Abstract
The North Temperate Lakes Long-Term Ecological Research site includes seven lakes in northern Wisconsin that vary in hydrology, trophic status, and landscape position. We examine the molecular composition of dissolved organic matter (DOM) within these lakes using Fourier transform-ion cyclotron resonance mass spectrometry (FT-ICR MS) and quantify DOM photochemical activity using probe compounds. Correlations between the relative intensity of individual molecular formulas and reactive species production demonstrate the influence of DOM composition on photochemistry. For example, highly aromatic, tannin-like formulas correlate positively with triplet formation rates, but negatively with triplet quantum yields, as waters enriched in highly aromatic formulas exhibit much higher rates of light absorption, but only slightly higher rates of triplet production. While commonly utilized optical properties also correlate with DOM composition, the ability of FT-ICR MS to characterize DOM subpopulations provides unique insight into the mechanisms through which DOM source and environmental processing determine composition and photochemical activity.
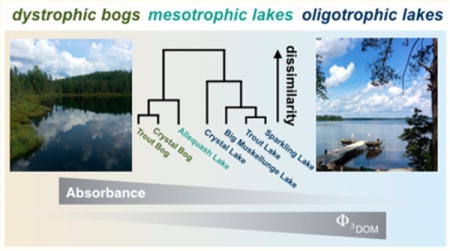
Graphical abstract
INTRODUCTION
Dissolved organic matter (DOM) is a compositionally diverse assembly of molecules that is ubiquitous in natural waters. DOM contributes to numerous environmental processes including carbon transport,1 redox cycling,2 and reactions with environmental contaminants.3 Of special interest to lacustrine systems is the ability of DOM to degrade xenobiotic compounds through the photochemical production of reactive triplet states (3DOM)4 and reactive intermediates such as singlet oxygen (1O2)5 and hydroxyl radicals.6,7
DOM derives from dissimilar sources and, accordingly, is diverse in composition and photochemical behavior.8–11 Broadly, DOM is considered allochthonous when derived from degraded terrestrial plant material or autochthonous when derived from aquatic microorganisms. Allochthonous DOM is typically higher in molecular weight,12 lower in heteroatom content (e.g., N and S),13 and more aromatic than autochthonous DOM.14 DOM is further differentiated in natural systems by physical,15 chemical,16 and biological17–19 processing. While it is challenging to apportion individual mechanisms to DOM modification in environmental systems, DOM tends to become more aliphatic, less oxidized, more diverse in elemental composition, and less chromophoric as it moves from uplands to oceans.20–22 Similarly, formulas that are more aliphatic, less oxidized, and N-rich are more persistent in lakes.23
DOM photochemistry also varies with source and environmental processing. 3DOM and 1O2 quantum yields are generally higher in autochthonous DOM than allochthonous DOM.24,25 Similarly, 1O2 quantum yields increase while 3DOM and 1O2 steady-state concentrations decrease as DOM moves from headwaters to the ocean.26,27 DOM photochemistry is commonly evaluated with the 3DOM probes sorbic acid (HDA) and 2,4,6-trimethylphenol (TMP), and the 1O2 probe furfuryl alcohol (FFA).28 While HDA and TMP directly measure 3DOM and 1O2 is formed from the reaction of 3DOM and O2, there is evidence that these probes measure distinct 3DOM subpopulations.15,29 Recent reviews have proposed using a combination of multiple probes to better describe 3DOM photoreactivity.30,31
The molecular composition of DOM is increasingly assessed with Fourier transform-ion cyclotron resonance mass spectrometry (FT-ICR MS).32,33 The ability of FT-ICR MS to identify individual molecular formulas in DOM has increased our understanding of how physical,34 chemical,35 and biological processes36 modify DOM and how DOM changes across environmental transects.20,21,37 For example, FT-ICR MS can determine the composition of heteroatom-containing formulas and identify the source of specific DOM subpopulations.16 Additionally, FT-ICR MS can detect bimodal distributions in aromaticity and heteroatom composition that are not apparent using bulk measurements.20 However, as with other methods for determining DOM composition, FT-ICR MS is subject to analytical biases, such as the preferential detection of readily ionized formulas.38
There is growing interest in relating DOM photochemistry and composition, as xenobiotic compounds are increasingly identified in remote waters.39,40 However, 3DOM production has not been previously related to molecular composition of DOM. Therefore, we combine photochemical analyses with FT-ICR MS to investigate how DOM composition controls photochemical activity in seven diverse lakes. Samples were collected from the North Temperate Lakes Long-Term Environmental Research (NTL-LTER) site in northern Wisconsin. Little is known about the DOM composition and photoreactivity of NTL-LTER lakes, and the diversity of DOM sources and distinct UV–vis absorbance spectra make them an ideal site to relate these attributes.
MATERIALS AND METHODS
Sample Location
The NTL-LTER site includes two dystrophic (Crystal and Trout Bogs), one mesotrophic (Allequash Lake), and four oligotrophic lakes (Big Muskellunge, Crystal, Sparkling, and Trout Lakes). The NTL-LTER lakes vary in surface area and water source, as well as in DOM source and concentration (Supporting Information (SI) Table S1).41–44 The site is in a forested region of northern Wisconsin that is dominated by lakes.45
Samples were collected from the center of the NTL-LTER lakes between 02/1990 and 11/2014 and analyzed within 2–3 weeks for the concentration of dissolved organic carbon ([DOC]) and by UV–visible spectroscopy (UV–vis). Additional samples (1–4 L) were collected from the center of each lake in June 2015 and the edge of each lake in August 2016 for photochemical analysis. Detailed information about sample collection, analysis, and materials is available in SI Sections S1–S2.
UV–vis Spectroscopy
Absorbance of samples collected in 2015 and 2016 was determined using a Shimadzu UV-2401 PC, relative to a Milli-Q reference. SUVA254 is the ratio of absorbance at 254 nm (A254) to [DOC].14 E2:E3 is the ratio of absorbance at 250 to 365 nm.22
Mass Spectrometry
DOM was extracted from the NTL-LTER samples collected in August 2016 by solid phase extraction (SPE) and analyzed by FT-ICR MS. Details about the SPE protocol are included in SI Section S3.46 Sample extracts were diluted 1:10 in 1:1 acetonitrile:Milli-Q and aspirated with 0.3 psi pressure into an electrospray source with an applied voltage of −1.4 V. Analysis was performed with a SolariX XR 12T FT-ICR MS (Bruker) coupled to a Triversa NanoMate sample delivery system (Advion), as described previously.47 Details about instrument settings and formula identification are available in SI Section S3. Formula relative intensities were determined by dividing the intensity of each formula by the sum of intensities of all identified formulas in a sample. Weighted average compositional values (e.g., H:Cw.avg) are the average of that compositional value in all formulas, weighted by the relative intensity of each formula. It should be noted that these average compositional values are determined only from formulas identified by FT-ICR MS, rather than bulk elemental analysis. Average compositional values exhibit similar qualitative trends to bulk analysis (e.g., elevated H:C and N:C in autochthonous fulvic acid isolates)13 and provide insight into compositional differences between natural DOM samples.21 Double bond equivalents per carbon (DBE/C) was calculated as (C − 0.5(H) + 0.5(N+S) + 1)/C.48 Optical properties and photochemical measurements are correlated by Pearson’s coefficients with the relative intensities of individual formulas.
Photochemistry
Samples collected in 2015 and 2016 were irradiated in a Rayonet photoreactor with UV-A bulbs (365 ± 9 nm).25,49 The initial photon flux of the experimental apparatus was determined to be 7.35 × 10−8 einsteins cm−2 s−1 with p-nitroanisole (PNA)-pyridine actinomtery.25 Irradiation durations varied according to probe and sample (10–360 min), and all irradiations were conducted in triplicate. HDA was added at initial concentrations of 10, 100, 250, 500, and 1000 μM. 3DOM quantum yields (Φ3DOM), formation rates (F3DOM), steady-state concentrations ([3DOM]ss) and first-order loss rate constants (kd) were calculated from the isomerization rate of t, t-HDA and previously estimated rate constants.25,50 Additionally, the observed loss rates of the 3DOM probe TMP (initial concentration = 10 μM) were used to calculate 3DOM quantum yield coefficients (fTMP) and steady-state concentrations of 3DOM ([3DOM]ss, TMP) using estimated rate constants as described previously.51,52 The quantum yields (Φ1O2) and steady-state concentrations of 1O2([1O2]ss) were calculated from the observed loss rates of FFA (initial concentration = 10 μM) and previously measured rate constants.53,54 Quantum yields and fTMP were calculated with PNA-pyridine actinometry. All calculations are described in detail in SI Section S4.55 FFA, PNA, TMP, and HDA isomer concentrations were quantified by high-performance liquid chromatography as described previously.25
RESULTS AND DISCUSSION
[DOC] and Optical Properties
[DOC] and UV–vis measurements taken from 1990 through 2014 distinguish the seven NTL-LTER lakes according to trophic status and reflect the source and transformation of DOM within each lake. [DOC] represents the concentration of bulk DOM, whereas A254 quantifies light absorption by chromophoric DOM. DOM composition is described by SUVA254, which correlates with aromaticity,14 and E2:E3, which is inversely related to molecular weight.25,56 The bogs have the highest mean [DOC], A254, and SUVA254, and lowest mean E2:E3 (Figure 1; SI Table S2). The high SUVA254 and low E2:E3 values are typical of terrestrially-derived DOM,57–59 and are reflective of the high fraction of DOM from adjacent wetlands (i.e., ~65%), high DOC loading rates, shallow photic zones, and short hydraulic residence times (HRTs).44
Figure 1.
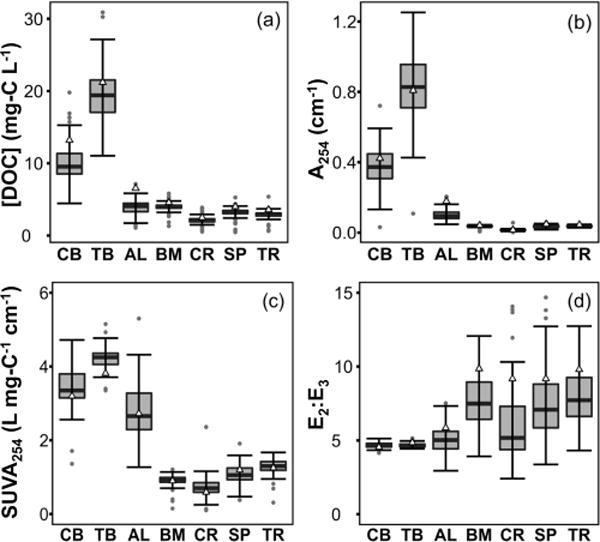
Measured (a) [DOC], (b) A254, (c) SUVA254, and (d) E2:E3 in NTL-LTER lakes. Values from samples collected from 1990–2014 are displayed as box-and-whisker plots; the mean is marked with a horizontal line, the edges of the box correspond to the 25th and 75th percentiles, and the whiskers mark 1.5 times the interquartile range. Outliers are marked with gray circles. Values from samples collected in 2016 are marked with triangles.
In contrast, optical properties cannot be used to distinguish the source of DOM in the oligotrophic lakes. These lakes have the lowest [DOC] and A254 due to longer HRTs and lower carbon loading rates (SI Table S1),44 but are highly variable in SUVA254 and E2:E3. The major sources of DOM to the oligotrophic lakes are precipitation, aerial deposition, surface waters, and groundwater, rather than terrestrially-derived DOM.44 Additionally, more DOM is lost to mineralization compared with export and sedimentation, indicating higher rates of DOM processing.44 The optical properties of the oligotrophic lakes could indicate the presence of autochthonous DOM, which typically has higher E2:E3 and lower SUVA254 than allochthonous DOM.14,22,59–61 However, photobleaching of terrestrially derived DOM decreases SUVA254 and increases E2:E3,15 and the presence of measurable autochthonous DOC in these lakes is debated.44,62 Collectively, the lower SUVA254 and higher E2:E3 values in oligotrophic lakes could reflect DOM from autochthonous sources or allochthonous DOM that has undergone extensive processing.
Mesotrophic Allequash Lake is unique among the seven NTL-LTER lakes. Although the DOC loading rate is similar to the bogs, Allequash Lake has the shortest HRT and, uniquely, exports more DOC than is lost to mineralization and sedimentation.44 Additionally, it receives more DOM from adjacent wetlands than the oligotrophic lakes, but less than the bogs (i.e., ~10%).44 These factors produce DOM that shares some properties with the bogs (high SUVA254, low E2:E3) and some properties with the oligotrophic lakes (low [DOC] and A254).
Samples were collected in 2016 for photochemistry and FT-ICR MS measurements, and have [DOC], A254, and E2:E3 slightly above historical means (Figure 1; SI Table S2). The elevated [DOC] and A254 of samples collected in 2016 may reflect their collection from the lake edges, rather than centers. However, UV–vis compositional measurements are generally within a standard deviation of historical means, indicating that the photochemistry and FT-ICR MS results are reflective of representative DOM for NTL-LTER lakes. Long-term trends in the optical properties of the NTL-LTER lakes have been discussed previously.63
Molecular Composition of DOM
[DOC] and UV–vis measurements differ between lakes according to trophic status, but the cause of these variations is unclear. Analysis of DOM by FT-ICR MS provides more detailed compositional information. 3037 unique CHON0–1P0–1S0–1 formulas are identified in the NTL-LTER lakes (Table 1), with 975–1791 unique formulas in individual lakes. In each lake, 60–81% of identified formulas contain only CHO. The predominance of CHO formulas is typical of allochthonous DOM.13,17 Similarly, the bulk compositional measurements of CHO formulas are characteristic of lignin-like, terrestrially-derived molecules (H:Cw.avg = 1.05–1.36; O:Cw.avg = 0.46–0.56; Figure 2 and SI Figure S2).21,64–66 While the terms “lignin-like” and “tannin-like” are not definitive (i.e., a lignin-like formula may not necessarily be derived from lignin), these elemental ratio cutoffs are useful in visualizing the FT-ICR MS data. H:Cw.avg increases and DBE/Cw.avg decreases from the bogs to Allequash Lake to the oligotrophic lakes, confirming that the DOM in the bogs is more aromatic (Table 1). O:Cw.avg is highest in the bogs and lower in Allequash Lake and the oligotrophic lakes.
Table 1.
Formulas Identified in NTL-LTER Lakes by FT-ICR MSa
| Total (#) | CHO (#) | CHON (#) | CHOP (#) | CHOS (#) | O:Cw.avg | H:Cw.avg | DBEw.avg | DBE/Cw.avg | |
|---|---|---|---|---|---|---|---|---|---|
| Dystrophic | |||||||||
| Crystal Bog (CB) | 1168 | 855 | 179 | 110 | 20 | 0.55 | 1.08 | 11.25 | 0.51 |
| Trout Bog (TB) | 975 | 793 | 113 | 44 | 18 | 0.56 | 1.06 | 11.63 | 0.52 |
| Mesotrophic | |||||||||
| Allequash L. (AL) | 1299 | 943 | 224 | 79 | 51 | 0.52 | 1.14 | 10.63 | 0.48 |
| Oligotrophic | |||||||||
| Big Muskellunge L. (BM) | 1581 | 962 | 404 | 90 | 109 | 0.50 | 1.30 | 8.26 | 0.41 |
| Crystal L. (CR) | 1523 | 915 | 466 | 55 | 78 | 0.44 | 1.39 | 7.05 | 0.36 |
| Sparkling L. (SP) | 1791 | 1174 | 463 | 54 | 84 | 0.52 | 1.22 | 9.22 | 0.44 |
| Trout L. (TR) | 1583 | 1101 | 334 | 59 | 81 | 0.50 | 1.24 | 9.01 | 0.43 |
Compositional information is calculated for all formulas identified in each lake.
Figure 2.
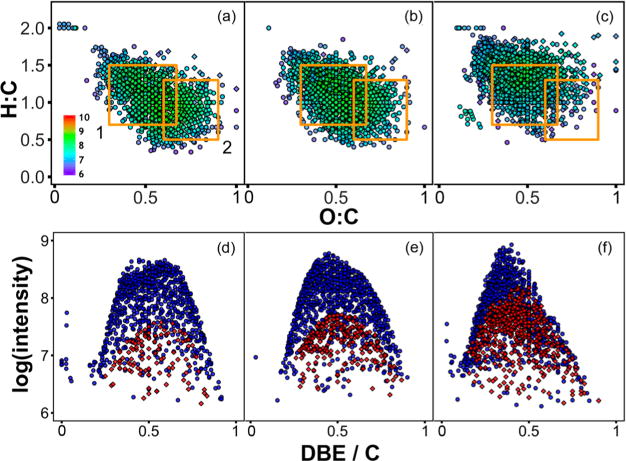
van Krevelen diagrams of CHO (circles) and CHON (diamonds) formulas identified in (a) Trout Bog, (b) Allequash Lake, and (c) Crystal Lake. Orange boxes indicate regions commonly defined as (1) lignin-like and (2) tannin-like.21,64–67 Marker color denotes the log-normalized intensity. The log-normalized intensity of CHO (blue circles) and CHON (red diamonds) formulas as a function of DBE/C in (d) Trout Bog, (e) Allequash Lake, and (f) Crystal Lake.
CHON formulas comprise 12–31% of all identified formulas (Table 1) and occupy similar van Krevelen space as CHO formulas from the same water body (Figure 2 and SI Figure S3; Table S5). Higher fractions of formulas containing N are observed in the oligotrophic lakes (21–31%) than in the bogs (12–15%) or Allequash Lake (17%). The enrichment of CHON formulas in oligotrophic lakes could derive from the extensive irradiation of allochthonous DOM or the presence of autochthonous DOM.17 However, the compositional similarity of CHON and CHO formulas suggests a terrestrial source.
Few CHOP and CHOS formulas are detected (SI Figures S4 and S5; Table 1 and SI Table S6). Crystal Bog and Allequash Lake have the highest abundance of P-containing formulas (9.4 and 6.1%, respectively), which could indicate preferential uptake of P-containing formulas in the nutrient-poor lakes. Phospholipid formulas have been identified in offshore coastal waters, but the CHOP formulas observed here have higher O:C than expected for phospholipids.21 While few CHOS formulas are identified in NTL-LTER lakes, some are observed at very high intensity, likely reflecting their ease of ionization.38
Molecular Composition Differences Among Lakes
Bray–Curtis dissimilarity analysis according to the log-weighted intensity of all identified formulas separates the lakes by trophic status (SI Figure S6). Crystal and Trout Bogs are highly similar, with Allequash Lake being slightly more dissimilar, while the oligotrophic lakes are highly dissimilar from the bogs. This trend reflects the differences in the 25-year record of optical properties. For example, SUVA254 measurements were highest in the bogs and lowest in Crystal Lake (Figure 1).
DOM compositional variation with trophic status is apparent in comparisons of CHON0–1 formulas that are commonly identified in five or more lakes (n = 844; SI Figure S7). The 481 CHO and 14 CHON formulas with higher relative intensity in the bogs and Allequash Lake have lower average H:C (1.01 ± 0.24) and higher average O:C (0.54 ± 0.15) than 250 CHO and 99 CHON formulas enhanced in the oligotrophic lakes (H:C = 1.37 ± 0.02; O:C = 0.47 ± 0.14). 25% of formulas enhanced in oligotrophic lakes have H:C values ≥1.5, which are typical of microbially derived compounds,64,67 compared with <1% of common formulas enhanced in the bogs and Allequash Lake. Interestingly, 59% of the 64 common CHON0–1 formulas with H:C < 1.2 that are enhanced in the oligotrophic lakes contain nitrogen. An enhancement of highly aromatic CHON formulas was observed during the irradiation of Congo River water.68
DBE/C describes the sum of double bonds or ring structures per carbon atom in a molecule. Dystrophic bogs show a bimodal distribution of CHO formulas with peaks centered on DBE/C ~ 0.4 and ~0.6 when log-normalized intensity is plotted against DBE/C (Figure 2 and SI Figure S8). These peaks correspond to H:C of 1.3 and 0.9, respectively, assuming an average of 20 carbon atoms per formula, which is reasonable given that the intensity-weighted average C of all observed formulas ranges from 19.6 in Crystal Lake to 22.4 in Trout Bog. The two peaks have similar maximum intensities in Trout Bog, while the more aromatic peak is visibly depressed in Allequash Lake and nearly absent in Crystal Lake. Therefore, the lower relative aromaticity (i.e., lower SUVA254 and higher H:Cw.avg) of oligotrophic lake DOM arises from the absence of a class of highly aromatic formulas. A similar bimodal distribution of DBE-O was observed in CHO formulas in samples collected near New Zealand, but was absent in waters collected farther offshore.20 The trends in intensity as a function of DBE/C are similar for CHON formulas, in agreement with the compositional similarity of CHO and CHON formulas in van Krevelen diagrams (Figure 2 and SI Figure S8). The presence of highly aromatic formulas in bogs and, to a lesser degree, Allequash Lake is attributable to the input of DOM from adjacent wetlands.44 Since irradiation preferentially removes highly aromatic formulas from terrestrial DOM,34,68,69 the absence of more aromatic DOM in the oligotrophic lakes may reflect their deeper photic zone and longer HRT. These formulas are related to long-wavelength absorption and therefore affect photoreactivity, as described below.
While highly aromatic formulas are largely absent in the oligotrophic lakes, the formulas with DBE/C of ~0.4 represent a ubiquitous class of aliphatic compounds (Figure 2d–f). The 307 CHO formulas observed in all seven lakes have an average DBE/C of 0.42 (SI Figure S9; Table S7). Furthermore, their average bulk compositional values (H:C = 1.22 ± 0.22; O:C = 0.47 ± 0.13) are similar to average values in the oligotrophic lakes. Aliphatic character was observed to correlate with environmental persistence in a previous comparison of DOM from diverse lakes.23 Additionally, the ubiquitous CHO formulas are compositionally similar to CHO formulas identified as universally present in terrestrial and marine samples (H:C ~ 1.4; O:C ~ 0.45) near New Zealand.20
Relating Optical Properties and Composition
UV–vis and FT-ICR MS measurements strongly correlate among the NTL-LTER lakes. For example, SUVA254 correlates with FT-ICR MS measurements of aromaticity (e.g., H:Cw.avg and DBE/Cw.avg; SI Figure S10). A linear correlation between DBEavg and SUVA254 was previously observed among the molecular weight fractions of a terrestrially derived DOM isolate.25 These correlations are noteworthy because the two techniques sample different populations of DOM; UV–vis spectroscopy only detects molecules that absorb light, while FT-ICR MS only detects molecules that ionize with the selected ionization source (i.e., negative mode electrospray).
Bulk optical properties also correlate with the relative intensity of individual molecular formulas. For this analysis, the Pearson’s correlation coefficient is calculated between the relative intensity of commonly identified CHON0–1 formulas and the optical properties in each lake. For data visualization, the formulas are divided into those enhanced in bogs and Allequash Lake and those enhanced in oligotrophic lakes (Figures 3a–d). The 477 CHON0–1 formulas that correlate positively with SUVA254 (r ≥ 0.5) are more aromatic and oxidized than the 217 formulas that show negative correlations (r ≤ −0.5). 91% of formulas that positively correlate with SUVA254 are enhanced in the bogs and Allequash Lake, while 82% of those that negatively correlate with SUVA254 are enhanced in the oligotrophic lakes. Conversely, 97% of the 173 formulas that correlate positively with E2:E3 have higher average relative intensity in the oligotrophic lakes; these formulas are more aliphatic and oxidized than the 452 formulas that correlate negatively with E2:E3. Negative correlations between formulas with high H:C and SUVA254 have been reported in diverse systems,23,47,70 but this is the first reported correlation between E2:E3 and the relative intensity of aliphatic formulas in lakes.
Figure 3.
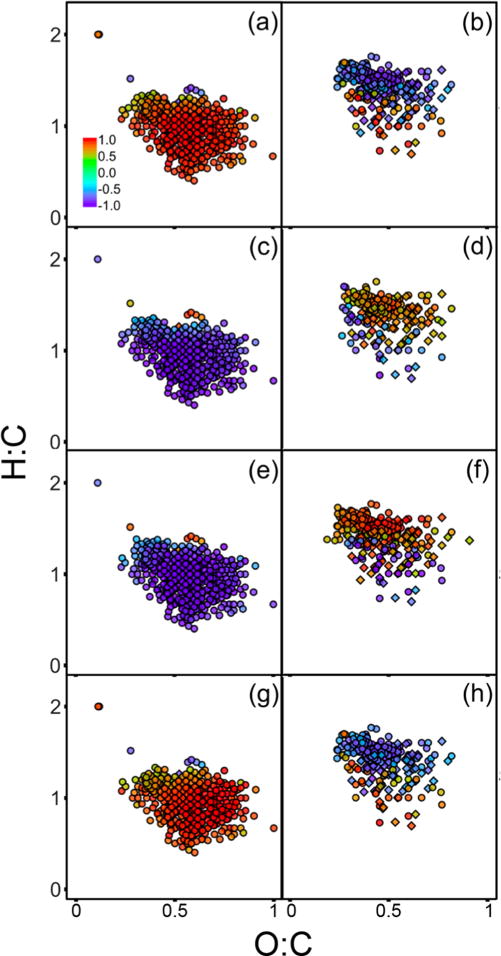
Commonly identified CHO (circles) and CHON (diamonds) formulas that have higher average relative intensity in (a, c, e, g) bogs and Allequash Lake or (b, d, f, h) oligotrophic lakes. Formulas have Pearson’s correlation coefficient ≤ −0.5 or ≥ 0.5 between relative intensity and (a, b) SUVA254, (c, d) E2:E3, (e, f) Φ3DOM, and (g, h) F3DOM. Marker fill denotes the Pearson’s coefficient for correlation between relative intensity and optical or photochemical properties.
Photochemistry
The photochemical activity of the NTL-LTER lakes gives further insight into DOM composition. Φ3DOM (0.002–0.0081), fTMP (5.4–68.3 M−1), and Φ1O2 (0.005–0.019) quantified in the 2016 samples are generally lower than previously reported for similar waters (Figure 4a; SI Table S8). For example, Φ3DOM values are slightly below previous measurements in allochthonous DOM isolates (~0.012),25,50 fTMP values are below previously reported values in river waters (60–100 M−1),60 and Φ1O2 values are below values reported in waters from Lake Superior and its tributaries (~0.02–0.035).26 However, the apparent discrepancy in fTMP is partially due to a revision in the quantum yield of the actinometer.55,71 For example, using historical constants for the PNA-pyridine system increases fTMP by 30% (SI Table S9).71 Lower Φ3DOM compared to Φ1O2 has been reported previously,25,52 and arises from differences in the concentration of 3DOM subpopulations that react with HDA and O2.4,29
Figure 4.
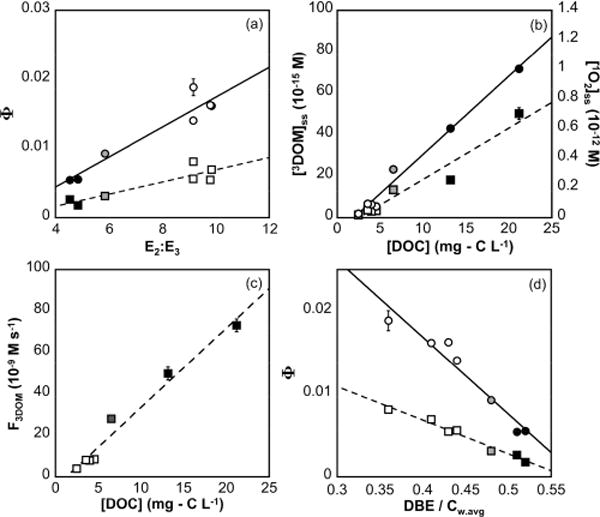
(a) Quantum yields of 3DOM determined with HDA (Φ3DOM; square markers) and 1O2 with FFA (round markers) plotted versus E2:E3 where Φ3DOM = 0.00088 × E2:E3 − 0.00187 (r2 = 0.81)2 and Φ1O2 = 0.00216 × E2:E3 − 0.00424 (r2 = 0.91). (b) Steady-state concentrations of 3DOM (square markers) and 1O2 (round markers) plotted versus [DOC], where [3DOM]ss (×10−15 M) = 4.31 × [DOC] + 0.26 (r2 = 0.94) and [1O2]ss (×10−15 M) = 52.9 × [DOC] − 102.8 (r2 = 0.99). (c) 3DOM formation rate plotted versus [DOC], where F3DOM (×10−9) = 3.82 × [DOC] − 4.76 (r2 = 0.97). (d) Φ3DOM (square markers; r2 = 0.97) and Φ1O2 (round markers; r2 = 0.94) versus DBE/Cw.avg. Oligotrophic lakes are indicated with hollow markers, Allequash Lake is indicated with gray markers, and bogs are indicated with black markers. Error bars represent the standard deviation of triplicate analysis. Trend lines show linear, least-squares regressions.
Φ3DOM, Φ1O2, and fTMP increase linearly with E2:E3, and are lowest in the bogs and highest in the oligotrophic lakes (Figure 4a and SI Figure S11a). While the slopes of Φ3DOM and fTMP are shallower than some previous reports,25,53,56 the observed slope of Φ1O2 vs E2:E3 is similar to one recorded in waters collected from Lake Superior and its tributaries.26 As with other compositional measurements, quantum yields of 3DOM and 1O2 vary with DOM source and environmental processing. Φ1O2 increases as terrestrial DOM undergoes photobleaching,15 whereas Φ3DOM and Φ1O2 are higher in autochthonous, rather than allochthonous, DOM.24,25
[3DOM]ss, measured with HDA (0.1320100335.0 × 10−14 M) and TMP (1.–11.7 × 10−14 M), and [1O2]ss (2.7–100 × 10−14 M) are highest in the bogs and lowest in the oligotrophic lakes (Figure 4b and SI Figure S11b; Table S10). While steady-state concentrations are sensitive to irradiation intensity, [3DOM]ss and [1O2]ss reported here are similar to values measured in surface waters.26,27,72 [3DOM]ss, HDA and [1O2]ss increase linearly with [DOC] (Figure 4b). A similar slope of [1O2]ss with [DOC] was observed in waters from Lake Superior and its tributaries.26
[3DOM]ss, TMP showed less variation than [3DOM]ss and [1O2]ss because [3DOM]ss, TMP levels off in samples with [DOC] > 5 mg-C L−1 (SI Figure S11b; Table S10). Similar results were also observed in NTL-LTER waters collected in 2015 (SI Figure S11c and Table S11). When Crystal and Trout Bog waters from 2015 were diluted to below 5 mg-C L−1, the linear correlation between [3DOM]ss, TMP and [DOC] was similar to the trend in the other NTL-LTER lakes (SI Figure S11d and Table S12). The suppression of TMP loss rates at high [DOC] has been observed previously,52,60 and may affect [3DOM]ss, TMP ranges in samples with varied [DOC].
Φ3DOM describes the ratio of F3DOM to light absorption, whereas [3DOM]ss describes the ratio of F3DOM to the 3DOM quenching rate constant (kd). The 3DOM probe HDA allows these parameters to be quantified separately.50 F3DOM is highest in the bogs, and increases linearly with [DOC] and SUVA254 (Figure 4c and SI Figure S12a). Similar F3DOM values and correlations between F3DOM and [DOC] have been reported for allochthonous DOM.27,50 While both F3DOM and rates of light absorbance (Rabs) are higher in the bogs than the oligotrophic lakes, Rabs varies by much more than F3DOM and results in lower quantum yields in the bogs (SI Table S8). Therefore, the trend in Φ3DOM across the NTL-LTER lakes is primarily driven by differences in Rabs, rather than F3DOM. In contrast, the consistency of kd among the lakes (SI Figure S13) demonstrates that 3DOM formation rates determine the differences seen in [3DOM]ss.
Relationships Between Photochemistry and Molecular Composition
Photochemical reactivity correlates with bulk FT-ICR MS measurements in the NTL-LTER lakes. For example, 3DOM and 1O2 quantum yields linearly correlate positively with H:Cw.avg and negatively with DBE/Cw.avg (Figure 4d and SI Figure S14). While previous studies have noted correlations between 3DOM production and UV–vis measurements,56 correlations between quantum yields and molecular composition determined by FT-ICR MS have not been previously reported.
As with optical properties, photochemical measurements correlate with the relative intensity of individual commonly identified CHON0–1 formulas (Figure 3). The 237 formulas that positively correlate with Φ3DOM are less aromatic (H:C = 1.45 ± 0.15) and oxidized (O:C = 0.45 ± 0.13) than the 466 formulas with negative Φ3DOM correlations (H:C = 0.98 ± 0.23; O:C = 0.56 ± 0.14). Similar trends are apparent when correlating the relative intensity of commonly identified CHON0–1 with Φ1O2 or fTMP (SI Figure S15). Conversely, the 449 formulas that correlate positively with F3DOM are more aromatic (H:C = 0.96 ± 0.23) and oxidized (O:C = 0.56 ± 0.14) than 196 formulas with negative F3DOM correlations (H:C = 1.45 ± 0.14; O:C = 0.47 ± 0.12). Formulas that correlate positively with F3DOM are more likely to be in the lignin- or tannin-like regions of the van Krevelen diagram (395/449) than formulas that correlate positively with Φ3DOM (123/237). Formulas enhanced in oligotrophic lakes are more likely to positively correlate with Φ3DOM (231/273) and negatively with F3DOM (190/233), while formulas enriched in bogs and Allequash Lake are more likely to correlate positively with F3DOM (406/412) and negatively with Φ3DOM (437/443).
There is extensive overlap between the commonly identified formulas that correlate positively with Φ3DOM and E2:E3, as well as between those that correlate positively with F3DOM and SUVA254. 97% of the formulas that positively correlate with Φ3DOM also positively correlate with E2:E3. Similarly, 99% of the formulas that correlate positively with F3DOM also correlate positively with SUVA254. These results agree with the linear correlations between bulk UV–vis and quantum yield measurements (Figure 4a and SI Figure S12a). Positive correlations between E2:E3 and Φ3DOM, and between absorbance and F3DOM, have been observed previously.25,56,60 However, this is the first study using FT-ICR MS to demonstrate that the same molecular formulas correlate with both optical properties and photochemical measurements.
No common formulas correlate positively with F3DOM and Φ3DOM, which is the ratio of F3DOM to Rabs. Instead, 627 common CHON0–1 formulas correlate positively with one and negatively with the other. This unintuitive result derives from the higher variation in Rabs than F3DOM, as described above. While F3DOM is highest in the bogs (Figure 4a), Rabs is even higher relative to the oligotrophic lakes (SI Table S8). This results in an inverse relationship between F3DOM and Φ3DOM in the NTL-LTER lakes (SI Figure S12b).
The charge-transfer model of DOM photochemistry describes long-wavelength absorbance as arising from intramolecular charge transfer interactions between electron-rich donor groups (e.g., hydroxy- or methoxy-aromatic moieties) and electron-poor acceptor groups (e.g., quinones or aldehydes) that are largely derived from the partial oxidation of lignins.9,73,74 These compounds should therefore be prevalent in highly aromatic, terrestrially derived DOM, such as the DOM collected from the bogs and Allequash Lake. Conversely, 3DOM production is related to the presence of borohydride-reducible carbonyls,75 which are found in compositionally diverse DOM formulas with H:C up to ~1.6.76
Although it is not possible to distinguish specific functional groups (e.g., carbonyls or phenols) using FT-ICR MS, the compositional and photochemical trends in NTL-LTER lakes support this model. For example, the increased intensity of aromatic formulas in the bogs and Allequash Lake, particularly the class of highly aromatic formulas apparent in plots of intensity versus DBE/C (Figure 2 and SI Figure S9), accompanies enhanced long-wavelength absorption (i.e., high SUVA254, low E2:E3). As a result, these waters exhibit both high rates of light absorbance (SI Table S8) and high 3DOM formation rates (Figure 4). Conversely, the lack of oxidized, aromatic formulas in oligotrophic lakes results in strongly reduced absorbance, but only moderately reduced F3DOM. Therefore, the oligotrophic lakes have higher 3DOM and 1O2 quantum yields (Figure 4). These results suggest that previous observations of higher 3DOM and 1O2 quantum yields in autochthonous DOM24,25 and increases in 1O2 quantum yields as DOM moves from headwaters to the ocean26,27 may be attributable to changes in DOM composition similar to those observed in the NTL-LTER lakes.
The correlations between relative formula intensity and photochemical measurements should be interpreted cautiously, as there are multiple possible explanations. For example, 1O2 is quenched within the DOM microenvironment77 and 3DOM may also be susceptible to intramolecular quenching. Intramolecular quenching is not apparent to hydrophilic probes, like the ones used here.77 Therefore, the apparently elevated Φ3DOM in oligotrophic lake waters could reflect decreased intramolecular quenching. Despite this caveat, this is the first direct link between 3DOM photochemistry and the molecular composition of DOM.
Environmental Implications
The NTL-LTER lakes represent a wide range of DOM source and hydrology. For example, Trout Bog receives DOM primarily from adjacent wetlands and water from precipitation, while Allequash Lake receives DOM and water from a mixture of surface and groundwater inputs.44 Despite these differences, DOM composition, optical properties, and photochemical activity are tightly correlated with lake trophic status. Further, there is a high correlation between FT-ICR MS measures of composition and both optical properties and photochemical reactivity. For example, highly aromatic formulas correlate with higher 3DOM production rates, while more aliphatic formulas correlate with higher quantum yields. These correlations suggest that straightforward models and UV–vis measurements can be used as a first approximation of DOM composition and photochemical reactivity in related water bodies. Future work should focus on relating FT-ICR MS measurements to the formation of other photochemically produced reactive intermediates, including hydrogen peroxide and hydroxyl radical.
However, while UV–vis measurements are useful in assessing DOM composition, FT-ICR MS provides uniquely detailed analysis of DOM composition and environmental processing. For example, here FT-ICR MS is used to identify a bimodal distribution in aromaticity and compare the composition of heteroatom-containing formulas. By relating compositional information about individual molecular formulas, FT-ICR MS provides insight into the processes that modify DOM composition. For example, while UV–vis measurements in NTL-LTER lakes show compositional dissimilarity between bogs and oligotrophic lakes, the decreased SUVA254 and increased E2:E3 in oligotrophic lakes could represent either autochthonous or photobleached allochthonous DOM. Conversely, FT-ICR MS results suggest that photoirradition, rather than biological processing, could produce the compositional distinctions between bogs and oligotrophic lakes.
Supplementary Material
Acknowledgments
We thank Emily Stanley and Noah Lottig for assistance with sample collection. NTL-LTER data collection was supported by NSF (DEB-1440297). DOC, UV–vis spectra, and ancillary data can be accessed at the NTL-LTER website: https://lter.limnology.wisc.edu/. A. Maizel was supported by the University of Wisconsin-Madison Graduate School. J. Li was supported as an NSF REU student (DEB-1440297).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b01270.
Information about NTL-LTER lakes, Figures S1–S15, and Tables S1–S12 are included in Supporting Information (PDF)
ORCID
Andrew C. Maizel: 0000-0002-2981-5241
Christina K. Remucal: 0000-0003-4285-7638
Notes
The authors declare no competing financial interest.
References
- 1.Opsahl S, Benner R. Distribution and cycling of terrigenous dissolved organic matter in the ocean. Nature. 1997;386:480–482. [Google Scholar]
- 2.Ratasuk N, Nanny MA. Characterization and quantification of reversible redox sites in humic substances. Environ Sci Technol. 2007;41(22):7844–7850. doi: 10.1021/es071389u. [DOI] [PubMed] [Google Scholar]
- 3.Remucal CK. The role of indirect photochemical degradation in the environmental fate of pesticides: A review. Environ Sci Processes Impacts. 2014;16(4):628–653. doi: 10.1039/c3em00549f. [DOI] [PubMed] [Google Scholar]
- 4.McNeill K, Canonica S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties. Environ Sci Processes Impacts. 2016;18(11):1381–1399. doi: 10.1039/c6em00408c. [DOI] [PubMed] [Google Scholar]
- 5.Haag WR, Gassman E. Singlet oxygen in surface waters—Part II: Quantum yields of its production by some natural humic materials as a function of wavelength. Chemosphere. 1984;13(5–6):641–650. [Google Scholar]
- 6.Mopper K, Zhou X. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science. 1990;250(4981):661–664. doi: 10.1126/science.250.4981.661. [DOI] [PubMed] [Google Scholar]
- 7.Page SE, Logan JR, Cory RM, McNeill K. Evidence for dissolved organic matter as the primary source and sink of photochemically produced hydroxyl radical in arctic surface waters. Environ Sci Processes Impacts. 2014;16(4):807–822. doi: 10.1039/c3em00596h. [DOI] [PubMed] [Google Scholar]
- 8.Chin YP, Aiken G, O’Loughlin E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ Sci Technol. 1994;28(11):1853–1858. doi: 10.1021/es00060a015. [DOI] [PubMed] [Google Scholar]
- 9.Boyle ES, Guerriero N, Thiallet A, Vecchio RD, Blough NV. Optical properties of humic substances and CDOM: Relation to structure. Environ Sci Technol. 2009;43(7):2262–2268. doi: 10.1021/es803264g. [DOI] [PubMed] [Google Scholar]
- 10.Cavani L, Halladja S, Ter Halle A, Guyot G, Corrado G, Ciavatta C, Boulkamh A, Richard C. Relationship between photosensitizing and emission properties of peat humic acid fractions obtained by tangential ultrafiltration. Environ Sci Technol. 2009;43(12):4348–4354. doi: 10.1021/es802964m. [DOI] [PubMed] [Google Scholar]
- 11.Jaffé R, Yamashita Y, Maie N, Cooper WT. Dissolved organic matter in headwater streams: Compositional variability across climatic regions of North America. Geochim Cosmochim Acta. 2012;94:95–108. [Google Scholar]
- 12.Appiani E, Page SE, McNeill K. On the use of hydroxyl radical kinetics to assess the number-average molecular weight of dissolved organic matter. Environ Sci Technol. 2014;48(20):11794–11802. doi: 10.1021/es5021873. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrilli J, Foreman CM, Marshall AG, McKnight DM. Characterization of IHSS Pony Lake fulvic acid dissolved organic matter by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and fluorescence spectroscopy. Org Geochem. 2013;65(C):19–28. [Google Scholar]
- 14.Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol. 2003;37(20):4702–4708. doi: 10.1021/es030360x. [DOI] [PubMed] [Google Scholar]
- 15.Sharpless CM, Aeschbacher M, Page SE, Wenk J, Sander M, McNeill K. Photooxidation-induced changes in optical, electrochemical, and photochemical properties of humic substances. Environ Sci Technol. 2014;48(5):2688–2696. doi: 10.1021/es403925g. [DOI] [PubMed] [Google Scholar]
- 16.Sleighter RL, Chin YP, Arnold WA. Evidence of incorporation of abiotic S and N into prairie wetland dissolved organic matter. Environ Sci Technol Lett. 2014;1:345–350. [Google Scholar]
- 17.Rossel PE, Vähätalo AV, Witt M, Dittmar T. Molecular composition of dissolved organic matter from a wetland plant (Juncus effusus) after photochemical and microbial decomposition (125 yr): Common features with deep sea dissolved organic matter. Org Geochem. 2013;60:62–71. [Google Scholar]
- 18.Kujawinski EB, Del Vecchio R, Blough NV, Klein GC, Marshall AG. Probing molecular-level transformations of dissolved organic matter: Insights on photochemical degradation and protozoan modification of DOM from electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Mar Chem. 2004;92(1–4):23–37. [Google Scholar]
- 19.Tfaily MM, Hamdan R, Corbett JE, Chanton JP. Investigating dissolved organic matter decomposition in northern peatlands using complimentary analytical techniques. Geochim Cosmochim Acta. 2013;112:116–129. [Google Scholar]
- 20.Gonsior M, Peake BM, Cooper WT, Podgorski DC, D’Andrilli J, Dittmar T, Cooper WJ. Characterization of dissolved organic matter across the subtropical convergence off the South Island, New Zealand. Mar Chem. 2011;123(1–4):99–110. [Google Scholar]
- 21.Sleighter RL, Hatcher PG. Molecular characterization of dissolved organic matter (DOM) along a river to ocean transect of the lower Chesapeake Bay by ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Mar Chem. 2008;110(3–4):140–152. [Google Scholar]
- 22.Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr. 2008;53(3):955–969. [Google Scholar]
- 23.Kellerman AM, Kothawala DN, Dittmar T, Tranvik LJ. Persistence of dissolved organic matter in lakes related to its molecular characteristics. Nat Geosci. 2015;8(6):454–457. [Google Scholar]
- 24.De Laurentiis E, Buoso S, Maurino V, Minero C, Vione D. Optical and photochemical characterization of chromophoric dissolved organic matter from lakes in Terra Nova Bay, Antarctica. Evidence of considerable photoreactivity in an extreme environment. Environ Sci Technol. 2013;47(24):14089–14098. doi: 10.1021/es403364z. [DOI] [PubMed] [Google Scholar]
- 25.Maizel AC, Remucal CK. Molecular composition and photochemical reactivity of size-fractionated dissolved organic matter. Environ Sci Technol. 2017;51(4):2113–2123. doi: 10.1021/acs.est.6b05140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson BM, McNally AM, Cory RM, Thoemke JD, Cotner JB, McNeill K. Spatial and temporal distribution of singlet oxygen in Lake Superior. Environ Sci Technol. 2012;46(13):7222–7229. doi: 10.1021/es301105e. [DOI] [PubMed] [Google Scholar]
- 27.Timko SA, Romera-Castillo C, Jaffé R, Cooper WJ. Photo-reactivity of natural dissolved organic matter from fresh to marine waters in the Florida Everglades, USA. Environ Sci Processes Impacts. 2014;16(4):866–878. doi: 10.1039/c3em00591g. [DOI] [PubMed] [Google Scholar]
- 28.Rosario-Ortiz FL, Canonica S. Probe compounds to assess the photochemical activity of dissolved organic matter. Environ Sci Technol. 2016;50(23):12532–12547. doi: 10.1021/acs.est.6b02776. [DOI] [PubMed] [Google Scholar]
- 29.Zepp RG, Schlotzhauer PF, Sink RM. Photosensitized transformations involving electronic energy transfer in natural waters: Role of humic substances. Environ Sci Technol. 1985;19(1):74–81. [Google Scholar]
- 30.McNeill K, Canonica S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties. Environ Sci Processes Impacts. 2016;18:1381–1399. doi: 10.1039/c6em00408c. [DOI] [PubMed] [Google Scholar]
- 31.Rosario-Ortiz FL, Canonica S. Probe compounds to assess the photochemical activity of dissolved organic matter. Environ Sci Technol. 2016;50(23):12532–12547. doi: 10.1021/acs.est.6b02776. [DOI] [PubMed] [Google Scholar]
- 32.Koch BP, Witt M, Engbrodt R, Dittmar T, Kattner G. Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Geochim Cosmochim Acta. 2005;69(13):3299–3308. [Google Scholar]
- 33.Hertkorn N, Frommberger M, Witt M, Koch BP, Schmitt-Kopplin P, Perdue EM. Natural organic matter and the event horizon of mass spectrometry. Anal Chem. 2008;80(23):8908–8919. doi: 10.1021/ac800464g. [DOI] [PubMed] [Google Scholar]
- 34.Gonsior M, Peake BM, Cooper WT, Podgorski D, D’Andrilli J, Cooper WJ. Photochemically induced changes in dissolved organic matter identified by ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry. Environ Sci Technol. 2009;43(3):698–703. doi: 10.1021/es8022804. [DOI] [PubMed] [Google Scholar]
- 35.Lavonen EE, Gonsior M, Tranvik LJ, Schmitt-Kopplin P, Köhler SJ. Selective chlorination of natural organic matter: Identification of previously unknown disinfection byproducts. Environ Sci Technol. 2013;47(5):2264–2271. doi: 10.1021/es304669p. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Fang Z, He C, Zhang Y, Xu C, Chung KH, Shi Q. Molecular characterization and transformation of dissolved organic matter in refinery wastewater from water treatment processes: Characterization by Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels. 2015;29(11):6956–6963. [Google Scholar]
- 37.Minor EC, Steinbring CJ, Longnecker K, Kujawinski EB. Characterization of dissolved organic matter in Lake Superior and its watershed using ultrahigh resolution mass spectrometry. Org Geochem. 2012;43:1–11. [Google Scholar]
- 38.Gonsior M, Zwartjes M, Cooper WJ, Song W, Ishida KP, Tseng LY, Jeung MK, Rosso D, Hertkorn N, Schmitt-Kopplin P. Molecular characterization of effluent organic matter identified by ultrahigh resolution mass spectrometry. Water Res. 2011;45(9):2943–2953. doi: 10.1016/j.watres.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Ferrey ML, Heiskary S, Grace R, Hamilton MC, Lueck A. Pharmaceuticals and other anthropogenic tracers in surface water: A randomized survey of 50 minnesota lakes. Environ Toxicol Chem. 2015;34(11):2475–2488. doi: 10.1002/etc.3125. [DOI] [PubMed] [Google Scholar]
- 40.Elliott SM, VanderMeulen DD. A regional assessment of chemicals of concern in surface waters of four Midwestern United States national parks. Sci Total Environ. 2017;579:1726–1735. doi: 10.1016/j.scitotenv.2016.11.114. [DOI] [PubMed] [Google Scholar]
- 41.Magnuson JJ, Frost TM. Trout Lake Station, a center for north temperate lake studies. Bull Ecol Soc Am. 1982;63(3):223–225. [Google Scholar]
- 42.Kratz TK, Webster KE, Bowser CJ, Magnuson JJ, Benson BJ. The influence of landscape position on lakes in northern Wisconsin. Freshwater Biol. 1997;37(1):209–217. [Google Scholar]
- 43.Magnuson JJ, Bowser CJ, Kratz TK. Long-term ecological research (LTER) on north temperate lakes of the United States. Verh Internat Verein Theor Angew Limnol. 1984;22:533–535. [Google Scholar]
- 44.Hanson PC, Buffam I, Rusak JA, Stanley EH. Quantifying lake allochthonous organic carbon budgets using a simple equilibrium model. Limnol Oceanogr. 2014;59(1):167–181. [Google Scholar]
- 45.Peterson GD, Beard TD, Jr, Beisner BE. Assessing future ecosystem services: A case study of the Northern Highlands Lake District, Wisconsin. Cons Ecol. 2003;7(3) [Google Scholar]
- 46.Dittmar T, Koch B, Hertkorn N. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol Oceanogr: Methods. 2008;6:230–235. [Google Scholar]
- 47.Maizel AC, Remucal CK. The effect of advanced secondary municipal wastewater treatment on the molecular composition of dissolved organic matter. Water Res. 2017;122:42–52. doi: 10.1016/j.watres.2017.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenson AC, Marshall AG, Cooper WT. Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra. Anal Chem. 2003;75(6):1275–1284. doi: 10.1021/ac026106p. [DOI] [PubMed] [Google Scholar]
- 49.Remucal CK, McNeill K. Photosensitized amino acid degradation in the presence of riboflavin and its derivatives. Environ Sci Technol. 2011;45(12):5230–5237. doi: 10.1021/es200411a. [DOI] [PubMed] [Google Scholar]
- 50.Grebel JE, Pignatello JJ, Mitch WA. Sorbic acid as a quantitative probe for the formation, scavenging and steady-state concentrations of the triplet- excited state of organic compounds. Water Res. 2011;45(19):6535–6544. doi: 10.1016/j.watres.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 51.Canonica S, Jans U, Stemmler K, Hoigné J. Transformation kinetics of phenols in water: Photosensitization by dissolved natural organic material and aromatic ketones. Environ Sci Technol. 1995;29(7):1822–1831. doi: 10.1021/es00007a020. [DOI] [PubMed] [Google Scholar]
- 52.Maizel AC, Remucal CK. The effect of probe choice and solution conditions on the apparent photoreactivity of dissolved organic matter. Environ Sci Processes Impacts. 2017 doi: 10.1039/C7EM00235A. [DOI] [PubMed] [Google Scholar]
- 53.Dalrymple RM, Carfagno AK, Sharpless CM. Correlations between dissolved organic matter optical properties and quantum yields of singlet oxygen and hydrogen peroxide. Environ Sci Technol. 2010;44(15):5824–5829. doi: 10.1021/es101005u. [DOI] [PubMed] [Google Scholar]
- 54.Appiani E, Ossola R, Latch DE, Erickson PR, McNeill K. Aqueous singlet oxygen reaction kinetics of furfuryl alcohol: Effect of temperature, pH, and salt content. Environ Sci Processes Impacts. 2017;19(4):507–516. doi: 10.1039/c6em00646a. [DOI] [PubMed] [Google Scholar]
- 55.Laszakovits JR, Berg SM, Anderson BG, O’Brien JE, Wammer KH, Sharpless CM. p-Nitroanisole/pyridine and p-nitroacetophenone/pyridine actinometers revisited: Quantum yield in comparison to ferrioxalate. Environ Sci Technol Lett. 2017;4(1):11–14. [Google Scholar]
- 56.McKay G, Couch KD, Mezyk SP, Rosario-Ortiz FL. Investigation of the coupled effects of molecular weight and charge-transfer interactions on the optical and photochemical properties of dissolved organic matter. Environ Sci Technol. 2016;50(15):8093–8102. doi: 10.1021/acs.est.6b02109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mostovaya A, Koehler B, Guillemette F, Brunberg A-K, Tranvik LJ. Effects of compositional changes on reactivity continuum and decomposition kinetics of lake dissolved organic matter. J Geophys Res: Biogeosci. 2016;121(7):1733–1746. [Google Scholar]
- 58.Müller MB, Schmitt D, Frimmel FH. Fractionation of natural organic matter by size exclusion chromatography–Properties and stability of fractions. Environ Sci Technol. 2000;34(23):4867–4872. [Google Scholar]
- 59.Minor E, Stephens B. Dissolved organic matter characteristics within the Lake Superior watershed. Org Geochem. 2008;39(11):1489–1501. [Google Scholar]
- 60.Bodhipaksha LC, Sharpless CM, Chin Y-P, Sander M, Langston WK, MacKay AA. Triplet photochemistry of effluent and natural organic matter in whole water and isolates from effluent-receiving rivers. Environ Sci Technol. 2015;49(6):3453–3463. doi: 10.1021/es505081w. [DOI] [PubMed] [Google Scholar]
- 61.Jin P, Jin X, Bjerkelund VA, Østerhus SW, Wang XC, Yang L. A study on the reactivity characteristics of dissolved effluent organic matter (EfOM) from municipal wastewater treatment plant during ozonation. Water Res. 2016;88(1):643–652. doi: 10.1016/j.watres.2015.10.060. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson GM, Pace ML, Cole JJ. Terrestrial dominance of organic matter in north temperate lakes. Global Biogeochem Cycles. 2013;27(1):43–51. [Google Scholar]
- 63.Jane SF, Winslow LA, Remucal CK, Rose KC. Long-term trends and synchrony in dissolved organic matter characteristics in Wisconsin, USA, lakes: Quality, not quantity, is highly sensitive to climate. J Geophys Res: Biogeosci. 2017;122(3):546–561. [Google Scholar]
- 64.Hockaday W, Purcell J, Marshall A, Baldock J, Hatcher PG. Electrospray and photoionization mass spectrometry for the characterization of organic matter in natural waters: A qualitative assessment. Limnol Oceanogr: Methods. 2009;7(1):81–95. [Google Scholar]
- 65.Kujawinski EB, Longnecker K, Blough NV, Del Vecchio R, Finlay L, Kitner JB, Giovannoni SJ. Identification of possible source markers in marine dissolved organic matter using ultrahigh resolution mass spectrometry. Geochim Cosmochim Acta. 2009;73(15):4384–4399. [Google Scholar]
- 66.Kim S, Kramer RW, Hatcher PG. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal Chem. 2003;75(20):5336–5344. doi: 10.1021/ac034415p. [DOI] [PubMed] [Google Scholar]
- 67.Sleighter RL, Hatcher PG. The application of electrospray ionization coupled to ultrahigh resolution mass spectrometry for the molecular characterization of natural organic matter. J Mass Spectrom. 2007;42(5):559–574. doi: 10.1002/jms.1221. [DOI] [PubMed] [Google Scholar]
- 68.Stubbins A, Spencer RGM, Chen H, Hatcher PG, Mopper K, Hernes PJ, Mwamba VL, Mangangu AM, Wabakanghanzi JN, Six J. Illuminated darkness: Molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol Oceanogr. 2010;55(4):1467–1477. [Google Scholar]
- 69.Ward CP, Cory RM. Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils. Environ Sci Technol. 2016;50(7):3545–3553. doi: 10.1021/acs.est.5b05354. [DOI] [PubMed] [Google Scholar]
- 70.Wagner S, Jaffé R, Cawley K, Dittmar T, Stubbins A. Associations between the molecular and optical properties of dissolved organic matter in the Florida Everglades, a model coastal wetland system. Front Chem. 2015;3(66):155–14. doi: 10.3389/fchem.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dulin D, Mill T. Development and evaluation of sunlight actinometers. Environ Sci Technol. 1982;16(11):815–820. doi: 10.1021/es00105a017. [DOI] [PubMed] [Google Scholar]
- 72.Teng Z, Arnold WA. Pesticide photolysis in prairie potholes: Probing photosensitized processes. Environ Sci Technol. 2013;47(13):6735–6745. doi: 10.1021/es3030808. [DOI] [PubMed] [Google Scholar]
- 73.Sharpless CM, Blough NV. The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties. Environ Sci Processes Impacts. 2014;16(4):654–671. doi: 10.1039/c3em00573a. [DOI] [PubMed] [Google Scholar]
- 74.Del Vecchio R, Blough NV. On the origin of the optical properties of humic substances. Environ Sci Technol. 2004;38(14):3885–3891. doi: 10.1021/es049912h. [DOI] [PubMed] [Google Scholar]
- 75.Golanoski KS, Fang S, Del Vecchio R, Blough NV. Investigating the mechanism of phenol photooxidation by humic substances. Environ Sci Technol. 2012;46(7):3912–3920. doi: 10.1021/es300142y. [DOI] [PubMed] [Google Scholar]
- 76.Baluha DR, Blough NV, Del Vecchio R. Selective mass labeling for linking the optical properties of chromophoric dissolved organic matter to structure and composition via ultrahigh resolution electrospray ionization mass spectrometry. Environ Sci Technol. 2013;47(17):9891–9897. doi: 10.1021/es402400j. [DOI] [PubMed] [Google Scholar]
- 77.Latch DE, McNeill K. Microheterogeneity of singlet oxygen distributions in irradiated humic acid solutions. Science. 2006;311(5768):1743–1747. doi: 10.1126/science.1121636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


