2005;41:465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hohenberger W, Merkel S, Matzel K, Bittorf B, Papadopoulos T, Gohl J. The influence of abdomino‐perianal (intersphincteric) resection of lower third rectal carcinoma on the rates of sphincter preservation and locoregional recurrence. Colorectal Dis. 2006;8:23–33. [DOI] [PubMed] [Google Scholar]
- 26. Chin CC, Yeh CY, Huang WS, Wang JY. Clinical outcome of intersphincteric resection for ultra‐low rectal cancer. World J Gastroenterol. 2006;12:640–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saito N, Moriya Y, Shirouzu K, et al. Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience. Dis Colon Rectum. 2006;49:S13–22. [DOI] [PubMed] [Google Scholar]
- 28. Chamlou R, Parc Y, Simon T, et al. Long‐term results of intersphincteric resection for low rectal cancer. Ann Surg. 2007;246:916–21. [DOI] [PubMed] [Google Scholar]
- 29. Portier G, Ghouti L, Kirzin S, Guimbaud R, Rives M, Lazorthes F. Oncological outcome of ultra‐low coloanal anastomosis with and without intersphincteric resection for low rectal adenocarcinoma. Br J Surg. 2007;94:341–5. [DOI] [PubMed] [Google Scholar]
- 30. Krand O, Yalti T, Tellioglu G, Kara M, Berber I, Titiz MI. Use of smooth muscle plasty after intersphincteric rectal resection to replace a partially resected internal anal sphincter: long‐term follow‐up. Dis Colon Rectum. 2009;52:1895–901. [DOI] [PubMed] [Google Scholar]
- 31. Han JG, Wei GH, Gao ZG, Zheng Y, Wang ZJ. Intersphincteric resection with direct coloanal anastomosis for ultralow rectal cancer: the experience of People's Republic of China. Dis Colon Rectum. 2009;52:950–7. [DOI] [PubMed] [Google Scholar]
- 32. Weiser MR, Quah HM, Shia J, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236–42. [DOI] [PubMed] [Google Scholar]
- 33. Kuo LJ, Hung CS, Wu CH, et al. Oncological and functional outcome of intersphincteric resection for low rectal cancer. J Surg Res. 2011;170:e93–8. [DOI] [PubMed] [Google Scholar]
- 34. Gong X, Jin Z, Zheng Q. Anorectal function after partial intersphincteric resection in ultra‐low rectal cancer. Colorectal Dis. 2012;14:e802–6. [DOI] [PubMed] [Google Scholar]
- 35. Akagi Y, Shirouzu K, Ogata Y, Kinugasa T. Oncologic outcomes of intersphincteric resection without preoperative chemoradiotherapy for very low rectal cancer. Surg Oncol. 2013;22:144–9. [DOI] [PubMed] [Google Scholar]
- 36. Tokoro T, Okuno K, Hida J, et al. Analysis of the factors associated with anal function after intersphincteric resection for very low rectal cancer. World J Surg Oncol. 2013;11:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saito N, Ito M, Kobayashi A. Nishizawa Yusuke, Kojima M, Nishizawa Yuji, Sugito M. Long‐term outcomes after intersphincteric resection for low‐lying rectal cancer. Ann Surg Oncol. 2014;21:3608–15. [DOI] [PubMed] [Google Scholar]
- 38. Rullier E, Sa‐Cunha A, Couderc P, Gontier R, Saric J. Laparoscopic intersphincteric resection with coloplasty and coloanal anastomosis for mid and low rectal cancer. Br J Surg. 2003;90:445–51. [DOI] [PubMed] [Google Scholar]
- 39. Park JS, Choi GS, Jun SH, Hasegawa S, Sakai Y. Laparoscopic versus open intersphincteric resection and coloanal anastomosis for low rectal cancer; Intermediate‐term oncologic outcomes. Ann Surg. 2011;254:941–6. [DOI] [PubMed] [Google Scholar]
- 40. Laurent C, Paumet T, Leblanc F, Denost Q, Rullier E. Intersphincteric resection for low rectal cancer: laparoscopic vs open approach. Colorectal Dis. 2011;14:35–43. [DOI] [PubMed] [Google Scholar]
- 41. Kuo LJ, Hung CS, Wang W, et al. Intersphincteric resection for very low rectal cancer: clinical outcomes of open versus laparoscopic approach and multidimensional analysis of the learning curve for laparoscopic surgery. J Surg Res. 2013;183:524–30. [DOI] [PubMed] [Google Scholar]
- 42. Kanso F, Maggiori L, Debove C, Chau A, Ferron M, Panis Y. Perineal or abdominal approach first during intersphincteric resection for low rectal cancer: which is the best strategy? Dis Colon Rectum. 2015;58:637–44. [DOI] [PubMed] [Google Scholar]
- 43. Tilney HS, Tekkis PP. Extending the horizons of restorative rectal surgery: intersphincteric resection for low rectal cancer. Colorectal Dis. 2008;10:3–15. [DOI] [PubMed] [Google Scholar]
- 44. Shelygin YA, Vorobiev GI, Pikunov DY, Markova EV, Djhanaev YA, Fomenko OY. Intersphincteric resection with partial removal of external anal sphincter for low rectal cancer. Acta Chir Iugosl. 2008;55:45–53. [DOI] [PubMed] [Google Scholar]
- 45. Ito M, Saito N, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis Colon Rectum. 2009;52:64–70. [DOI] [PubMed] [Google Scholar]
- 46. Barisic G, Markovic V, Popovic M, Dimitrijevic I, Gavrilovic P, Krivokapic Z. Function after intersphincteric resection for low rectal cancer and its influence on quality of life. Colorectal Dis. 2011;13:638–43. [DOI] [PubMed] [Google Scholar]
- 47. Denost Q, Laurent C, Capdepont M, Zerbib F, Rullier E. Risk factors for fecal incontinence after intersphincteric resection for rectal cancer. Dis Colon Rectum. 2011;54:963–8. [DOI] [PubMed] [Google Scholar]
- 48. Hashimoto H, Shiokawa H, Funahashi K, et al. Development and validation of a modified fecal incontinence quality of life scale for Japanese patients after intersphincteric resection for very low rectal cancer. J Gastroenterol. 2010;45:928–35. [DOI] [PubMed] [Google Scholar]
- 49. Shirouzu K, Ogata Y. Oncologic and functional outcomes of external sphincter resection In: Schiessel R, Metzger P, editors. Intersphincteric resection for low rectal tumors. New York: Springer‐Verlag/Wien, 2012; p. 121–9. [Google Scholar]
- 50. Lee SY, Jo JS, Kim HJ, Kim CH, Kim YJ, Kim HR. Prognostic factors for low rectal cancer patients undergoing intersphincteric resection after neoadjuvant chemoradiation. J Surg Oncol. 2015;111:1054–8. [DOI] [PubMed] [Google Scholar]
- 51. Kim CH, Lee SY, Kim HR, Kim YJ. Factors associated with oncologic outcomes following abdominoperineal or intersphincteric resection in patients treated with preoperative chemotherapy. A propensity score analysis. Medicine. 2015;94:e2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saito N, Sugito M, Ito M, et al. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg. 2009;33:1750–6. [DOI] [PubMed] [Google Scholar]
- 53. Kim HS, Ko S, Oh NG. Long‐term results of extended intersphincteric resection for very low rectal cancer: a retrospective study. BMC Surg. 2016;16:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ware JE Jr, Sherboume CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 55. Rockwood TH, Church JM, Fleshman JW, et al. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16. discussion ‐7. [DOI] [PubMed] [Google Scholar]
- 56. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- 57. Akagi Y, Kinugasa T, Shirouzu K. Intersphincteric resection for very low rectal cancer: a systematic review. Surg Today. 2013;43:838–47. [DOI] [PubMed] [Google Scholar]
- 58. Orkin BA, Sinykin SB, Lloyd PC. The digital examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656–60. [DOI] [PubMed] [Google Scholar]
- 59. Dobben AC, Terra MP, Deutekom M, et al. Anal inspection and digital rectal examination compared to anorectal physiology tests and endoanal ultrasonography in evaluating fecal incontinence. Int J Colorectal Dis. 2007;22:783–90. [DOI] [PubMed] [Google Scholar]
- 60. Holzer B, Urban M, Hölbling N, et al. Magnetic resonance imaging predicts sphincter invasion of low rectal cancer and influences selection of operation. Surgery. 2003;133:656–61. [DOI] [PubMed] [Google Scholar]
- 61. Shirouzu K, Ogata Y. Histopathologic tumor spread in very low rectal cancer treated with abdominoperineal resection. Dis Colon Rectum. 2009;52:1887–94. [DOI] [PubMed] [Google Scholar]
- 62. Salerno GV, Daniels IR, Moran BJ, Heald RJ, Thomas K, Brown G. Magnetic resonance imaging prediction of an involved surgical resection margin in low rectal cancer. Dis Colon Rectum. 2009;52:632–9. [DOI] [PubMed] [Google Scholar]
- 63. Urban M, Rosen HR, Hölbling N, et al. MR imaging for the preoperative planning of sphincter‐saving surgery for tumors of the lower third of the rectum: use of intravenous and endorectal contrast materials. Radiology. 2000;214:503–8. [DOI] [PubMed] [Google Scholar]
- 64. Beets‐Tan RG, Lettinga T, Beets GL. Pre‐operative imaging of rectal cancer and its impact on surgical performance and treatment outcome. Eur J Surg Oncol. 2005;31:681–8. [DOI] [PubMed] [Google Scholar]
- 65. Dent OF, Chapuis PH, Haboubi N, Bokey L. Magnetic resonance imaging cannot predict histological tumour involvement of a circumferential surgical margin in rectal cancer. Colorectal Dis. 2011;13:974–81. [DOI] [PubMed] [Google Scholar]
- 66. Bamba Y, Itabashi M, Kameoka S. Preoperative evaluation of the depth of anal canal invasion in very low rectal cancer by magnetic resonance imaging and surgical indications for intersphincteric resection. Surg Today. 2012;42:328–33. [DOI] [PubMed] [Google Scholar]
- 67. Rullier E, Laurent C, Carles J, Saric J, Michel P, Parneix M. Local recurrence of low rectal cancer after abdominoperineal and anterior resection. Br J Surg. 1997;84:525–8. [PubMed] [Google Scholar]
- 68. Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. for the Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. [DOI] [PubMed] [Google Scholar]
- 69. Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: a meta‐analysis. JAMA. 2000;284:1008–15. [DOI] [PubMed] [Google Scholar]
- 70. Hassan I, Larson DW, Wolff BG, et al. Impact of pelvic radiotherapy on morbidity and durability of sphincter preservation after coloanal anastomosis for rectal cancers. Dis Colon Rectum. 2008;51:32–7. [DOI] [PubMed] [Google Scholar]
- 71. Bonnel C, Parc YR, Pocard M, et al. Effects of preoperative radiotherapy for primary resectable rectal adenocarcinoma on male sexual and urinary function. Dis Colon Rectum. 2002;45:934–9. [DOI] [PubMed] [Google Scholar]
- 72. Peeters KC, Marijnen CA, Nagtegaal ID, et al. Dutch Colorectal Cancer Group. The TME trial after a median follow‐up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. [DOI] [PubMed] [Google Scholar]
- 73. Nagamatsu Y, Shirouzu K, Isomoto H, Ogata Y, Tsuchida I, Akagi Y. Surgical treatment of lower rectal cancer with sphincter preservation using handsewn coloanal anastomosis. Surg Today. 1998;28:696–700. [DOI] [PubMed] [Google Scholar]
- 74. Kirwan WO, Turnbull RB Jr, Fazio VW, Weakley FL. Pullthrough operation with delayed anastomosis for rectal cancer. Br J Surg. 1978;65:695–8. [DOI] [PubMed] [Google Scholar]
- 75. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–9. [DOI] [PubMed] [Google Scholar]
- 76. Beersiek F, Parks AG, Swash M. Pathogenesis of ano‐rectal incontinence. A histometric study of the anal sphincter musculature. J Neurol Sci. 1979;42:111–27. [DOI] [PubMed] [Google Scholar]
- 77. Rockwood TH, Church JM, Fleshman JW, et al. Patients and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–32. [DOI] [PubMed] [Google Scholar]
- 78. Hallböök O, Påhlman L, Krog M, Wexner SD, Sjödahl R. Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Ann Surg. 1996;224:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vironen JH, Kairaluoma M, Aalto AM, Kellokumpu IH. Impact of functional results on quality of life after rectal surgery. Dis Colon Rectum. 2006;49:568–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Operative techniques of intersphincteric resection for lower rectal cancer, http://www.wiley.co.jp/journals/AGS1-1_Shirozu_Video1.html


