Abstract
Objective
Genetic counseling (GC) and germline genetic testing (GT) for BRCA1 and BRCA2 are considered standard of care for patients with high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancers (HGOC). We describe a universal genetic testing initiative to increase the rates of recommendation and acceptance of GC and GT to greater than 80% for patients with HGOC at our institution.
Methods
Data from a consecutive cohort of patients seen in our gynecologic oncology clinics between 9/1/2012 and 8/31/2015 for evaluation of HGOC were retrospectively analyzed. Data were abstracted from the tumor registry, medical records, and research databases. Descriptive statistics were used to evaluate patient characteristics and GC, GT, and PARP inhibitor use. Various clinic interventions were developed, influenced by the Plan-Do-Study-Act cycle method, which included physician-coordinated GT, integrated GC, and assisted GC referrals.
Results
A cohort of 1636 patients presented to the gynecologic oncology clinics for evaluation of HGOC during our study period, and 1423 (87.0%) were recommended to have GC and GT. Of these, 1214 (85.3%) completed GT and 217 (17.9%) were found to have a BRCA1 or BRCA2 mutation. Among BRCA-positive patients, 167 had recurrent or progressive disease, and 56 of those received PARP inhibitor therapy.
Conclusions
The rates of GC and GT recommendation and completion among patients with HGOC at our institution exceeded 80% following the implementation of a universal genetic testing initiative. Universal genetic testing of patients with HGOC is one strategy to identify those who may benefit from PARP inhibitor therapy.
Keywords: Genetic counseling, Genetic testing, Ovarian cancer, PARP Inhibitors, BRCA1, BRCA2
Introduction
Approximately 10–20% of high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancers (HGOC) are hereditary, primarily due to germline mutations in the BRCA1 or BRCA2 genes.(1–3) A mutation in BRCA1 or BRCA2 confers a 40–66% lifetime risk of breast cancer and a 13–46% lifetime risk of ovarian cancer in women.(4) Identification of a BRCA mutation has implications for the treatment of HGOC and the management of inherited cancer risks in patients and their families.
The National Comprehensive Cancer Network (NCCN) BRCA1 and BRCA2 genetic testing guidelines were revised in 2007 to state that all women with epithelial ovarian, fallopian tube, and primary peritoneal cancers meet criteria for genetic testing, regardless of their age at diagnosis or family history of cancer.(5) The same statement was later reflected in the consensus guidelines of several professional organizations.(6–8) Despite these recommendations, fewer than 25% of patients with HGOC in the United States are referred for genetic counseling and testing.(9–11) Studies have suggested that physician recommendation and referral patterns may influence patients’ access to standard of care cancer genetics services.(12–15)
In 2007, less than 12% of patients with invasive epithelial ovarian cancer seen in the gynecologic oncology clinics at our institution were referred for genetic counseling.(16) In 2013, as part of an institution-wide research program, we implemented a universal genetic testing initiative in our gynecologic oncology clinics. This initiative was implemented with the goal of ensuring that at least 80% of patients with HGOC received a recommendation for standard of care genetic counseling and testing for BRCA1 and BRCA2. Here we describe our experience implementing the initiative, including the development and assessment of clinic interventions used to reach our goal.
Patients and Methods
Approval for the initiation and conduct of the quality improvement project was obtained from The University of Texas MD Anderson Cancer Center’s Quality Improvement Assessment Board. Subsequently, for this retrospective data analysis, MD Anderson Cancer Center Institutional Review Board approval was obtained with a waiver of informed consent.
This was a cohort study of female patients who initially presented to the gynecologic oncology clinics for evaluation of suspected or confirmed diagnosis of HGOC from September 1, 2012, through August 31, 2015. All patients were seen by a gynecologic oncologist or medical oncologist within the gynecologic oncology clinics located at our main campus and/or our regional clinic locations. Patients under 18 years of age and those with ovarian tumors other than HGOC were excluded from analysis.
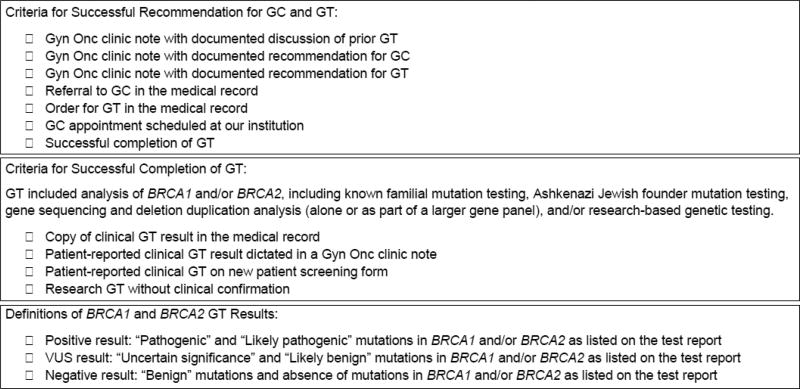
Data were collected from the institutional tumor registry, electronic medical records, and departmental databases, and were stored in a password-protected REDCap database.(17) Data included clinical documentation between September 1, 2012 and August 31, 2016, allowing for capture of disease status, and uptake of genetic counseling, genetic testing, and Poly (ADP-Ribose) Polymerase inhibitor (PARPi) use, within at least one year from the date of initial presentation to the gynecologic oncology clinics. The quality improvement metrics captured included rates of recommendation for genetic counseling and genetic testing, rates of completion of genetic counseling, rates of completion of genetic testing, and the outcomes of genetic testing (positive, negative, or variant), as defined in Figure 1. Retrospective data included: patient demographics, vital status, prior and current cancer diagnoses, cancer treatment (including the use of PARPi therapy), genetic testing methodology, genes analyzed, dates of genetic counseling and genetic testing, clinic interventions used to promote genetic counseling and testing, and documented reasons for lack of genetic counseling and/or genetic testing.
Figure 1. Criteria for Universal Genetic Testing Metrics.
For the task to be counted as “successfully completed,” at least one item must have been completed in the category’s check list. The criteria could be met prior to or following the patient’s initial presentation to our institution’s gynecologic oncology clinics. Genetic testing may have been coordinated by our institution or outside our institution.
Abbreviations: GC, genetic counseling; GT, genetic testing; Gyn Onc, gynecologic oncology; VUS, variant of uncertain significance
Universal Genetic Testing Initiative Methods
A working group of gynecologic oncology stakeholders, including physicians, genetic counselors, advanced practice providers, nurses, clinical managers, and physician trainees, was assembled in 2008 to study and improve the rates of genetic counseling and genetic testing referral. The Plan-Do-Study-Act (PDSA) cycle method guided the initial quality improvement project design, but due to changing genetic testing guidelines, limited staffing, and lack of funding to support the project, the initiative was not fully implemented.(18) An institution-wide research program was announced in 2012, launched in 2013, and allowed the universal genetic testing initiative to be fully implemented in our gynecologic oncology clinics.
The working group reviewed gynecologic oncology clinic practice patterns and identified barriers that affected patients’ access to genetic counseling and genetic testing. Clinic interventions were developed with the intention of reducing or eliminating these barriers, targeting issues within the control of the working group members, and minimizing clinic workflow disruptions. A variety of clinic interventions were created and implemented during the course of the initiative and are described in Table 1. The three “key” interventions were considered the most measurable, consistently implemented, and well received by patients and providers, and included: physician-coordinated genetic testing, integrated genetic counseling, and assisted genetic counseling referral.
Table 1.
Universal Genetic Testing Clinic Interventions
| Intervention Category |
Name of Intervention |
Initiated | Location and (Duration) |
Intervention Description | Metrics |
|---|---|---|---|---|---|
| I: Physician | Physician-coordinated genetic testing (PCGT) | 2013 | Main campus (for 5 months) and regional locations (ongoing) | Gyn Onc physician coordinated GT process independent of genetic counselor. | Number of patients who received GT coordinated by a physician |
| Physician education | Ongoing | All locations (ongoing) | Attended national meetings and conferences discussing hereditary cancer. Gyn Onc genetic counselors provided education as needed. | Rates of GC/GT recommendation | |
| Tumor board/case review discussions | Ongoing | All locations (ongoing) | Discussed indications for GC/GT at weekly meeting, including verbal reminders by Gyn Onc department chair. | Rates of GC/GT recommendation | |
| Institution-wide research program | 2013 | All locations (ongoing) | Institution-wide research effort included support for various Gyn Onc projects, including the QI initiative. | Rates of GC/GT recommendation | |
| II: Clinic | Integrated genetic counseling (IGC) | 2006 and 2014 | Main campus (ongoing) |
|
Rate of GC completion and time between initial Gyn Onc visit and GC completion |
| New-patient screening form | 2013 | Main campus (limited use by 2015 due to changes in new patient processes) | Form provided at initial Gyn Onc visit collected information regarding prior GT and family history of cancer. Completed form was scanned into the patient’s medical record. | Number of patients who completed the screening form | |
| Patient education document | 2008 | Main campus (limited use by 2015) | Patient education document described the purpose of Moon Shots programs, including GC/GT. Provided to the patient by advanced practice providers or other clinic staff. | Rates of GC/GT completion | |
| III: Process | Assisted genetic counseling referral (AGCR) | 2015 | All locations (occurred 6/2015, 9/2015, 2/2016) | Electronic referral to GC drafted for patients with HGOC not previously offered GC/GT. | Number of referrals drafted, number of referrals signed within 1 month, and GC completion |
| Clinic patient tracking | 2012 | All locations (through 2016) | Research data coordinator collected data from Gyn Onc clinic schedules and medical record to determine whether patients received GC/GT. | Not applicable: clinic patient tracking provided all data for all other metrics. | |
| Provider email notifications | 2015 | Main campus (occurred 3/2015, 4/2015, 5/2015, and 7/2016) | Research data coordinator and/or genetic counselor notified physician and care teams of upcoming patients not previously seen or referred for GC/GT. | Number of email notifications, referrals made within 1 month of email, and GC/GT completion |
Bold text denotes the “key” interventions. Abbreviations: Gyn Onc, Gynecologic Oncology; GT, genetic testing; GC, genetic counseling; HGOC, high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancer
I. Physician-coordinated genetic testing (PCGT)
The PCGT intervention, initiated in 2013, was an alternative to standard clinic practice, developed to address the barrier that regional clinic locations did not have on-site genetic counselors. To date, the standard clinical practice at our institution is consistent with a traditional genetic counseling model: a provider identifies a patient for genetic counseling and genetic testing, makes a referral to genetic counseling, a genetic counselor sees the patient for consultation and coordinates genetic testing (including informed consent and paperwork), and when the results are available, the genetic counselor discloses the results. In contrast, in PCGT a gynecologic oncology physician (with assistance of advanced practice providers, as needed) performed pre-test counseling, obtained informed consent from the patient for genetic testing, coordinated genetic testing sample collection, completed all paperwork, and disclosed the results to the patient. Prior to PCGT initiation, genetic counselors provided education and training regarding: the components of a standard informed consent, genetic testing options, laboratory billing policies, health insurance coverage guidance, instructions for specimen collection processes and test requisition completion, and provided examples of possible test results. All genetic tests performed via PCGT included full assessment of the BRCA1 and BRCA2 genes. Additional genes tested (multi-gene panel testing) varied by physician preference, institutional laboratory contracts, clinic location processes, and patient’s clinical and family history indications. Referrals for genetic counseling could be made at any time during the PCGT process for any patient, and patients with a mutation (positive and/or variant of uncertain significance results) identified by PCGT were referred for post-test counseling with a genetic counselor.
II. Integrated genetic counseling (IGC)
The IGC intervention was intended to address the barriers: lack of knowledge about the genetic counseling referral process, lack of knowledge of genetic counselor availability, and the limited number of urgent and same-day genetic counseling appointments. Beginning in 2006, genetic counselors became integrated within the gynecologic oncology clinic and academic department. Integration included the delivery of genetic counseling services within the gynecologic oncology clinic, provision of an office for genetic counselors within the department, and inclusion of genetic counselors in research project collaborations and in routine clinical meetings with gynecologic oncology physicians and advanced practice providers, such as tumor board conferences. Beginning in 2014 as part of IGC, the genetic counseling schedule was optimized, which prioritized appointments for patients with a gynecologic cancer diagnosis (over appointments for individuals with only a family history of gynecologic cancer). During the universal genetic testing initiative, 2.5 full-time genetic counselors were available to complete 20–25 genetic counseling appointments in the gynecologic oncology clinic per week. Of these appointments, 75% were designated for patients with a diagnosis of gynecologic cancer, and the remainder were used for individuals with only a family history of gynecologic cancer, patient follow-up, urgent, or same-day appointments. Also as part of IGC, to standardize the urgent and same-day appointment request process, a group email address was created. All genetic counselors in the gynecologic oncology clinic received urgent or same-day appointment requests from gynecologic oncology physicians and advanced practice providers through the group email address and responded if available to see the patients.
III. Assisted genetic counseling referral (AGCR)
The AGCR intervention was developed following the implementation of patient tracking, and was intended to address the barrier of inconsistent documentation by physicians and clinic staff of recommendations for genetic counseling and genetic testing. Patients without documentation of genetic counseling or genetic testing were identified through patient tracking, performed by one full-time research data coordinator. After being identified and scheduled to return to the gynecologic oncology clinic, a referral to genetic counseling for the patient was drafted in the electronic medical record system. An email was sent to the gynecologic oncology physician and advanced practice providers to notify them of the referral and to request their signature if the referral was deemed appropriate. After a referral was signed, the patient was scheduled for genetic counseling per usual clinic procedures.
Statistical Analysis
Descriptive statistics were used to characterize patient clinical and demographic characteristics, genetic testing results, and PARPi treatment. Statistical analyses were performed using IBM SPSS Statistics (version23, Armonk, NY). Chi-square analysis was used to identify associations between categorical variables. P-values less than or equal to 0.05 were considered statistically significant.
Results
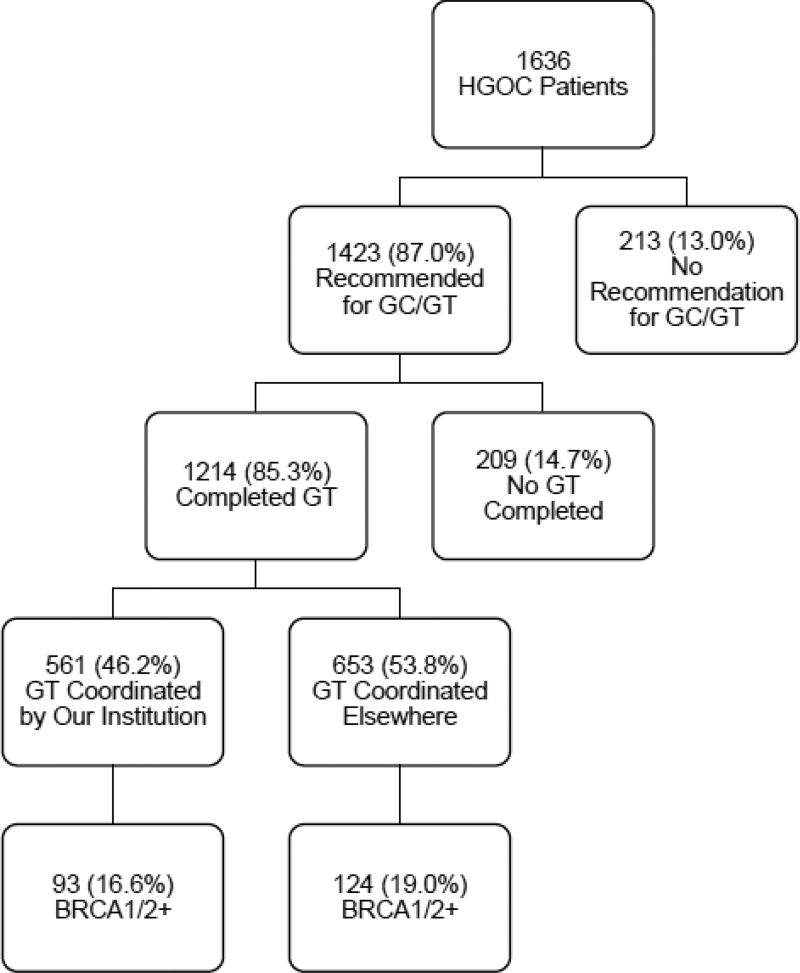
During the 3-year study period, a total of 1636 women with HGOC presented as patients to our gynecologic oncology clinics. Patient demographic information is found in Table 2. Of these patients, 1423 (87.0%) were recommended for genetic counseling and genetic testing, 1214 (85.3%) of those completed genetic testing, and 217 (17.9%) of those tested were identified to have a BRCA1 or BRCA2 mutation (see Figure 2). Our cohort of 1636 patients was composed of women presenting to the gynecologic oncology clinic to receive cancer treatment (44.1%), a onetime second opinion (40.1%), or treatment planning and coordination (15.8%). Patients presenting for a one-time second opinion visit were less likely to have received a recommendation for genetic counseling and genetic testing (83.7% recommended), than were patients receiving cancer treatment (89.8%), or treatment planning and coordination (87.6%, P=0.004). Race was not a statistically significant factor in the receipt of a recommendation for genetic counseling and genetic testing (P=0.14); however, black patients had the lowest rate of recommendation (80.4%) among racial groups in our cohort.
Table 2.
Patient Demographics
| HGOC Patients (N=1636) | ||
|---|---|---|
| N | % | |
| Race | ||
| White | 1266 | 77.4 |
| Asian | 100 | 6.1 |
| Black | 97 | 5.9 |
| American Indian/Alaska native | 2 | 0.1 |
| Native Hawaiian/Pacific Islander | 1 | 0.1 |
| Other/not reported | 170 | 10.4 |
| Religion | ||
| Christian | 1252 | 76.5 |
| Jewish | 37 | 2.3 |
| Muslim | 27 | 1.6 |
| Buddhist | 16 | 1.0 |
| Hindu | 21 | 1.3 |
| Other/not reported | 283 | 17.3 |
| Age at HGOC diagnosis, years | ||
| <20 | 0 | 0.0 |
| 20–29 | 10 | 0.6 |
| 30–39 | 58 | 3.6 |
| 40–49 | 255 | 15.6 |
| 50–59 | 527 | 32.2 |
| 60–69 | 506 | 30.9 |
| 70–79 | 227 | 13.9 |
| ≥80 | 53 | 3.2 |
| HGOC histology | ||
| Serous component | 1323 | 80.9 |
| Clear cell component | 141 | 8.6 |
| Endometrioid component | 96 | 5.9 |
| Mullerian carcinoma, NOS | 76 | 4.6 |
| Type of care received | ||
| Cancer treatment | 722 | 44.1 |
| Treatment planning and coordination | 258 | 15.8 |
| One-time second opinion only | 656 | 40.1 |
Abbreviations: HGOC, high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancers; NOS, not otherwise specified
Figure 2. Patient CONSORT Diagram.
Abbreviations: HGOC, high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancers; GC, genetic counseling; GT, genetic testing; BRCA1/2+, positive BRCA1 and/or BRCA2 genetic testing result
Two hundred-nine patients were recommended to undergo, but did not complete, genetic testing. The most common documented reasons for failure to complete testing were: patient elected to pursue genetic testing elsewhere with no results reported back to our institution (n=45, 21.5%), patient declined genetic testing (n=22, 10.5%), patient declined genetic counseling (n=18, 8.6%), and financial concerns or lack of health insurance coverage for testing (n=14, 6.7%).
Of those 1214 patients who successfully completed genetic testing, 561 (46.2%) had testing coordinated by a genetic counselor or physician at our institution, and for 94.7%, a copy of the results was available in our medical records. Six hundred fifty-three (53.8%) patients had genetic testing coordinated outside of our institution, but for only 45.6% of these patients was a copy of the results available in our medical records. The types of genetic testing completed, and result outcomes in Table 3, demonstrate the variety of BRCA1 and BRCA2 genetic testing methodologies utilized during our initiative.
Table 3.
Genetic Testing
| Type of Genetic Test | n |
BRCA1 Positive |
BRCA2 Positive |
BRCA1/2 VUS |
Negative | Unknownǂ |
|---|---|---|---|---|---|---|
| Single site/Known familial mutation | 25 | 19 | 5 | 0 | 1 | 0 |
| Ashkenazi Jewish founder mutations | 9 | 4 | 0 | 0 | 5 | 0 |
| BRCA1/2 Sequencing only | 80 | 18 | 5 | 3 | 54 | 0 |
| BRCA1/2 Sequencing + Del/Dup | 568 | 72 | 28 | 29 | 439 | 0 |
| Gene panel including BRCA1/2 Sequencing + Del/Dup § | 164 | 13 | 6 | 6 | 136 | 3 |
| Research testing only (no clinical testing) | 29 | 1 | 0 | 0 | 28 | 0 |
| Unspecified clinical testing | 339 | 35 | 11 | 6 | 258 | 29 |
| Total: | 1214 | 162 | 55 | 44 | 921 | 32 |
Abbreviations: VUS: Variant of uncertain significance, Del/Dup: Deletion and Duplication, also known as Large Rearrangement analysis
All unknown results were genetic tests performed outside of MD Anderson, with a copy of the results not available in the MD Anderson medical records.
Various genetic testing laboratories and their respective gene panel offerings were included together in this category.
Identification of a BRCA mutation has implications for cancer risks, as well as for HGOC treatment. Forty-nine (22.6%) patients with a BRCA-positive result had a second primary breast cancer diagnosis: 41 were diagnosed prior to HGOC, 3 had synchronous diagnoses, and 5 were diagnosed after their HGOC diagnosis. During the study period, PARPi therapy was approved by the FDA for the treatment of patients with a germline BRCA mutation and recurrent HGOC after three prior lines of treatment have failed.(19) During our study period, of the 217 BRCA-positive patients, 167 (77.0%) had recurrent or progressive disease, and 56 (33.5%) of those were noted to have received PARPi therapy.
Intervention Results
The rates of recommendation and completion of genetic testing were similar between our main campus and regional gynecologic oncology clinic locations. Of 197 patients seen at a regional clinic location, 84 of the 151 (55.6%) patients who completed genetic testing had physician-coordinated genetic testing. These patients may not have otherwise completed genetic testing due to the lack of genetic counselors at their clinic location or inability to travel to the main campus location for genetic counseling.
Meyer et al. reported that in 2007, the median time between a patient’s initial gynecologic oncology visit and a genetic counseling referral at our institution was greater than 3 years.(16) Notably, during the course of the universal genetic testing initiative, the time between a patient’s initial gynecologic oncology visit and their completion of genetic counseling, for those who pursued it at our institution, declined from an average of 197 days in 2012 to 78 days by 2015. This decrease may represent a combination of improved identification and referral of patients at the time of their initial gynecologic oncology clinic visit and the integrated genetic counseling intervention.
The assisted genetic counseling referral intervention resulted in placement of 34 electronic referrals for genetic counseling, 33 (97.1%) were signed, and 28 genetic counseling appointments were subsequently completed. The provider email notification intervention was considered ineffective, as only 14 of 72 (19.4%) emails resulted in a completed referral which led to 13 subsequent genetic counseling appointments. New patient screening forms, a clinic-level intervention, were completed by 1137 (69.5%) patients, with 707 (62.2%) patients noting no prior genetic testing at the time of their initial visit to the gynecologic oncology clinic. Upon review, 331 (46.8%) of these 707 patients completed genetic testing, coordinated by our institution, following their initial clinic visit. It is unclear whether the screening form improved the rates of genetic testing among these patients; however, it may have improved the documentation of patients’ genetic testing status.
Discussion
Following the implementation of a 3-year universal genetic testing initiative, we successfully improved the rates of recommendation and completion of genetic counseling and genetic testing to greater than 80% among patients with HGOC at our institution. At the conclusion of our study, 56 patients had received PARPi therapy following the identification of a germline BRCA mutation. Universal genetic testing of patients with HGOC is one strategy to identify patients who may benefit from PARPi therapy.
The clinic interventions were developed to address specific barriers to patients receiving standard of care genetic counseling and genetic testing in our gynecologic oncology clinics. Varied clinic interventions used simultaneously during the course of our initiative may have had a greater impact on clinical practice patterns in our gynecologic oncology clinic than had we implemented single, discrete interventions. Our experience suggests that there may be no single preferred or optimal delivery model in the provision of genetic counseling and genetic testing for patients with ovarian cancer. At larger institutions, incorporating genetic counselors into the ovarian cancer care team is effective. Physician-coordinated genetic testing is also a reasonable option for ovarian cancer patients, in part because these patients meet genetic testing criteria regardless of their family history of cancer, and because the results can be used to guide cancer treatment and management. Ultimately, delivery care models should designed and implemented with the primary goals of patient-centered care and guideline based practice; but optimized to work within the constraints of available resources, clinical facilitators and barriers, within any oncology or gynecologic oncology clinic.
Through our retrospective data collection and review, we noted a disparity in genetic testing reports’ availability in our institution’s electronic medical record. The delayed or absent transfer of genetic testing reports between electronic medical record systems can complicate the genetic counseling referral process, lead to unnecessary and costly duplicate testing, and hinder access to PARPi therapy due to lack of available results. Future research and health technology innovation should seek to improve the sharing of medical records between health systems and identify how to best integrate the increasing quantity of genetic and genomic data within electronic medical record systems.
There are several limitations to our study. This study details the experiences and patients seen at a single institution. A large number of patients were seen for one-time second-opinion consults, which required rapid identification, referral, and access to genetic counselors and genetic testing. Additionally, patients seen at our institution often travel across the country or from outside the United States to seek cancer diagnosis and treatment, and therefore may not be representative of the United States’ HGOC patient population. We also recognize that the dedicated institution-wide research program resources, research and clinical staff, and genetic counselor support at our institution during the course of this project may not be characteristic of oncology practices across the United States, and therefore, replicating our initiative or interventions in other oncology settings may be challenging. The interventions used, while designed to be low-risk to patients and clinicians, were not rigorously studied or validated during our initiative. Future studies should include the assessment of patient satisfaction, knowledge, and fulfillment of psychosocial needs when using alternate care delivery models such as physician-coordinated genetic testing.
The outcomes of our initiative may reflect the influence of external events beyond our control. Several major events occurred during the course of our initiative, including the United States Supreme Court decision regarding the prior BRCA patent law, Angelina Jolie’s public announcement of her genetic testing results and risk-reducing surgeries, and published genetic testing guidelines by the Society of Gynecologic Oncology, American College of Medical Genetics, and the American Society of Clinical Oncology (6–8, 20, 21). Another major event was the FDA approval of PARPi therapy in 2014, which added a new incentive for patients with ovarian cancer to undergo genetic testing for BRCA1 and BRCA2 (19). The impact of these events is difficult to quantify in the context of our study but likely contributed to our improved genetic counseling and testing rates during this period.
Since we have not yet reached 100% adherence with national guidelines, our universal genetic testing initiative will continue, with increased awareness of efficiency and sustainability. We plan to disseminate our universal genetic testing initiative to other oncology settings to identify barriers to patients accessing genetics services, determine the current rates of recommendation and completion of genetic counseling and genetic testing, and determine the feasibility of implementing our process in different settings. Future studies should include the assessment of healthcare costs and savings related to the implementation of universal genetic testing, quality improvement-based clinical interventions, and genetic testing strategies (single gene versus multi-gene panel) in the ovarian cancer patient population. Additionally, Kwon et al. have modeled the potential downstream benefits of providing BRCA testing to all patients with HGOC and the implications for cancer prevention in BRCA-positive families.(22) We plan to assess the impact on the families identified to have a BRCA mutation during our universal genetic testing initiative to determine whether cascade testing has been performed and whether relatives have changed their medical management and cancer screening practices.
Manuscript Highlights.
<25% of ovarian cancer patients in the U.S. receive recommended genetics services
We increased the rates of genetic counseling and testing to over 85% in our clinic
Various interventions were used to increase rates of genetic counseling and testing
Physician-coordinated genetic testing of ovarian cancer patients is an option
Genetic testing results can impact ovarian cancer treatment options
Acknowledgments
The study was funded by The University of Texas MD Anderson Cancer Center Funding was provided by The MD Anderson Cancer Center Moon Shot™ targeting breast and ovarian cancers and Phillips 66. This work was also supported by the NIH/NCI under award number P30CA016672 (used the Clinical Trials Support Resource and the Biostatistics Resource Group). We would like to acknowledge the Department of Scientific Publications for their review of the manuscript, and everyone who helped with the universal genetic testing effort, including gynecologic oncology clinic administrators, staff, nurses, genetic counselors, advanced practice providers, and physicians of the Gynecologic Oncology and Reproductive Medicine department and the Moon Shots Program leadership.
This project was funded by The University of Texas MD Anderson Cancer Center Breast and Ovarian Cancer Moon Shot™ Program and Phillips 66. This work was also supported by the NIH/NCI under award number P30CA016672 (used the Clinical Trials Support Resource and the Biostatistics Resource Group). Dr. Shannon Westin is supported by the Andrew Sabin Family Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors: Ms. Bednar, Ms. Oakley, Dr. Sun, Ms. Burke, and Dr. Lu have no conflicts of interest to disclose. Mr. Munsell reports salary support from grants from National Institutes of Health, the Cancer Prevention Research Institute of Texas, and Stand Up 2 Cancer. He also reports salary support paid to his institution from Novadaq Technologies, Inc. and from Pacira Pharmaceuticals, Inc. for work on sponsored studies. Dr. Westin reports grants, personal fees and non-financial support from AstraZeneca, personal fees from ACI Clinical/ Xenetic BioSciences, personal fees and non-financial support from Clovis Oncolgy, personal fees and non-financial support from Genentech, personal fees and non-financial support from Medivation, grants from COTI, grants from Novartis, outside the submitted work.
References
- 1.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 2.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. Journal of the National Cancer Institute. 2006;98(23):1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 3.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(21):2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Parmigiani G. Meta-Analysis of BRCA1 and BRCA2 Penetrance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(11):1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian [Internet] National Comprehensive Cancer Network, Inc; 2008. [Google Scholar]
- 6.Lancaster JM, Powell CB, Chen LM, Richardson DL Committee SGOCP. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecologic oncology. 2015;136(1):3–7. doi: 10.1016/j.ygyno.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Hampel H, Bennett RL, Buchanan A, Pearlman R, Wiesner GL, Guideline Development Group ACoMG et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genetics in medicine : official journal of the American College of Medical Genetics. 2015;17(1):70–87. doi: 10.1038/gim.2014.147. [DOI] [PubMed] [Google Scholar]
- 8.Lu KH, Wood ME, Daniels M, Burke C, Ford J, Kauff ND, et al. American Society of Clinical Oncology Expert Statement: collection and use of a cancer family history for oncology providers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(8):833–40. doi: 10.1200/JCO.2013.50.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Febbraro T, Robison K, Wilbur JS, Laprise J, Bregar A, Lopes V, et al. Adherence patterns to National Comprehensive Cancer Network (NCCN) guidelines for referral to cancer genetic professionals. Gynecologic oncology. 2015;138(1):109–14. doi: 10.1016/j.ygyno.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell CB, Littell R, Hoodfar E, Sinclair F, Pressman A. Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling? Int J Gynecol Cancer. 2013;23(3):431–6. doi: 10.1097/IGC.0b013e318280f2b4. [DOI] [PubMed] [Google Scholar]
- 11.Wright JD, Chen L, Tergas AI, Accordino M, Ananth CV, Neugut AI, et al. Underuse of BRCA testing in patients with breast and ovarian cancer. American journal of obstetrics and gynecology. 2016;214(6):761–3. doi: 10.1016/j.ajog.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Cragun D, Bonner D, Kim J, Akbari MR, Narod SA, Gomez-Fuego A, et al. Factors associated with genetic counseling and BRCA testing in a population-based sample of young Black women with breast cancer. Breast cancer research and treatment. 2015;151(1):169–76. doi: 10.1007/s10549-015-3374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy AM, Bristol M, Domchek SM, Groeneveld PW, Kim Y, Motanya UN, et al. Health Care Segregation, Physician Recommendation, and Racial Disparities in BRCA1/2 Testing Among Women With Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(22):2610–8. doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichmeyer JN, Burnham C, Sproat P, Tivis R, Beck TM. The value of a genetic counselor: improving identification of cancer genetic counseling patients with chart review. Journal of genetic counseling. 2014;23(3):323–9. doi: 10.1007/s10897-013-9664-5. [DOI] [PubMed] [Google Scholar]
- 15.Kurian AW, Griffith KA, Hamilton AS, et al. Genetic testing and counseling among patients with newly diagnosed breast cancer. Jama. 2017;317(5):531–4. doi: 10.1001/jama.2016.16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer LA, Anderson ME, Lacour RA, Suri A, Daniels MS, Urbauer DL, et al. Evaluating Women with Ovarian Cancer for BRCA1 and BRCA2 Mutations. Obstetrics and Gynecology. 2010;115:945–52. doi: 10.1097/AOG.0b013e3181da08d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Associates in Process Improvement. [[updated 4/10/2013; cited 2017 1/25/2017]];Plan-Do-Study-Act (PDSA) Cycle AHRQ: Agency for Healthcare Research and Quality: U.S. Department of Health and Human Services. 2008 Available from: https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle.
- 19.Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, et al. FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(19):4257–61. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 20.Evans DGR, Barwell J, Eccles DM, Collins A, Izatt L, Jacobs C, et al. The Angelina Jolie effect: How high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Research. 2014;16(5) doi: 10.1186/s13058-014-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conley JM, Cook-Deegan R, Lazaro-Munoz G. MYRIAD AFTER MYRIAD: THE PROPRIETARY DATA DILEMMA. North Carolina journal of law & technology. 2014;15(4):597–637. [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon JS, Daniels MS, Sun CC, Lu KH. Preventing future cancers by testing women with ovarian cancer for BRCA mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(4):675–82. doi: 10.1200/JCO.2008.21.4684. [DOI] [PubMed] [Google Scholar]