Abstract
Purpose of review
We reviewed and evaluated recently published scientific studies that explored the role of the intestinal microbiota in eating disorders.
Recent findings
Studies have demonstrated that the intestinal microbiota is a contributing factor to both host energy homeostasis and behavior—two traits commonly disrupted in patients with eating disorders. To date, intestinal microbiota research in eating disorders has focused solely on anorexia nervosa (AN). Initial studies have reported an atypical intestinal microbial composition in patients with AN compared to healthy controls. However, the impact of these AN-associated microbial communities on host metabolism and behavior remains unknown.
Summary
The intriguing pattern of findings in patients with AN encourages further investigation of the intestinal microbiota in eating disorders. Elucidating the specific role(s) of these microbial communities may yield novel ideas for augmenting current clinical therapies to promote weight gain, decrease gastrointestinal distress, and even reduce psychological symptomatology.
Keywords: Eating disorders, intestinal microbiota, metabolism, brain-gut-microbiota axis
Introduction
Eating disorders
Eating disorders encompass a range of debilitating psychiatric illnesses broadly characterized by extreme weight and appetite dysregulation [1]. Of the three major eating disorders—anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) —AN is the only eating disorder to date that has been investigated in relation to the intestinal microbiota [2–6]. AN is specifically characterized by extreme weight loss or failure to gain expected weight accompanied by fear of weight gain. The disorder typically—but not exclusively—presents during adolescence and affects 0.9% of females and 0.3% of males in the United States [7, 8]. AN has the highest mortality rate of any psychiatric illness with a standardized mortality ratio of 5.86, and only half of patients experience long-term recovery [9, 10]. Moreover, patients with AN often present with other psychiatric and physiological disturbances including anxiety, depression, and gastrointestinal (GI) distress, further complicating the treatment of this disorder [8, 11].
Treatments for acute AN generally involve a combination of clinical renourishment to promote weight gain and psychotherapy to address disordered eating cognitions and behaviors [12, 13]. The evidence base for psychotherapeutic interventions is weak, especially in adults, and clinical protocols for refeeding vary considerably. Refeeding is often associated with GI distress including pain, bloating, and constipation as well as abnormal body fat deposition [14, 15]. Weight relapse (the re-loss of weight after refeeding) is common and contributes to recurrent presentations [16].
Like all eating disorders, the etiology of AN remains incompletely understood, but as with other complex traits, AN is influenced by an array of genetic and environmental factors [17–19]. The poor understanding of the underlying biology of eating disorders has hampered the development of optimal evidenced-based practices to guide clinicians in their approach. Deeper insight into the biological underpinnings of AN has the potential to significantly improve the standard of care and advance the development of effective pharmaceuticals or other treatments for AN. Although many biological factors merit investigation, the intestinal microbiota has recently emerged as a potential target for treatment during clinical renourishment to ameliorate GI distress and improve treatment outcomes.
This review provides an overview of the roles that the intestinal microbiota plays in eating disorders (Figure 1). The review first focuses on characterizing the intestinal microbiota and then explores the avenues through which these enteric (i.e., intestinal) communities may contribute to the persistence, recovery, or relapse from eating disorders.
Figure 1.
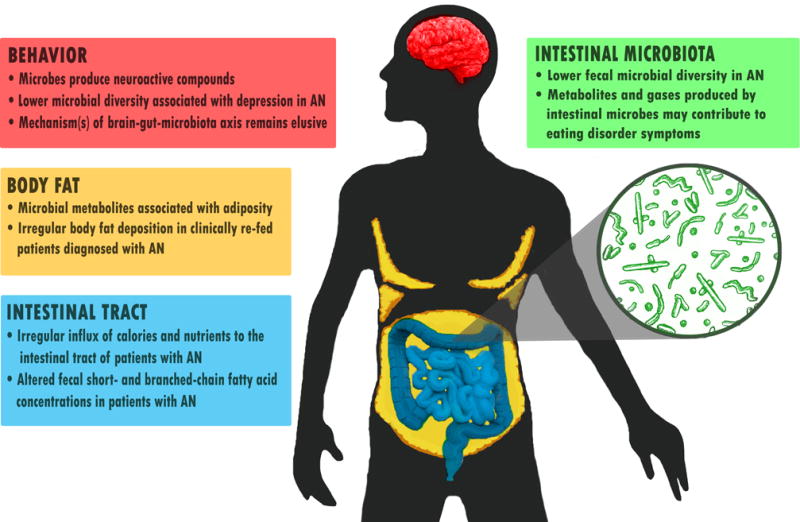
Microbial influences in anorexia nervosa (AN)
The intestinal microbiota
The intestinal microbiota is defined as the community of microorganisms, including bacteria, archaea, fungi, parasites, and viruses, that reside within the human GI tract [20]. It has been estimated that this complex community comprises trillions of microbes, equating to a 1:1 ratio of human-to-bacterial cells, with the greatest density and diversity found in the lower GI tract [21]. The specific collection of microorganisms is unique to each individual and the composition of the intestinal microbiota is influenced by myriad host factors including genetics, diet, health status, age, sex, geographical location, and drug exposure [22–30]. Microbial dysbiosis—an imbalance in the expected prevalence of microbial species in the intestinal niche—is often associated with various diseases [27]. The vast majority and most well researched of these microbes are bacteria, which are the focus of this review. However, the role of fungi and viruses should not be overlooked, as these kingdoms are emerging as relevant to other GI diseases, such as inflammatory bowel diseases (IBD) [31, 32].
Perhaps more impressive than the sheer number of microorganisms are the robust and significant relationships this community has with human health and disease. The intestinal microbiota is pivotal for detoxifying ingested drugs, training the human immune system to distinguish between pathogens and commensal organisms, and synthesizing vitamins including B vitamins and vitamin K [30, 33, 34]. Recently, the gut microbiota has been implicated in substantially influencing host weight regulation and energy harvest from the diet (i.e., extracting calories from food ingested) as well as modulating host behavior via direct and indirect pathways [35, 36]. As a result of these findings, attention to the intestinal microbiota has increased over the past two decades in metabolic and GI disorders including obesity, malnutrition, IBD, and colorectal cancer [37–40]. There is also nascent interest in the intestinal microbiota’s role in Parkinson’s disease and neurodevelopmental disorders such as autism [41, 42]. Given that gut microbiotas influence both weight regulation and behavior, two hallmarks of AN, initial investigations into the intestinal microbiotas of patients with AN have yielded intriguing preliminary results.
Energy homeostasis and the intestinal microbiota
Accumulating evidence from both animal studies and, more recently, human clinical trials, supports the notion that the intestinal microbiota plays a substantial role in nutrient extraction and host metabolism. The majority of intestinal microbiota research has focused on mechanisms by which gut microbiotas either directly produce metabolites or indirectly regulate host metabolic pathways to influence host energy homeostasis. It is highly plausible that the metabolic functions of these microbial communities are affected by the dysregulated influx of nutrients and calories to the GI tract in patients with eating disorders.
Evidence for a role of the intestinal microbiota in energy homeostasis
Germ-free (GF) rodents—mice and rats born and living without any microorganisms—are a powerful animal model to investigate both the causal role of the intestinal microbiota in human diseases and its direct effect on host physiology and metabolism. Compared with conventionally raised rodents (i.e., rodents living with microorganisms), GF rodents display slower GI transit time and an enlarged cecum (a pouch located between the small and large intestines) caused by accumulation of mucous glycoproteins [43, 44]. GF rodents also have less body fat and consume approximately 30% more daily calories of chow to maintain normal growth compared with conventionally raised rodents [45]. These unique phenotypic characteristics suggest that the intestinal microbiota substantially interacts with its host to promote intestinal transit, digest nutrients, and assimilate energy to influence host metabolism.
Transplantation studies, in which GF mice are colonized with human fecal microbiotas (as a proxy for intestinal microbiotas), permit investigators to observe metabolic, physiological, and behavioral outcomes resulting from the introduced microorganisms. In a seminal study by Ridaura et al., investigators colonized GF mice with fecal microbiotas from either obese or normal-weight human twins [46]. Over a two-week colonization period, the GF mice colonized with microbiotas from obese humans developed more adiposity despite no significant difference in food intake, suggesting a greater capacity for the obese-associated intestinal microbiotas to extract calories from the standard chow diet. This basic study design has since been replicated to probe into functions of other microbial communities implicated in a variety of metabolic diseases. In one such recent study, GF mice were colonized with stool provided by women who had undergone either Roux-en-Y gastric bypass or vertical banded gastroplasty ten years prior or who were obese controls matched to the pre-surgery BMI of the women in the surgical groups [47]. Notably, formerly GF mice colonized with fecal microbiotas from both bariatric surgery patient groups (i.e., Roux-en-Y gastric bypass and vertical banded gastroplasty) displayed less fat mass compared to mice colonized with the obese participants’ stool, indicating that the decreased fat deposition was driven by these surgically altered microbial communities. These findings also demonstrate that clinical interventions can indeed effect lasting compositional and functional changes to intestinal microbial communities. Although compelling and highly supportive of the gut microbiota as a major contributor to host metabolism, these human transplantation studies must be interpreted cautiously within the context of a small number of donor samples (i.e., 2-5 human donors per group) and/or the almost exclusive use of male GF mice [38, 46–48]. Replications and extensions using both male and female GF mice and more donor samples will contribute valuable data to this field.
Initial attempts to translate these animal studies into clinical investigations are underway. Fecal microbiota transplantations, by which a liquid preparation of stool from a healthy human donor is introduced following a bowel lavage to the GI tract of a recipient, has been shown to improve insulin sentivity in a group of obese males (n=9) six weeks after treatment [49]. In contrast, a randomized double-blind placebo-controlled trial (RCT) evaluated changes to metabolic parameters in prediabetic obese men (n=57) after a seven-day course of antibiotics in order to investigate the effects of depletion, rather than augmentation, of the intestinal microbiota. The investigators reported decreased microbial diversity and secondary bile acid concentrations in the vancomycin antibiotic group at seven days, but saw no changes in insulin sensitivity at either seven days or the eight-week follow-up as compared to the placebo group [50]. Although no study of antibiotics in AN has been conducted that analyzed the intestinal microbiota, antibiotics such as erythromycin and other prokinetic agents have been used clinically to accelerate gastric transit time and weight gain and reduce GI distress [51, 52]. Repeating such clinical trials and including pre- and post-measures of the intestinal microbiota and other metabolic indices could be a valuable addition to the AN treatment literature and a first step in understanding whether alterations to the intestinal microbiota play a role in recovery and relapse.
Mechanisms
Crosstalk between the microbes and host intestinal epithelial cells has emerged as an exciting area of research to explore mechanisms by which specific microbes, and/or the production of specific microbial metabolites, may influence host physiology and metabolism. A currently popular hypothesis proposes that certain microbial communities driven by environmental stressors alter GI physiology to increase host energy assimilation [53]. To investigate this hypothesis, Chevalier et al. colonized GF mice with fecal microbiotas from mice subjected to either room temperature or cold (6°C) housing conditions [54]. The authors reported that the cold microbiota-colonized mice displayed an increased capacity to absorb calories via greater small intestinal and microvilli length resulting from reduced intestinal epithelial cell apoptosis (programmed cell death). This intestinal epithelial adaptation to increase the total GI absorptive surface is a potential mechanism orchestrated by the intestinal microbiota to improve caloric harvest for fat deposition and mitigation of the cold stressor.
Another area of research investigating the crosstalk between enteric microbes and host intestinal epithelial cells pertains to the metabolites those microbes produce. Enteric microbial-derived metabolites, namely short-chain fatty acids (SCFAs) and secondary bile acids, have also been shown to be significant contributors to host energy homeostasis. SCFAs, specifically acetate, propionate, and butyrate, are derived from bacterial fermentation of complex polysaccharides and supply up to 10% of the host’s daily caloric intake [55]. Indeed, butyrate is the primary energy source for colonocytes while acetate and propionate are both substrates for hepatic lipogenesis and gluconeogenesis, respectively, to produce lipids and glucose for host utilization [56, 57]. In addition to providing energy, SCFAs can bind to specific distal ileum and colonic G-protein coupled receptors (GPCRs; GPR41 and GPR43) to induce the secretion of gut hormones from intestinal enteroendocrine cells. These hormones, such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), stimulate insulin secretion and inhibit gastric motility, respectively [58, 59]. Secondary bile acids are produced in a two-step process by which bacteria in the distal ileum and colon first deconjugate and then dehydroxylate unabsorbed primary bile acids to create secondary bile acids. Both primary and secondary bile acids aid in lipid digestion and cholesterol metabolism and can also function as signaling molecules to alter glucose homeostasis and brown adipose tissue metabolism [60].
Behavior modulation and the intestinal microbiota
In addition to their role in energy homeostasis, enteric microbes and their metabolites can modulate mood and behavior. The knowledge that the central nervous system (CNS) interacts with our digestive tract (the “brain-gut axis”) has existed since the discovery of the enteric nervous system, a collection of 200-600 million neurons that line the GI tract, over a century ago [36]. However, the discovery that intestinal microbes can influence neurological function is much more recent, and has come to be known as the “brain-gut-microbiota axis” [36, 61]. Elucidating the mechanism behind this phenomenon is an active area of research, and one that is of particular relevance to eating disorders given their clear relationship with psychological function, eating, and behavior.
Evidence for a brain-gut-microbiota axis
As with research into the intestinal microbiota’s role in energy homeostasis, the use of GF rodents has greatly benefited preclinical studies investigating the brain-gut-microbiota axis. A pioneering study by Sudo et al. demonstrated that there are basal differences in various biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis stress response between GF and microbe-colonized mice, with GF mice experiencing more aggressive stress responses [62]. This exaggerated response in GF mice was reversible when the mice were colonized with microbes at an adolescent age (4 weeks old), but not when they were first colonized with microbes as adults (greater than 6 weeks of age). Subsequent studies have demonstrated that compared to mice with “normal” intestinal microbiotas, GF mice exhibit a number of differences in brain and neuron morphology, anxiety-like behavior, and levels of serotonin and brain-derived neurotrophic factor [63–68].
One powerful approach to observe the effect that enteric microbial presence has on disease symptoms is the manipulation of the intestinal microbiotas of mouse models for particular neurological diseases. For example, Sampson et al. recently demonstrated that GF conditions ameliorate the motor deficits displayed by a murine model for Parkinson’s disease [41]. Additionally, when those GF mice were colonized with microbiotas from individuals with Parkinson’s disease, their motor deficiencies worsened compared with genetically identical GF mice colonized with microbiotas from healthy humans. Similarly, Hsiao et al. reported that targeted treatment of a mouse model for autism spectrum disorder (ASD) with Bacteroides fragilis improved both behavioral and gut permeability symptoms [69]. They also observed that when wild-type mice were given a particular metabolite (4-ethylphenylsulfate) that is typically elevated in the ASD mouse model and modulated by B. fragilis, they developed some of the anxiety-like behavioral symptoms characteristic of the ASD mouse.
Another intriguing line of evidence to support the existence of a brain-gut-microbiota axis pertains to prebiotics, which are compounds that support the growth of particular microbes. Recent evidence in mice demonstrates that serial administration of fructooligosaccharides (an artificial sweetener) and galactooligosaccharides significantly alters bacterial abundances in the intestinal microbiota, and decreases both anxiety-like and depressive-like behavior [61].
These converging lines of preclinical evidence, combined with studies that establish dysbioses in the intestinal microbiotas of patients with certain disorders, have encouraged a number of human clinical trials investigating the therapeutic application of microbes for psychiatric disorders. Many such trials—using so-called “psychobiotics,” or living organisms that offer mental health benefits upon ingestion [70]—are currently underway. While the popular media tend to focus on psychobiotic clinical trials that achieve positive results, negative results are also quite common. For example, a recent double-blind, placebo-controlled RCT investigating the efficacy of probiotics in the treatment of depression found no marked difference in outcomes between the placebo and probiotic groups [71]. A meta-analysis of RCTs investigating the efficacy of psychobiotics in treating anxiety and depression revealed that many RCTs report different results, with overall preliminary evidence existing to tentatively support the use of psychobiotics in treating these disorders [72]. Importantly, many of the RCTs employed different strains of bacteria, complicating efforts to pool and summarize the results.
Mechanisms
Hypotheses explaining the mechanisms by which enteric microbes influence mood and behavior abound, and at present, propose many distinct pathways for this complex, multifaceted process. Generally, the hypothesized mechanisms focus on two aspects of the brain-gut-microbiota axis: 1) which compounds (either produced directly by bacteria or whose production bacteria promote) have the ability to influence mood and behavior, and 2) how those compounds might interface with other elements of the nervous system.
Enteric bacteria either directly produce or stimulate the production of an expansive list of bioactive compounds, to such an extent that the intestinal microbiota has been referred to as a “neglected endocrine organ” [73]. The most notable compounds produced or promoted by enteric microbes in both human and murine hosts that may influence mood are neurotransmitters (including dopamine, serotonin, acetylcholine, and γ-aminobutyric acid) and some of their precursors (e.g., tryptophan, kynurenine) [74–78]. Certain bacteria also exhibit increased growth in the presence of catecholamines, suggesting a potential for enteric bacteria to modulate behavior by removing neuroactive compounds [79].
Where these molecules travel after their production in the gut and how they induce a behavioral effect remain active areas of inquiry. One proposed mechanism involves the vagus nerve. Bravo et al. demonstrated that the positive emotional effects of colonization with Lactobacillus rhamnosus (JB-1) were negated after vagotomy in mice, suggesting that the vagus nerve (the tenth pair of cranial nerves, involved in controlling the upper digestive tract and other organs of the chest and abdomen) may serve as a conduit in the brain-gut-microbiota axis [80]. It is also uncertain whether any of the metabolites or neuroactive compounds produced by bacteria can cross the blood-brain barrier (BBB) to influence neurological functioning. This remains to be established, though it is possible that they may be able to reach circumventricular organs lacking a BBB. Complicating this hypothesis, it has been shown in mice that the presence of enteric microbes results in a less permeable BBB, compared to the BBB of GF mice [64].
Intestinal microbial communities in eating disorders
Animal studies have demonstrated that the intestinal microbiota is intimately linked to traits exhibited by individuals with eating disorders, such as dysregulated energy homeostasis and behavior. However, characterization of enteric microbial communities from individuals with eating disorders is a necessary step toward establishing a clinical link between those communities and these illnesses. To date, most of the literature characterizing the intestinal microbiota in patients with eating disorders has focused on AN.
Evidence for a role of the intestinal microbiota in patients with eating disorders
Initially, the microbial profiles of a small number of patients with AN (n=9) were compared to obese (n=20) and control (n=20) groups [2]. Using polymerase chain reaction (PCR), this study found significantly higher levels of Methanobrevibacter smithii (a commensal enteric microbe belonging to the Archaea domain) in patients with AN compared to controls. As M. smithii can reduce CO2 in the presence of H2 to produce methane, a gas that is associated with delayed intestinal motility, the authors speculated that this microbe may promote constipation, a symptom frequently observed in patients with AN [81]. Given that the intestinal microbiota harbors up to 1,150 different bacterial species, and this study only investigated four microbial groups using a relatively narrow approach, a broader characterization was warranted [82]. Using a culturomics approach (large-scale culturing of microorganisms combined with molecular identification of cultured microbial colonies), investigators identified 11 new bacterial species in a stool sample from one individual with AN [3]. However, because the main objective of the study was to develop a novel technology, the researchers only used one stool sample as a template and therefore could not draw any direct association between the 11 novel bacterial strains and the clinical status of the donor.
Although these studies collectively suggest an altered intestinal microbiota in patients with eating disorders, broad molecular methods provide a more comprehensive and unbiased characterization of these complex communities. Kleiman et al. was the first group to characterize the intestinal microbiota of patients with AN using high-throughput sequencing of the 16S rRNA gene comparing female patients with AN before (n=16) and after (n=10) clinical refeeding at an inpatient specialist unit to healthy controls (n=12) [4]. The authors reported lower microbial diversity in patients with AN at both time points compared with controls. Interestingly, higher levels of self-reported depression in patients with AN at hospital admission were significantly associated with lower microbial diversity, suggesting a brain-gut-microbiota interaction in this population.
Another PCR-based investigation (employing reverse transcription quantitative PCR) collected stool samples from patients with restricting type AN (n=14), binge-eating type AN (n=11), and controls (n=21) [5]. Compared with controls, patients with AN had lower abundances of specific taxa belonging to Streptococcus, Clostridium, and Bacteroides genera and lower concentrations of the fecal SCFAs acetate and propionate. Most recently, results from the largest recruited cohort of patients with AN to date replicated the previously reported dysbiotic enteric microbial communitiy in patients with AN (n=55) which also changed following clinical refeeding (n=44). The authors also measured specific microbial-derived metabolites and found elevated concentrations of fecal branched-chain fatty acids (BCFAs, products of protein fermentation) in patients with AN which did not return to levels measured in the controls (n=55) following clinical refeeding [6]. Collectively, these results indicate that the intestinal microbiota of clinically refed patients with AN remains metabolically abnormal.
Mechanisms
Although these studies establish the presence of a dysbiotic intestinal microbiota in patients with AN, the mechanism by which an abnormal enteric microbial community influences either the persistence or the treatment of eating disorders has not yet been fully elucidated. One possible mechanism is via the host immune system within the context of “molecular mimicry,” wherein bacteria produce compounds that mimic those native to the host. Auto-antibodies that recognize alpha-Melanocyte-stimulating hormone (α-MSH) and contribute to regulation of food intake and behavior have become an intriguing avenue of research into the molecular mechanisms behind disordered eating [83]. Proteomics has revealed that the caseinolytic protease B (ClpB) protein produced by commensal Escherichia coli is an antigenic mimic of α-MSH [84]. Mice immunized with bacterial ClpB have lower bodyweights, food consumption, and anxiety than controls, and patients with AN, BN, and BED have elevated levels of plasma ClpB protein [85]. Together, these studies suggest a role for the intestinal microbiota in the initiation or persistence of eating disorders. However, the influence of an eating disorder-associated gut microbiota on its host both prior to and during clinical refeeding is yet to be determined.
Clinical relevance and conclusions
Will research on the intestinal microbiota truly yield revolutionary perspectives on illnesses including eating disorders, or will we look back on it as a blind alley in science? Chances are good that the reality will be somewhere in between. Flexible skepticism is a safe stance, but should not impede attempts to detail and clarify the role of the intestinal microbiota in AN and other eating disorders. It is logical to assume that severe alterations in energy consumption and availability (as in AN, BN, and BED) would have effects on the intestinal ecosystem. Living in a competitive environment, intestinal bacteria (and presumably other microorganisms) that are well suited to either a low-energy environment (such as in AN) or a variable-energy environment (such as BN and BED) may be more likely to survive and dominate. Whether dysbioses exist that predispose to extreme appetite imbalance is unknown and is a difficult scientific puzzle whose solution will require prospective studies. More tractable are studies in which we determine whether intestinal dysbioses contribute to persistence, recovery, or relapse from eating disorders. Though it is unlikely that the intestinal microbiota will be the sole therapeutic target in treating AN, it is possible that augmenting treatment with agents that target the intestinal microbiota may facilitate weight gain, decrease GI distress associated with renourishment, and perhaps even reduce anxiety and depression via the brain-gut-microbiota axis. Future work branching beyond AN to the other eating disorders—not only BN and BED, but also perplexing childhood illnesses such as avoidant/restrictive food intake disorder (ARFID) and pica—may expand the clinician’s toolbox for treating these debilitating illnesses.
Footnotes
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington, DC: 2013. Feeding and Eating Disorders. [Google Scholar]
- 2.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLOS ONE. 2009;4(9):e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfleiderer A, Lagier JC, Armougom F, Robert C, Vialettes B, Raoult D. Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology. 2013;32(11):1471–81. doi: 10.1007/s10096-013-1900-2. [DOI] [PubMed] [Google Scholar]
- 4•.Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, et al. The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosomatic Medicine. 2015;77(9):969–81. doi: 10.1097/psy.0000000000000247. The first study to report a microbial dysbiosis in patients with AN using high-throughput sequencing techniques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, et al. Gut dysbiosis in patients with anorexia nervosa. PLOS ONE. 2015;10(12):e0145274. doi: 10.1371/journal.pone.0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack I, Cuntz U, Gramer C, Niedermaier S, Pohl C, Schwiertz A, et al. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Scientific Reports. 2016;6:26752. doi: 10.1038/srep26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61(3):348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U. Anorexia nervosa: Aetiology, assessment, and treatment. The Lancet Psychiatry. 2015;2(12):1099–111. doi: 10.1016/s2215-0366(15)00356-9. [DOI] [PubMed] [Google Scholar]
- 9.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: A meta-analysis of 36 studies. Archives of General Psychiatry. 2011;68(7):724–31. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 10.Zipfel S, Lowe B, Reas DL, Deter HC, Herzog W. Long-term prognosis in anorexia nervosa: Lessons from a 21-year follow-up study. Lancet (London, England) 2000;355(9205):721–2. doi: 10.1016/s0140-6736(99)05363-5. [DOI] [PubMed] [Google Scholar]
- 11.Waldholtz BD, Andersen AE. Gastrointestinal symptoms in anorexia nervosa. A prospective study. Gastroenterology. 1990;98(6):1415–9. doi: 10.1016/0016-5085(90)91070-m. [DOI] [PubMed] [Google Scholar]
- 12.National Collaborating Centre for Mental Health. National Institute for Health and Clinical Excellence: Guidance. Eating Disorders: Core Interventions in the Treatment and Management of Anorexia Nervosa, Bulimia Nervosa and Related Eating Disorders. Leicester (UK): The British Psychological Society & The Royal College of Psychiatrists; 2004. [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Treatment of patients with eating disorders, third edition. The American Journal of Psychiatry. 2006;163(7 Suppl):4–54. [PubMed] [Google Scholar]
- 14.Sato Y, Fukudo S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clinical Journal of Gastroenterology. 2015;8(5):255–63. doi: 10.1007/s12328-015-0611-x. [DOI] [PubMed] [Google Scholar]
- 15.Mayer L, Walsh BT, Pierson RN, Jr, Heymsfield SB, Gallagher D, Wang J, et al. Body fat redistribution after weight gain in women with anorexia nervosa. The American Journal of Clinical Nutrition. 2005;81(6):1286–91. doi: 10.1093/ajcn/81.6.1286. [DOI] [PubMed] [Google Scholar]
- 16.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10-15 years in a prospective study. The International Journal of Eating Disorders. 1997;22(4):339–60. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Boraska V, Franklin CS, Floyd JA, Thornton LM, Huckins LM, Southam L, et al. A genome-wide association study of anorexia nervosa. Molecular Psychiatry. 2014;19(10):1085–94. doi: 10.1038/mp.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinney A, Kesselmeier M, Jall S, Volckmar AL, Focker M, Antel J, et al. Evidence for three genetic loci involved in both anorexia nervosa risk and variation of body mass index. Molecular Psychiatry. 2017;22(2):321–2. doi: 10.1038/mp.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner DM, Garfinkel PE. Socio-cultural factors in the development of anorexia nervosa. Psychological Medicine. 1980;10(4):647–56. doi: 10.1017/s0033291700054945. [DOI] [PubMed] [Google Scholar]
- 20.Proctor LM. The National Institutes of Health Human Microbiome Project. Seminars in Fetal & Neonatal Medicine. 2016;21(6):368–72. doi: 10.1016/j.siny.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–40. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–20. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 27.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflammatory Bowel Diseases. 2016;22(5):1137–50. doi: 10.1097/mib.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLOS ONE. 2015;10(4):e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152(1–2):39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160(3):447–60. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ott SJ, Kuhbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, et al. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scandinavian Journal of Gastroenterology. 2008;43(7):831–41. doi: 10.1080/00365520801935434. [DOI] [PubMed] [Google Scholar]
- 33.Hill MJ. Intestinal flora and endogenous vitamin synthesis. European Journal of Cancer Prevention: The Official Journal of the European Cancer Prevention Organisation (ECP) 1997;6(Suppl 1):S43–5. doi: 10.1097/00008469-199703001-00009. [DOI] [PubMed] [Google Scholar]
- 34.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nature Reviews Immunology. 2016;16(6):341–52. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 36.Mayer E. Gut feelings: The emerging biology of gut-brain communication. Nature Reviews Medicine. 2011;12:453–66. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science (New York, NY) 2016;351(6275) doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. The ISME Journal. 2012;6(2):320–9. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s Disease. Cell. 2016;167:1469–80. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLOS ONE. 2013;8(10):e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustafsson BE, Midtvedt T, Strandberg K. Effects of microbial contamination on the cecum enlargement of germfree rats. Scandinavian Journal of Gastroenterology. 1970;5(4):309–14. [PubMed] [Google Scholar]
- 44.Strandberg K, Sedvall G, Midtvedt T, Gustafsson B. Effect of some biologically active amines on the cecum wall of germfree rats. Proceedings of the Society for Experimental Biology and Medicine (New York, NY) 1966;121(3):699–702. doi: 10.3181/00379727-121-30864. [DOI] [PubMed] [Google Scholar]
- 45.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science (New York, NY) 2013;341(6150):1241214. doi: 10.1126/science.1241214. A seminal investigation demonstrating that obesity is a phenotype transmissible by the intestinal microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tremaroli V, Karlsson F, Werling M, Stahlman M, Kovatcheva-Datchary P, Olbers T, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metabolism. 2015;22(2):228–38. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 49.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 50•.Reijnders D, Goossens GH, Hermes GD, Neis EP, van der Beek CM, Most J, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: A randomized double-blind placebo-controlled trial. Cell Metabolism. 2016;24(1):63–74. doi: 10.1016/j.cmet.2016.06.016. A human clinical trial reporting that short-term administation of antibiotics decreases intestinal microbial diversity but does not result in improved whole-body energy homeostasis in obese individuals at an 8-week follow-up. Other human clinical trials aimed at manipulating the intestinal microbiota include pre- or probiotic supplementation and fecal microbial transplants. [DOI] [PubMed] [Google Scholar]
- 51.Hiyama T, Yoshihara M, Tanaka S, Haruma K, Chayama K. Effectiveness of prokinetic agents against diseases external to the gastrointestinal tract. Journal of Gastroenterology and Hepatology. 2009;24(4):537–46. doi: 10.1111/j.1440-1746.2009.05780.x. [DOI] [PubMed] [Google Scholar]
- 52.Stacher G, Peeters TL, Bergmann H, Wiesnagrotzki S, Schneider C, Granser-Vacariu GV, et al. Erythromycin effects on gastric emptying, antral motility and plasma motilin and pancreatic polypeptide concentrations in anorexia nervosa. Gut. 1993;34(2):166–72. doi: 10.1136/gut.34.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 54.Chevalier C, Stojanovic O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163(6):1360–74. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews. 1990;70(2):567–90. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 56.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabolism. 2011;13(5):517–26. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2013;305(12):G900–10. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- 58.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–71. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(43):16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabolism. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 61•.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biological Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.12.031. Continuing this group’s research into the brain-gut-microbiota axis, Burokas et al. treated male wildtype mice with two different kinds of prebiotics (fructooligosaccharides and galactooligosaccharides) for three weeks, then assessed changes to a number of biochemical and psychological metrics. The central finding was that prebiotics reduced stress-related symptoms, even in chronically stressed mice. [DOI] [PubMed] [Google Scholar]
- 62.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of Physiology. 2004;558(Pt 1):263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 64.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014;6(263) doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology & Motility. 2011;23(3):255–65. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 67.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79(1):132–37. [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry. 2013;18(6):666–73. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 69•.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–63. doi: 10.1016/j.cell.2013.11.024. Research in a mouse model of autism spectrum disorder (ASD) demonstrating the positive effect of treatment with Bacteroides fragilis on numerous characteristics of ASD. The authors also found that treating wildtype mice with a serum metabolite, 4EPS, that is increased in the ASD mouse model induces behavioral traits similar to those in the ASD mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dinan TG, Stanton C, Cryan JF. Psychobiotics: A novel class of psychotropic. Biological Psychiatry. 2013;74(10):720–6. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Australian & New Zealand Journal of Psychiatry. 2017 doi: 10.1177/0004867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pirbaglou M, de Souza RJ, Stearns JC, Motamed M, Ritvo P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutrition Research. 2016;36(9):889–98. doi: 10.1016/j.nutres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Clarke G, Stilling R, Kennedy P, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: The neglected endocrine organ. Molecular Endocrinology. 2014;28(8):1221–38. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. Journal of Applied Microbiology. 2012;113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, et al. Cerebral low-molecular metabolites influenced by intestinal microbiota: A pilot study. Frontiers in Systems Neuroscience. 2013;7 doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Özogul F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. European Food Research and Technology. 2004;219:465–9. doi: 10.1007/s00217-004-0988-0. [DOI] [Google Scholar]
- 77.Stanaszek PM, Snell JF, O’Neill JJ. Isolation, extraction, and measurement of acetylcholine from Lactobacillus plantarum. Applied and Environmental Microbiology. 1977;34(2):237–9. doi: 10.1128/aem.34.2.237-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yano J, Yu K, Donaldson G, Shastri G, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sciences. 1992;50(3):203–12. doi: 10.1016/0024-3205(92)90273-r. [DOI] [PubMed] [Google Scholar]
- 80.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(38):16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2006;290(6):G1089–95. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 82.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinno MH, Do Rego JC, Coeffier M, Bole-Feysot C, Ducrotte P, Gilbert D, et al. Regulation of feeding and anxiety by alpha-MSH reactive autoantibodies. Psychoneuroendocrinology. 2009;34(1):140–9. doi: 10.1016/j.psyneuen.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 84.Tennoune N, Chan P, Breton J, Legrand R, Chabane YN, Akkermann K, et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide alpha-MSH, at the origin of eating disorders. Translational Psychiatry. 2014;4:e458. doi: 10.1038/tp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Breton J, Legrand R, Akkermann K, Jarv A, Harro J, Dechelotte P, et al. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. The International Journal of Eating Disorders. 2016;49(8):805–8. doi: 10.1002/eat.22531. [DOI] [PubMed] [Google Scholar]


