Abstract
We describe a case of acute vertebral osteomyelitis with associated prevertebral abscess due to Erysipelothrix rhusiopathiae in an immunocompetent adult with recent known traumatic inoculation from the barb of a fish.
Keywords: Erysipelothrix rhusiopathiae, Osteomyelitis, Abscess, Zoonosis, Occupational injury
Case report
A 48-year-old man presented to the emergency department with back pain and chills. He had a history of asthma, spinal stenosis, and multiple traumatic injuries to his extremities requiring orthopedic hardware placement in his right shoulder, left hip, and right knee; he also endorsed a previous anaphylactic reaction to penicillin. Two weeks prior to presentation he sustained a puncture injury to his left index finger from the barb of a sea robin (Prionotus carolinus), a fish with fan-like fins containing stiff rays commonly found in shallow waters of the western Atlantic Ocean, that he caught while fishing recreationally. Within four days he developed an elevated painful lesion at the puncture site that his primary care doctor diagnosed as an abscess, which was incised and drained; the patient then completed a 10-day course of trimethoprim-sulfamethoxazole. Six days prior to presentation he sustained a minor mechanical fall onto his buttocks that resulted in lower back pain and right-sided sciatica. His lower back pain became severe over the following days with the addition of chills and night sweats.
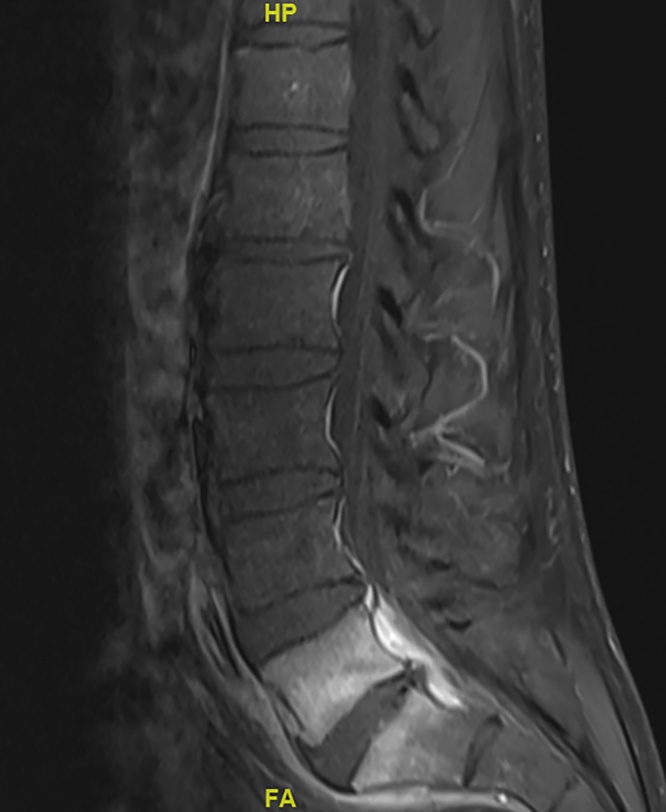
In the emergency room, his temperature was 37.8 °C and other vital signs were unremarkable. His exam revealed a healing 5-mm circular lesion on his left index finger, paraspinal lumbar tenderness that was more pronounced on the right, and decreased sensation to light touch in the distal right lower extremity. CBC revealed a WBC count of 11.0 × 10^9/L (neutrophils, 69.9%; lymphocytes, 19.9%; monocytes, 9.3%; eosinophils, 0.6%), hemoglobin of 12.4 g/dL, and platelets of 280 × 10^9/L; BMP revealed sodium of 134 mEq/L, potassium of 3.3 mEq/L, and chloride of 95 mEq/L, but was otherwise unremarkable; CRP was 147.3 mg/L and ESR was >130 mm/h. MRI of the spine revealed disc protrusion at L5-S1 with right S1 nerve impingement, L5-S1 osteomyelitis with surrounding phlegmon extending into the prevertebral and epidural space, and a 9 × 2 mm abscess within the prevertebral space (Fig. 1).
Fig. 1.
MRI spine with contrast demonstrates L5-S1 osteomyelitis with surrounding phlegmon extending into the prevertebral and epidural space, and a 9 × 2 mm abscess within the prevertebral space.
Neurosurgery performed a right L5-S1 microdiscectomy and drainage of the adjacent purulent fluid. Microdiscectomy wound cultures grew Erysipelothrix rhusiopathiae (2 of 2 specimens) and coagulase-negative Staphylococcus species (1 of 2 specimens). Blood cultures remained negative. Transthoracic echocardiography, which was performed in light of the high incidence of endocarditis in patients with invasive E. rhusiopathiae infections, was unremarkable and showed no evidence of endocarditis. Susceptibility testing was not performed. The patient was treated with an 8-week course of ceftriaxone, which he tolerated well despite his reported penicillin allergy, and had gradual resolution of his back pain.
Discussion
E. rhusiopathiae is a facultative anaerobic gram-positive rod that is a rare cause of human disease, occurring primarily as an occupationally-acquired zoonosis in individuals with exposure to contaminated animal and fish products. There are three well-defined manifestations in humans: a localized cellulitic form known as erysipeloid, a diffuse cutaneous form, and a septicemic form that is associated with endocarditis [1]. Rare manifestations include abscess formation, septic arthritis, and osteomyelitis [2], [3], [4], [5], [6], [7].
Apart from the present case, there are only two previous confirmed reports of E. rhusiopathiae osteomyelitis in the literature (Table 1): left hip osteomyelitis in a child without known exposures or previous injury [3] and pubic osteomyelitis in an adult who was thought to have suffered traumatic inoculation of the bacterium approximately 19 years earlier [4]. Two additional cases in which imaging suggested osteomyelitis but tissue cultures were negative occurred in adults with likely but unspecified occupational exposure [5], [6].
Table 1.
Characteristics of E. rhusiopathiae osteomyelitis as described by published case reports.
| Case report authors | Patient age (years), sex | Notable past medical history | Type of infection | Timing of inoculation | Contact with animals | Diagnosis of osteomyelitis | Treatment |
|---|---|---|---|---|---|---|---|
| This report | 48, M | Spinal stenosis, orthopedic injuries | Digital skin abscess, prevertebral abscess, lumbar osteomyelitis | 14 days prior (digital trauma from fish barb) | Recreational fishing | Tissue isolate | Surgery, ceftriaxone |
| Mukhopadhyay et al. [3] | 5, M | None | Hip osteomyelitis | Unknown | Unknown | Tissue isolate | Surgery, amoxicillin-clavulanic acid |
| Denes et al. [4] | 57, M | None | Pubic osteomyelitis | 19 years prior (pelvic trauma from cow horn) | Farming | Tissue isolate | Rifampicin, levofloxacin |
| Upapan and Chayakulkeeree [5] | 62, M | Diabetes mellitus, cirrhosis | Psoas abscess, paravertebral abscess, bacteremia | Unknown | Farming | Suspected on imaging, but tissue isolate negative | Surgery, levofloxacin |
| Romney et al. [6] | 67, F | Diabetes mellitus | Lumbar spondylitis, bacteremia | Unknown | Raw fish preparation | Suspected on imaging, but tissue isolate negative | Penicillin-G |
Our case appears to be unique in light of the acute onset of vertebral osteomyelitis following distant traumatic skin inoculation, in this case a finger puncture wound from a fish barb, consistent with hematogenous seeding. The acute formation of abscesses and osteomyelitis in this case is particularly interesting as E. rhusiopathiae is known to be a minimally-aggressive slow-growing organism [1], [8]. Furthermore, our patient had no predisposing factor for systemic disease, including no evidence of alcohol or drug dependence, immunosuppression, chronic liver disease, or diabetes mellitus [2]; however, as spinal trauma is a known risk factor for vertebral infections, our patient’s acutely herniated intervertebral disc may have contributed to the adjacent development of his abscess and osteomyelitis.
E. rhusiopathiae can be isolated on standard culture media, including blood agar plates. Growth is slow, often taking two days. There are two distinct colony types: smooth colonies, which appear as Gram-positive rods or coccobacilli, and rough colonies, which are larger and appear as long filaments. Gram stain is not distinctive as it may feature either colony morphology; rough colonies can decolorize and appear Gram negative. E. rhusiopathiae is catalase negative, non-motile, and distinctively produces hydrogen sulfide on triple sugar iron agar [9]. Isolates may be misinterpreted as Lactobacillus spp. or Enterococcus spp. due to similarities in their colony morphology [10]. Accurate and rapid identification is possible through molecular identification as well as mass spectrometry [2], [11].
Clinical & Laboratory Standards Institute (CLSI) interpretive criteria exist for susceptibility testing of E. rhusiopathiae and isolates are generally susceptible to penicillin, cephalosporins, fluoroquinolones, and carbapenems. It is intrinsically resistant to vancomycin, aminoglycosides, and sulfonamides, and resistance to clindamycin and erythromycin can also occur [9]. Resistance to sulfonamides is notable as trimethoprim-sulfamethoxazole is a common choice for empirical treatment of abscesses, which our patient initially received following incision and drainage of a presumed abscess on his index finger. Similarly, the intrinsic resistance of E. rhusiopathiae to vancomycin should be taken into consideration when patients receive empiric treatment for osteomyelitis, particularly in patients with recent traumatic exposure to animal or fish products.
Conclusion
E. rhusiopathiae is a rare cause of invasive infections in humans. It may be under-diagnosed in light of its slow growth and potentially misdiagnosed due to the similarities in its colony morphology to more common laboratory isolates. Nevertheless recent exposures can lead to invasive infections even in patients without identifiable risk factors, as demonstrated in this case. Thus a high index of suspicion for E. rhusiopathiae infection is necessary when patients present with characteristic exposure histories regardless of timeline.
Disclosure statement
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Acknowledgements
We would like to thank the patient described in this case and the staff of Alpert Medical School at Brown University and Rhode Island Hospital who supported this research.
References
- 1.Brooke C.J., Riley T.V. Erysipelothrix rhusiopathiae: bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J Med Microbiol. 1999;48(9):789–799. doi: 10.1099/00222615-48-9-789. [DOI] [PubMed] [Google Scholar]
- 2.Principe L., Bracco S., Mauri C., Tonolo S., Pini B., Luzzaro F. Erysipelothrix rhusiopathiae bacteremia without endocarditis: rapid identification from positive blood culture by MALDI-TOF mass spectrometry. A case report and literature review. Infect Dis Rep. 2016;8(March (1)):6368. doi: 10.4081/idr.2016.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukhopadhyay C., Shah H., Vandana K.E., Munim F., Vijayan S. A child with Erysipelothrix arthritis-beware of the little known. Asian Pac J Trop Biomed. 2012;2(June (6)):503–504. doi: 10.1016/S2221-1691(12)60085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denes E., Camilleri Y., Fiorenza F., Martin C. First case of osteomyelitis due to Erysipelothrix rhusiopathiae: pubic osteomyelitis in a gored farmer. Int J Infect Dis. 2015;30(January):133–134. doi: 10.1016/j.ijid.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Upapan P., Chayakulkeeree M. Erysipelothrix rhusiopathiae bacteremia without endocarditis associated with psoas abscess: the first case report in Thailand. J Med Assoc Thai. 2014;97(March (Suppl. 3)):S232–S236. [PubMed] [Google Scholar]
- 6.Romney M., Cheung S., Montessori V. Erysipelothrix rhusiopathiae endocarditis and presumed osteomyelitis. Can J Infect Dis. 2001;12(July (4)):254–256. doi: 10.1155/2001/912086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feasi M., Bacigalupo L., Cappato S., Pontali E., Usiglio D., Rollandi G.A. Erysipelothrix rhusiopathiae intra-abdominal abscess. Int J Infect Dis. 2010;14(January (1)):e81–e83. doi: 10.1016/j.ijid.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Reboli A.C., Farrar W.E. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin Microbiol Rev. 1989;2(October (4)):354–359. doi: 10.1128/cmr.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen J., Pfaller M. ASM Press; 2015. Manual of clinical microbiology [e-book] Available from: eBook Academic Collection (EBSCOhost). (Accessed 1 November 2017) [Google Scholar]
- 10.Dunbar S.A., Clarridge J.E., 3rd Potential errors in recognition of Erysipelothrix rhusiopathiae. J Clin Microbiol. 2000;38(March (3)):1302–1304. doi: 10.1128/jcm.38.3.1302-1304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q., Chang B.J., Riley T.V. Erysipelothrix rhusiopathiae. Vet Microbiol. 2010;140(January (3–4)):405–417. doi: 10.1016/j.vetmic.2009.08.012. [DOI] [PubMed] [Google Scholar]