Abstract
Background
The fecal occult blood test (FOBT) is widely accepted as the most economic and non‐invasive screening method for colorectal cancer (CRC). However, the FOBT is inconvenient because it requires a fecal sample and shows limited accuracy. Alternatively, we hypothesized that fecal gas compounds from bowel movements may be a non‐invasive biomarker for CRC.
Methods
Gas compounds were collected from the bowel movements of 30 patients with CRC and from 26 healthy controls. The patient group comprised 17 males and 13 females, and the average age was 68 years. Additionally, 22 patients had colon cancer, and eight patients had rectal cancer. Gas compounds were analyzed using gas chromatography and compared with those from healthy controls.
Results
In the gas analysis, methyl mercaptan was significantly higher in the CRC group than in the control group. Hydrogen was significantly lower in the CRC group than in the control group and was correlated with tumor depth and advanced disease stage. Sensitivity, specificity, and accuracy of detection by a discriminant formula were 90%, 57.7%, and 75%, respectively.
Conclusion
Gas compounds from defecation constitute a promising, novel non‐invasive approach for CRC screening. (UMIN000028256)
Keywords: accuracy, colorectal cancer, diagnosis, gas
Short abstract
This study analyzed the gas compounds of colorectal cancer (CRC) patients and healthy controls at defecation in toilet using an extremely non‐invasive technique. The volatile organic compounds from defecation are different compared with CRC patients and healthy controls. The sensitivity, specificity, and accuracy of the discriminant formula for colorectal cancer were 90%, 57.7%, and 75%, respectively. We concluded that gas compounds from defecation constitute a promising, novel non‐invasive approach for CRC screening.
1. INTRODUCTION
Colorectal cancer (CRC) is the second most common cancer in both genders in Western countries, with more than 70% of such cases involving patients over 65 years of age.1 In Japan, CRC was ranked the second and fourth most common type of cancer in 2015 in women and men, respectively.2
The fecal occult blood test (FOBT) is widely accepted as the most economic and non‐invasive screening method for CRC and, because many patients with positive FOBT results subsequently undergo a colonoscopy, there is a reduced incidence and mortality of CRC.3, 4, 5 Moreover, the sensitivity of the FOBT for CRC is high, ranging from 73.3% to 88.9%.6, 7 However, the FOBT detects blood in the stool that is not always associated with CRC, with the positive predictive value (PPV) of the FOBT for CRC being 10.6%‐12.0%.8, 9 Therefore, an easier, more convenient and more effective method for CRC screening must be developed.
Recently, several authors reported a relationship between cancer and odor gas components. Seaman et al reported that odorous gas dimethyl trisulfide is associated with fungating wounds in skin and breast cancer,10 and several authors reported that detection dogs can detect skin cancer.11, 12 The first analysis of volatile organic compounds (VOC) was reported in 1971 by Pauling et al,13 who identified several hundred VOC in human breath and urine by using gas chromatography‐mass spectrometry. Over the course of a quarter‐century, several authors reported the association of VOC with lung cancer,14 breast cancer,15 skin cancer,16 and prostate cancer.17 Regarding CRC, several studies have shown the reliability of VOC in detecting CRC in different substances, including urine,18, 19, 20 exhaled breath,21, 22 blood,23 and feces.24, 25 However, sample collection was inconvenient in these studies. Therefore, the development of a more convenient method is necessary.
In the present study, we examined gas components in bowel movements from patients with CRC using a novel method and evaluated the diagnostic value of this method for CRC.
2. METHODS
2.1. Patients
This was a prospective observational study at Yokohama City University Hospital and Yokohama City University Medical Center. Inclusion criteria were age 20 years or older, histologically proven adenocarcinoma, any clinical stage, and elective operation for CRC. Exclusion criteria were concurrent malignancy in another organ, inflammatory bowel disease, infectious enteritis, bowel obstruction or the inability to tolerate surgery under general anesthesia. A total of 30 patients with CRC who were scheduled for surgery were enrolled in this study from July 2014 to April 2015. Gas samples were collected from the patients before starting preoperative bowel preparation. Twenty‐six healthy adult volunteers were enrolled as a control group.
This study was approved by the Yokohama City University Ethics Committee and was conducted in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects (IRB number B140703028), and all patients provided informed consent before any study procedure was carried out. This study is registered on the University Hospital Medical Information Network (UMIN) Center (UMIN000028256).
2.2. Gas analysis
2.2.1. Apparatus for gas sampling
As shown in Figure 1, a gas sampling apparatus was placed in a toilet in the hospital ward. Defecation gas samples from patients were collected from the toilet into a 25‐L sampling bag (500 mm × 700 mm). The sampling bag was made of fluorinated vinyl resin (Tedlar bag, DuPont, Wilmington, WA, USA), and a Teflon flexible pipe was connected to a fan and the Tedlar bag. A commercial gas sensor (semiconductor gas sensor, TGS2600; FIGARO Engineering, Osaka, Japan) was arranged at the rear of the fan, and a stopper was used at the junction between the Tedlar bag and Teflon flexible pipe.
Figure 1.
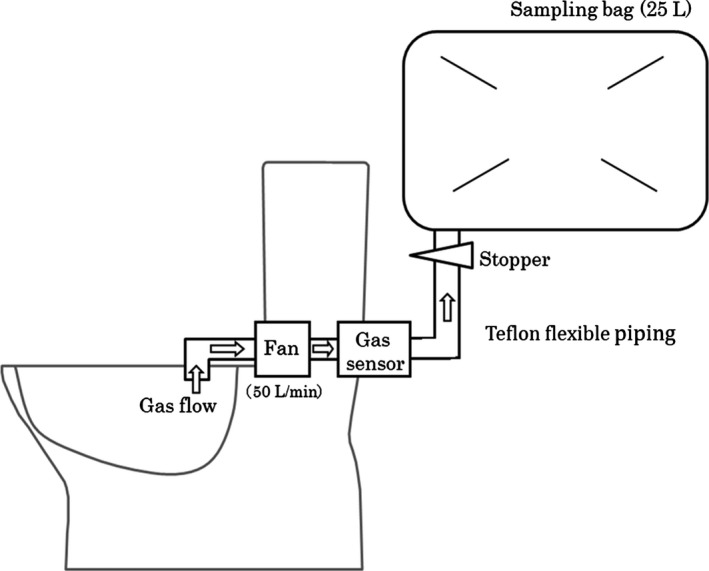
Gas sampling system
2.2.2. Collecting gas components during defecation
Gas components were collected during defecation as follows: (i) Air in the toilet was aspirated with a fan (50 L/min) and passed through a commercial gas sensor. (ii) The stopper automatically opened immediately after the gas sensor detected gas components. (iii) The stopper closed after 30 seconds. (iv) The Tedlar bag was sealed with a large clip. To measure background gas, air in the toilet was aspirated when the toilet was not in use.
2.2.3. Measurement of methyl mercaptan and hydrogen sulfide concentrations
In the defecation gas samples, we measured the content of sulfur‐containing compounds methyl mercaptan (MM) and hydrogen sulfide (H2S). Volatile sulfur‐containing compounds were analyzed by gas chromatography using a Sulfur Chemiluminescence Detector (GC/SCD) (Agilent Technologies, Santa Clara, CA, USA.). Briefly, gases collected in the Tedlar bag were aspirated into a cold‐trapping tube, injected into the gas chromatography system by an Entech 7100A Preconcentrator (Entech Instruments Inc., Simi Valley, CA, USA) and then analyzed.
2.2.4. Measurement of hydrogen, carbon dioxide, and methane concentrations
Hydrogen (H2), methane (CH4) and carbon dioxide (CO2) were analyzed by gas chromatography using a thermal conductivity detector (GC/TCD) (Agilent 490 Micro GC; Agilent Technologies). Then, gases collected in the Tedlar bag were injected into the gas chromatography system and analyzed.
2.2.5. Calculation of gas amounts during defecation
Concentrations of background gases were ignored because the concentration of background gas (except for CO2) was sufficiently lower than the concentration of gas during defecation. The amount of gas during defecation was calculated by multiplying the gas concentration and the aspirated gas volume.
2.3. Statistical analysis
When samples were recorded more than once, mean and standard deviation (SD) were calculated and used for subsequent analyses. Patient characteristics are expressed as numbers and percentages or means ± SD as appropriate. A t test was used to evaluate the significance of the differences in continuous variables. Using a multivariate logistic regression model, we developed a formula to discriminate between CRC patients and healthy volunteers by using gas data from study participants. A formula was calculated by discriminant analysis using factors that had a significant difference by multivariate analysis. All analyses were carried out using IBM SPSS, version 21 (SPSS Inc., Chicago, IL, USA) and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
Characteristics of the CRC group are shown in Table 1, and 17 males and 13 females were included, with the average age being 68 years. Twenty‐two patients had colon cancer, and eight patients had rectal cancer. Although the most common tumor site was the rectum (n = 8), other primary tumors were located in the sigmoid colon (n = 7), cecum (n = 6), rectosigmoid colon (n = 5), transverse colon (n = 2), ascending colon (n = 1), and descending colon (n = 1). Mean tumor size was 3.8 cm. There were nine cases of stage I disease, 12 stage II disease, six stage III disease, and three stage IV disease. The control group included 22 males and four females, and the mean age was 37 years.
Table 1.
Characteristics of colorectal cancer patients
| Factor | N = 30 (%) | |
|---|---|---|
| Gender | Male | 17 (56.7) |
| Female | 13 (43.3) | |
| Age (years) | mean ± SD | 68 ± 11 |
| Tumor location | Cecum | 6 (20) |
| Ascending | 1 (3.3) | |
| Transverse | 2 (6.7) | |
| Descending | 1 (3.3) | |
| Sigmoid | 7 (23.3) | |
| Rectosigmoid | 5 (16.7) | |
| Rectum | 8 (26.7) | |
| Tumor size (mm) | Mean ± SD | 38 ± 24 |
| Tumor depth | T1 | 7 (23.3) |
| T2 | 4 (13.4) | |
| T3 | 14 (46.6) | |
| T4 | 5 (16.7) | |
| LN involvement | N0 | 21 (70) |
| N1 | 7 (23.3) | |
| N2 | 2 (6.7) | |
| Stage (UICC) | I | 9 (30) |
| II | 12 (40) | |
| III | 6 (20) | |
| IV | 3 (10) |
LN, lymph node; UICC, Union for International Cancer Control.
3.2. Gas compound measurements obtained from the sampling apparatus
For both groups, gas compound measurements obtained from the sampling apparatus are shown in Figure 2. Mean amount of MM in the defecation gas was 4.25 μg in the CRC group and 1.62 μg in the control group. The amount of MM was significantly higher in the CRC group than in the control group (P = .036). However, although the mean amount of H2S was 12.36 μg in the CRC group and 4.74 μg in the control group, there was no significant difference between the groups (P = .163). The mean amount of H2 was 0.49 mg in the CRC group and 1.55 mg in the control group, resulting in a significantly lower amount of H2 in the CRC than in the control group (P = .008). Mean amounts of CH4 and CO2 were not significantly different between the groups.
Figure 2.
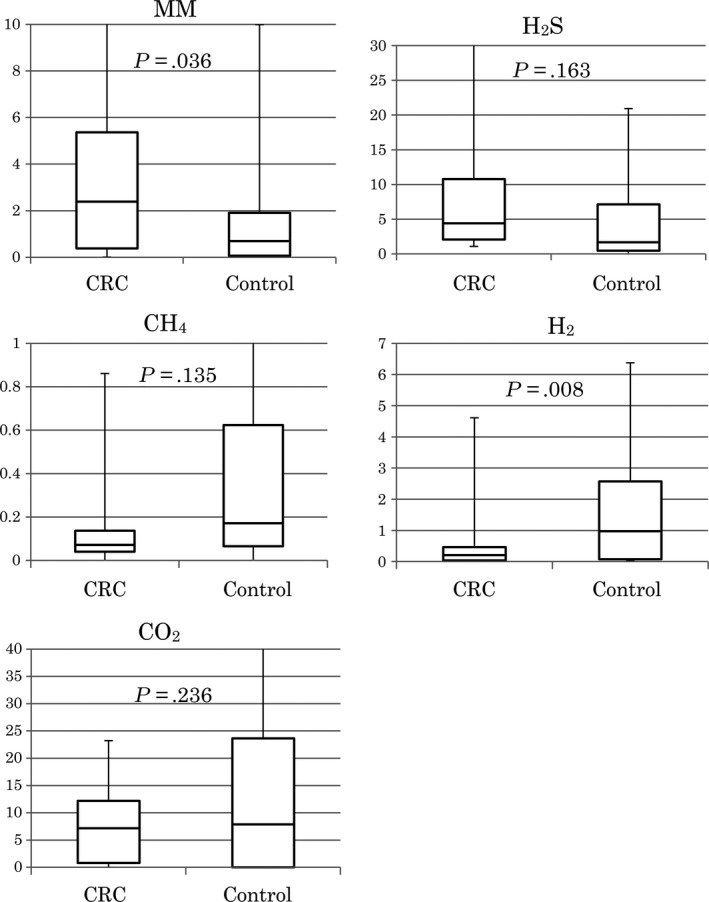
Comparison between gas compounds in the CRC and control groups (box plot). CH4, methane; CO2, carbon dioxide; CRC, colorectal cancer; H2, hydrogen; H2S, hydrogen sulfide; MM, methyl mercaptan
The control group was younger than the CRC group. To consider the effect of age, we compared the findings from the younger and older healthy controls and found that there were no significant differences in the amount of any gas compound between the two groups (data not shown).
3.3. Correlations between gas compound amounts and clinicopathological status of CRC patients
Correlations between gas compound amounts and clinicopathological status of CRC patients are shown in Table 2. The amount of H2 was significantly lower in patients with T3/4 tumors and advanced‐stage disease than that in other CRC patients. For the other gas compounds, no significant correlations were observed with the clinicopathological status of CRC patients.
Table 2.
Correlations between gas compound amounts and clinicopathological status of CRC patients
| Factor | N | MM (μg) | H2S (μg) | CH4 (mg) | CO2 (mg) | H2 (mg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | P‐value | Mean (SD) | P‐value | Mean (SD) | P‐value | Mean (SD) | P‐value | Mean (SD) | P‐value | ||
| Gender | |||||||||||
| M | 17 | 5.66 (7.32) | .134 | 17.75 (34.96) | .214 | 0.67 (1.33) | .134 | 9.19 (7.96) | .703 | 0.26 (0.34) | .144 |
| F | 13 | 2.41 (2.18) | 5.30 (4.42) | 0.10 (0.09) | 10.33 (8.13) | 0.80 (1.44) | |||||
| Location | |||||||||||
| Colon | 22 | 3.14 (3.64) | .113 | 7.65 (8.59) | .113 | 0.51 (1.19) | .485 | 8.75 (7.66) | .296 | 0.44 (0.97) | .664 |
| Rectum | 8 | 7.28 (9.35) | 25.3 (50.0) | 0.18 (0.22) | 12.2 (8.54) | 0.63 (1.11) | |||||
| CEA (mg/dL) | |||||||||||
| <5 | 22 | 4.51 (6.58) | .695 | 13.62 (31.14) | .678 | 0.53 (1.19) | .338 | 11.24 (8.16) | .073 | 0.55 (1.14) | .587 |
| ≥5 | 8 | 3.54 (3.29) | 8.89 (7.99) | 0.12 (0.18) | 5.40 (5.56) | 0.33 (0.40) | |||||
| Tumor size (mm) | |||||||||||
| <40 | 15 | 4.24 (7.36) | .991 | 14.92 (37.37) | .610 | 0.20 (0.25) | .239 | 10.06 (7.61) | .799 | 0.59 (1.15) | .611 |
| ≥40 | 15 | 4.26 (4.08) | 9.79 (9.23) | 0.64 (1.42) | 9.30 (8.46) | 0.40 (0.84) | |||||
| Tumor depth (mm) | |||||||||||
| <T3 | 11 | 3.87 (8.32) | .793 | 17.5 (43.6) | .426 | 0.23 (0.29) | .437 | 13.2 (7.83) | .059 | 0.99 (1.51) | .035 |
| ≥T3 | 19 | 4.46 (4.06) | 9.32 (8.73) | 0.53 (1.27) | 7.62 (7.38) | 0.21 (0.29) | |||||
| Lymph node metastasis | |||||||||||
| N | 21 | 4.44 (6.43) | .79 | 14.7 (31.7) | .776 | 0.56 (1.21) | .259 | 11.4 (8.10) | .66 | 0.62 (1.16) | .215 |
| N+ | 9 | 3.80 (4.49) | 6.70 (6.81) | 0.09 (0.07) | 5.64 (6.06) | 0.14 (0.16) | |||||
| UICC Stage | |||||||||||
| 0‐I | 9 | 4.66 (9.09) | .802 | 21.3 (47.9) | .236 | 0.25 (0.31) | .562 | 13.1 (8.38) | .119 | 1.12 (1.65) | .020 |
| II‐IV | 21 | 4.07 (4.05) | 8.49 (8.69) | 0.49 (1.21) | 8.20 (7.41) | 0.22 (0.28) | |||||
CEA, carcinoembryonic antigen; CH4, methane; CO2, carbon dioxide; CRC, colorectal cancer; H2, hydrogen; H2S, hydrogen sulfide; MM, methyl mercaptan; UICC, Union for International Cancer Control.
3.4. Logistic regression analysis and discriminant analysis
Multivariate logistic regression analysis was carried out to identify CRC patients (Table 3), and a total of 56 data sets (30 CRC patients and 26 controls) were used. When there were two or more samples, an average value was used for logistic regression analysis.
Table 3.
Multivariate logistic regression analysis
| Factor | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| MM | 1.224 | 0.978‐1.532 | .078 |
| H2 | 0.557 | 0.325‐0.952 | .032 |
H2, hydrogen; MM, methyl mercaptan.
The discriminant formula is as follows:
(positive: more than 0)
The receiver operating characteristic (ROC) curve of the discriminant formula is shown in Figure 3. Sensitivity, specificity, negative predictive value (NPV), PPV, and accuracy of the discriminant formula were 90%, 57.7%, 83.3%, 75%, and 75%, respectively.
Figure 3.
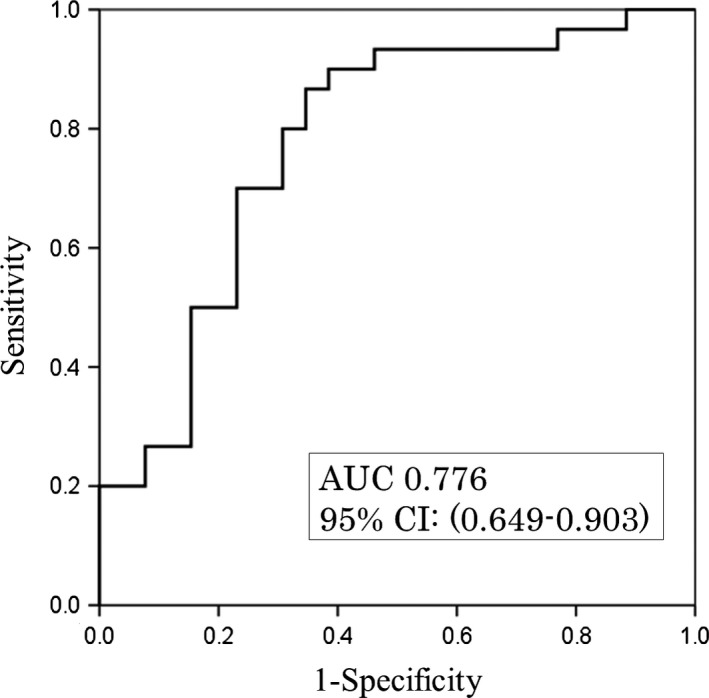
Receiver operating characteristic curve of the discriminant formula
4. DISCUSSION
To our knowledge, our study is the first to analyze fecal gas compounds from CRC patients using an extremely non‐invasive technique. Our data show that the amount of gas compounds obtained during defecation was significantly different between CRC patients and controls.
The first analysis of VOC was reported in 1971 by Pauling et al,13 who identified several hundred VOC in human breath and urine by using gas chromatography‐mass spectrometry. Moreover, several authors have shown the usefulness of VOC for detecting CRC in different substances. These studies showed fair reliability, with sensitivities ranging from 30% to 94% and specificities ranging from 60% to 94%. In our study, the sensitivity was 90%, the specificity was 57.7%, and the accuracy was 75%. In contrast, the sensitivity and specificity of FOBT in previous studies were 73.3%–88.9% and 95.8%–97.6%.6, 7, 26 However, the analysis of VOC is still not as useful as FOBT in CRC screening.
In the present study, MM concentration was higher in the CRC group than in the control group. MM is a sulfur‐containing gas, and Yamagishi et al showed that sulfur‐containing gas can be produced by reacting sulfur‐containing amino acids with glucose or lactic acid. Thus, it can be presumed that an accumulation of lactic acid and glucose results in the production of sulfur‐containing gas in tumor tissues.27 It is well known that lactic acid is produced by cancer cells by the Warburg effect.28 Another hypothesis for the mechanism of increasing sulfur‐containing gas is related to colonic flora. Recently, Fusobacterium in the colonic flora was implicated in periodontitis, inflammatory bowel disease and CRC.29 By genomic analysis, Kostic et al showed that Fusobacterium sequences were enriched in colorectal carcinoma.30 Nakano et al31 reported that Fusobacterium nucleatum is one of the most potent producers of MM from l‐methionine by l‐methionine‐α‐deamino‐γ‐mercaptomethane‐lyase.
In the present study, amounts of CH4 were not significantly different between the groups. CH4 is produced by methanogen, which is common in wetlands, where it is responsible for marsh gas, and in the digestive tracts of animals such as ruminants and humans. A previous study32 showed that patients with CRC produced significantly more CH4 than healthy controls. However, other studies could not confirm an association between CH4 and colon cancer by breath methane analysis33 or animal experiments.32 Moreover, although the mechanism involved in the increased CH4 in CRC is unclear, tumors may increase CH4 by obstructing the bowel.32
We analyzed the concentrations of H2 and CO2 relative to the pH of the intestinal environment. In the CRC group, the amount of H2 was significantly lower and the concentration of CO2 was lower (not significant) than in the control group. Ohigashi et al reported that CRC patients had different intestinal environments, including alterations in microbial flora, decreased short‐chain fatty acids, and increased pH. They also showed that organic acids, particularly acetic, propionic, butyric and valeric acids, were decreased in the CRC group.35 Carbohydrates in the intestine are fermented by bacteria to form organic acids and gases including CO2 and H2. When organic acids decrease, the pH increases, and the concentration of CO2 decreases.36 Therefore, we speculated that the concentrations of H2 and CO2 were lower in the CRC group.
The FOBT is now a standard screening method for CRC.3, 4, 5 However, our method of collecting gas compounds during defecation is more convenient because stool collection is not necessary. Nevertheless, cost‐effectiveness is one of the most important factors for medical check‐up, and although the cost of gas compound analysis by gas chromatography is expensive, approximately 30 000 yen per analysis, FOBT costs approximately 2000 yen. However, if toilets are equipped with a gas sensor in the future, gas compounds can be detected without using gas chromatography. This will allow more people to be routinely screened, and early detection of CRC may increase.
The first limitation of the present study was its small sample size and, as this was a cross‐sectional study of a CRC group and a control group, the second limitation was that the predictive value of the gas compound for screening an unselected population is not yet known. Moreover, we could not compare data from the gas compound analysis and FOBT because we did not have data from FOBT of patients and healthy volunteers. The third limitation was that the reason for the differences in gas compounds between CRC patients and healthy controls was unclear. Gas composition is probably affected by meals and, unfortunately, information regarding the food of CRC patients and controls was not accessible. Thus, further study of the association between consumed food, colonic flora and gas compound production is warranted. Furthermore, a large‐scale trial comparing our method with the FOBT, which is a standard screening method, is necessary to confirm the usefulness of our method for CRC screening.
In conclusion, our study showed that analyzing gas compounds from defecation might provide a complete and non‐invasive metabolomics biomarker profile that could be used as a diagnostic tool.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Ishibe A, Ota M, Takeshita A, et al. Detection of gas components as a novel diagnostic method for colorectal cancer. Ann Gastroenterol Surg. 2018;2:147–153. https://doi.org/10.1002/ags3.12056
REFERENCES
- 1. Group CCC . Surgery for colorectal cancer in elderly patients: a systematic review. Lancet. 2000;356:968–74. [PubMed] [Google Scholar]
- 2. Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan.
- 3. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. [DOI] [PubMed] [Google Scholar]
- 4. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal‐occult‐blood test. Lancet. 1996;348:1467–71. [DOI] [PubMed] [Google Scholar]
- 5. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal‐occult‐blood screening for colorectal cancer. Lancet. 1996;348:1472–7. [DOI] [PubMed] [Google Scholar]
- 6. Iwase T. The evaluation of an immunochemical occult blood test by reversed passive hemagglutination compared with Hemoccult II in screening for colorectal cancer In: Young GP, Saito H, editors, Fecal occult blood tests: current issues and new tests. San Jose, CA: Smith Kline Diagnostics Inc, 1992; p. 90–5. [Google Scholar]
- 7. Nakama H, Yamamoto M, Kamijo N, et al. Colonoscopic evaluation of immunochemical fecal occult blood test for detection of colorectal neoplasia. Hepatogastroenterology. 1999;46:228–31. [PubMed] [Google Scholar]
- 8. UK Colorectal Cancer Screening Pilot Group . Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ. 2004;329:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manfredi S, Piette C, Durand G, Plihon G, Mallard G, Bretagne JF. Colonoscopy results of a French regional FOBT‐based colorectal cancer screening program with high compliance. Endoscopy. 2008;40:422–7. [DOI] [PubMed] [Google Scholar]
- 10. Seaman S. Management of malignant fungating wounds in advanced cancer. Semin Oncol Nurs. 2006;22:185–93. [DOI] [PubMed] [Google Scholar]
- 11. Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734. [DOI] [PubMed] [Google Scholar]
- 12. Church J, Williams H. Another sniffer dog for the clinic? Lancet. 2001;358:930. [DOI] [PubMed] [Google Scholar]
- 13. Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas‐liquid partition chromatography. Proc Natl Acad Sci USA. 1971;68:2374–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phillips M, Gleeson K, Hughes JM, et al. Volatile organic compounds in breath as markers of lung cancer: a cross‐sectional study. Lancet. 1999;353:1930–3. [DOI] [PubMed] [Google Scholar]
- 15. Mangler M, Freitag C, Lanowska M, Staeck O, Schneider A, Speiser D. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol Pol. 2012;83:730–6. [PubMed] [Google Scholar]
- 16. Abaffy T, Duncan R, Riemer DD, et al. Differential volatile signatures from skin, naevi and melanoma: a novel approach to detect a pathological process. PLoS ONE. 2010;5:e13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khalid T, Aggio R, White P, et al. Urinary volatile organic compounds for the detection of prostate cancer. PLoS ONE. 2015;10:e0143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silva CL, Passos M, Camara JS. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid‐phase microextraction in combination with gas chromatography‐mass spectrometry. Br J Cancer. 2011;105:1894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westenbrink E, Arasaradnam RP, O'Connell N, et al. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens Bioelectron. 2015;67:733–8. [DOI] [PubMed] [Google Scholar]
- 20. Arasaradnam RP, McFarlane MJ, Ryan‐Fisher C, et al. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PLoS ONE. 2014;9:e108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altomare DF, Di Lena M, Porcelli F, et al. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg. 2013;100:144–50. [DOI] [PubMed] [Google Scholar]
- 22. Amal H, Leja M, Funka K, et al. Breath testing as potential colorectal cancer screening tool. Int J Cancer. 2016;138:229–36. [DOI] [PubMed] [Google Scholar]
- 23. Wang C, Li P, Lian A, et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol Ther. 2014;15:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Batty CA, Cauchi M, Lourenco C, Hunter JO, Turner C. Use of the analysis of the volatile faecal metabolome in screening for colorectal cancer. PLoS ONE. 2015;10:e0130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Meij TG, Larbi IB, van der Schee MP, et al. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: proof of principle study. Int J Cancer. 2014;134:1132–8. [DOI] [PubMed] [Google Scholar]
- 26. Hisamichi S, Fukao A, Fujii Y, et al. Mass screening for colorectal cancer in Japan. Cancer Detect Prev. 1991;15:351–6. [PubMed] [Google Scholar]
- 27. Yamagishi K, Onuma K, Chiba Y, et al. Generation of gaseous sulfur‐containing compounds in tumour tissue and suppression of gas diffusion as an antitumour treatment. Gut. 2012;61:554–61. [DOI] [PubMed] [Google Scholar]
- 28. Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. [DOI] [PubMed] [Google Scholar]
- 29. Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Can Res. 2014;74:1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakano Y, Yoshimura M, Koga T. Methyl mercaptan production by periodontal bacteria. Int Dent J. 2002;52(Suppl 3):217–20. [DOI] [PubMed] [Google Scholar]
- 32. Pique JM, Pallares M, Cuso E, Vilar‐Bonet J, Gassull MA. Methane production and colon cancer. Gastroenterology. 1984;87:601–5. [PubMed] [Google Scholar]
- 33. Sivertsen SM, Bjorneklett A, Gullestad HP, Nygaard K. Breath methane and colorectal cancer. Scand J Gastroenterol. 1992;27:25–8. [DOI] [PubMed] [Google Scholar]
- 34. Flick JA, Hamilton SR, Rosales FJ, Perman JA. Methane excretion and experimental colonic carcinogenesis. Dig Dis Sci. 1990;35:221–4. [DOI] [PubMed] [Google Scholar]
- 35. Ohigashi S, Sudo K, Kobayashi D, et al. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci. 2013;58:1717–26. [DOI] [PubMed] [Google Scholar]
- 36. Hirayama K, Hashimoto H, Takeshita A, et al. Correlation between indicators of intestinal environment and amount of carbon dioxide in gas excreted during defecation. Biosci Microflora. 2010;29:135–41. [Google Scholar]


