Abstract
Despite recent advances in chemotherapy, outcomes of patients with peritoneal metastases (PM) from gastric cancer are still very poor and standard treatment has not been established. Although oral S‐1 appears to be effective for patients with PM, the effects of systemic chemotherapy are limited. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) yield fewer benefits in patients with PM from gastric cancer than in patients with PM from other malignancies. In comparison, repeated intraperitoneal chemotherapy (RIPEC) with taxanes using an implantable peritoneal access port has a pharmacokinetic advantage for the control of peritoneal lesions and in combination with systemic chemotherapy can result in surprisingly long‐term survival in patients with PM from gastric cancer. Herein, we review the results of recent clinical studies specifically targeting PM from gastric cancer and discuss future prospects for an intraperitoneal approach to the ideal treatment of patients with gastric cancer with peritoneal involvement.
Keywords: gastric cancer, HIPEC, intraperitoneal chemotherapy, peritoneal metastasis
1. INTRODUCTION
Gastric cancer is the fifth most common malignancy worldwide, and the third leading cause of cancer‐related deaths.1 The peritoneum is the most frequent site of metastases and recurrences in patients with gastric cancer.2, 3 Although various approaches have been attempted such as extended resections, combination chemotherapy, heated intraperitoneal chemotherapy and immunotherapy, the prognosis of patients with peritoneal metastases (PM) is still very poor and there is no established standard treatment.
In general, these patients are treated with systemic chemotherapy similar to patients with other distant metastases. Based on the results of Asian phase III trials,4, 5 fluoropyrimidine plus platinum agents are considered to be the standard regimens for advanced or recurrent gastric cancer, although docetaxel or anthracyclines are combined to treat patients in Western countries.6, 7 However, the efficacy of these regimens for patients with PM is still unclear. Patients with PM with massive ascites are often excluded from clinical trials because of their poor general condition. Although the survival of patients with PM is supposed to be worse compared to patients without PM from gastric cancer who received chemotherapy,8 there are few clinical trials specifically targeting patients with PM, probably because of the lack of measurable disease.
Intraperitoneal (IP) infusion of anticancer drugs was intended to enable an increased dose and time of exposure of intra‐abdominal tumor cells to anticancer drugs with minimal systemic toxic effects. In fact, IP administration has been shown to result in a higher drug concentration and longer half‐life in the peritoneal cavity compared with intravenous administration, although this is affected by a variety of biophysical parameters, including molecular weight, charge and solubility.9, 10 Hyperthermia has been shown to increase the beneficial effects of anticancer agents by augmenting cytotoxicity and/or increasing the penetration of the drugs into tissue.11 Based on this theoretical background, hyperthermic intraperitoneal chemotherapy (HIPEC) combined with cytoreductive surgery (CRS) have been used mainly in Western countries as a general treatment for PM from various malignancies.
Recently, repeated intraperitoneal chemotherapy (IPC) has been used, administered with a subcutaneous infusion port connected to an intraperitoneal catheter. Once the port is implanted, anticancer drugs can be repeatedly injected into the abdominal cavity without additional surgical stress. This method has been most vigorously evaluated in patients with ovarian cancer and, based on the results of phase III studies,12, 13 repeated IPC is now recommended for patients with Stage III epithelial ovarian cancer after optimal debulking surgery according to the National Comprehensive Cancer Network Guideline.14 In this review, we summarize current clinical data on the multidisciplinary treatment of this disease, mainly focused on repeated IPC and then refer to recent developments in drug modification and delivery systems which may achieve better clinical results for patients with PM from gastric cancer.
2. SYSTEMIC CHEMOTHERAPY
Although previous phase III trials have established standard systemic therapy regimens for patients with metastatic gastric cancer, some anticancer drugs such as cisplatin or irinotecan, cannot be safely given to patients with PM, because of severe and sustained toxicity causing intestinal stenosis or ascites. Therefore, until recently, large‐scale trials have not been conducted for patients with PM and a standard chemotherapy regimen for these patients has yet to be established.
Table 1 shows the results of recent studies (within 10 years) of systemic chemotherapy targeted specifically for patients with PM from gastric cancer. Historically, 5‐fluorouracil (5‐FU) has been used as the key drug for patients with PM, and many regimens using other drugs combined with 5‐FU have been evaluated. Median survival time (MST) of patients in these studies was 8.0~13.2 months.15, 16, 17, 18, 19 Paclitaxel (PTX), which is generally used as second‐line treatment for patients with metastatic gastric cancer, was expected to be effective for PM because of favorable pharmacokinetics.20, 21 However, the effects of systemic PTX alone seems to be limited for patients with PM.17, 22 Recently, nanoparticle albumin‐bound paclitaxel (Nab‐PTX) has been shown to elicit a higher antitumor effect in a peritoneal xenograft model23 and used for clinical trials, although the results are still premature.24
Table 1.
Clinical outcomes of systemic chemotherapy for gastric cancer with PM
| Author, Year | Regimen | Study | n | MST (mo) | 1y‐OS (%) | RR (%) |
|---|---|---|---|---|---|---|
| Imazawa, 200915 | 5Fu + MTX | P2 | 31 | 9 | 16 | 25 |
| Oh, 200716 | FOLFOX‐4 | P2 | 48 | 8.4 | 27 | 33 |
| Iwasa, 201217 | 5Fu + leukovorin + PTX | P1/2 | 25 | 8.0 | – | – |
| Shirao, 201318 |
5Fu + MTX 5Fu continuous infusion |
P3 | 103 102 | 10.6, 9.4 | 40.7, 37 | – |
| Masuishi, 201719 | FOLFOX‐4 | R/S | 10 | 13.2 | – | |
| Imamoto, 2011 22 | PTX | R/S | 64 | 5.2 | – | 39 |
| Ishizone, 200625 | S‐1 | P2 | 16 | 18 | – | |
| Shitara, 201326 | (S1/Cap) + CDDP | R/S | 120 | 15.9 | 60< | – |
| Shigeyasu, 201327 | S1 + docetaxel | P2 | 19 | 15.3 | 58 | – |
| Shitara, 201724 | PTX | P3 | 248 | 10.9 | – | – |
| Nab‐PTX (triweekly) | 247 | 10.3 | ||||
| Nab‐PTX (weekly) | 246 | 11.1 |
Cap, capecitabine; CDDP, cisplatin; FOLFOX‐4, oxaliplatin, leucovorin and 5‐fluorouracil; 5Fu, 5‐fluorouracil; MST, median survival time; MTX, methotrexate; Nab‐PTX, nanoparticle albumin‐bound paclitaxel; OS, overall survival; PM, peritoneal metastases; PTX, paclitaxel; P1, P2, P3; phase I, II, III; R/S, retrospective study; ‐, not described.
S‐1 is an oral fluoropyrimidine derivative, combining tegafur with two modulators, and is considered to be a pivotal agent for the treatment of patients with gastric cancer in Japan. Small‐scale studies, with notable efficacy for S‐1 alone25 or in combination with cisplatin26 or docetaxel27 have been reported, suggesting superior results for S‐1 in the control of PM compared with other 5‐FU derivatives. Taken together, however, these results suggest that the effects of systemic administration are considered to be limited presumably as a result of the so‐called “peritoneal‐plasma barrier” which prevents effective drug delivery from the systemic circulation to peritoneal lesions.
3. CYTOREDUCTIVE SURGERY AND HIPEC
Hyperthermic intraperitoneal chemotherapy combined with total peritonectomy was originally developed by Sugarbaker based on the concept that peritoneal metastasis is a localized disease in the abdominal cavity.28 At specialized centers in Western countries, this aggressive approach has been carried out mainly in patients with pseudomyxoma, mesothelioma, ovarian and colorectal cancers, which suggested considerable efficacy for PM as a result of these malignancies. However, evidence for this strategy against PM from gastric cancer is relatively scarce because of the low frequency of this condition in Western countries. In Asia, HIPEC combined with modified surgery has been used to treat patients with gastric cancer in specialized centers for many years, but large‐scale comparative studies have not been done, probably because of the high toxicity associated with these regimens.
Table 2 summarizes recent reports on cytoreductive surgery (CRS) and HIPEC in the treatment of patients with PM from gastric cancer including two phase III studies. As in ovarian and colorectal cancer, mitomycin (MMC), cisplatin (CDDP) and, more recently, oxaliplatin, have been used for HIPEC.29, 30, 31, 32, 33, 34 However, MST of the patients was 9.2~11.5 months and the 1‐year survival did not exceed 50%, except in a small‐scale phase II study that shows a MST of 19 months in patients treated with HIPEC followed by systemic treatment with FLOT (5Fu + leukovorin + oxaliplatin + docetaxel) regimen.35 Even in patients who received optimal cytoreduction, MST remains at 15~25 months, which is significantly worse than the survival of patients with ovarian cancer or colorectal cancer and does not exceed the survival in patients treated with systemic chemotherapy.
Table 2.
Clinical outcomes of CRS and HIPEC for gastric cancer with PM
| Author, Year | Regimen | Study | n | MST (mo) | Survival (%) | Morbidity % (Mortality %) |
|---|---|---|---|---|---|---|
| Hall, 200429 | MMC | P2 | 34 | 11.2 | 1 y OS, 45 (CC0/1) 16 (CC2) | 35 (–) |
| Yonemura, 200530 | MMC + CDDP | R/S | 107 | 11.5 | 5 y OS, 6.7 | 21.5 (2.8) |
| Glehen, 201031 | MMC + CDDP or L‐OHP + CPT‐11 | R/S | 159 | 9.2 | 1 y OS, 43 (CC0; 65) | 27.8 (6.7) |
| Yang, 201132 | MMC + CDDP | P3 | 34 | 11.0 | 1 y OS, 41 | 14.7 (–) |
| Magge, 201433 | MMC + CDDP | P2 | 23 | 9.5 | 1 y OS, 50 | 52.2 (4.3) |
| Muller, 201435 | L‐OHP + DTX | P2 | 26 | 19 | 2 y OS, 38 | 23 (0) |
| Rudloff, 201434 | L‐OHP | P3 | 9 | 11.3 | 1 y OS, 44 | 88 (11) |
CDDP, cisplatin; CPT‐11, irinotecan; CRS, cytoreductive surgery; DTX, docetaxel; 5Fu, 5‐fluorouracil; HIPEC, hypothermic intraperitoneal chemotherapy; L‐OHP, oxaliplatin; MMC, mitomycin; MST, median survival time; OS, overall survival; PM, peritoneal metastases; P2, P3, phase II, phase III; R/S, retrospective study; CC‐0, CC‐1, complete resection‐0, ‐1;‐, not described.
The lack of appreciable increase in survival is presumably caused by a higher malignant potential of disseminated gastric cancer cells. Moreover, morbidity was 14.7%~88% with significant mortality. Although other drug combinations may improve outcomes, it is suggested that HIPEC results in less benefit for patients with PM from gastric cancer compared with patients with PM from other malignancies. A recent review suggests this aggressive treatment should be used only in patients with a low peritoneal carcinoma index (PCI <6) and a good response with negative cytology after HIPEC in patients with gastric cancer.36
4. REPEATED INTRAPERITONEAL CHEMOTHERAPY USING TAXANES
The most significant shortcoming of HIPEC is that a single dose, even with hyperthermia, is not sufficient to allow the anticancer drugs to infiltrate into the deep portion of metastatic lesions on the peritoneal surfaces, and therefore multiple IP doses are needed to result in marked antitumor effects on the PM. Repeat IP infusion of anticancer drugs using an implantable port system was carried out over a decade ago. To treat patients with PM from gastric cancer, neoadjuvant MMC and CDDP were used. However, the clinical effects of those series were disappointing, probably because those drugs are readily absorbed into the systemic circulation and do not stay in the abdominal cavity for a long time. Indeed, pharmacokinetic studies have shown relatively low ratios using area under the curve analysis comparing peritoneal cavity levels to the systemic compartment after IP doasage of MMC or CDDP.10, 37 Therefore, this approach has long been abandoned in clinical trials.
In this century, however, attention is again focused on repeated intraperitoneal chemotherapy (RIPEC) using taxanes (Figure 1). Taxanes such as PTX and docetaxel (DTX) are insoluble in water and solubilized with a specific agent, Cremophor EL, and ethanol (Taxol®, BMS, New York, USA) or polysorbate 80 (Taxotare®, Sanofi, Paris, France), respectively. These form relatively large particles (10‐12 nm in diameter) in solution and are gradually absorbed through the lymphatic system only, which results in prolonged retention in the peritoneal cavity.38, 39 After IP infusion, taxanes show much higher area under the curve ratios than other hydrophilic drugs when comparing levels in the peritoneum to the plasma.10, 40 Even if they are infused many times, taxanes rarely result in peritoneal adhesions which may prevent drug diffusion to PM, probably because of their strong antiproliferative effects. These two biological characteristics are a major advantage for RIPEC.
Figure 1.

Left: Repeated intraperitoneal chemotherapy using an implantable port system. The catheter is placed in the pouch of Douglas (arrows) and taxanes dissolved in 500~1000 mL saline infused over 60 min. Right: Representative laparoscopic and X‐ray views of intraperitoneal port and catheter
Based on these basic findings, RIPEC using PTX or DTX at normothermic conditions has been attempted for the treatment of patients with PM from gastric cancer mainly in Japan. In those studies, IP taxanes were combined with systemic chemotherapy as neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) and the results were already summarized in a previous review.41 As shown in Table 3, this approach resulted in a MST of 15.1~24.6 months and 1‐year survival of over 70%,42, 43, 44, 45, 46, 47, 48, 49 which are considerably better than results with systemic chemotherapy or HIPEC.
Table 3.
Clinical outcomes of repeated IPC with systemic chemotherapy for gastric cancer with PM
| Author, Year | IP regimen | Systemic regimen | Study | n | MST (mo) | 1 y OS (%) | Cytology negative conversion rate (%) |
|---|---|---|---|---|---|---|---|
| Ishigami, 201042 | PTX (20 mg/m2) | S‐1 + PTX | P2 | 40 | 22.5 | 78 | 86 |
| Fujiwara, 201243 | DTX (40~60 mg/m2) | S‐1 | R/S | 18 | 24.6 | 76 | 78 |
| Fushida, 201344 | DTX (45 mg/m2) | S‐1 | P1/2 | 39 | 16.2 | 70.4 | 81 |
| Yamaguchi, 201345 | PTX (20 mg/m2) | S‐1 + PTX | P1 | 35 | 17.6 | 77.1 | 97 |
| Ishigami, 201646 | PTX (20 mg/m2) | S‐1 + PTX | P3 | 114 | 17.7 | 71.9 | 95 |
| Fujiwara, 201647 | PTX (40 mg/m2) | S‐1 + L‐OHP | P2 | 60 | NR | 71.5 | 71 |
| Fukushima, 201748 | DTX (10 mg/m2) | Cap + CDDP | P2 | 48 | NR | 75 | 76 |
| Cho, 201749 | DTX (100 mg/m2) | Cap + CDDP | P1/2 | 39 | 15.1 | – | – |
Cap, capecitabine; CDDP, cisplatin; DTX, docetaxel; IPC, intraperitoneal chemotherapy; L‐OHP, oxaliplatin; MST, median survival time; NR, not reached; OS, overall survival; PM, peritoneal metastases; PTX, paclitaxel. P1, 2, 3, phase I, II, III; R/S, retrospective study; ‐, not described.
In fact, second‐look laparoscopy showed a drastic macroscopic change in the appearance of PM in many patients (Figure 2). The mechanism for marked shrinkage of peritoneal tumors has not been fully elucidated. However, murine studies have shown that PTX, given as a single IP infusion, directly infiltrates up to several hundred micrometers beneath the surface of peritoneal nodules and induces massive destruction of tumor cells as well as microvessels in the tumor periphery.50, 51 Thus, repeated doses of IP PTX might eradicate deeper tumor cells. Drastic changes in the peripheral structure of each nodule may reduce intratumor pressure and enhance the delivery efficiency of systemically infused anticancer drugs, which might be another important mechanism to explain the remarkable antitumor effects against PM. If gastrectomy is carried out in patients with a good response, MST reached 26.5~30.5 months.52, 53 Determining the appropriate criteria and timing for conversion surgery is an important clinical subject for the future.
Figure 2.
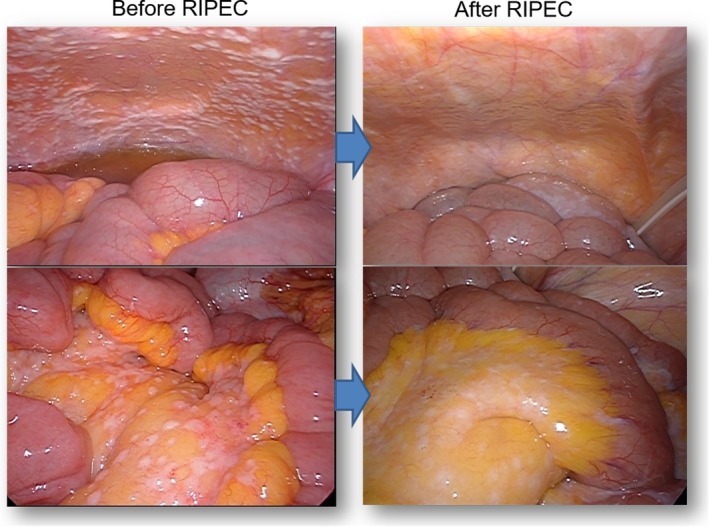
Representative laparoscopic views of peritoneal metastases before and after repeated intraperitoneal chemotherapy
Another important advantage of RIPEC is relatively mild toxicity compared with HIPEC. According to one series, grade 3/4 neutropenia occurred in 21%~50%42, 45, 46, 47, 48 and port‐related complications such as infection, occlusion and reflux occurred in 20.6%.54 However, non‐hematological toxicities were relatively rare with no treatment‐related deaths. It is notable that abdominal pain, which is often considered to be a dose‐limiting toxicity in IPC, was rarely observed probably because of the low dose of IP taxanes.
Patients with only isolated tumor cells in the peritoneal cavity (P0CY1) also have dismal long‐term outcomes and there is no established consensus to direct treatment.55 As this strategy can cause remarkable shrinkage of macroscopic tumors, RIPEC can be expected to be more effective against P0CY1 cases. In fact, the cytology converted to be negative in 71%~97% of patients with PM,42, 43, 44, 45, 46, 47, 48 which has never been achieved in other methods in previous reports. Moreover, when used for P0CY1 cases, 1‐year OS rate was 84.2% with negative change of cytology in 94.7% patients.56 This suggests that RIPEC with taxanes has the splendid power to control peritoneal micrometastases and thus may be a promising strategy for the prevention of peritoneal recurrence for gastric cancer with serosal exposure.57
As pharmaceutical companies are not interested in clinical trials of drugs with an already expired patent, a reasonable drug‐approval system needs to be established for the sake of patients with this dismal condition.
5. OTHER NOVEL INTRAPERITONEAL TREATMENTS
Catumaxomab is a trifunctional monoclonal antibody with two different antigen‐binding sites, EpCAM, CD3 and a functional Fc domain, thereby activating a complex antitumor immune reaction.58 Heiss et al reported that IP injection of catumaxomab improved puncture‐free survival and had a better trend in survival in patients with malignant ascites.59 Based on these results, catumaxomab has been licensed for clinical use in the European Union since 2009 for patients with malignant effusions. Goere et al have shown clinical efficacy for the treatment of patients with PM from gastric cancer.60
Bevacizumab, a humanized variant of an antivascular endothelial growth factor (VEGF) antibody, might be another useful drug for the treatment of malignant ascites.61 Fushida et al have shown that systemic infusion of anti‐VEGF antibody is effective in patients with malignant ascites.62 More recently, the effectiveness of immune checkpoint blocking antibodies has been reported in patients with metastatic gastric cancer.63 Although no clinical trials are targeted for patients with PM from gastric cancer, the use of these antibody preparations with IPC might be promising.
Nanodrugs, a new drug formulation, measuring 20‐100 nm in molecular diameter are another promising approach for patients with PM. Nanodrugs are preferentially accumulated in tumor tissue as a result of the enhanced permeability and retention (EPR) effect.64 PMB‐30W is a water‐soluble, amphiphilic polymer composed of 2‐methacryloxyethyl phosphorylcholine and n‐butyl methacrylate and enables the construction of PTX‐containing nanoparticles.65 IP administration of PTX formulated with PMB‐30W resulted in deeper penetration into peritoneal nodules and showed enhanced antitumor effects against peritoneal xenografts of human gastric cancer compared with conventional cremophor‐conjugated PTX in murine models.50, 66 In the same model, the intraperitoneal administration of another PTX‐incorporating polymeric micellar nanoparticle, NK105, was shown to have significantly greater antitumor effects compared with IP Taxol®.67 As NK105 was already used as a second‐line treatment for patients with recurrent gastric cancer with an excellent response,68 IP chemotherapy with NK105 might be useful for clinical trial.
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel technique which delivers anticancer drugs into the closed abdominal cavity as an aerosol under pressure.69 This unique drug delivery technique is based on the concept that generating an artificial pressure gradient with a laparoscopic procedure can enhance tissue uptake with homogeneous distribution of vaporized drugs within the closed abdominal cavity. According to a recent report by Nadiradze et al, 24 patients with PM from gastric cancer treated with PIPAC using low‐dose cisplatin and doxorubicin had a MST of 15.4 months.70 Although these are early results, this method may be another promising strategy for the treatment of PM.
Cell‐free and concentrated ascites reinfusion therapy (CART) was originally developed for patients with cirrhotic ascites, but is fairly effective for palliation in patients with massive ascites. After recent technological improvements, autologous ascites which contains a large number of proteins and nutrients, can be reinfused without severe toxicity, often resulting in a drastic improvement of the general condition of cachectic patients with PM.71, 72 In fact, induction of CART enables patients with highly advanced PM and massive ascites to receive IPC with improved survival.73
6. CONCLUSION
For many years, PM from gastric cancer have been considered a terminal condition, and treatment has typically been palliative. Repeated IPC with taxanes enables delivery of a high concentration of drugs to the tumor cells in the peritoneal nodules, and seems to be the best approach for the treatment of PM so far. However, the effectiveness of IPC critically depends on how homogeneously the drug can be distributed in the entire abdomen and how deeply the drug can infiltrate into the peritoneal tumors. Many factors are related to the distance of drug penetration in solid tumors and the mechanisms are still largely unknown.74 Drug modification as well as improved delivery systems to enhance drug infiltration in peritoneal tumors should further prolong the survival of these patients. The era is coming when PM of gastric cancer are manageable.
DISCLOSURE
Conflicts of Interest: The author has no financial and personal relationships with other people or organizations that could inappropriately influence their work.
ACKNOWLEDGEMENTS
This study was funded by Japan Society for Promotion of Science and Japan Agency of Medical Research and Development.
Kitayama J, Ishigami H, Yamaguchi H, et al. Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2018;2:116–123. https://doi.org/10.1002/ags3.12060
REFERENCES
- 1. Stomach cancer estimated incidence, mortality and prevalence, worldwide. 2012 GLOBOCAN. 2012.
- 2. Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta‐analysis of randomized trials. Eur J Surg Oncol. 2014;40:12–26. [DOI] [PubMed] [Google Scholar]
- 4. Koizumi W, Narahara H, Hara T, et al. S‐1 plus cisplatin versus S‐1 alone for first‐line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. [DOI] [PubMed] [Google Scholar]
- 5. Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5‐fluorouracil/cisplatin as first‐line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73. [DOI] [PubMed] [Google Scholar]
- 6. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. [DOI] [PubMed] [Google Scholar]
- 7. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. [DOI] [PubMed] [Google Scholar]
- 8. Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago‐gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–403. [DOI] [PubMed] [Google Scholar]
- 9. Dedrick RL, Myers CE, Bungay PM, DeVita VT Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 10. Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277–83. [DOI] [PubMed] [Google Scholar]
- 11. Oleson JR, Calderwood SK, Coughlin CT, et al. Biological and clinical aspects of hyperthermia in cancer therapy. Am J Clin Oncol. 1988;11:368–80. [DOI] [PubMed] [Google Scholar]
- 12. Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard‐dose intravenous cisplatin plus paclitaxel versus moderately high‐dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small‐volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. [DOI] [PubMed] [Google Scholar]
- 14. Morgan RJ Jr, Alvarez RD, Armstrong DK, et al. Epithelial ovarian cancer. J Natl Compr Canc Netw. 2011;9:82–113. [DOI] [PubMed] [Google Scholar]
- 15. Imazawa M, Kojima T, Boku N, et al. Efficacy of sequential methotrexate and 5‐fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer. 2009;12:153–7. [DOI] [PubMed] [Google Scholar]
- 16. Oh SY, Kwon HC, Lee S, et al. A Phase II study of oxaliplatin with low‐dose leucovorin and bolus and continuous infusion 5‐fluorouracil (modified FOLFOX‐4) for gastric cancer patients with malignant ascites. Jpn J Clin Oncol. 2007;37:930–5. [DOI] [PubMed] [Google Scholar]
- 17. Iwasa S, Goto M, Yasui H, et al. Multicenter feasibility study of combination therapy with fluorouracil, leucovorin and paclitaxel (FLTAX) for peritoneal disseminated gastric cancer with massive ascites or inadequate oral intake. Jpn J Clin Oncol. 2012;42:787–93. [DOI] [PubMed] [Google Scholar]
- 18. Shirao K, Boku N, Yamada Y, et al. Randomized Phase III study of 5‐fluorouracil continuous infusion vs. sequential methotrexate and 5‐fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn J Clin Oncol. 2013;43:972–80. [DOI] [PubMed] [Google Scholar]
- 19. Masuishi T, Kadowaki S, Kondo M, et al. FOLFOX as First‐line Therapy for Gastric Cancer with Severe Peritoneal Metastasis. Anticancer Res. 2017;37:7037–42. [DOI] [PubMed] [Google Scholar]
- 20. Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47:2486–93. [PubMed] [Google Scholar]
- 21. Kobayashi M, Sakamoto J, Namikawa T, et al. Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients. World J Gastroenterol. 2006;12:1412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imamoto H, Oba K, Sakamoto J, et al. Assessing clinical benefit response in the treatment of gastric malignant ascites with non‐measurable lesions: a multicenter phase II trial of paclitaxel for malignant ascites secondary to advanced/recurrent gastric cancer. Gastric Cancer. 2011;14:81–90. [DOI] [PubMed] [Google Scholar]
- 23. Kinoshita J, Fushida S, Tsukada T, et al. Comparative study of the antitumor activity of Nab‐paclitaxel and intraperitoneal solvent‐based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol Rep. 2014;32:89–96. [DOI] [PubMed] [Google Scholar]
- 24. Shitara K, Takashima A, Fujitani K, et al. Nab‐paclitaxel versus solvent‐based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open‐label, randomised, non‐inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:277–87. [DOI] [PubMed] [Google Scholar]
- 25. Ishizone S, Maruta F, Saito H, et al. Efficacy of S‐1 for patients with peritoneal metastasis of gastric cancer. Chemotherapy. 2006;52:301–7. [DOI] [PubMed] [Google Scholar]
- 26. Shitara K, Mizota A, Matsuo K, et al. Fluoropyrimidine plus cisplatin for patients with advanced or recurrent gastric cancer with peritoneal metastasis. Gastric Cancer. 2013;16:48–55. [DOI] [PubMed] [Google Scholar]
- 27. Shigeyasu K, Kagawa S, Uno F, et al. Multicenter phase II study of S‐1 and docetaxel combination chemotherapy for advanced or recurrent gastric cancer patients with peritoneal dissemination. Cancer Chemother Pharmacol. 2013;71:937–43. [DOI] [PubMed] [Google Scholar]
- 28. Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8:454–63. [DOI] [PubMed] [Google Scholar]
- 30. Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370–5. [DOI] [PubMed] [Google Scholar]
- 31. Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi‐institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370–7. [DOI] [PubMed] [Google Scholar]
- 32. Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol. 2014;21:1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muller H, Hotopp T, Tofeili A, Wutke K. Systemic Chemotherapy using FLOT ‐ Regimen Combined with Cytoreductive Surgery plus HIPEC for Treatment of Peritoneal Metastasized Gastric Cancer. Hepatogastroenterology. 2014;61:703–6. [PubMed] [Google Scholar]
- 36. Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol. 2016;42:1123–31. [DOI] [PubMed] [Google Scholar]
- 37. Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7:108–15. [DOI] [PubMed] [Google Scholar]
- 38. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332:1004–14. [DOI] [PubMed] [Google Scholar]
- 39. Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–92. [DOI] [PubMed] [Google Scholar]
- 40. Ishigami H, Kitayama J, Otani K, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S‐1 for advanced gastric cancer. Oncology. 2009;76:311–4. [DOI] [PubMed] [Google Scholar]
- 41. Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017;20(Suppl 1):111–21. [DOI] [PubMed] [Google Scholar]
- 42. Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S‐1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. [DOI] [PubMed] [Google Scholar]
- 43. Fujiwara Y, Takiguchi S, Nakajima K, et al. Intraperitoneal docetaxel combined with S‐1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2012;105:38–42. [DOI] [PubMed] [Google Scholar]
- 44. Fushida S, Kinoshita J, Kaji M, et al. Phase I/II study of intraperitoneal docetaxel plus S‐1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2013;71:1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S‐1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–8. [DOI] [PubMed] [Google Scholar]
- 46. Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III study of intraperitoneal paclitaxel plus S‐1/paclitaxel compared with S‐1/cisplatin in gastric cancer patients with peritoneal metastasis: PHOENIX‐GC trial. J Clin Oncol. 2016;15(suppl):4014. [DOI] [PubMed] [Google Scholar]
- 47. Fujiwara Y, Ishigami H, Miwa H, et al. Phase II study of intraperitoneal paclitaxel plus S‐1/oxaliplatin for gastric cancer with peritoneal metastasis: SOX+IP PTX trial. J Clin Oncol. 2016;15(suppl):4040. [Google Scholar]
- 48. Fukushima R, Ishigami H, Miwa H, et al. Phase II study of intraperitoneal docetaxel plus capecitabine/cisplatin for gastric cancer with peritoneal metastasis: XP+IP DOC trial.. J Clin Oncol. 2017;15(suppl):4039. [Google Scholar]
- 49. Cho H, Ryu MH, Kim KP, et al. Phase I/II study of a combination of capecitabine, cisplatin, and intraperitoneal docetaxel (XP ID) in advanced gastric cancer patients with peritoneal metastasis. Gastric Cancer. 2017;20:970–7. [DOI] [PubMed] [Google Scholar]
- 50. Soma D, Kitayama J, Konno T, et al. Intraperitoneal administration of paclitaxel solubilized with poly(2‐methacryloxyethyl phosphorylcholine‐co n‐butyl methacrylate) for peritoneal dissemination of gastric cancer. Cancer Sci. 2009;100:1979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kitayama J, Emoto S, Yamaguchi H, Ishigami H, Watanabe T. Intraperitoneal paclitaxel induces regression of peritoneal metastasis partly by destruction of peripheral microvessels. Cancer Chemother Pharmacol. 2014;73:605–12. [DOI] [PubMed] [Google Scholar]
- 52. Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage Gastrectomy After Intravenous and Intraperitoneal Paclitaxel (PTX) Administration with Oral S‐1 for Peritoneal Dissemination of Advanced Gastric Cancer with Malignant Ascites. Ann Surg Oncol. 2014;21:539–46. [DOI] [PubMed] [Google Scholar]
- 53. Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20(Suppl 1):128–34. [DOI] [PubMed] [Google Scholar]
- 54. Emoto S, Ishigami H, Hidemura A, et al. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Jpn J Clin Oncol. 2012;42:1013–9. [DOI] [PubMed] [Google Scholar]
- 55. Cabalag CS, Chan ST, Kaneko Y, Duong CP. A systematic review and meta‐analysis of gastric cancer treatment in patients with positive peritoneal cytology. Gastric Cancer. 2015;18:11–22. [DOI] [PubMed] [Google Scholar]
- 56. Aizawa M, Ishigami H, Yabusaki H, et al. Phase II study of intraperitoneal paclitaxel plus S‐1/paclitaxel for gastric cancer with positive peritoneal cytology: CY‐PHOENIX trial. J Clin Oncol. 2017;4(Suppl):96. [Google Scholar]
- 57. Kitayama J, Ishigami H, Yamaguchi H, Emoto S, Watanabe T. Intraperitoneal Paclitaxel is useful as adjuvant chemotherapy for advanced gastric cancer with serosal exposure. Case Rep Oncol. 2014;7:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bokemeyer C. Catumaxomab–trifunctional anti‐EpCAM antibody used to treat malignant ascites. Expert Opin Biol Ther. 2010;10:1259–69. [DOI] [PubMed] [Google Scholar]
- 59. Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goere D, Gras‐Chaput N, Auperin A, et al. Treatment of gastric peritoneal carcinomatosis by combining complete surgical resection of lesions and intraperitoneal immunotherapy using catumaxomab. BMC Cancer. 2014;14:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kobold S, Hegewisch‐Becker S, Oechsle K, Jordan K, Bokemeyer C, Atanackovic D. Intraperitoneal VEGF inhibition using bevacizumab: a potential approach for the symptomatic treatment of malignant ascites? Oncologist. 2009;14:1242–51. [DOI] [PubMed] [Google Scholar]
- 62. Fushida S, Oyama K, Kinoshita J, et al. VEGF is a target molecule for peritoneal metastasis and malignant ascites in gastric cancer: prognostic significance of VEGF in ascites and efficacy of anti‐VEGF monoclonal antibody. Onco Targets Ther. 2013;6:1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro‐oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- 64. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 65. Konno T, Watanabe J, Ishihara K. Enhanced solubility of paclitaxel using water‐soluble and biocompatible 2‐methacryloyloxyethyl phosphorylcholine polymers. J Biomed Mater Res A. 2003;65:209–14. [DOI] [PubMed] [Google Scholar]
- 66. Kamei T, Kitayama J, Yamaguchi H, et al. Spatial distribution of intraperitoneally administrated paclitaxel nanoparticles solubilized with poly (2‐methacryloxyethyl phosphorylcholine‐co n‐butyl methacrylate) in peritoneal metastatic nodules. Cancer Sci. 2011;102:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Emoto S, Yamaguchi H, Kishikawa J, Yamashita H, Ishigami H, Kitayama J. Antitumor effect and pharmacokinetics of intraperitoneal NK105, a nanomicellar paclitaxel formulation for peritoneal dissemination. Cancer Sci. 2012;103:1304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kato K, Chin K, Yoshikawa T, et al. Phase II study of NK105, a paclitaxel‐incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Invest New Drugs. 2012;30:1621–7. [DOI] [PubMed] [Google Scholar]
- 69. Solass W, Kerb R, Murdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nadiradze G, Giger‐Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low‐Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J Gastrointest Surg. 2016;20:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Japanese CSG, Matsusaki K, Ohta K, Yoshizawa A, Gyoda Y. Novel cell‐free and concentrated ascites reinfusion therapy (KM‐CART) for refractory ascites associated with cancerous peritonitis: its effect and future perspectives. Int J Clin Oncol. 2011;16:395–400. [DOI] [PubMed] [Google Scholar]
- 72. Ito T, Hanafusa N, Iwase S, et al. Effects of cell‐free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy‐related ascites. Int J Clin Oncol. 2015;20:623–8. [DOI] [PubMed] [Google Scholar]
- 73. Yamaguchi H, Kitayama J, Emoto S, et al. Cell‐free and concentrated ascites reinfusion therapy (CART) for management of massive malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S‐1. Eur J Surg Oncol. 2015;41:875–80. [DOI] [PubMed] [Google Scholar]
- 74. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. [DOI] [PubMed] [Google Scholar]


