Abstract
We used positron emission tomography to study neural mechanisms underlying intensely pleasant emotional responses to music. Cerebral blood flow changes were measured in response to subject-selected music that elicited the highly pleasurable experience of “shivers-down-the-spine” or “chills.” Subjective reports of chills were accompanied by changes in heart rate, electromyogram, and respiration. As intensity of these chills increased, cerebral blood flow increases and decreases were observed in brain regions thought to be involved in reward/motivation, emotion, and arousal, including ventral striatum, midbrain, amygdala, orbitofrontal cortex, and ventral medial prefrontal cortex. These brain structures are known to be active in response to other euphoria-inducing stimuli, such as food, sex, and drugs of abuse. This finding links music with biologically relevant, survival-related stimuli via their common recruitment of brain circuitry involved in pleasure and reward.
The ubiquity of music in human culture is indicative of its ability to produce pleasure and reward value. Many people experience a particularly intense, euphoric response to music which, because of its frequent accompaniment by an autonomic or psychophysiological component, is sometimes described as “shivers-down-the-spine” or “chills” (1–3). Because such chills are a clear, discrete event and are often highly reproducible for a specific piece of music in a given individual (2), they provide a good model for objective study of emotional responses to music.
In a prior positron emission tomography (PET) study, we observed that activity in paralimbic brain regions correlated with unpleasant or mildly pleasant emotions elicited by varying amounts of musical dissonance (4). These regions, including parahippocampal gyrus, orbitofrontal, subcallosal, and frontal polar cortex, have been previously implicated in emotional responses more generally (5–10). For example, regional cerebral blood flow (rCBF) increases in parahippocampal gyrus have been observed during unpleasant emotional states evoked by pictures with negative emotional valence (8), and patients with lesions in subcallosal and other ventral medial prefrontal cortex (VMPF) regions are impaired in identification of emotional expression (7). In contrast, the paralimbic regions associated with musical dissonance differed from neocortical regions known to be involved in music perception and cognition, such as right superior temporal and right prefrontal cortices (11–14).
While the previous PET study focused primarily on unpleasant responses to music, the present study was designed to investigate neural correlates of intensely pleasurable responses to music. Animal studies have shown that reinforcement and motivation in relation to administration of a variety of drugs of abuse involve recruitment of brain regions such as the ventral striatum [particularly the nucleus accumbens (NAc) and ventral pallidum], ventral tegmental area (VTA), amygdala, hippocampus, VMPF, hypothalamus, and dorsal midbrain areas, such as periaqueductal gray (PAG) and pedunculopontine tegmental nucleus (PPT) (15). Animal studies of endogenous reward in response to natural stimuli, such as food and sex, show involvement of brain activity in similar regions. For example, both food and sexual activity have been shown to increase dopamine activity in NAc (16, 17), although the exact site of activity in NAc may differ between drug and natural reward (18).
Human studies of rewarding stimuli, such as drugs of abuse and food, have also suggested that intensely pleasurable emotions are accompanied by activity in neural systems underlying reward/motivation, emotional (limbic), and arousal processes. For example, fMRI activity changes in NAc, VTA, basal forebrain, thalamus, insula, cingulate, hippocampus, and amygdala have been observed during cocaine craving or cocaine rush in cocaine-dependent humans (19). The pleasant experience of chocolate consumption in humans has been found to be correlated with activity in midbrain, insula, the subcallosal region, and orbitofrontal cortex (OfC) (20).
In the present study, PET was used to measure rCBF changes while subjects listened to music that they selected to predictably elicit the euphoric experience of chills. We hypothesized that activity changes in reward/motivation, limbic, paralimbic, and arousal brain regions would correlate with the intensity of these chills. We also wished to determine whether any of these regions were similar to those recruited by mildly pleasant emotion in the dissonance PET study (4).
Methods
Subjects.
Subjects were McGill University students (age range: 20–30), five female and five male, with at least 8 years of music training. Musicians were used in this experiment based on the premise that this population is more likely to experience strong emotional responses to music; however, music training is not necessary to experience these responses. Individual subjects were selected on the basis of their reports of frequent, reproducible experiences of chills in response to certain pieces of music. All subjects gave informed consent to participate after the procedures and possible consequences of the study were explained.
Stimuli.
Each subject selected one piece of music that consistently elicited intensely pleasant emotional responses, including chills. Because music preference is highly individual, subject-selected music was the most reliable way to produce intense emotional responses (21). All music selections were of the classical genre, and included pieces such as Rachmaninoff's Piano Concerto No. 3 in D Minor, Opus 30, Intermezzo Adagio [Fig. 1, subject 1 (Chills)], and Barber's Adagio for Strings [Fig. 1, subject 2 (Chills) and subject 1 (Ctrl)]. No words were associated with the selected or any other version of the pieces. Subjects reported that their emotional responses were intrinsic to the music itself, producing minimal personal associations and/or memories. Because chills are known to occur consistently in the same part of a music selection for a given subject, we determined these times before scanning. One 90-sec excerpt, including the section that elicited chills, was taken from each subject's music selection and used as “subject-selected music” for that subject.
Figure 1.
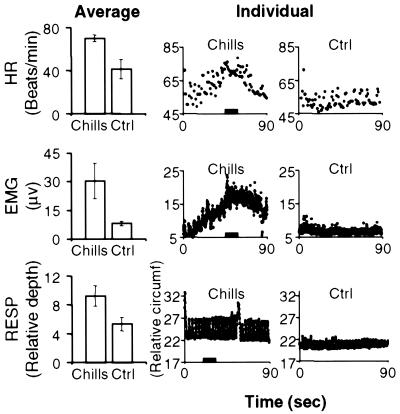
Average measurements (Left) of HR (beats/min), EMG (μV), and RESP (relative depth) for subject-selected (Chills) and control (Ctrl) music conditions, with corresponding examples of individual measurements (Center and Right) for each condition over the timecourse of selected scans (90 sec). Error bars indicate standard error for each average. The same subject/scan is shown for individual measurements of HR and EMG (subject 1); a different subject (subject 2) is shown for RESP. Note that subject 1's control music was subject 2's selected music. Black bars on the abscissa of graphs in the center column indicate time periods corresponding to chills. In all three examples, the chills rating was 7 on a scale of 0 to 10.
Each subject's selected music was used as another subject's emotionally “neutral” control, such that group-averaged data analysis involved comparison of identical sets of stimuli. For example, if stimulus A evoked chills in subject 1, and stimulus B evoked chills in subject 2, then stimulus B might serve as control music for subject 2, and stimulus A might serve as control music for subject 2. Each music stimulus was used only once as a control. Subjects were asked to rate the emotional intensity of their responses to each of the other nine music selections; to qualify as a neutral control, emotional intensity ratings were required to be ≤3 on a scale of 0 to 10 (10 = most intense). Subjects were familiarized with their control music before scanning to minimize responses attributable to effects of novelty.
Scanning Procedures.
PET scans were performed and registered with MRI scans as described in our previous PET study (4). During each 60-sec PET scan, subjects listened passively to one of four stimuli [selected music, control music, and two baseline conditions: amplitude-matched noise (4, 14) and silence]. Stimulus onset occurred ≈15 sec before scan onset to establish and stabilize subjects' responses to the stimulus. Each condition was repeated three times; stimulus presentation order was pseudorandomized.
Measurements of heart rate (HR), electromyogram (EMG), respiration depth (RESP), electrodermal response, and skin temperature were made during PET scans by using an F1000 polygraph instrumentation System [Biofeedback Instrument Corp. (New York); manufactured by Focused Technology (Ridgecrest, CA)]. After each PET scan, subjects rated their emotional reactions to each stimulus by using analog rating scales. Ratings were acquired for “chills intensity” (0 to 10), “emotional intensity” (0 to 10), and “unpleasant versus pleasant” (−5 to +5).
Data Analysis.
Regression maps (22) were calculated to assess the significance of the relationship between chills intensity rating and CBF (i.e., their linear regression). The dataset for this analysis consisted of normalized CBF values obtained in each subject during each of the subject-selected and control music scans, yielding a total of 60 image volumes. Because subjects did not experience chills during the control music condition (see Results), chills intensity ratings were set to zero for all control scans. The effect of the variation in chills response was assessed by means of analysis of covariance (23), with subjects as a main effect and the chills intensity rating as a covariate. The following model was fitted: E(yij) = ai + βPrij, were yij is the normalized CBF of subject i on scan j, and rij is the rating for subject i at scan j. The subject effect (ai) is removed and the parameter of interest is the slope βP of the effect of the chills rating on CBF. The subject effect regressor was subtracted from the analysis to remove the influence of individual differences in response that would have been implicitly removed in a comparison of means model (task-baseline) (24). Removal of this effect allowed grouping of within and between subject data in the regression analysis. Values equal to or exceeding a criterion of t = 3.53 were considered significant (P < 0.01, two-tailed), yielding a false-positive rate of 0.58 in 182 resolution elements (each of which has dimensions 14 × 14 × 14 mm), if the volume of brain gray matter is 500 cm3 (24).
To control for within-subject differences in familiarity that may have existed between the subject-selected and control music conditions, the regression with chills intensity was recalculated to include scans from only the subject-selected music condition. Because specific regions were hypothesized to be active in this analysis, significance (P < 0.05) was determined by using a standard one-tailed t test analysis.
In addition, rCBF values from left ventral striatum, left dorsal midbrain, left hippocampus/amygdala, right amygdala, and VMPF were extracted from individual scans (subject-selected music condition only) and plotted against subject ratings of chills intensity. A Pearson correlation was used to determine the correlation coefficient for each of these regions. For these regional plots and correlations only, rCBF values were extracted before normalization; to correct for this, both rCBF values and chills intensity ratings were normalized to the lowest value for each subject, such that lowest values were set to zero.
As a complementary analysis, we performed a subtraction of the control music condition from the subject-selected music condition. Because each stimulus was used twice across subjects (once in the subject-selected music condition and once in the control music condition), across the entire group, the stimuli used in these two conditions differed in emotional valence but not in physical features such as musical style or tempo. Therefore, when these two conditions were averaged separately and subtracted from each other, these potential confounding factors were eliminated. Subtraction of baseline conditions (noise, silence) from music conditions was also used to confirm that rCBF decreases were actually decreases from baseline and not merely differences between subject-selected and control music conditions.
Two further regression analyses were used to investigate the relationship between psychophysiological activity and rCBF. In the first, rCBF was covaried with HR, EMG, and RESP measurements. In the second, the regression with chills intensity was recalculated to remove effects of all individual psychophysiological measurements (22), including HR, EMG, RESP, electrodermal response, and skin temperature.
Results
Subjects reported experiencing chills during 77% of scans when their own selected music was played. HR (t = 3.02, P < 0.01), EMG (t = 2.41, P < 0.05), and RESP (t = 3.82, P < 0.001) increased significantly during the highest rated chills music condition relative to the control music condition (Fig. 1), while electrodermal and skin temperature measurements did not differ significantly between these conditions. Chills intensity ratings ranged from 1 to 9 (see Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org), varying both within and between subjects. Within subjects, ratings did not vary systematically over time (see Fig. 4); there was no significant effect of stimulus repetition number on chills intensity ratings (repeated measures ANOVA; F = 0.57, P = 0.57). Chills were never reported for control music, noise, or silence conditions. Ratings of pleasantness and emotional intensity tended to be higher than chills intensity, suggesting that pleasantness and emotional intensity must reach a certain level before chills are experienced. The average rating of chills intensity for subject-selected music was 4.5 out of 10, whereas the average ratings for emotional intensity and pleasantness were, respectively, 7.4 out of 10 and 4.4 out of 5.0 (see Tables 3 and 4, which are published as supporting information on the PNAS web site, for correlations of these ratings with CBF and see supporting Results, which are published on the PNAS web site, for further information).
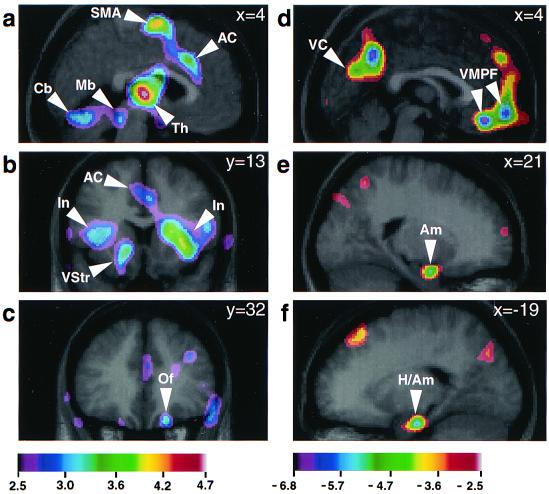
Regression analysis correlating rCBF with increasing chills intensity ratings in the subject-selected and control music conditions identified rCBF increases in left ventral striatum, dorsomedial midbrain, bilateral insula, right OfC, thalamus, anterior cingulate cortex (AC), supplementary motor area (SMA), and bilateral cerebellum (Table 1, all music, and Fig. 2). rCBF decreases with increasing chills intensity were observed in right amygdala, left hippocampus/amygdala, VMPF, and in widespread, bilateral posterior neocortical regions, particularly in cuneus/precuneus regions (Table 1, all music, and Fig. 2). Subtraction of noise and silent baseline conditions from subject-selected and control music conditions verified that right amygdala, left hippocampus/amygdala, and VMPF activity decreased from baseline during subject-selected music, whereas no rCBF changes were observed in these structures during control music as compared with the noise and silent baselines.
Table 1.
Regressions correlating rCBF with ratings of chills intensity
| Region | Coordinates
|
t value (all music) | t value* (s-s music) | Distance†, mm | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive correlations | ||||||
| L. ventral striatum | −13 | 12 | −5 | 3.86 | 2.54 | 2 |
| L. dorsomedial midbrain | −3 | −33 | −17 | 3.70 | 2.36 | 2 |
| R. thalamus | 4 | −16 | 5 | 6.18 | 2.53 | 9 |
| M. anterior cingulate (BA 24/32) | 5 | 22 | 33 | 4.03 | 2.21 | 25 |
| R. orbitofrontal cortex (BA 14) | 17 | 32 | −23 | 3.52‡ | ||
| R. insula | 32 | 13 | 2 | 4.78 | ||
| L. insula | −27 | 5 | 12 | 3.63 | ||
| M. suppl motor area (BA 6) | 0 | −6 | 65 | 5.80 | 3.23 | 12 |
| L. cerebellum | −15 | −68 | −20 | 4.82 | 2.99 | 5 |
| R. cerebellum | 30 | −66 | −21 | 3.71 | ||
| Negative correlations | ||||||
| L. hippocampus/amygdala | −19 | −16 | −23 | −4.04 | −4.02 | 6 |
| R. amygdala | 21 | −4 | −21 | −3.68 | −3.39 | 3 |
| M. prefrontal cortex (BA 32) | 1 | 37 | −18 | −4.55 | ||
| M. prefrontal cortex (BA 10) | 4 | 56 | −14 | −4.49 | −4.47 | 3 |
| M. cuneus (BA 18) | 0 | −73 | 24 | −3.52‡ | ||
| R. precuneus (BA 7) | 5 | −57 | 41 | −4.84 | ||
| L. precuneus (BA 7) | −5 | −61 | 29 | −3.85 | ||
t values are shown for the regression including data from both subject-selected and control music conditions (all music, column 5), and for the regression including data from only the subject-selected music condition (s-s music, column 6). Positive t values denote correlation with increasing ratings of chills intensity; negative t values denote correlation with decreasing ratings of chills intensity. L, left; R, right; M, medial.
t values were significant at P < 0.05 using a standard one-tailed t test (n = 9). Coordinates refer to location in stereotaxic space (25) for t values in the “all music” regression.
Distance indicates the vector distance between the response peaks in the subject-selected music regression as compared with the all-music condition.
t value just below significance threshold.
Figure 2.
Neuroanatomical regions demonstrating significant rCBF correlations with chills intensity ratings. Regression analyses were used to correlate rCBF from averaged PET data for combined subject-selected and control music scans with ratings of chills intensity (0 to 10). Correlations are shown as t-statistic images superimposed on corresponding average MRI scans (see Table 1, all music). The t-statistic ranges for each set of images are coded by color scales below each column, corresponding to a–c (positive correlations with increasing chills intensity), and d–f (negative correlations). a (sagittal section, x = 4 mm) shows positive rCBF correlations in left dorsomedial midbrain (Mb), right thalamus (Th), AC, SMA, and bilateral cerebellum (Cb). b (coronal section, y = 13 mm) shows left ventral striatum (VStr) and bilateral insula (In; also AC). c (coronal section, y = 32 mm) shows right orbitofrontal cortex (Of). d (sagittal section, x = 4 mm) shows negative rCBF correlations in VMPF and visual cortex (VC). e (sagittal section, x = 21 mm) shows right amygdala (Am). f (sagittal section, x = −19 mm) shows left hippocampus/amygdala (H/Am).
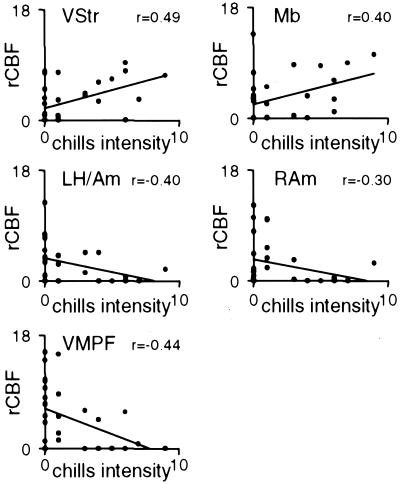
When the regression with chills intensity was recalculated to include scans from only the subject-selected music condition, increases in left ventral striatum, left dorsomedial midbrain, right thalamus, AC, SMA, and left cerebellum, and decreases in right amygdala, left hippocampus/amygdala, and VMPF remained significant (Table 1, s-s music; see also Fig. 5, which is published as supporting information on the PNAS web site). The locations of peak values for this regression were within 9 mm of the locations identified in the all-music regression, except for AC and SMA. When rCBF was measured in left ventral striatum, left dorsal midbrain, left hippocampus/amygdala, right amygdala, and VMPF, correlation coefficients were similar for left ventral striatum (0.49) and dorsomedial midbrain (0.40), as well as for left hippocampus/amygdala (−0.40) and right amygdala (−0.30) (Fig. 3).
Figure 3.
rCBF versus subject ratings of chills intensity for regions significantly correlated with chills intensity (subject-selected music only). rCBF values were calculated from subject-selected music scans (10 subjects; 3 scans per subject) for left ventral striatum, left dorsal midbrain, left hippocampus/amygdala, right amygdala, and VMPF. rCBF was calculated for volumes of interest in a 5-mm radius around peak response coordinates reported in Table 1 and plotted here. Trendlines on each plot indicate a least squares fit. A Pearson correlation was used to calculate the correlation coefficient (r) for each region, which is displayed in the top right corner of each plot. VStr, ventral striatum; Mb, dorsomedial midbrain; LH/Am, left hippocampus/amygdala; RAm, right amygdala.
A complementary subtraction analysis comparing the subject-selected and control music conditions (subject-selected minus control music) revealed CBF increases in regions similar to those observed in the regressions: left ventral striatum, dorsomedial midbrain, bilateral insula, right OfC, thalamus, AC, SMA, and bilateral cerebellum (Table 2; see also Fig. 6, which is published as supporting information on the PNAS web site). rCBF decreases were observed in right amygdala, left hippocampus/amygdala, VMPF, and in widespread, bilateral neocortical regions, including occipital, parietal, and temporal cortices (Table 2 and Fig. 6). Because certain regions were already hypothesized to be active, some t values below 3.53 are included here.
Table 2.
Subtraction analysis: subject-selected music minus control music
| Region | Coordinates
|
t value* | ||
|---|---|---|---|---|
| x | y | z | ||
| CBF increases | ||||
| L. ventral striatum | −13 | 1 | −5 | 2.72 |
| R. dorsomedial midbrain | 4 | −40 | −17 | 2.92 |
| R. thalamus | 3 | −16 | −2 | 4.61 |
| M. anterior cingulate (BA 24/32) | −1 | 32 | 15 | 2.63 |
| R. orbitofrontal cortex (BA 14) | 20 | 34 | −23 | 2.78 |
| R. insula | 32 | 15 | 3 | 5.41 |
| L. insula | −39 | 12 | 11 | 3.75 |
| M. suppl motor area (BA 6) | 1 | −2 | 63 | 6.26 |
| L. cerebellum | −8 | −66 | −18 | 5.03 |
| R. cerebellum | 9 | −62 | −18 | 3.75 |
| CBF decreases | ||||
| L. hippocampus/amygdala | −23 | −14 | −23 | −3.11 |
| R. amygdala | 21 | −6 | −21 | −2.95 |
| M. prefrontal cortex (BA 32) | 1 | 39 | −17 | −3.24 |
| M. prefrontal cortex (BA 10) | 0 | 56 | −6 | −3.18 |
| M. cuneus (BA 18) | 0 | −73 | 24 | −3.52 |
| R. precuneus (BA 7) | 5 | −57 | 41 | −4.84 |
| L. precuneus (BA 7) | −5 | −61 | 29 | −3.85 |
Positive t values denote CBF increases; negative t values denote CBF decreases. Coordinates refer to location in stereotaxic space (25). L, left; R, right; M, medial.
t values were significant at P < 0.05 using a standard one-tailed t test (n = 9).
To determine whether rCBF activity in certain regions correlated with changes in psychophysiological activity, regression analyses were used to correlate rCBF with individual measurements of HR, EMG, and RESP. Increases in psychophysiological activity correlated with rCBF increases in several structures, including thalamus, AC, OfC, insula, cerebellum, and SMA. None of these psychophysiological measurements correlated significantly with rCBF changes in ventral striatum, dorsomedial midbrain, amygdala, hippocampus/amygdala, or VMPF. When effects of all psychophysiological activity were removed from the chills intensity regression, significant rCBF changes remained, not only in ventral striatum, dorsomedial midbrain, amygdala, and hippocampus/amygdala, but also in thalamus and AC.
Discussion
Subjects experienced chills of varying intensity while listening to their selected music. These chills were associated with increases in HR, EMG, and RESP relative to the control music condition, indicating changes in autonomic and other psychophysiological activity. Regression analysis assessing the relationship between increasing chills intensity ratings and PET measurements of rCBF identified changes in brain structures that have been associated with brain reward circuitry (refs. 15, 26, 27; Table 1 and Fig. 2). These included rCBF increases in left ventral striatum and dorsomedial midbrain, and rCBF decreases in right amygdala, left hippocampus/amygdala, and VMPF. These structures remained active when control music was removed from the regression analysis, and in the subtraction analysis, verifying that these structures were active specifically in correlation with chills, and not simply due to differences in attention, familiarity, or acoustic features between subject-selected and control music. rCBF increases with chills intensity were also observed in paralimbic regions (bilateral insula, right OfC) and in regions associated with arousal (thalamus and AC) and motor (SMA and cerebellum) processes.
The pattern of activity observed here in correlation with music-induced chills is similar to that observed in other brain imaging studies of euphoria and/or pleasant emotion (19, 20, 28). For example, activity in NAc, VTA, thalamus, insula, and AC has been reported to increase, and in left amygdala and VMPF to decrease, in response to cocaine administration in cocaine-dependent subjects (19). In addition, animal studies support a critical role for ventral striatum (NAc, in particular), several midbrain areas (e.g., VTA, PAG, and PPT), amygdala, hippocampus, and medial prefrontal cortex in circuitry underlying reward processes, including hedonic impact, reward learning, and motivation (15, 26, 27). Activity in these regions in relation to reward processes is known to involve dopamine and opioid systems, as well as other neurotransmitters.
Dopaminergic activity in either NAc and/or VTA appears to be the common mechanism underlying reward response to all naturally rewarding stimuli (e.g., food and sex) (16, 27, 29), and to drugs with euphorogenic properties and/or abuse potential (27). For example, self-administration of i.v. drugs, such as cocaine or heroin, in rats correlates strongly with activity increases in NAc as measured by microdialysis (30, 31), as well as behavioral indices of satiety, while consequent declines in NAc dopamine levels were highly correlated with onset of further self-stimulation. NAc is also rich in opiate receptors and is modulated by enkephalins (15). Efferent projections from NAc also are largely opioid, and are thought to be specifically responsible for reward-related behavior (27). Although the resolution and registration methods of PET do not allow us to conclude specifically that the ventral striatum activity observed in the present study is in the NAc, the coordinates of this activity peak do, indeed, overlie the NAc coordinates in the Talairach atlas.
Similarly, although PET methods do not allow us to determine definitively which specific midbrain nuclei were active in the present study, the dorsomedial location of the response suggests that this activity may be either in PAG or in PPT. Because activity in this region did not covary with psychophysiological activity, it is unlikely that the midbrain response was in the reticular formation, part of the arousal system. Both PAG and PPT play an integral role in reward responses. PAG is rich in opioid receptors and endogenous opioids such as endorphin and enkephalin, and is involved in opioid-mediated reward (32) as well as analgesia (33). Support for involvement of opioid systems specifically in response to music comes from a preliminary study that demonstrated that blocking opioid receptors with naloxone decreased or inhibited the chills response in some subjects (1). The PPT is thought to be involved specifically in the acquisition of drug-rewarded behavior (15), receiving descending NAc projections via the ventral pallidum, and sending projections to structures such as substantia nigra, VTA, thalamus, amygdala, and cerebellum.
The observation of rCBF decreases in the amygdala and hippocampus during music-induced chills is compatible with the key role played by these structures in both reward and emotion (10, 15, 26, 34–36). The amygdala sends glutamatergic afferents to both NAc and VTA (27) and, along with hippocampus, PAG, and NAc, is a key structure in opioid-mediated responses (33, 37, 38). The possibility of a direct functional interaction between hippocampus/amygdala and midbrain in the present study is supported by the exactly opposite correlation of dorsomedial midbrain and left hippocampus/amygdala rCBF with chills intensity (Fig. 3). Decreases in the left amygdala have been previously observed in fMRI studies of cocaine and procaine administration (19, 28). In the cocaine study, amygdala decreases correlated with ratings of “craving” rather than “rush,” suggesting that the amygdala decreases observed in the present study may have occurred more in relation to anticipation of the chills response than to the chills response itself. In other studies, however, amygdala activity has been shown to increase during states of euphoria (17, 39), indicating that modulation of amygdala may be complex.
Amygdala decreases accompanied by ventral striatum increases may also indicate gating between behaviorally antagonistic “approach” and “withdrawal” systems. The amygdala is known to be involved in fear and other aversive emotions, as well as evaluative processes associated with socially and biologically relevant emotions (10, 34–36), whereas ventral striatum mediates evaluative processes associated with reward and motivation/approach behavior. Thus, activation of the reward system by music may maximize pleasure, not only by activating the reward system but also by simultaneously decreasing activity in brain structures associated with negative emotions. The amygdala and hippocampus both receive inhibitory presynaptic input from cholinergic neurons intrinsic to NAc (15), suggesting a possible mechanism for decreased activity in these regions as a consequence of activity increases in ventral striatum.
Significant increases in psychophysiological activity were observed during the chills, relative to the control music condition, consistent with previous reports of psychophysiological activity changes during emotional responses to music (40). Although activity changes in ventral striatum, dorsomedial midbrain, amygdala, and hippocampus may be involved in producing states of reward and motivation associated with music-induced chills, other structures may have been active more in relation to the autonomic/psychophysiological component of the chills response. Specifically, thalamus and AC are thought to be centrally involved in mechanisms of general arousal and attentional processes (41). rCBF increases in both thalamus and AC were found to correlate positively with increasing psychophysiological activity, indicating a possible role for these regions in psychophysiological processes. However, because rCBF increases in thalamus and AC were not eliminated when effects of psychophysiological activity were removed from the chills intensity regression analysis, it appears that these structures may have mediated other arousal and/or reward processes as well.
Brain structures correlating with intensely pleasant emotion in the present study differed considerably from those observed during unpleasant or pleasant responses to musical dissonance or consonance in our previous study (4). In particular, right parahippocampal activity previously observed to correlate with unpleasant responses to dissonance did not correlate with chills intensity here, supporting the notion that parahippocampal activity may be specifically related to negative emotion. In addition, regions associated with reward/motivation circuitry, such as ventral striatum, dorsomedial midbrain, amygdala, and hippocampus, were found to correlate with chills intensity but not with the more mildly pleasant emotion associated with consonance. These discrepancies provide further evidence that different emotions are associated with activity in different groups of brain structures. In contrast, VMPF and OfC activity changes were seen in correlation with pleasant emotion in both this and the previous music study, although VMPF subregions differed between studies. Lesion and imaging studies have implicated medial prefrontal and OfC regions in integration of reward and/or punishment information to make behavioral judgments about stimuli (6, 9). This integrative capacity suggests that these regions may subserve multiple emotional functions and therefore may respond to more than one type of emotion.
We have shown here that music recruits neural systems of reward and emotion similar to those known to respond specifically to biologically relevant stimuli, such as food and sex, and those that are artificially activated by drugs of abuse. This is quite remarkable, because music is neither strictly necessary for biological survival or reproduction, nor is it a pharmacological substance. Activation of these brain systems in response to a stimulus as abstract as music may represent an emergent property of the complexity of human cognition. Perhaps as formation of anatomical and functional links between phylogenically older, survival-related brain systems and newer, more cognitive systems increased our general capacity to assign meaning to abstract stimuli, our capacity to derive pleasure from these stimuli also increased. The ability of music to induce such intense pleasure and its putative stimulation of endogenous reward systems suggest that, although music may not be imperative for survival of the human species, it may indeed be of significant benefit to our mental and physical well-being.
Supplementary Material
Acknowledgments
We are grateful to Dr. Alan C. Evans for making available the facilities of the McConnell Brain Imaging Centre. We thank Drs. Pascal Belin and Alain Dagher for editorial comments, and Marc Bouffard, Pierre Ahad, and Sylvain Milot for their technical expertise. We also thank the technical staff of the McConnell Brain Imaging Unit and of the Medical Cyclotron Unit for their assistance. This work was supported by a National Institute of Mental Health postdoctoral (National Research Service Award) fellowship and the Jeanne Timmins Costello fellowship in Neuroscience to A.J.B. and by grants from the Canadian Institutes of Health Research and the McDonnell-Pew Cognitive Neuroscience Program to R.J.Z.
Abbreviations
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- VMPF
ventral medial prefrontal cortex
- NAc
nucleus accumbens
- VTA
ventral tegmental area
- OfC
orbitofrontal cortex
- HR
heart rate
- EMG
electromyogram
- RESP
respiration depth
- AC
anterior cingulate
- SMA
supplementary motor area
- PAG
periaqueductal gray
- PPT
pedunculopontine nucleus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Goldstein A. Physiol Psychol. 1980;8:126–129. [Google Scholar]
- 2.Sloboda J A. Psychol Music. 1991;19:110–120. [Google Scholar]
- 3.Panksepp J. Music Perception. 1995;13:171–207. [Google Scholar]
- 4.Blood A J, Zatorre R J, Bermudez P, Evans A C. Nat Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler R E, Davidson R J, Tomarken A J. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 6.Rolls E T, Hornak J, Wade D, McGrath J. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornak J, Rolls E T, Wade D. Neuropsychologia. 1996;34:247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 8.Lane R D, Reiman E M, Bradley M M, Lang P J, Ahern G L, Davidson R J, Schwartz G E. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 9.Lane R D, Reiman E M, Ahern G L, Schwartz G E, Davidson R J. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 10.Zald D H, Pardo J V. Proc Natl Acad Sci USA. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatorre R J. J Acoust Soc Am. 1988;84:566–572. doi: 10.1121/1.396834. [DOI] [PubMed] [Google Scholar]
- 12.Zatorre R J, Samson S. Brain. 1991;114:2403–2417. doi: 10.1093/brain/114.6.2403. [DOI] [PubMed] [Google Scholar]
- 13.Zatorre R J, Halpern A R. Neuropsychologia. 1993;31:221–232. doi: 10.1016/0028-3932(93)90086-f. [DOI] [PubMed] [Google Scholar]
- 14.Zatorre R J, Evans A C, Meyer E. J Neurosci. 1994;14:1908–1919. doi: 10.1523/JNEUROSCI.14-04-01908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardo M T. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 16.Pfaus J G, Damsma G, Wenkstern D, Fibiger H C. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- 17.Schilstrom B, Svensson H M, Svensson T H, Nomikos G G. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- 18.Carelli R M, Ijames S G, Crumling A J. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breiter H C, Gollub R L, Weisskoff R M, Kennedy D N, Makris N, Berke J D, Goodman J M, Kantor H L, Gastfriend D R, Riorden J P, et al. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 20.Small D M, Zatorre R J, Dagher A, Evans A C, Jones-Gotman M. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 21.Thaut M H, Davis W B. J Music Therapy. 1993;30:210–223. [Google Scholar]
- 22.Paus T, Zatorre R J, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans A C. J Cognit Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- 23.Sokal R R, Rohlf F J. Biometry. 2nd Ed. San Francisco: Freeman; 1981. [Google Scholar]
- 24.Worsley K J, Marrett S, Neelin P, Vandal A C, Friston K J, Evans A C. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 26.Berridge K C, Robinson T E. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 27.Gardner E L, Vorel S R. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- 28.Ketter T A, Andreason P J, George M S, Lee C, Gill D S, Parekh P I, Willis M W, Herscovitch P, Post R M. Arch Gen Psychiatry. 1996;53:59–69. doi: 10.1001/archpsyc.1996.01830010061009. [DOI] [PubMed] [Google Scholar]
- 29.Terry P, Gilbert D B, Cooper S J. Obesity Res. 1995;3:515S–523S. doi: 10.1002/j.1550-8528.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 30.Wise R A, Leone P, Rivest R, Leeb K. Synapse. 1995;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- 31.Wise R A, Newton P, Leeb K, Burnette B, Pocock D J, Justice J B., Jr Psychopharmacology. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 32.Olmstead M C, Franklin K B. Behav Neurosci. 1997;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- 33.Pavlovic Z W, Bodnar R J. Brain Res. 1998;779:158–169. doi: 10.1016/s0006-8993(97)01115-3. [DOI] [PubMed] [Google Scholar]
- 34.Adolphs R, Tranel D, Damasio H, Damasio A R. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris J S, Frith C D, Perrett D I, Rowland D, Young A W, Calder A J, Dolan R J. Nature (London) 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 36.Rogan M T, LeDoux J E. Cell. 1996;85:469–475. doi: 10.1016/s0092-8674(00)81247-7. [DOI] [PubMed] [Google Scholar]
- 37.Bot G, Chahl L A. Brain Res. 1996;731:45–56. doi: 10.1016/0006-8993(96)00457-x. [DOI] [PubMed] [Google Scholar]
- 38.McBride W J, Murphy J M, Ikemoto S. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 39.Kling M A, Garner D L, Calogero A E, Coppola R, Trettau J, Kellner C H, Lefter L, Hart M J, Cowdry R W, Post R M. J Pharmacol Exp Ther. 1994;268:1548–1564. [PubMed] [Google Scholar]
- 40.Krumhansl C L. Can J Exp Psychol. 1997;51:336–352. doi: 10.1037/1196-1961.51.4.336. [DOI] [PubMed] [Google Scholar]
- 41.Paus T. Prog Brain Res. 2000;126:65–77. doi: 10.1016/S0079-6123(00)26007-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.