Abstract
Purpose
The impact of postoperative complications on survival after radical surgery for esophageal, gastric, and colorectal cancers remains controversial. We conducted a systematic review of recent publications to examine the effect of postoperative complications on oncological outcome.
Methods
A literature search of PubMed/MEDLINE was performed using the keywords “esophageal cancer,” “gastric cancer,” and “colorectal cancer,” obtaining 27 reports published online up until the end of April 2016. Articles focusing on (i) postoperative morbidity and oncological outcome; and (ii) body mass index (BMI), postoperative morbidity, and oncological outcome, were selected. Univariate and multivariate analyses (Cox proportional hazards model) were performed.
Results
Patients with postoperative complications had significantly poorer long‐term survival than those without complications. Complications were associated with impaired oncological outcomes. The hazard ratios for overall survival were 1.67 (95% confidence interval [CI], 1.31‐2.12), 1.59 (95% CI, 1.13‐2.24), and 1.55 (95% CI, 1.28‐1.87) in esophageal, gastric, and colorectal cancers, respectively. High BMI was associated with postoperative morbidity rate but not with poor oncological outcome. Low BMI was significantly associated with inferior oncological outcome.
Conclusions
Complications after radical surgery for esophageal, gastric, and colorectal cancers are associated with patient prognosis. Avoiding such complications might improve the outcomes.
Keywords: colorectal cancer, esophageal cancer, gastric cancer, oncological outcome, postoperative complication
1. Introduction
Albeit recent advancements in surgical techniques and perioperative care, the postoperative morbidity rate is as high as approximately 40% after esophageal cancer surgery,1, 2 and 20–30% after both gastric cancer surgery3, 4, 5 and colorectal surgery.6, 7, 8 Various reports have shown that such postoperative complications frequently reduce the overall survival (OS) as well as cancer‐specific survival after major surgery for cancer.9 In particular, severe postoperative complications are associated with impaired long‐term survival after gastroesophageal and pancreatic cancer surgery.10 One possible explanation for this phenomenon is that the changes in patient immunological responses trigger the progression of residual disease into a clinically manifest recurrence.11 Some research has shown a negative impact of postoperative complications on survival outcomes. However, several other studies have concluded that surgical complications have no negative effect on survival rates and that these rates depend exclusively on the pathological stage of the tumor.12 Lerut et al.13 have reported that the modified Clavien classification, in addition to the microscopic residual tumor and extracapsular lymph node involvement, is a useful prognostic indicator of early recurrence and its timing. They have noted that achieving esophagectomy without postoperative complications is of utmost importance because of their potential negative effect on early oncological outcomes.13 Recent advances in endoscopic diagnosis and clinical radiology in Japan allow early detection of gastric cancer, and therapeutic strategies have been established in some clinical trials. D2 lymph node dissection is safely carried out with low mortality and morbidity and provides favorable oncological outcomes.12, 13 However, in clinical practice, postoperative complications do occur, causing some practical problems including longer hospital stay, excessive weight loss with sarcopenia, psychological damage, and delay of adjuvant chemotherapy in advanced cases. The incidence of postoperative complications as a detrimental prognostic factor has recently attracted considerable attention. The impact of postoperative complications on oncological outcomes has also been investigated in patients undergoing colorectal cancer resection. However, the results have been inconsistent. In 2011, Mirnezami et al.14 published a meta‐analysis of the effects of anastomotic leakage on oncological outcomes. They concluded that anastomotic leakage has a negative prognostic impact on local recurrence and cancer‐specific survival.
Acute lung injury induced by the overproduction of inflammatory cytokines can lead to pneumonia after esophageal surgery.15, 16 For the improvement of long‐term survival, it is essential to minimize mortality by optimizing surgical techniques and perioperative care.17, 18, 19 In addition, severe infections, pulmonary complications, and liver dysfunction require extended intensive care and long hospital stays for some patients.9, 20 A history of such postoperative complications increases the likelihood of poor survival. The effect of body mass index (BMI) on oncological outcomes after major resection for cancer has also been investigated.20, 21, 22, 23 Although the majority of the studies show a significant association between high BMI and postoperative morbidity, the association of BMI with long‐term oncological outcomes is still controversial. The effects of BMI and postoperative complications might differ depending on the type of operation and/or the type of cancer. Esophageal cancer surgery is probably one of the most stressful types of surgery. Moreover, postoperative complications after esophagectomy can sometimes be fatal.
In the present study, we re‐evaluated the clinical impact of postoperative morbidity on oncological outcome by systematically reviewing recent publications (from 2011 onward), mainly focusing on long‐term patient survival. In addition, we discuss possible mechanisms of this phenomenon. With the present review, we hope to develop a foundation for future guidelines of Japanese Association of Gastroenterological Surgery for perioperative care and postoperative complication management.
2. Methods
2.1. Research themes and study selection criteria
The present review was based on three types of surgery: (i) esophageal cancer surgery; (ii) gastric cancer surgery; and (iii) colorectal cancer surgery. Articles including information related to these research themes were searched by H.S., Y.H., T.F., and K.O. independently using PubMed and MEDLINE in December 2015. In PubMed, ‘esophageal cancer’, ‘gastric cancer’, ‘colorectal cancer’, and ‘postoperative complication’ were used as search terms. In MEDLINE, the following search terms were used (advanced search system): 1. incidence.sh.; 2. Mortality.sh.; 3. Follow‐Up Studies.sh.; 4. “prognos*.”tw.; 5. “predict*.”tw.; 6. 2 or 3 or 4 or 5; 7. “esophag*.”ab. (or “gastric.”ab. or “colorectal.”ab.); 8. “postoperative complication*.”ab.; 9. “postoperative morbid*.”ab.; 10. 8 or 9; 11. “esophag*.”ti. (or “gastric.”ab. or “colorectal.”ab.); 12. 10 and 11; 13. 6 and 12. Authors (H.S., Y.H., and T.F.) evaluated the relevance of each article and categorized it as relevant or irrelevant. Irrelevant articles were excluded from the review.
2.2. Data extraction
Key messages and information were extracted from each article and organized by the authors. In order to evaluate the impact of postoperative complications on long‐term survival, we conducted a publication‐based meta‐analysis. The following information from the eligible articles was used: authors, title, countries of origin, publication year, total sample size, study design, study period, variables used for the statistical adjustment, definition of complication, conclusion, and the summary statistics (hazard ratios and their 95% confidence intervals [CI]) for outcomes. Primary outcome measure of the meta‐analysis was OS. Postoperative complications were evaluated using the Clavien‐Dindo classification.24 Complications with Clavien‐Dindo grade II or higher were defined as severe complications.
2.3. Statistical analysis
For the meta‐analysis, quantitative data were pooled using the random effects inverse variance weighted meta‐analysis in STATA 13 (StataCorp, College Station, TX, USA). If the adjusted hazard ratio was not reported or it was missing, we treated it as missing and did not include it in the summary statistics calculations. The hazard ratio and the corresponding 95% CI for OS were calculated for each study to compare patients with and without postoperative complications. Heterogeneity between the trials and groups of studies was measured using the I 2 statistics, which indicate the percentage of variance in a meta‐analysis that is attributable to the study heterogeneity.25 All reported P‐values are two‐tailed and P‐values <0.05 were considered statistically significant.
3. Results
3.1. Studies included in the present review
Our systematic search identified 372 articles using PubMed and 871 articles using MEDLINE. We manually found one additional eligible paper and included it in our analysis. We considered 80 studies (41 studies of esophageal cancer, 21 studies of gastric cancer, and 18 studies of colorectal cancer) that were eligible based on title and abstract. After a full‐text search, a final set of 23 studies (six studies of esophageal cancer, six studies of gastric cancer, and 11 studies of colorectal cancer) was used for the meta‐analysis of the impact of postoperative complications on long‐term patient survival. The PRISMA flow diagram26 for the present study is shown in Figure S1.
3.2. Prognostic impact of postoperative complications after esophageal cancer surgery
Ten studies evaluated the prognostic impact of postoperative complications on long‐term survival. Four out of the 10 eligible studies did not report the hazard ratios of postoperative complications because it was not a statistically significant factor (based on univariate analysis using a variable selection process).27, 28, 29, 30 Thus, we combined the hazard ratios of six remaining studies which adjusted for several confounders in the multivariate model. The overall hazard ratio for postoperative OS was 1.67 (95% CI = 1.31–2.12), as illustrated in Figure 1 (statistically significant heterogeneity among the studies is shown [P = 0.001]). The information found in each study is listed in Table 1.
Figure 1.
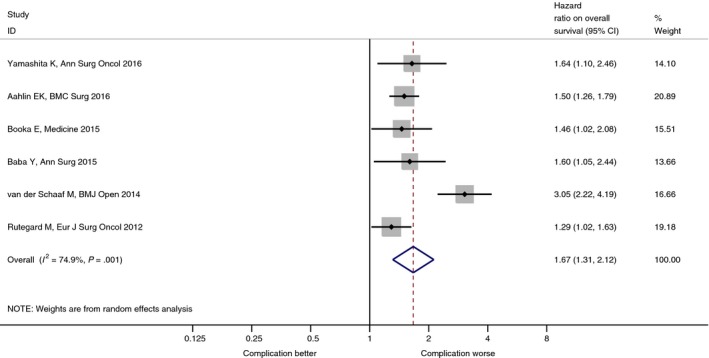
Postoperative morbidity and long‐term survival after radical surgery for esophageal cancer. BMI, body mass index.
Table 1.
Multivariate analyses of prognostic factors for patients after esophageal cancer surgery
| Author | Year | No. patients | Definition of complication | Conclusion |
|---|---|---|---|---|
| Yamashita et al.1 | 2016 | 255 | Infectious complication | Pulmonary infection is associated with unfavorable prognosis. |
| Aahlin et al.10 | 2016 | 1965 | Deep infection, deep hemorrhage, anastomotic dehiscence, reoperation for other causes | Major postoperative complications are associated with impaired long‐term survival. |
| Booka et al.33 | 2015 | 402 | Increased Clavien‐Dindo classification 2, pneumonia | Pneumonia has a negative impact on overall survival after esophagectomy. |
| Baba et al.2 | 2015 | 502 | Increased Clavien‐Dindo classification 2, pneumonia | Postoperative pulmonary complications might be an independent predictor of poor long‐term survival in patients undergoing resection of esophageal squamous cell carcinoma. |
| van der Schaaf et al.32 | 2014 | 1822 | Re‐operation within 30 days | Re‐operation within 30 days of primary esophageal resection is associated with increased mortality. |
| Rutegård et al.31 | 2012 | 567 | Respiratory complication | Occurrence of surgical complications might be an independent predictor of poor long‐term survival. |
D'Annoville et al.30 reported that when postoperative mortality is excluded, postoperative complications did not affect disease‐free survival in patients with complete resection. This deserves substantial information regarding the prognosis of a subgroup of patients in critical situations where incrementing intensive care is debated. In addition, Xia et al.29 have reported that major perioperative morbidity does not have a negative impact on long‐term survival and that tumor characteristics at the time of resection are the most important determinants of long‐term survival. Based on the patient population at a center with a long experience of esophageal cancer surgery, Lindner et al. examined the occurrence of general and esophageal cancer surgery‐specific perioperative complications.28 Their results have demonstrated that these complications did not affect the long‐term survival of esophageal cancer patients.
On the basis of the data from a Swedish national database cohort study, Rutegård et al. have concluded that surgical complications might be independent predictors of poor long‐term survival in patients undergoing esophageal cancer resection, including patients who survived the postoperative period.31, 32 This large, population‐based, nationwide cohort study has shown that re‐operation within 30 days of primary esophageal resection is associated with increased mortality, even when the initial 3 months after surgery is excluded. Similarly, three independent single institutes in high‐volume centers of Japan have shown that pneumonia has a negative impact on OS after esophagectomy. The incidence of postoperative infectious complications and, in particular, pulmonary infections, is associated with unfavorable prognosis in patients with esophageal cancer undergoing preoperative chemotherapy.1, 2, 33
Intense postoperative inflammatory response frequently observed in patients with severe postoperative pneumonia is significantly correlated with poor postoperative survival. Therefore, the oncological benefit of reducing postoperative inflammation in esophageal cancer should be investigated.27 Based on risk stratification for esophagectomy using a Japanese nationwide database, Takeuchi et al.34 observed that the 30‐day and operative mortality rates were lower than those in previously published reports. The risk models developed in their study might contribute to improvements in procedure quality control and the establishment of a novel scoring system.34
3.3. Prognostic impact of postoperative morbidity after gastric cancer surgery
Several studies have reported a negative impact of postoperative complications on patient prognosis after gastric cancer surgery using multivariate analysis.35, 36, 37, 38, 39 Table 2 and Figure 2 show the summarized results of studies evaluating the prognostic impact of postoperative complications. All eligible studies reported adjusted hazard ratios obtained using multivariate analysis; the overall hazard ratio was 1.59 (95% CI, 1.13–2.24), with a statistically significant heterogeneity (P < 0.0001). In each study, postoperative complications were defined by a Clavien‐Dindo grade higher than II,34, 35, 36, 37 which was observed in 10.3–14.5% of the studied patients. There were no apparent differences between the incidences of postoperative complications among large‐volume institutions in Japan. It is not clear from these reports if the occurrence of such complications was limited to Clavien‐Dindo grades III or higher (more severe complications). The prognoses of OS,35, 39 relapse‐free survival (RFS),36 both OS and oncological outcome,37 and both OS and RFS38 were evaluated. We found reports on the negative impact of postoperative complications on patient prognosis for every stage of gastric cancer,36 stage III gastric cancer,37 and stages II and III gastric cancers.38 Cancer‐related death is not commonly observed in the early stages of gastric cancer; thus, its negative impact on RFS might be characteristic of stage III gastric cancer patients.
Table 2.
Multivariate analyses of prognostic factors for patients after gastric cancer surgery
| Author | Year | Country | Study type | Period | Tumor | No. patients | Complications | Survival | Impact on survival | HR | P‐value | Other factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al.35 | 2013 | China | RCS | 2005–2006 | GC | 432 |
All postoperative complications 12.5% (54/432) |
OS | Negative | 2.5 (1.8–3.6) | <0.001 | Stage |
| Hayashi et al.36 | 2015 | Japan | RCS | 2000–2005 | GC | 502 |
Infectious complications 10.3% (52/502) > CDII |
RFS | Negative | 1.958 (1.154–3.289) | 0.013 | ASA, age, stage |
| Kubota et al.37 | 2014 | Japan | RCS | 2005–2008 | GC | 1395 |
Complications 14.5% (202/1395) > CDII |
OS, DSM | Negative | 1.88 (1.26–2.80) | 0.0018 | Age, T, N, blood loss |
| Tokunaga et al.38 | 2013 | Japan | RCS | 2002–2006 | GC | 765 |
Intra‐abdominal infection 10.6% (81/765) > CDII |
OS, RFS | Negative | 2.448 (1.475–4.060) | <0.001 | Stage |
| Jiang et al.39 | 2014 | China | RCS | 2003–2008 | GC | 386 |
Complications 21.5% (83/386) |
OS | Negative | 1.453(1.079–1.956) | 0.014 | PNI, BMI, blood loss, T, N |
| Migita et al.40 | 2013 | Japan | RCS | 2003–2009 | GC | 548 |
Complications 28.6% (157/548) |
OS, RFS | No impact | 1.31 (0.89–1.94) | 0.172 | PNI |
| Climent et al.41 | 2015 | Spain | RCS | 1990–2009 | GC | 271 |
Complications 59.8% (162/271) > CDII |
OS | No impact | 0.76 (0.51–1.12) | 0.167 | |
| Saito et al.42 | 2015 | Japan | RCS | 2001–2012 | GC | 305 |
Complications 28.2% (86/305) > CDII |
RFS | No impact | 1.10 (0.70–1.73) | 0.682 | CRP, adjuvant therapy, blood loss |
ASA, American Society of Anesthesiologists; BMI, body mass index; CDII, Clavien‐Dindo grade II; CRP, C‐reactive protein; DSM, disease‐free survival; GC, gastric cancer; HR, hazard ratio; N, nodal staging; OS, overall survival; PNI, prognostic nutritional index; RCS, retrospective cohort study; RFS, relapse‐free survival; T, tumor depth.
Figure 2.

Postoperative morbidity and long‐term survival after radical surgery for gastric cancer.
However, some studies have found that postoperative complications do not always have a negative impact on prognosis.40, 41, 42 Migita et al. have focused on the prognostic nutritional index, and found that a reduction in the value of this index had a negative impact on the long‐term outcomes of gastric cancer patients. Climent et al. have analyzed the impact of postoperative complications on recurrence and survival after gastric cancer resection. In this study conducted in Spain, the incidence of postoperative complications (Clavien‐Dindo grade II or higher) was reported as 59.8%, which is somewhat higher than that reported elsewhere.36, 37, 38 They have concluded that these complications do not have a negative impact on oncological outcome. However, regional differences and patient background might be important in the interpretation of the incidence of postoperative complications.
In some reports, the incidence of postoperative complications is evaluated as a negative prognostic factor that affects not only the OS, but also the RFS of gastric cancer patients after curative surgery. In gastric cancer surgery, the major postoperative complications are anastomotic leakage and intra‐abdominal abscess as a result of pancreatic fistula and pneumonia. Adverse effects of postoperative complications on patient survival might be a result of excessive inflammation caused by the complications, stimulating the growth of residual cancer cells through some soluble factors. They might also be caused by reduced immune reaction against cancer cells. Presently, these speculations have not been confirmed. Saito et al.42 suggested that the actual event that affects patient survival might not be postoperative complications, but excessive inflammation after surgery. They have reported that a high level of postoperative C‐reactive protein is a more reliable indicator of survival after surgery than postoperative complications.
Another hypothesis must also be considered. If particularly frail patients with potentially poor prognoses easily develop postoperative complications, the incidence of these complications might not be an independent negative prognostic factor. Preoperative nutritional statuses of patients might contribute to this ‘frailty’. Prognostic nutritional index I has been evaluated as a predictor of postoperative complications and poor prognosis.39 The hazard ratio of preoperative nutritional index for prognosis might be offset by the hazard ratio of postoperative complications. Jiang et al. have reported that postoperative complications are significant negative prognostic factors despite the negative impact of prognostic nutritional index shown in a multivariate analysis.39 In order to elucidate this phenomenon, the effect of prognostic nutritional index on the incidence of postoperative complications and patient survival should be studied in a large‐scale prospective setting.
The popularity of minimally invasive surgery for gastric cancer has been growing. The feasibility and non‐inferiority of laparoscopic gastrectomy have been compared with those of conventional open surgery. A phase‐II clinical trial of early‐stage gastric cancer (JCOG 07033) has shown that the incidences of anastomotic leakage and pancreatic fistula formation were acceptably low. Kim et al. have reported a decrease in morbidity after laparoscopic distal gastrectomy for stage I gastric cancer.43 However, the only significant factor was wound complication. Minimally invasive surgery might confer a benefit of low surgical stress (a small wound). However, the superiority of minimally invasive surgery is not definitively proven by a low incidence of postoperative complications correlating with good oncological outcomes. Sufficient level of surgical intervention, including appropriate extent of dissection, should be maintained. Robot‐assisted gastrectomy, an alternative to minimally invasive surgery, might reduce the incidence of postoperative complications;44 however, the benefits of this procedure, including its effect on oncological outcomes, has not been established to date.
3.4. Prognostic impact of postoperative morbidity after colorectal cancer surgery
Selected papers, published between 2011 and 2016, are summarized in Table 3, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55. These studies were cohort studies that conducted risk adjustment using multivariate analysis. Overall hazard ratio for postoperative OS was 1.55 (95% CI, 1.28–1.87) with a statistically significant heterogeneity (P = 0.0001) as shown in Figure 3. These results are consistent with those reported in a previous systematic review by Mirnezami et al.14 Mirnezami et al. have examined the effects of anastomotic leakage, obtaining odds ratios of 2.9 (95% CI, 1.78–4.71) for local recurrence, 1.38 (95% CI, 0.96–1.99) for distant recurrence, and 1.75 (95% CI, 1.47–2.1) for cancer‐specific survival. As the papers used in the two reviews do not overlap, these results might be reproducible.
Table 3.
Summary of studies reporting postoperative complications and oncological survival after colorectal cancer surgery
| Author | Publication year | Country | Study design | Study period | Type of surgery | No. patients | Definition of complications | Endpoint |
|---|---|---|---|---|---|---|---|---|
| Artinyan A45 | 2015 | USA | PBS | 1999–2009 | C + R | 12 075 | Infectious postoperative complications | OS |
| Odermatt M46 | 2014 | UK | PCS | 2003–2012 | C + R | 844 | Major complications (CDIIIb or IV) | OS, DFS |
| Xia X47 | 2014 | China | RCS | 2006–2009 | C (Laparosc.) | 224 | Postoperative complications (CDII or higher) | OS, RFS |
| Nachiappan S48 | 2015 | UK | RCS | 2004–2013 | C + R | 1048 | Anastomotic leak | OS, DFS |
| Krarup PM49 | 2014 | Denmark | PBS | 1988–2015 | C | 8589 | Anastomotic leak | OS, LR, DR |
| Lin JK50 | 2011 | Taiwan | RCS | 1993–2003 | R | 999 | Anastomotic leak | OS, DFS, CSS |
| Smith JD51 | 2012 | USA | RCS | 1991–2010 | R | 1127 | Anastomotic leak | LR, DFS, OS |
| Espín E52 | 2015 | Spain | PBS | 2006–2008 | R | 1181 | Anastomotic leak | OS, CSS, LR, OR |
| Kang J53 | 2015 | S. Korea | RCS | 2006–2009 | R (Laparosc.) | 1083 | Anastomotic leak | LR, DFS, OS |
| Park EJ54 | 2016 | S. Korea | RCS | 2005–2012 | R (Laparosc.) | 686 | Complications CDI or higher | LR, DFS, OS |
| Jörgren F55 | 2011 | Sweden | PBS | 1995–1997 | R | 250 | Anastomotic leak | OS, CSS |
C, colectomy; CSS, cancer‐specific survival; DFS, disease‐free survival; DR, distant recurrence; LR, local recurrence; OR, overall recurrence; OS, overall survival; PBS, population‐based study; PCS, prospective cohort study; R, rectal resection; RCS, retrospective cohort study.
Figure 3.

Postoperative morbidity and long‐term survival after radical surgery for colorectal cancer
There are several speculations that could explain the negative impact of postoperative complications on survival outcome. One of the most popular theories is that inflammatory cytokines, including interleukin (IL)‐1, IL‐6, and tumor necrosis factor (TNF), might promote tumor proliferation, survival, avoidance of apoptosis, progression of metastasis, and resistance to drug therapy. For example, Miki et al.56 have demonstrated that intense surgical stress and presence of an acute‐phase reactant were independently associated with the overexpression of IL‐6 in tumors. Another possible mechanism might be impairment in cell‐mediated immunity by systemic inflammation, resulting in the proliferation of metastatic tumor cells.57 It has also been proposed that intraluminal neoplastic cells might escape into the extraluminal space during an anastomotic leak, leading to implantation and local recurrence. Salvans et al.58 have conducted an interesting in vitro study using a colon cancer cell line to determine the effects of infected peritoneal fluid on migration and invasion of tumor cells. They have demonstrated that the fluid enhanced both cell migration and cell invasion compared with the non‐infected control.
3.5. Prognostic impact of BMI on postoperative morbidity and long‐term survival (Table 4)
Table 4.
Prognostic impact of body mass index in gastroenterological cancer surgery
| Author | Year | Type of cancer | No. patients | Conclusion | |
|---|---|---|---|---|---|
| Wang et al.60 | 2015 | Esophageal SCC | 424 | Preoperative BMI was an independent prognostic factor for OS and DFS. The proposed new prognostic model with the pN classification supplemented by BMI might improve the ability to predict ESCC patient outcome. | |
| Zogg et al.62 | 2015 | Various types of cancer | 529 955 | Obese patients should be treated following the optimal oncological standards without being hindered by a misleading perception of prohibitively increased perioperative risk. Underweight and certain types of morbidly obese patients require targeted provision of appropriate care. | |
| Melis et al.21 | 2015 | Esophageal cancer | 510 | BMI did not affect the number of harvested lymph nodes, rates of negative margins, or morbidity and mortality after esophagectomy for cancer. Esophagectomy can be carried out safely and efficiently in mildly obese patients. | |
| Chen et al.63 | 2015 | Gastric cancer | 1249 | Despite an increased risk of mild postoperative complications, the high‐BMI patients exhibited paradoxically ‘superior’ survival outcomes in comparison with the normal‐BMI patients. These findings confirm the ‘obesity paradox’ in GC patients undergoing gastrectomy. | |
| Levolger et al.73 | 2015 | Gastrointestinal cancer and hepatobiliary cancer | 2884 | Sarcopenia identified before surgery is associated with impaired overall survival in gastrointestinal and hepatopancreatobiliary malignancies, and increases postoperative morbidity in patients with colorectal cancer with or without hepatic metastases. | |
| Pan et al.64 | 2015 | Esophageal cancer and gastric cancer | 4823 | H‐BMI has distinctly different effects on the postoperative survival of EAC and ESCC patients. H‐BMI is a potential predictor for improved prognosis in EC patients overall, and particularly in EAC patients, treated with curative esophagectomy. However, in ESCC patients, H‐BMI is a potential predictor for a poor prognosis of postoperative survival. | |
| Miao et al.68 | 2015 | . | Esophageal cancer | 1342 | A high BMI is not associated with increased overall morbidity following esophagectomy; moreover, it is associated with a decreased incidence of chylothorax. The improved overall survival of patients with high BMI in comparison with those with low BMI might be as a result of a relatively low pathological stage. A high BMI should not be a relative contraindication for esophagectomy. |
| Ida et al.65 | 2015 | Esophageal cancer | 138 | Sarcopenia might be a predictor of pulmonary complications after esophagectomy. Further analysis is needed to clarify whether nutritional intervention improves skeletal muscle mass and thus contributes to reduction in postoperative respiratory complications in sarcopenic patients. | |
| Eom et al.66 | 2014 | Gastric cancer | 4813 | ABSI shows a good correlation with surgical complications in patients with gastric cancer. Further studies are needed to clarify the clinical significance of ABSI, and the results could help to determine the effect of abdominal obesity on gastric cancer surgery and the clinical usefulness of ABSI. | |
| Bickenbach et al.67 | 2013 | Gastric cancer | 1853 | Increased BMI is a predictor of increased postoperative complications, including anastomotic leak, but it is not a predictor of survival in gastric cancer. | |
| Zhang et al.59 | 2013 | Esophageal cancer | 2031 | Preoperative BMI is an independent prognostic factor for survival, strongly associated with postoperative complications in esophageal cancer. | |
| Hayashi Y91 | 2010 | Cancer. 116(24):5619–27, 2010 Dec 15. | Esophageal cancer | 301 | High BMI is common in EC patients. The improved OS/DFS noted in patients with high BMI might be a result of a low baseline clinical stage. Confirmation of these findings is warranted. |
| Grotenhuis et al.61 | 2010 | Esophageal cancer | 556 | BMI has no prognostic value for short‐term and long‐term outcome in patients who undergo esophagectomy for cancer. It is not an independent predictor for radical R0 resection. Patients oncologically eligible for esophagectomy should not be denied surgery on the basis of their BMI class. |
ABSI, A Body Shape Index; BMI, body mass index; DFS, disease‐free survival; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; GC, gastric cancer; H‐BMI, high BMI; OS, overall survival; SCC, squamous cell carcinoma.
Multivariate analyses carried out in various studies have shown that preoperative BMI is an independent prognostic factor for reduced survival, and that it is strongly associated with postoperative complications in esophageal cancer.59 Wang et al.60 have reported that preoperative BMI is an independent prognostic factor for OS and disease‐free survival (DFS). Their proposed new prognostic model with the pN classification supplemented by BMI might improve the ability to predict outcomes for esophageal squamous cell carcinoma patients.
However, we identified several studies showing that high BMI does not worsen the long‐term oncological outcome. Grotenhuis et al.61 reported that BMI was not of prognostic value for short‐ and long‐term outcomes in patients who underwent esophagectomy for cancer and it is not an independent predictor for radical (R0) resection. Patients oncologically eligible for esophagectomy should not be denied surgery on the basis of their BMI class. Zogg et al.62 also suggested that outcomes after major resection for cancer suggest that obese patients should be treated according to optimal oncological standards. Their treatment should not be hindered by a misleading perception of prohibitively high perioperative surgical risk. However, the authors have noted that underweight patients and certain types of morbidly obese individuals require targeted provision of appropriate care.
Interestingly, Chen et al.63 have concluded that high‐BMI patients exhibit paradoxically ‘superior’ survival outcomes compared with normal‐BMI patients despite the higher risk of mild postoperative complications. These findings confirm the ‘obesity paradox’ in gastric cancer patients undergoing gastrectomy. Pan et al.64 reported that high BMI has distinctly different effects on postoperative survival of esophageal adenocarcinoma and esophageal squamous cell carcinoma patients. Overall, high BMI is a potential predictor of improved prognosis in esophageal cancer patients, particularly in esophageal adenocarcinoma patients treated with curative esophagectomy. However, in patients with esophageal squamous cell carcinoma, a high BMI is a predictor of poor prognosis of postoperative survival. Ida et al.65 reported that sarcopenia might be a predictor of pulmonary complications after esophagectomy. Further analysis is required to elucidate whether nutritional intervention improves skeletal muscle mass and thus contributes to the reduction of postoperative respiratory complications in sarcopenic patients.
Eom et al.66 have reported that the A Body Shape Index (ABSI), rather than BMI, correlates with surgical complications in patients with gastric cancer. Further studies are required to elucidate the clinical significance of ABSI; the results might help determine the effect of abdominal obesity on gastric cancer surgery outcome and the clinical usefulness of this index.67 Enhanced BMI is a predictor of increased postoperative complications, including anastomotic leak, but it is not a predictor of survival in gastric cancer. Melis et al.21 have reported that BMI does not affect the number of harvested lymph nodes, rates of negative margins, and morbidity and mortality after esophagectomy for cancer. In their experience, esophagectomy can be carried out safely and efficiently in mildly obese patients. Miao et al.68 have shown that high BMI is not associated with increased overall morbidity following esophagectomy; however, it is associated with a decreased incidence of chylothorax. However, better OS observed in patients having high BMI compared with those having low BMI might be attributed to a relatively low pathological stage. In summary, a high BMI should not be a relative contraindication for esophagectomy.
It has been shown that after colorectal resection, low BMI has a detrimental effect on long‐term survival. Toiyama et al. have revealed that a BMI <20 is associated with reduced OS and DFS after laparoscopic resection.69 Adachi et al.70 demonstrated in elderly (≥80 years) colorectal cancer patients (stage 0 to III) that a BMI <18.5 is associated with decreased OS and cancer‐specific survival. Uratani et al.71 studied stage I–III patients undergoing laparoscopic resection and found that a BMI <20 is correlated with reduced DFS and OS. Doleman et al. have recently conducted a meta‐analysis on the effects of BMI following the diagnosis of colorectal cancer. They have reported that a low BMI is associated with increased all‐cause mortality and cancer‐specific mortality.72 This suggests that underweight patients might have lower nutritional statuses and lower body muscle content than individuals with normal bodyweight.
In addition to the low BMI, sarcopenia, defined as decreased muscle content, also has a negative impact on oncological outcome. Levolger et al.73 have reported that sarcopenia identified before surgery using single‐slice computed tomography, is associated with impaired OS in gastrointestinal and hepatopancreatobiliary malignancies. Sarcopenia also increases postoperative morbidity in patients with colorectal cancer with or without hepatic metastases.73
3.6. Strategy for reducing the morbidity rate for improved oncological outcome
We found that postoperative sepsis was the only major postoperative event associated with long‐term mortality. Postoperative sepsis might reflect a deep impairment in the immune response, which might increase cancer recurrence and mortality.20 Therefore, minimizing surgical stress and/or levels of inflammatory mediators might reduce postoperative complications and improve oncological outcome. Following this working hypothesis, perioperative steroid therapy has been evaluated in patients who had undergone esophagectomy. Perioperative steroid therapy reduces postoperative morbidity but does not improve long‐term survival in patients with thoracic esophageal cancer.15, 16, 74 Early administration of sivelestat in patients receiving radical surgery for esophageal cancer can inhibit postoperative systemic inflammatory reactions, and it might also have a beneficial effect on prognosis.75, 76 Some of the examined factors did not differ between the treated and control groups, including IL‐8 on postoperative day 1, IL‐6 before the surgery and on postoperative day 5, PaO2/FiO2 following the surgery, mortality, anastomotic leakage, severe infection, and renal and hepatic failure. Giving prophylactic methylprednisolone during the perioperative period might reduce the incidence of specific types of postoperative complications and inhibit the postoperative inflammatory reaction. However, additional randomized controlled trials should be done to evaluate this strategy.77 Ulinastatin prevents postoperative complications and immunosuppression in esophagectomy patients, thereby prolonging RFS.78 Yamana et al.79 have shown that an intensive preoperative respiratory rehabilitation program can reduce postoperative pulmonary complications in esophageal cancer patients. Giving postoperative ghrelin can effectively inhibit the activity of inflammatory mediators and improve postoperative clinical course in patients with esophageal cancer.80 Furthermore, treatment with IL‐1β or lipopolysaccharide enhances the expression of IL‐6 protein in a human colonic cancer cell line, Caco‐2. This overexpression is abrogated by additional presupplementation of IL‐1RA. Moreover, Elaraj et al.81 have demonstrated that IL‐1RA inhibits the growth of colonic adenocarcinoma cell line xenografts in nude mice. Application of anti‐inflammatory therapy as described above is one of the promising strategies for future cancer treatment, with a likelihood of reducing morbidity rates and improving oncological outcomes.
3.7. Future perspectives
Although postoperative complications have been associated with impaired long‐term survival after gastrointestinal cancer resections, we must also consider their indirect effects. Adjuvant chemotherapy might sometimes be terminated or delayed in patients who develop postoperative complications. Furthermore, patients with multiple comorbidities or with poor nutritional statuses might have a tendency to develop postoperative complications as well as late mortality. Therefore, the preoperative physiological status can be a confounding factor in the evaluation of postoperative complications and late mortality. Richards et al.82 have analyzed the effects of various perioperative factors on disease recurrence in patients with colorectal cancer. In their prediction model of postoperative complications, they used the Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (POSSUM) system and the modified Glasgow Prognostic Score for systemic inflammatory response markers.83 POSSUM comprises a physiological score and an operative score. The physiological score includes 11 variables of vital signs and laboratory data. The operative score includes six variables of the operation and tumor stage. The modified Glasgow Prognostic Score ranges from 0 to 2 depending on the abnormality of preoperative C‐reactive protein and serum albumin levels. The authors found that the POSSUM physiological score and the modified Glasgow Prognostic Score, but not postoperative complications, are the independent predictors of DFS. In patients with gastric cancer, the Glasgow Prognostic Score has also been associated with long‐term survival.37 Similarly, the Estimation of Physiological Ability and Surgical Stress scores84 were associated with OS following gastrointestinal cancer resection.85, 86, 87
The association between postoperative complications and reduced long‐term survival in gastrointestinal cancer patients, whether or not these complications are detrimental for long‐term survival, remains to be established owing to unmeasured confounders. It is possible that compromised statuses (potential confounders) simply cause postoperative complications as well as late mortality (Fig. 4). For example, a diabetes mellitus patient with interstitial pneumonitis will have an increased chance of postoperative complications and a higher risk of tumor‐related and unrelated death. To clarify the doubts persisting in this field, a large‐scale prospective cohort study should be conducted. Such a study should use a statistical method (e.g. propensity score adjustment) accounting for preoperative physiological status as well as systemic inflammatory response. As shown in Figure 4, scanty adjuvant therapy is also considered one of the key intermediate factors affecting postoperative complications and long‐term prognosis. Greenleaf et al. have found that adjuvant therapy improves patient survival compared with patients not undergoing such therapy. However, the period before the initiation of adjuvant therapy did not affect survival among the treated patients.88 Adjustments of intermediate variables in the standard statistical models should be conducted with caution because of a potential over‐adjustment bias.89 Recently, we carried out a mediation analysis to establish whether the effect of exposure on a particular outcome is mediated by a hypothesized intermediate variable.90 Using such methods might shed some light on the causal relationship between postoperative complications and reduced long‐term survival.
Figure 4.

Relationship between postoperative complications and long‐term survival following gastrointestinal cancer resection
In conclusion, our literature review suggests that severe postoperative morbidities are associated with impaired long‐term prognosis. Avoiding such complications after radical surgery might improve oncological outcomes. Because there are no large‐scale prospective cohort studies in this field, further multi‐institutional prospective studies should be carried out.
Conflicts of Interest
Authors declare no conflicts of interest for this article.
Supporting information
PRISMA flow diagram
Acknowledgments
We thank Ms Akemi Hayashi for preparing the data of the selected papers. This work has been partly supported by a research grant of Toho University School of Medicine.
Shimada H, Fukagawa T, Haga Y, Oba K. Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann Gastroenterol Surg. 2017;1:11–23. https://doi.org/10.1002/ags3.12002
References
- 1. Yamashita K, Makino T, Miyata H, et al. Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol. 2016;23:2106–14. [DOI] [PubMed] [Google Scholar]
- 2. Baba Y, Yoshida N, Shigaki H, et al. Prognostic impact of postoperative complications in 502 patients with surgically resected esophageal squamous cell carcinoma: A retrospective single‐institution study. Ann Surg. 2016;264:305–11. [DOI] [PubMed] [Google Scholar]
- 3. Katai H, Sasako M, Fukuda H, et al. Safety and feasibility of laparoscopy‐assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13:238–44. [DOI] [PubMed] [Google Scholar]
- 4. Sano T, Sasako M, Yamamoto S, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para‐aortic lymphadenectomy–Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–73. [DOI] [PubMed] [Google Scholar]
- 5. Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vries S, Jeffe DB, Davidson NO, Deshpande AD, Schootman M. Postoperative 30‐day mortality in patients undergoing surgery for colorectal cancer: development of a prognostic model using administrative claims data. Can Causes Cont. 2014;25:1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Govaert JA, Fiocco M, van Dijk WA, et al. Dutch value based healthcare study group. Multicenter stratified comparison of hospital costs between laparoscopic and open colorectal cancer resections: influence of tumor location and operative risk. Ann Surg. 2016. [Epub ahead of print]. http://doi.org/10.1097/SLA00000000000002000. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki H, Gotoh M, Sugihara K, et al. Nationwide survey and establishment of a clinical database for gastrointestinal surgery in Japan: targeting integration of a cancer registration system and improving the outcome of cancer treatment. Cancer Sci. 2011;102:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pucher PH, Aggarwal R, Qurashi M, Darzi A. Meta‐analysis of the effect of postoperative in‐hospital morbidity on long‐term patient survival. Br J Surg. 2014;101:1499–508. [DOI] [PubMed] [Google Scholar]
- 10. Aahlin EK, Olsen F, Uleberg B, Jacobsen BK, Lassen K. Major postoperative complications are associated with impaired long‐term survival after gastro‐esophageal and pancreatic cancer surgery: a complete national cohort study. BMC Surg. 2016;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lagarde SM, Reitsma JB, Ten Kate FJW, et al. Predicting individual survival after potentially curative esophagectomy for adenocarcinoma of the esophagus or gastroesophageal junction. Ann Surg. 2008;248:1006–13. [DOI] [PubMed] [Google Scholar]
- 12. Ancona E, Cagol M, Epifani M, et al. Surgical complications do not affect longterm survival after esophagectomy for carcinoma of the thoracic esophagus and cardia. J Am Coll Surg. 2006;203:661–9. [DOI] [PubMed] [Google Scholar]
- 13. Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250:798–807. [DOI] [PubMed] [Google Scholar]
- 14. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta‐analysis. Ann Surg. 2011;253:890–899. [DOI] [PubMed] [Google Scholar]
- 15. Shimada H, Ochiai T, Okazumi S, et al. Clinical benefits of steroid therapy on surgical stress in patients with esophageal cancer. Surgery. 2000;128:791–8. [DOI] [PubMed] [Google Scholar]
- 16. Shimada H, Okazumi S, Matsubara H, et al. Effect of steroid therapy on postoperative course and survival of patients with thoracic esophageal carcinoma. Esophagus. 2004;1:89–94. [Google Scholar]
- 17. Okazumi S, Ochiai T, Shimada H, et al. Development of less invasive surgical procedures for thoracic esophageal cancer. Dis Esophagus. 2004;17:159–63. [DOI] [PubMed] [Google Scholar]
- 18. Palazzo F, Rosato EL, Chaudhary A, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagectomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg. 2015;220:672–9. [DOI] [PubMed] [Google Scholar]
- 19. Hyun M‐H, Lee C‐H, Kwon Y‐J, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258–65. [DOI] [PubMed] [Google Scholar]
- 20. Mokart D, Giaoui E, Barbier L, et al. Postoperative sepsis in cancer patients undergoing major elective digestive surgery is associated with increased long‐term mortality. J Crit Care. 2015;31:48–53. [DOI] [PubMed] [Google Scholar]
- 21. Melis M, Meredith KL, Weber J, et al. Body mass index and perioperative complications after esophagectomy for cancer. Ann Surg. 2015. [Epub ahead of print]. http://doi.org/10.1097/SLA.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 22. Kamachi K, Ozawa S, Hayashi T, Kazuno A, Ito E, Makuuchi H. Impact of body mass index on postoperative complications and long‐term survival in patients with esophageal squamous cell cancer. Dis Esophagus. 2016;29:229–35. [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi M, Ishii K, Seki H, et al. Excessive visceral fat area as a risk factor for early postoperative complications of total gastrectomy for gastric cancer: a retrospective cohort study. BMC Surg. 2016;16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;89:873–80. [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuda S, Takeuchi H, Kawakubo H, et al. Correlation between intense postoperative inflammatory response and survival of esophageal cancer patients who underwent transthoracic esophagectomy. Ann Surg Oncol. 2015;22:4453–60. [DOI] [PubMed] [Google Scholar]
- 28. Lindner K, Fritz M, Haane C, Senninger N, Palmes D, Hummel R. Postoperative complications do not affect long‐term outcome in esophageal cancer patients. World J Surg. 2014;38:2652–61. [DOI] [PubMed] [Google Scholar]
- 29. Xia BT, Rosato EL, Chojnacki KA, Crawford AG, Weksler B, Berger AC. Major perioperative morbidity does not affect long‐term survival in patients undergoing esophagectomy for cancer of the esophagus or gastroesophageal junction. World J Surg. 2013;37:408–15. [DOI] [PubMed] [Google Scholar]
- 30. D'Annoville T, D'Journo XB, Trousse D, et al. Respiratory complications after oesophagectomy for cancer do not affect disease‐free survival. Eur J Cardio‐thoracic Surg. 2012;41:66–73. [DOI] [PubMed] [Google Scholar]
- 31. Rutegård M, Lagergren P, Rouvelas I, Mason R, Lagergren J. Surgical complications and long‐term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur J Surg Oncol. 2012;38:555–61. [DOI] [PubMed] [Google Scholar]
- 32. van der Schaaf M, Derogar M, Johar A, et al. Reoperation after oesophageal cancer surgery in relation to long‐term survival: a population‐based cohort study. BMJ Open. 2014;4:e004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Booka E, Takeuchi H, Nishi T, et al. The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine (Baltimore). 2015;94:e1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web‐based database. Ann Surg. 2014;260:259–66. [DOI] [PubMed] [Google Scholar]
- 35. Li Q‐G, Li P, Tang D, Chen J, Wang D‐R. Impact of postoperative complications on long‐term survival after radical resection for gastric cancer. World J Gastroenterol. 2013;19:4060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayashi T, Yoshikawa T, Aoyama T, et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer. 2015;18:368–74. [DOI] [PubMed] [Google Scholar]
- 37. Kubota T, Hiki N, Sano T, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21:891–8. [DOI] [PubMed] [Google Scholar]
- 38. Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra‐abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83. [DOI] [PubMed] [Google Scholar]
- 39. Jiang N, Deng J‐YY, Ding X‐WW, et al. Prognostic nutritional index predicts postoperative complications and long‐term outcomes of gastric cancer. World J Gastroenterol. 2014;20:10537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Migita K, Takayama T, Saeki K, et al. The prognostic nutritional index predicts long‐term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647–54. [DOI] [PubMed] [Google Scholar]
- 41. Climent M, Hidalgo N, Vidal O, et al. Postoperative complications do not impact on recurrence and survival after curative resection of gastric cancer. Eur J Surg Oncol. 2015;42:132–9. [DOI] [PubMed] [Google Scholar]
- 42. Saito T, Kurokawa Y, Miyazaki Y, et al. Which is a more reliable indicator of survival after gastric cancer surgery: Postoperative complication occurrence or C‐reactive protein elevation? J Surg Oncol. 2015;112:894–9. [DOI] [PubMed] [Google Scholar]
- 43. Kim W, Kim H‐H, Han S‐U, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short‐term outcomes from a multicenter randomized controlled trial (KLASS‐01). Ann Surg. 2016;263:28–35. [DOI] [PubMed] [Google Scholar]
- 44. Seo HS, Shim JH, Jeon HM, Park CH, Song KY. Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res. 2015;194:361–6. [DOI] [PubMed] [Google Scholar]
- 45. Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long‐term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261:497–505. [DOI] [PubMed] [Google Scholar]
- 46. Odermatt M, Miskovic D, Flashman K, et al. Major postoperative complications following elective resection for colorectal cancer decrease long‐term survival but not the time to recurrence. Colorectal Dis. 2015;17:141–509. [DOI] [PubMed] [Google Scholar]
- 47. Xia X, Wu W, Zhang K, et al. Prognostic significance of complications after laparoscopic colectomy for colon cancer. PLoS One. 2014;9:e108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nachiappan S, Askari A, Malietzis G, et al. The impact of anastomotic leak and its treatment on cancer recurrence and survival following elective colorectal cancer resection. World J Surg. 2015;39:1052–58. [DOI] [PubMed] [Google Scholar]
- 49. Krarup PM, Nordholm‐Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long‐term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930–8. [DOI] [PubMed] [Google Scholar]
- 50. Lin JK, Yueh TC, Chang SC, et al. The influence of fecal diversion and anastomotic leakage on survival after resection of rectal cancer. J Gastrointest Surg. 2011;15:2251–61. [DOI] [PubMed] [Google Scholar]
- 51. Smith JD, Paty PB, Guillem JG, Temple LK, Weiser MR, Nash GM. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg. 2012;256:1034–38. [DOI] [PubMed] [Google Scholar]
- 52. Espín E, Ciga MA, Pera M, et al. Oncological outcome following anastomotic leak in rectal surgery. Br J Surg. 2015;102:416–22. [DOI] [PubMed] [Google Scholar]
- 53. Kang J, Choi GS, Oh JH, et al. Multicenter analysis of long‐term oncologic impact of anastomotic leakage after laparoscopic total mesorectal excision: the Korean laparoscopic colorectal surgery study group. Medicine (Baltimore). 2015;94:e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park EJ, Baik SH, Kang J, et al. The impact of postoperative complications on long‐term oncologic outcomes after laparoscopic low anterior resection for rectal cancer. Medicine (Baltimore). 2016;95:e3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jörgren F1, Johansson R, Damber L, Lindmark G. Anastomotic leakage after surgery for rectal cancer: a risk factor for local recurrence, distant metastasis and reduced cancer‐specific survival? Colorectal Dis. 2011;13:272–83. [DOI] [PubMed] [Google Scholar]
- 56. Miki C, Konishi N, Ojima E, Hatada T, Inoue Y, Kusunoki M. C‐reactive protein as a prognostic variable that reflects uncontrolled up‐regulation of the IL‐1‐IL‐6 network system in colorectal carcinoma. Dig Dis Sci. 2004;49:970–6. [DOI] [PubMed] [Google Scholar]
- 57. Biswas SK, Lopez‐Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–87. [DOI] [PubMed] [Google Scholar]
- 58. Salvans S, Mayol X, Alonso S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg. 2014;260:939–43. [DOI] [PubMed] [Google Scholar]
- 59. Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical‐based cohort and meta‐analysis. Br J Cancer. 2013;109:2894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang F, Duan H, Cai M, et al. Prognostic significance of the pN classification supplemented by body mass index for esophageal squamous cell carcinoma. Thorac Cancer. 2015;6:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grotenhuis BA, Wijnhoven BPL, Hötte GJ, van der Stok EP, Tilanus HW, van Lanschot JJB. Prognostic value of body mass index on short‐term and long‐term outcome after resection of esophageal cancer. World J Surg. 2010;34:2621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zogg CK, Mungo B, Lidor AO, et al. Influence of body mass index on outcomes after major resection for cancer. Surgery (United States). 2015;158:472–85. [DOI] [PubMed] [Google Scholar]
- 63. Chen H‐N, Chen X‐Z, Zhang W‐H, et al. The impact of body mass index on the surgical outcomes of patients with gastric cancer: a 10‐year, single‐institution cohort study. Medicine (Baltimore). 2015;94:e1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pan W, Sun Z, Xiang Y, Fang W. The correlation between high body mass index and survival in patients with esophageal cancer after curative esophagectomy: evidence from retrospective studies. Asia Pac J Clin Nutr. 2015;24:480–8. [DOI] [PubMed] [Google Scholar]
- 65. Ida S, Watanabe M, Yoshida N, et al. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol. 2015;22:4432–7. [DOI] [PubMed] [Google Scholar]
- 66. Eom BW, Joo J, Yoon HM, Ryu KW, Kim Y‐W, Lee JH. A body shape index has a good correlation with postoperative complications in gastric cancer surgery. Ann Surg Oncol. 2014;21:1115–22. [DOI] [PubMed] [Google Scholar]
- 67. Bickenbach KA, Denton B, Gonen M, Brennan MF, Coit DG, Strong VE. Impact of obesity on perioperative complications and long‐term survival of patients with gastric cancer. Ann Surg Oncol. 2013;20:780–7. [DOI] [PubMed] [Google Scholar]
- 68. Miao L, Chen H, Xiang J, Zhang Y. A high body mass index in esophageal cancer patients is not associated with adverse outcomes following esophagectomy. J Cancer Res Clin Oncol. 2015;141:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Toiyama Y, Hiro J, Shimura T, et al. The impact of body mass index on oncological outcomes in colorectal cancer patients with curative intent. Int J Clin Oncol. 2016;21:1102–10. [DOI] [PubMed] [Google Scholar]
- 70. Adachi T, Hinoi T, Kinugawa Y, et al. Lower body mass index predicts worse cancer‐specific prognosis in octogenarians with colorectal cancer. J Gastroenterol. 2016;51:779–87. [DOI] [PubMed] [Google Scholar]
- 71. Uratani R, Toiyama Y, Shimura T, et al. Preoperative lower body mass index correlates with poorer prognosis in patients undergoing curative laparoscopic surgery for colorectal cancer. Anticancer Res. 2015;35:5639–48. [PubMed] [Google Scholar]
- 72. Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta‐analysis. Tech Coloproctol. 2016;20:517–35. [DOI] [PubMed] [Google Scholar]
- 73. Levolger S, vanVugt JLA , deBruin RWF , IJzermans JNM. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–58. [DOI] [PubMed] [Google Scholar]
- 74. Sato N, Koeda K, Ikeda K, et al. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002;236:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nishiyama J, Matsuda M, Ando S, Hirasawa M, Suzuki T, Makuuchi H. The effects of the early administration of sivelestat sodium, a selective neutrophil elastase inhibitor, on the postoperative course after radical surgery for esophageal cancer. Surg Today. 2012;42:659–65. [DOI] [PubMed] [Google Scholar]
- 76. Nagai Y, Watanabe M, Baba Y, et al. Preventive effect of sivelestat on postoperative respiratory disorders after thoracic esophagectomy. Surg Today. 2013;43:361–6. [DOI] [PubMed] [Google Scholar]
- 77. Gao Q, Mok HP, Wang WP, Xiao‐Feizuo, Chen LQ. Effect of perioperative glucocorticoid administration on postoperative complications following esophagectomy: a meta‐analysis. Oncol. Lett. 2014;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang L, Wang N, Zhou S, et al. Preventive effect of ulinastatin on postoperative complications, immunosuppression, and recurrence in esophagectomy patients. World J Surg Oncol. 2013;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yamana I, Takeno S, Hashimoto T, et al. Randomized controlled study to evaluate the efficacy of a preoperative respiratory rehabilitation program to prevent postoperative pulmonary complications after esophagectomy. Dig Surg. 2015;32:331–7. [DOI] [PubMed] [Google Scholar]
- 80. Takata A, Takiguchi S, Miyazaki Y, et al. Randomized phase II study of the anti‐inflammatory effect of ghrelin during the postoperative period of esophagectomy. Ann Surg. 2015;262:230–6. [DOI] [PubMed] [Google Scholar]
- 81. Elaraj DM, Weinreich DM, Varghese S, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–96. [DOI] [PubMed] [Google Scholar]
- 82. Richards CH, Platt JJ, Anderson JH, McKee RF, Horgan PG, McMillan DC. The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Ann Surg. 2011;254:83–9. [DOI] [PubMed] [Google Scholar]
- 83. Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–60. [DOI] [PubMed] [Google Scholar]
- 84. Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E‐PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29:219–25. [DOI] [PubMed] [Google Scholar]
- 85. Tominaga T, Takeshita H, Takagi K, et al. E‐PASS score as a useful predictor of postoperative complications and mortality after colorectal surgery in elderly patients. Int J Colorectal Dis. 2016;31:217–25. [DOI] [PubMed] [Google Scholar]
- 86. Ariake K, Ueno T, Takahashi M, et al. E‐PASS comprehensive risk score is a good predictor of postsurgical mortality from comorbid disease in elderly gastric cancer patients. J Surg Oncol. 2014;109:586–92. [DOI] [PubMed] [Google Scholar]
- 87. Ishino Y, Saigusa S, Ohi M, et al. Preoperative C‐reactive protein and operative blood loss predict poor prognosis in patients with gastric cancer after laparoscopy‐assisted gastrectomy. Asian J Endosc Surg. 2014;7:287–94. [DOI] [PubMed] [Google Scholar]
- 88. Greenleaf EK, Kulaylat AN, Hollenbeak CS, Almhanna K, Wong J. Timing of adjuvant chemotherapy and impact on survival for resected gastric cancer. Ann Surg Oncol. 2016;23:4203–13. [DOI] [PubMed] [Google Scholar]
- 89. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oba K, Morita S, Rahman M, Sakamoto J. Factors associated with compliance and non‐compliance by physicians in a large‐scale randomized clinical trial. Trials. 2006;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hayashi Y, Correa AM, Hofstetter WL, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer. 2010;116:5619–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA flow diagram


