Abstract
The evolutionarily-conserved FOXO family of transcription factors has emerged as a significant arbiter of neural cell fate and function. From the neural stem cell state through mature neurons under both physiological and pathological conditions they have been found to modulate neural cell survival, stress responses, lineage commitment and neuronal signaling. Lineage-specific FOXO knockout mice have provided an invaluable tool for the dissection of FOXO biology in the nervous system. Within the neural stem cell compartments of the brain FOXOs are required for the maintenance of NSC quiescence and for the clearance of reactive oxygen species. Within mature neurons FOXO transcriptional activity is essential for the prevention of age-dependent axonal degeneration. Acutely, FOXO3 has been found to cause axonal degeneration upon withdrawal of neurotrophic factors. In more active neural signaling, FOXO6 promotes increased dendritic spine density of hippocampal neurons and is required for the consolidation of memories. In addition to the central nervous system (CNS) FOXOs also influence the functionality of the peripheral nervous system (PNS). FOXO1 knockout within the PNS results in a reduction of sympathetic tone and decreased levels of brain-derived norepinephrine and lower energy expenditure. FOXO3 knockout mice have impaired hearing which may be due to defects in synapse localization within the ear. Given the scope of FOXO activities in both the CNS and PNS it will be of interest to study FOXOs within the context of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, Huntington’s and Amyotrophic Lateral Sclerosis. From within the nervous system FOXOs may also regulate important parameters such as whole-body metabolism, motor function and catecholamine production; making FOXOs key players in physiologic homeostasis.
1. INTRODUCTION
In recent years mammalian FOXO transcription factors have arisen as crucial regulators of cell fate and function in the nervous system. Of the four mammalian homologs FOXO1, 3 and 6 are the ones most implicated in neuronal function (Hoekman et al., 2006). FOXOs have roles ranging from neural progenitor cell maintenance, reactive oxygen species (ROS) suppression, induction of apoptosis, promotion of survival by engagement of autophagy and regulation of catecholamine biosynthesis (Paik et al., 2009; Renault et al., 2009; Gilley, Coffer & Ham, 2003; Xu, Das, Reilly & Davis, 2011; Kajimura, Paone, Mann & Karsenty, 2014; Doan et al., 2016). The response of FOXOs to a given input (i.e. growth factor withdrawal or oxidative stress) is typically governed by post-translational modifications of the proteins which in-turn control FOXO subcellular localization and/or transcriptional activity. Due to their diversity of function gain or loss of FOXO activity in the mammalian nervous system can have a number of consequences under both physiologic and pathophysiologic conditions. In this review we will explore these context-dependent roles of FOXO in the mammalian nervous system and the phenotypic consequences they elicit.
2. NEURAL DEVELOPMENT & STEM CELL MAINTENANCE
The neural stem cells (NSCs) of the subventricular zone (SVZ) and the subgranular zone (SGZ) are a pool of brain stem cells capable of giving rise to new neurons throughout the lifespan of a mammal (Zhao, Deng & Gage, 2008). In order to accomplish this task without premature depletion (they have a limited replicative lifespan) their cell cycle and lineage commitment must be tightly regulated. Knockout of FOXO1/3/4 or even FOXO3 alone is sufficient to reduce the quiescence of NSCs causing them to hyperproliferate and therefore strongly deplete this stem cell pool early in life (Paik et al., 2009; Renault et al., 2009). In addition to hyperproliferation FOXO knockout NSCs also exhibit decreased self-renewal, increased apoptosis, decreased glycolytic flux, decreased glutathione, increased oxidative metabolism and high ROS levels (Paik et al., 2009; Yeo et al., 2013). Increased oxidative metabolism in conjunction with the decrease in glutathione are the likely causes of the increased ROS. Treatment with the ROS scavenging antioxidant N-acetylcysteine (NAC) can rescue the apoptosis and self-renewal defects but it cannot rescue the hyperproliferation. FOXO3 knockout NSCs from adult mice also have a bias in lineage commitment towards the astrocyte and away from the oligodendrocyte and neuronal lineages (Renault et al., 2009). In normal neural progenitors, FOXO3 inhibits ASCL1-dependent neurogenesis and also restrains neurogenesis in vivo (Webb et al., 2013). Similarly, constitutively active FOXO1 inhibits while its knockdown increases neurogenic differentiation of neural progenitors (Kim, Hwang, Muller & Paik, 2015). These findings together raise the possibility that FOXOs suppress lineage commitment and such activity may help maintaining the NSC reserves throughout the lifetime.
A phenotypic consequence of the above described NSC dysregulation is that FOXO1/3/4 and FOXO3 knockout mice have enlarged brains due to increased cell number and a limited ability to generate new neurons later in life (Paik et al., 2009; Renault et al., 2009). Although FOXO1/3/4 knockout mice have enlarged brains they are developmentally and histopathologically normal otherwise (Paik et al., 2009). The same is mainly true of the FOXO6 knockout mouse although FOXO6 knockout neurons do have a somewhat decreased dendritic spine density (Salih et al., 2012). They also have defects in the radial migration of cortical neurons (Paap et al., 2016). Interestingly, combined knockdown of FOXO1/3/6 by shRNA in vitro and in vivo results in Pak1 loss and severe defects in the establishment of neural polarity (de la Torre-Ubieta et al., 2010). This phenotype was only observed upon targeting FOXO1/3/6 simultaneously and not caused by knockdown of any FOXO alone; consistent with the normal brain architecture and development seen in other brain FOXO knockout mouse models. However, the polarity defect was best rescued by overexpression of FOXO6 rather than FOXO1 or FOXO3 which could not rescue as well. It may be that FOXO6 has a specialized role in the establishment of neuronal polarity although its loss is compensated by FOXO1 or FOXO3 to a certain extent.
3. NEURONAL SURVIVAL & STRESS RESPONSES
The FOXO family is well-known for its responsiveness to extracellular cues and execution of stress resistance or apoptotic programs; in the nervous system this is no exception. One of the prime regulators of FOXO activity is the PI3K/AKT pathway (Brunet et al., 1999; Kops et al., 1999; Nakae, Park & Accili, 1999). In response to a wide swath of growth factors that include insulin and neurotrophic factors (i.e. NGF and BDNF) AKT is activated which then phosphorylates FOXOs at three different sites (Fig. 1A & 1B). This phosphorylation results in their nuclear exclusion and cytoplasmic sequestration; rendering them unable to regulate gene expression. The relevance of this signaling pathway was first shown in PC12-derived and primary cultured sympathetic spinal cord neurons where overexpression of a dominant-negative FOXO3 construct could rescue BIM-induced cell death triggered by NGF withdrawal (Gilley, Coffer & Ham, 2003). Shortly thereafter this pathway was also described in motor neurons and it was suggested that FasL and JNK signaling might be partially responsible for FOXO3-induced death (Barthelemy, Henderson & Pettmann, 2004). Most recently AKT/FOXO3 signaling has been shown to be of central importance in neuronal cell death triggered from the axon that signals back to the cell body to commence apoptosis following NGF withdrawal (Simon et al., 2016). FOXO1 has also been shown to regulate apoptosis downstream of AKT in neurons. Synapse activity-dependent NMDA receptor (NMDAR) signaling activates PI3K/AKT which was shown to be neuroprotective under conditions of reactive oxygen species (ROS) accumulation (Papadia et al., 2008). Mechanistically, AKT-mediated inhibition of FOXO1 reduced TXNIP levels which resulted in increased thioredoxin activity and enhanced ROS detoxification.
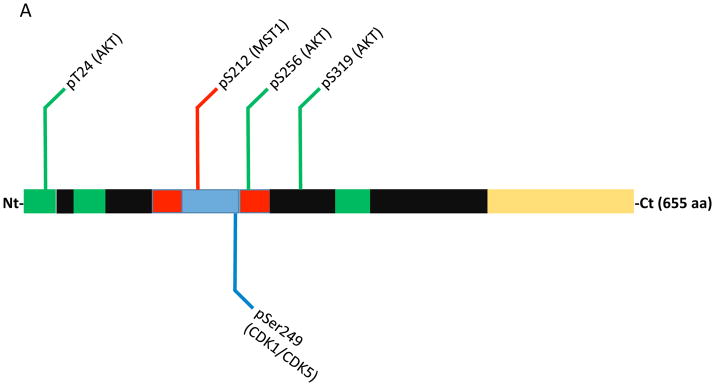
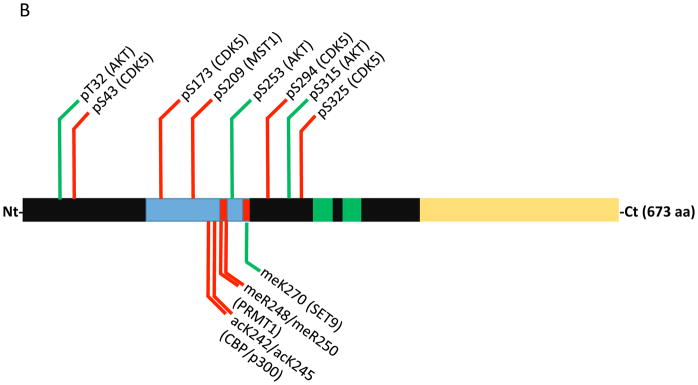
Figure 1.
Domain map and post-translaional modifications that influence FOXO1 & FOXO3 apoptotic activity within neurons. (A) Annotated FOXO1 protein numbered vs. the human sequence. Within the domain structure the forkhead domain is colored blue, nuclear localization signals are red, nuclear export signals are green and the transactivation domain is yellow. Modifications that promote cell death are marked with red lines, green lines mark modifications that promote survival and blue lines mark modifications with conflicting reports on survival. (B) Annotated FOXO3 protein numbered vs. the human sequence. Same color scheme as A.
FOXOs also regulate apoptosis in neurons in response to activity deprivation and ROS via the activation of alternative signaling routes. FOXO3 and FOXO1 have been found to be phosphorylated by the MST1 kinase in response to either ROS or neuronal activity deprivation (depolarization), respectively (Lehtinen et al., 2006; Yuan et al., 2009; Fig. 1A & 1B). MST1/MST2 are core components of the so-called Hippo pathway which regulates organ size by coordinating differentiation, proliferation and apoptosis (Qin, Tian, Zhou & Chen, 2013). In cerebellar granular neurons (CGNs) ROS triggers MST1 to phosphorylate FOXO3 on Ser209 (mislabeled as Ser207 in Lehtinen et al., 2006; Fig. 1B) which causes it to translocate to the nucleus and induce cell death (Lehtinen et al., 2006). In a related study FOXO1 was found to be phosphorylated by MST1 on Ser212 (equivalent of FOXO3 Ser209) in response to depolarization or growth factor withdrawal which also caused apoptosis (Yuan et al., 2009; Fig. 1A). Surprisingly, the cell cycle regulatory kinase CDK1 is activated in post-mitotic CGNs by depolarization and phosphorylates FOXO1 on Ser249 causing its nuclear translocation and apoptosis (Yuan et al., 2008; Fig. 1A). In cortical neurons under oxidative stress CDK5 was also found to phosphorylate Ser249 on FOXO1 (Zhou et al., 2015). In contrast to the CDK1 report this event resulted in the cytosolic sequestration of FOXO1 and attenuation of apoptosis. Interestingly, it was also found that Ser249 phosphorylation was dominant to AKT-mediated phosphorylation in that Ser249 phosphorylation could localize FOXO1 to the cytoplasm even when all three AKT sites were mutated to alanine. Increased CDK activity and Ser249 phosphorylation of FOXO1 have also been associated with the promotion of autophagy and neuronal survival. JNK triple knockout CGNs exhibited increased autophagic flux that is CDK and FOXO1-dependent (Xu, Das, Reilly & Davis, 2011). In this JNK-deficient background knockdown of FOXO1 by shRNA or pharmacological CDK inhibition resulted in inhibition of autophagy and increased CGN death. Additional studies will be needed to resolve the discrepant actions of FOXO1 in these distinct contexts.
In addition to phosphorylation methylation was another modification recently identified as modulating FOXO’s apoptotic activity. In response to ROS the methyltransferase SET9 was found to methylate FOXO3 on Lys270 which impaired its transcriptional activity resulting in reduced apoptosis in CGNs (Xie et al., 2012; Fig. 1B). It has also been shown that SIRT1-mediated deacetylation of FOXO3 at Lys242 and Lys245 by the class III histone deacetylase SIRT1 (this deacetylation also occurs on FOXO1 at the same sites) can attenuate FOXO3 apoptotic activity in CGNs (Brunet et al., 2004; Daitoku et al., 2004; Fig. 1B). SIRT1 is known to be activated by ROS as well as other stress stimuli such as DNA damage (Houtkooper, Pirinen & Auwerx, 2012). It will be of interest to further explore the interplay of these modifications and how they undoubtedly collaborate to regulate FOXO function and neuronal survival.
4. PHENOTYPIC CONSEQUENCES OF FOXO LOSS
Although it is clear that FOXOs can induce death in neurons in response to a variety of stressors they are also essential for the long-term maintenance of established neurons under physiologic conditions. Deletion of FOXO in neurons results in an age-dependent degeneration (Hwang and Paik, unpublished data).
Whole-body FOXO3 null mice have adult onset auditory neuropathy rendering them partially deaf (Gilels, Paquette, Zhang, Rahman & White, 2013). This was traced to the synapses being not correctly positioned within the cochlea. In this study it was also found that FOXO3 may function cell autonomously within spiral ganglion neurons where auditory stimulation could actually induce its nuclear localization. This activity-dependent response has also been seen with FOXO6 in the process of memory consolidation (Salih et al., 2012). FOXO6 knockout mice are capable of learning but have a reduced ability to form long-term contextual and object recognition memories. Furthermore, specific inhibition of FOXO6 in the hippocampus of wild-type mice by a dominant negative construct is sufficient to recapitulate this phenotype. Molecularly, learning tasks actually activate FOXO6 which then binds and activates the promoters of genes involved in synapse formation.
More restricted deletions of FOXOs have also provided insight into their nervous system function. FOXO1 brain-specific deletion increased the anxiety of mice in a forced swim test while deletion of FOXO3 decreased their anxiety (Polter et al., 2009). Strikingly, FOXO3 knockout anxiety was decreased similarly to the level of the antidepressant imipramine and the combination of FOXO3 knockout and imipramine gave the least anxiety; suggesting that imipramine may actually function in-part through FOXO3. In agreement imipramine treatment was found to inactivate FOXO3 in the cerebral cortex by cytosolic sequestration. Together with the FOXO1 results this study suggests that FOXOs may have distinct effects on depressive behavior.
FOXO1 has also been investigated by specific deletion in the sympathetic nervous system. Deletion of FOXO1 in the sympathetic nervous system by crossing FOXO1Flox mice with dopamine-β-hydroxylase (DBH) Cre mice resulted in reduced catecholamine (adrenaline) biosynthesis, reduced sympathetic nervous system activity (sympathetic tone), reduced energy expenditure, improved glucose clearance and increased bone mass (Kajimura, Paone, Mann & Karsenty, 2014). Notably, catecholamine production was reduced in the brain but was normal in the adrenaline-producing chromaffin cells of the adrenal medulla (adrenal gland); in these cells FOXO1 is not expressed so deletion had no direct effect. This effect on catecholamine biosynthesis was attributed to reduced levels of DBH expression due to loss of FOXO1 binding to the DBH promoter. When challenged with glucose FOXO1 sympathetic knockout mice exhibited enhanced insulin secretion which may explain their enhanced glucose clearance. FOXO1 has also been specifically knocked out in dopaminergic neurons using DAT-IRES-Cre (Doan et al., 2016). These mice demonstrated resistance to weight gain, improved glucose clearance, increased serum levels of norepinephrine, increased midbrain levels of dopamine and increased interscapular brown adipose tissue thermogenesis. The effect on dopamine biosynthesis was attributed to direct suppression of the tyrosine hydroxylase promoter by FOXO1 binding. These results are additionally supported by the finding that FOXO1 deletion using synapsin Cre has a similar metabolic profile including resistance to weight gain on a high fat diet (Ren et al., 2013). From these studies it is clear that FOXO1 is capable of regulating whole-body metabolism from its actions within the nervous system. However, it remains to be explained what role if any catecholamine metabolism actually plays in the common increased glucose clearance phenotype given the point that catecholamine biosynthesis is decreased in the DBH-Cre model while it is increased in the DAT-IRES-Cre model.
5. NEURODEGENERATIVE DISEASE
Huntington’s, Parkinson’s and Alzheimer’s Disease are all caused by the accumulation of insoluble protein aggregates (mHtt, α-synuclein/Lewy Bodies and amyloid beta plaques, respectively) that generate considerable oxidative and proteotoxic stress within neurons. This chronic stress eventually leads to axonal degeneration and/or apoptosis of the affected neurons (Montie & Durcan, 2013). Given the pivotal role FOXOs can play in triggering either neuronal apoptosis or survival in response to stress they are potentially very relevant players in the pathologies of these afflictions.
In Huntington’s disease (HD) mutant huntingtin protein (mHtt) was found to specifically activate the XBP1 transcription factor (Vidal et al., 2012). XBP1 knockout in the background of the mHtt mouse model reduced mHtt levels, enhanced neuronal survival and improved motor performance. These improvements were attributed to an increase in autophagy. Loss of XBP1 caused FOXO1 to accumulate on the protein level in the nucleus and co-expression of wild-type FOXO1 with mHtt in a cell line model was also able to increase autophagy and clearance of mHtt. Increased expression and nuclear localization has also been observed for FOXO3 in HD but the functional meaning of it has not yet been determined (Kannike, Sepp, Zuccato, Cattaneo & Timmusk, 2014).
FOXO3’s pro-apoptotic and survival activities have been evaluated in a Parkinson’s disease (PD) model by overexpression of wild-type, constitutively active and dominant-negative constructs in dopaminergic neurons of the substantia nigra (Pino et al., 2014). In agreement with many findings in other neuronal types overexpression of constitutively active FOXO3 induced acute apoptosis in DA neurons. However, high-level overexpression of dominant-negative FOXO3 was also toxic in DA neurons and this was accompanied by signs of oxidative damage; indicating the necessity of FOXO3 for basic neuronal survival and resistance to oxidative stress. When co-injected with α-synuclein constitutively active FOXO3 was still acutely toxic while dominant-negative FOXO3 was completely protective of DA neurons. There was no explanation offered for this striking level of protection provided by dominant-negative FOXO3 in light of the fact that α-synuclein levels and autophagic vacuole formation were largely unchanged by dominant-negative FOXO3 in DA neurons. FOXO1 and putative FOXO1 target genes (i.e. SMOX) are increased in the frontal cortex of PD patients which is a region of the brain also pathologically affected in PD but not as degenerated by the time of autopsy (Dumitriu et al., 2012). Whether this is also true in the DA neurons of the substantia nigra and the implications of this are unclear.
So far only FOXO3 has been studied in Alzheimer’s disease (AD) models and seems to largely be an inducer of cell death in response to amyloid beta (Aβ) plaques. Injection of Aβ1-42 into the hippocampus and cortex of adult rats resulted in a series of post-translational modifications to FOXO3 followed by its nuclear translocation and induction of cell death by BIM which could be rescued by FOXO3-directed shRNA (Sanphui & Biswas, 2013). Aβ1-42 induced a decrease in AKT-mediated phosphorylation, increased MST1 phosphorylation at Ser209 and increased methylation by PRMT1; the methylation at Arg248 and Arg250 being known to promote the nuclear retention and activation of FOXOs (Yamagata et al., 2008; Fig. 1B). Additional modifications of FOXO3 have been uncovered that are particularly relevant in the pathogenesis of Alzheimer’s. CDK5 can phosphorylate FOXO3 at Ser43, Ser173, Ser294 and Ser325 in response to glutamate-induced excitotoxicity in immortalized mouse hippocampal (HT22) cells or primary cortical neurons (Shi, Viccaro, Lee & Shah, 2016; Fig. 1B). These phosphorylations can induce FOXO3 nuclear translocation and initially promote stress resistance through upregulation of MnSOD. At later time points FOXO3 drives BIM expression and cell death and also promotes neurotoxic cleavage and excretion of Aβ1-42. Knockdown of CDK5 or FOXO3 is sufficient to prevent these events. In a p25-induced mouse model of Alzheimer’s early activation of both CDK5 and FOXO3 were observed, preceding Aβ plaque formation and cell death. Further support for FOXO3 as a pivotal player in early AD pathogenesis comes from the discovery that the miR-132/212 microRNAs are strongly suppressed in early and late stage AD neurons and that this corresponds with upregulation of PTEN and FOXO3 (Wong et al., 2013). These microRNAs were found to directly bind to the 3pUTRs of PTEN and FOXO3 inhibiting their expression. Experimentally, inhibition of miR-132/212 increased baseline apoptosis in hippocampal neurons and also enhances apoptosis in response to oxidative stress. These effects could be rescued by knockdown of PTEN and FOXO3.
6. FUTURE DIRECTIONS
Although much has been revealed about the role of FOXOs in the mammalian nervous system much remains to be understood. Physiologically, FOXO1 and FOXO3 seem to be necessary for the maintenance of neuronal integrity from mid-life on. They may work to counteract and/or prevent the cumulative damage caused by proteotoxic stress and reactive oxygen species. It will be of interest to study the neuron-specific FOXO knockout model further to identify additional signaling and metabolic pathways FOXO may regulate that offer neuronal protection. This will increase our understanding of exactly how neurons age and what may be done to maintain their functionality. A mammalian genetic model that could enhance FOXO activity throughout the nervous system would be most welcome in order to assess whether or not FOXO can slow or prevent the onset of age-related neuropathies and accompanying motor and cognitive decline. From a developmental perspective further exploration of FOXO6 loss in conjunction with the deletion of other FOXO isoforms will be necessary to establish the full meaning of FOXO in neural development. This may lead to new understandings about the pathways and signaling cues that drive mammalian neurogenesis and neural circuit construction. The extensive post-translational modifications that regulate FOXO activity indicate that FOXOs act as hubs for the integration of signaling outputs from diverse pathways. A deeper understanding of the interplay of these modifications and how they influence FOXO-mediated gene regulation will be a necessary step towards understanding the full range of their contextual functions.
Further study of the roles of FOXOs within the full spectrum of neurodegenerative diseases is warranted. It may prove fruitful to delete FOXOs within the backgrounds of established PD, HD and AD models to clarify their functions in these pathologies. Studies should also be extended to the role of FOXOs in Amyotrophic Lateral Sclerosis (ALS) given the progressive degeneration observed in the FOXO1/3/4 knockout model. Finally, efforts should be made to evaluate pharmacological interventions to restore or inhibit FOXO functionality as appropriate. This could be accomplished by targeting known or novel FOXO regulatory enzymes as this may lead to potent treatments for these destructive conditions.
References
- Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neuroscience. 2004;5(48):1–9. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownawell AM, Kops GJ, Macara IG, Burgering BM. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Molecular and Cellular Biology. 2001;21(10):3534–3546. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, et al. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. The Journal of Cell Biology. 2002;156(5):817–828. doi: 10.1083/jcb.200112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(27):10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan KV, Kinyua AW, Yang DJ, Ko CM, Moh SH, Shong KE, et al. FoxO1 in dopaminergic neurons regulates energy homeostasis and targets tyrosine hydroxylase. Nature Communications. 2016;7:12733. doi: 10.1038/ncomms12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu A, Latourelle JC, Hadzi TC, Pankratz N, Garza D, Miller JP, et al. Gene expression profiles in Parkinson disease prefrontal cortex implicate FOXO1 and genes under its transcriptional regulation. PLOS Genetics. 2012;8(6):e1002794. doi: 10.1371/journal.pgen.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilels F, Paquette ST, Zhang J, Rahman I, White PM. Mutation of Foxo3 causes adult onset auditory neuropathy and alters cochlear synapse architecture in mice. The Journal of Neuroscience. 2013;33(47):18409–18424. doi: 10.1523/JNEUROSCI.2529-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. The Journal of Cell Biology. 2003;162(4):613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expression Patterns. 2006;6(2):134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nature Reviews Molecular Cell Biology. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. The Journal of Biological Chemistry. 2003;278(38):35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- Kajimura D, Paone R, Mann JJ, Karsenty G. Foxo1 regulates Dbh expression and the activity of the sympathetic nervous system in vivo. Molecular Metabolism. 2014;3(7):770–777. doi: 10.1016/j.molmet.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannike K, Sepp M, Zuccato C, Cattaneo E, Timmusk T. Forkhead transcription factor FOXO3a levels are increased in Huntington disease because of overactivated positive autofeedback loop. The Journal of Biological Chemistry. 2014;289(47):32845–32857. doi: 10.1074/jbc.M114.612424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Hwang I, Muller FL, Paik JH. Functional regulation of FoxO1 in neural stem cell differentiation. Cell Death and Differentiation. 2015;22(12):2034–2045. doi: 10.1038/cdd.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398(6728):630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125(5):987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Montie HL, Durcan TM. The cell and molecular biology of neurodegenerative diseases: an overview. Frontiers in Neurology. 2013;4:194. doi: 10.3389/fneur.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. The Journal of Biological Chemistry. 1999;274(23):15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- Nasrin N, Ogg S, Cahill CM, Biggs W, Nui S, Dore J, et al. DAF-16 recruits the CREB-binding protein coactivator complex to the insulin-like growth factor binding protein 1 promoter in HepG2 cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(19):10412–10417. doi: 10.1073/pnas.190326997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paap RH, Oosterbroek S, Wagemans CM, von OL, Schellevis RD, Vastenhouw-van der Linden AJ, et al. FoxO6 affects Plxna4-mediated neuronal migration during mouse cortical development. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(45):E7087–E7096. doi: 10.1073/pnas.1609111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nature Neuroscience. 2008;11(4):476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino E, Amamoto R, Zheng L, Cacquevel M, Sarria JC, Knott GW, et al. FOXO3 determines the accumulation of alpha-synuclein and controls the fate of dopaminergic neurons in the substantia nigra. Human Molecular Genetics. 2014;23(6):1435–1452. doi: 10.1093/hmg/ddt530. [DOI] [PubMed] [Google Scholar]
- Polter A, Yang S, Zmijewska AA, van GT, Paik JH, DePinho RA, et al. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biological Psychiatry. 2009;65(2):150–159. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Tian J, Zhou D, Chen L. Mst1 and Mst2 kinases: regulations and diseases. Cell & Bioscience. 2013;3(1):31. doi: 10.1186/2045-3701-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Plum-Morschel L, Gutierrez-Juarez R, Lu TY, Kim-Muller JY, Heinrich G, et al. Blunted refeeding response and increased locomotor activity in mice lacking FoxO1 in synapsin-Cre-expressing neurons. Diabetes. 2013;62(10):3373–3383. doi: 10.2337/db13-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochemical Journal. 2001;354(Pt 3):605–612. doi: 10.1042/0264-6021:3540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih DA, Rashid AJ, Colas D, Torre-Ubieta L, Zhu RP, Morgan AA, et al. FoxO6 regulates memory consolidation and synaptic function. Genes & Development. 2012;26(24):2780–2801. doi: 10.1101/gad.208926.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanphui P, Biswas SC. FoxO3a is activated and executes neuron death via Bim in response to beta-amyloid. Cell Death and Disease. 2013;4:e625. doi: 10.1038/cddis.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Viccaro K, Lee HG, Shah K. Cdk5-Foxo3 axis: initially neuroprotective, eventually neurodegenerative in Alzheimer’s disease models. Journal of Cell Science. 2016;129(9):1815–1830. doi: 10.1242/jcs.185009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, et al. Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling. Cell. 2016;164(5):1031–1045. doi: 10.1016/j.cell.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So CW, Cleary ML. MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function. Molecular and Cellular Biology. 2002;22(18):6542–6552. doi: 10.1128/MCB.22.18.6542-6552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Ubieta L, Gaudilliere B, Yang Y, Ikeuchi Y, Yamada T, DiBacco S, et al. A FOXO-Pak1 transcriptional pathway controls neuronal polarity. Genes & Development. 2010;24(8):799–813. doi: 10.1101/gad.1880510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, et al. Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Human Molecular Genetics. 2012;21(10):2245–2262. doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Marshall CB, Yamamoto K, Li GY, Gasmi-Seabrook GM, Okada H, et al. Structures of KIX domain of CBP in complex with two FOXO3a transactivation domains reveal promiscuity and plasticity in coactivator recruitment. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(16):6078–6083. doi: 10.1073/pnas.1119073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Pollina EA, Vierbuchen T, Urban N, Ucar D, Leeman DS, et al. FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell Reports. 2013;4(3):477–491. doi: 10.1016/j.celrep.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Veremeyko T, Patel N, Lemere CA, Walsh DM, Esau C, et al. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Human Molecular Genetics. 2013;22(15):3077–3092. doi: 10.1093/hmg/ddt164. [DOI] [PubMed] [Google Scholar]
- Xie Q, Hao Y, Tao L, Peng S, Rao C, Chen H, et al. Lysine methylation of FOXO3 regulates oxidative stress-induced neuronal cell death. EMBO Reports. 2012;13(4):371–377. doi: 10.1038/embor.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Das M, Reilly J, Davis RJ. JNK regulates FoxO-dependent autophagy in neurons. Genes & Development. 2011;25(4):310–322. doi: 10.1101/gad.1984311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, et al. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Molecular Cell. 2008;32(2):221–231. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Yeo H, Lyssiotis CA, Zhang Y, Ying H, Asara JM, Cantley LC, et al. FoxO3 coordinates metabolic pathways to maintain redox balance in neural stem cells. EMBO Journal. 2013;32(19):2589–2602. doi: 10.1038/emboj.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Becker EB, Merlo P, Yamada T, DiBacco S, Konishi Y, et al. Activation of FOXO1 by Cdk1 in cycling cells and postmitotic neurons. Science. 2008;319(5870):1665–1668. doi: 10.1126/science.1152337. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Lehtinen MK, Merlo P, Villen J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. The Journal of Biological Chemistry. 2009;284(17):11285–11292. doi: 10.1074/jbc.M900461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochemical Journal. 2004;378(Pt 3):839–849. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li H, Li X, Zhang G, Niu Y, Yuan Z, et al. The roles of Cdk5-mediated subcellular localization of FOXO1 in neuronal death. Journal of Neuroscience. 2015;35(6):2624–2635. doi: 10.1523/JNEUROSCI.3051-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]