Abstract
Diphtheroids are gram-positive pleomorphic bacilli in the family of Coryneform bacteria. These organisms are present as part of the human flora. Past practice habits had been to consider them as contaminants when isolated from clinical samples. Corynebacterium jeikeium is one of the most clinically important nondiphtherial Corynebacteria that can cause different forms of infections specifically in patients with underlying risk factors and co-morbidities including immunocompromised subjects. Through this article, we present a 67-year-old gentleman with extensive co-morbidities including heart failure with reduced ejection fraction and ESRD on hemodialysis through a femoral catheter who presented with chest pain and fatigue. Further investigation confirmed diagnosis of C. jeikeium endocarditis. We go on to review previously reported cases of C. jeikeium endocarditis and we will discuss different aspects of C. jeikeium infection with a focus on microbiology, pathophysiology, and treatment.
Keywords: Corynebacterium, Jeikeium, Endocarditis
1 Case report
We report a case of a 67-year-old African-American man who presented with an achy, non-radiating pain in his chest and acute left foot pain. He reported these symptoms had been present for three days prior to admission and were associated with fatigue and malaise in the last few preceding months. His chest pain was aggravated by deep breathing, coughing, sitting up, and moving. He denied any nausea, vomiting, or sweating.
He had an extensive past medical history including being diagnosed with End Stage Renal Disease (ESRD) 18 years ago and being on hemodialysis via a right femoral catheter during the last three years due to several fistula failures, non-ischemic heart failure with reduced ejection fraction, pericardial effusion, low-grade follicular cell lymphoma (non-compliant with outpatient chemotherapy, last chemotherapy 2 years ago), stroke, deep vein thrombosis (DVT) and pulmonary embolism (PE), paroxysmal atrial fibrillation not on anticoagulation, chronic obstructive pulmonary disease (COPD) (on 4 L home oxygen), hepatitis C infection (not currently on treatment), chronic anemia, and medication non-adherence. The patient’s past surgical history included multiple dialysis catheter placements and AV fistula creation, atrial flutter ablation, and hernia repair. His last nuclear stress test, performed a year earlier, revealed ejection fraction of 27% with no evidence of ischemia.
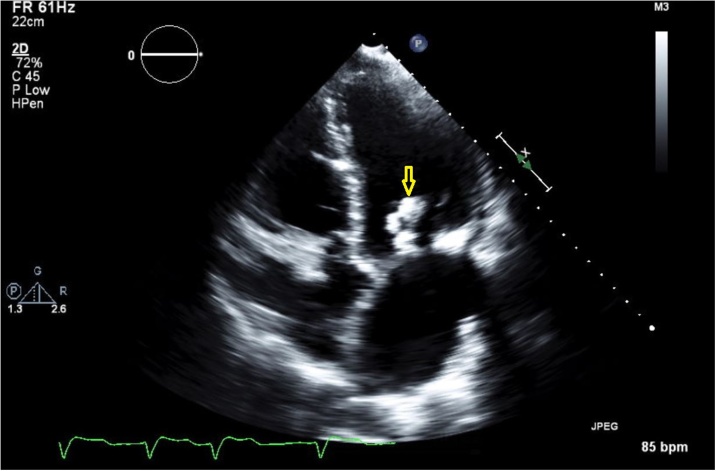
On admission, lab results included an elevated troponin of 0.965 which later decreased to 0.674, White Blood Cell (WBC) count of 9.0 × 103/mL with 71.3% neutrophils. An ECG did not show changes compared to previous studies. On the third day, a nuclear stress test showed LV ejection fraction of 17% with moderate size, mild to moderate severity perfusion defect in the inferior wall with extension to infero-septal wall and reversible apical inferior wall ischemic changes. The patient had two episodes of low-grade fever during the first 48 h of admission with a maximum temperature of 38.1 °C. Therefore, blood cultures were drawn from peripheral veins which reported a growth of gram-positive rods (i.e., diphtheroid) in both samples. Suspecting endocarditis, a transthoracic echocardiogram was performed which showed a mobile echo-density measuring 2.9 cm in length attached to the mitral valve chordae which most likely represented vegetation (Picture 1 Fig. 1, yellow arrow). This was not seen in the previous study, completed 10 months earlier. There was severe mitral annular calcification and moderate mitral regurgitation with mild mitral stenosis. Aortic valve leaflets were mildly thickened with aortic annular calcification extending into the anterior mitral leaflet. There was mild to moderate tricuspid regurgitation. Repeat blood cultures from both peripheral veins and femoral dialysis tunneled permcath reported isolation of the same organism (6 of 7 samples).
Fig. 1.
Mitral valve vegetation.
The patient was started on vancomycin during each hemodialysis session and femoral permcath was replaced by a temporary trialysis catheter. Samples from both peripheral vein and dialysis catheter were sent to the reference lab at the Mayo Clinic, Rochester, MN. The organism was identified as Corynebacterium jeikeium which was resistant to penicillin (MIC > 8 mcg/mL), ceftriaxone (MIC > 2 mcg/mL), and meropenem (MIC = 4 mcg/mL). Additionally, it was sensitive to vancomycin (MIC ≤ 1 mcg/mL). Therefore, treatment with vancomycin continued. Repeated blood cultures three days after starting antimicrobials were still positive for diphtheroid. Cultures became negative nine days after initiation of antimicrobial therapy. Therefore, a new femoral permcath was inserted and the patient was discharged with a primary diagnosis of Corynebacterium jeikeium endocarditis and a plan to continue vancomycin with each dialysis session for a total course of 6 weeks. The patient fully recovered from the infection through this course of treatment and was seen at the hospital for a dialysis catheter malfunction a few weeks after completing the antimicrobial regimen. Unfortunately, he did not follow up for an out-patient repeat echocardiogram.
2 Discussion
We found ten cases of confirmed Corynebacterium jeikeium (CJ) endocarditis based on our MEDLINE search of English literature for the key words of ‘Corynebacterium’, ‘Jeikeium’ and ‘Endocarditis’. The table below (Table 1) summarizes these cases.
Table 1.
Summary of C. jeikeium endocarditis reported cases. AS: Aortic Stenosis. AV: Aortic Valve. AVR: Aortic Valve Replacement. CABG: Coronary Artery Bypass Graft. ESRD: End-Stage Renal Disease. HD: Hemodialysis. MI: Mitral Insufficiency. LV: Left Ventricle. MS: Mitral Stenosis. MV: Mitral Valve. MVR: Mitral Valve Replacement. TI: Tricuspid Insufficiency.
| Reference | Patient | Age/Sex | Co-morbidity | History of valve replacement/site/type | Endo carditis site | Indwelling line/site/type | Antibiotic Treatment | Antibiotic duration | Surgical treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| B. Vanbosterhaut [1] | 1 | 77/F | AS, MS | AVR and MVR | MV | No | Vancomycin | 6 weeks | MVR | Recovery |
| B. Vanbosterhaut [1] | 2 | 51/M | MI, Dental carries | NA | MV | No | Vancomycin, Gentamycin | 6 weeks | MVR | Recovery |
| B. Vanbosterhaut [1] | 3 | 54/M | ESRD, HD, MI | No | MV | NA | Vancomycin | 10 weeks | No | Recovery |
| B. Vanbosterhaut [1] | 4 | 57/F | MS, TI, CABG | AVR, MVR, tricuspid annuloplasty | MV | No | Piperacillin, Netilmicin, Erythromycin | NA | No | Death |
| B. Vanbosterhaut [1] | 5 | 45/M | Mixed AS/AI, LV dilatation | AVR | AV | No | Vancomycin | 30 days | AVR | Recovery |
| C.A. David [2] | 6 | 56/F | Alcoholic, liver transplant, HD | No | AV | Dialysis catheter and central line | Vancomycin, Amphotericin | 2 weeks | AVR | Recovery |
| Mitchell J. Ross [3] | 7 | 63/F | CABG | No | AV | Repeated right femoral cannulation | Vancomycin, Gentamycin | NA | AVR | Death |
| F. Mookadam [4] | 8 | 84/M | AS | AVR (bioprosthetic) | AV | No | Vancomycin, Gentamycin, Rifampicin | 6 weeks | AVR | Recovery |
| C. Bechara [5] | 9 | 72/M | Permenant pacemaker | No | Pacemaker | No | Vancomycin, Doxycycline, Rifampicin | 6 weeks | Pacemaker change | Recovery |
| A. Lappa [6] | 10 | 57/M | AS | AVR (Mechanical) | AV | No | Daptomycin, Rifampicin, Ceftazidine | 6 weeks | AVR | Recovery |
The first case was a 77-year-old female who presented with oliguria, fatigue, and generalized weakness. She had two episodes of a urinary tract infection (UTI) in the last two months. She also had aortic valve (AV) and mitral valve (MV) replacement six months earlier due to aortic stenosis (AS) and mitral insufficiency (MI). Diagnosis of mitral valve infective endocarditis (IE) due to C. jeikeium was confirmed with a blood culture. She was treated by mitral valve replacement (MVR) and vancomycin for six weeks. The patient was discharged completely cured [1].
The second patient was a 51-year-old male who was admitted to the hospital due to a long history of fever, fatigue, weight loss, and dental caries. Patient had past medical history of mitral insufficiency. Blood culture showed growth of C. jeikeium. The patient was diagnosed with IE and underwent multiple teeth extractions. He was further treated with vancomycin and gentamycin for a total of six weeks. He was readmitted four months later with another episode of endocarditis and was retreated with the same antimicrobials for another six weeks. The patient recovered and discharged after two months [1].
The third case was a 54-year-old male who was admitted to the hospital after experiencing fever and chills for one day. He was on hemodialysis due to ESRD and also suffered from mitral insufficiency. Echocardiography showed MV vegetation and blood culture confirmed growth of C. jeikeium. Patient was treated with vancomycin for ten weeks and recovered uneventfully [1].
The fourth patient was a 57-year-old woman who underwent MV and AV replacement (MVR and AVR), tricuspid valve annuloplasty, and coronary artery bypass grafting (CABG) due to calcified MS, functional tricuspid insufficiency, and stenotic lesions of the coronary arteries. She developed septicemia after surgery and mitral insufficiency was noticed. Blood cultures confirmed diphtheroid group JK growth. Combined antimicrobials, including parenteral erythromycin, piperacillin, and netilmicin, were started, but the patient deteriorated and mitral valve vegetation remained unchanged. The patient also developed positive blood cultures for Staphylococcus epidermidis and died after three months due to cardiogenic shock [1].
Case number five was a 45-year-old man who underwent AVR due to severe aortic insufficiency (AI) following mixed AS and AI with signs of left ventricular dilatation. The patient developed fever and chills. Blood cultures showed JK diphtheroid growth. An echocardiography revealed severe AI due to paravalvular leakage. He was started on vancomycin and his prosthetic valve was replaced. He recovered after receiving vancomycin for a total of 30 days [1].
The sixth patient was a 56-year-old alcoholic woman who underwent liver transplantation due to fulminant hepatic failure. She also suffered from hepatorenal syndrome which required hemodialysis. The post-transplant course was complicated with respiratory insufficiency, renal failure, and sepsis. Different cultures from central line and indwelling dialysis catheter showed growth for C. jeikeium and the patient was treated with vancomycin and ceftazidime for four weeks. She recovered, but one month later, was re-admitted due to decreased urine output and orthopnea. Echocardiogram showed new incidence of AV vegetation and severe AI. Aortic valve replacement surgery was performed and the patient was treated with vancomycin and amphotericin for two weeks. She recovered and was discharged in good condition [2].
The seventh case was a 63-year-old woman with a history of CABG. She presented with fever, progressive malaise, fatigue, and generalized weakness six weeks after she underwent two coronary catheterizations with angioplasty and stent placement both performed through a right femoral access with a one week interval. The initial evaluation did not show any evidence of infective endocarditis. So a third cardiac catheterization was performed that showed AV vegetation with severe AI. She was treated with intravenous vancomycin and gentamicin and underwent emergent AVR with a bioprosthesis. Her post surgical course was complicated with multi-organ dysfunction and the patient’s family requested withdrawal of support, thus, the patient expired [3].
Case number eight was an 84-year-old man who presented with a low grade fever, left sided body weakness, and fatigue for ten days before admission. His past medical history was positive for bioprosthetic AVR due to severe aortic calcification. An EKG showed first degree AV block. TEE confirmed vegetation on the prosthetic aortic valve. Blood culture grew C. jeikeium on three cultures. His bioprosthetic AV was replaced and vancomycin, gentamycin, and rifampicin were given for a total of six weeks. The patient recovered and was discharged in good health [4].
The ninth case was a 72-year-old man who was admitted after six weeks of fever. He had a dual chamber pacemaker due to brady-tachy syndrome with bifasicular block. The TEE was unremarkable and one out of twelve sets of blood cultures showed growth of C. jeikeium. The patient received vancomycin and linezolid for two weeks followed by doxycycline for six weeks. Three weeks after discontinuation of antimicrobials, the patient was readmitted with recurrent fever and a positive blood culture. He was then treated with vancomycin for two weeks followed by doxycycline and rifampicin for four weeks. The pacemaker was replaced as well. He recovered in good condition [5].
The last case was a 57-year-old man who was admitted to the hospital due to third degree AV block; a temporary pacemaker was immediately inserted. The patient had previously undergone AVR. TTE revealed large vegetation on the prosthetic aortic valve. He was started on daptomycin, rifampicin, and ceftazidime after the blood culture was sent. The results showed C. jeikeium growth. The antimicrobials continued for six weeks. The aortic valve was replaced and the patient recovered [6].
Diphtheroids are gram-positive pleomorphic bacilli in the family of Coryneform bacteria. These organisms are present in the human flora and generally regarded as nonpathogenic contaminants of different samples including blood cultures due to low virulence, but they may cause opportunistic infections in immunocompromised patients [[7], [8]].
During the last four decades, there have been an increasing number of scientific publications related to the clinical microbiology and antimicrobial susceptibility of pathogenic corynebacteria and other gram positive rods. A few different features have contributed to this development: 1) as a result of advances in medical science, there is an increased number of patients with immunosuppressive disease or risk factors for opportunistic and nosocomial infection, 2) organisms whose pathogenic potential was initially underestimated, like nondiphtherial corynebacteria, are now recognized with increasing frequency as opportunistic human pathogens [9], and 3) improvement in microbiologic and genetic techniques like polymerase chain reaction (PCR) made it possible to differentiate between several species of these organisms.
3 History
In 1970, Johnson and Kaye [10] first described bacterial endocarditis following cardiac surgery with the isolation of nondiphtherial corynebacteria from blood cultures, which were typically multiresistant against antimicrobial agents. In 1976, Hande et al. [11] described sepsis of a previously undescribed Corynebacterium species in four patients, three of them had leukemia in relapse and one had a ventriculoatrial shunt. The isolates uniformly had a metallic sheen on blood agar and were resistant to most antimicrobials, except for vancomycin. One year later, in 1977, Pearson et al. [12] published twelve cases of Corynebacterium bacteremia on an oncology ward. Eight patients had mucocutaneous break and evidence of skin colonization with the same bacteria. Vancomycin was chosen as the treatment of choice. In 1979, Centers for Disease Control and Prevention (CDC) tentatively designated these bacteria as “group JK corynebacteria” [13]. In 1987, these bacteria were taxonomically delineated as C. jeikeium [14].
Subsequent to this, several reports established C. jeikeium as the causative agent of a variety of severe nosocomial infections including endocarditis, device infection, osteomyelitis, and cellulitis. It was most frequently associated with immunocompromised patients with malignancies, in-place medical devices, breaks in the skin barrier, and therapy with broad-spectrum antimicrobials [9]. A high mortality rate was documented in the case of C. jeikeium sepsis in hematological patients [15].
4 Microbiology and diagnosis
C. jeikeiums are pleomorphic gram-positive strict aerobic rods that exist as a part of the normal skin flora, particularly in the axillary, rectal, and inguinal regions of hospitalized patients [13]. Their shapes vary from short coccobacilli to long bacillary forms. They have white smooth colonies and are one of the most lipid requiring colonies among Corynebacterium [13].
More than 80 species of Corynebacterium have been identified and more than 50 species have been related to human diseases [[16], [17]]. Different molecular genetic studies are used to differentiate these species which include 16S rRNA gene sequencing, rpoB sequencing, and Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) [[9], [18], [19]]. Recent whole-gene sequencing by these methods revealed that C. jeikeium is a group of four genomospecies [20].
In clinical practice settings, different biochemical test systems have been developed to identify Corynebacterium species including API Coryne, RapID CB Plus, and Christie-Atkins-Munch-Petersen (CAMP) systems. These testes can correctly identify high numbers of strains to the species level [[9], [21]]. They have variable sensitivity and specificity in diagnosing C. jeikeium, and are based on C. jeikeium biochemical characteristics including acid production from glucose, negative nitrate reduction, and negative urease tests. For further confirmation, additional tests such as detection of pyrazinamidase and alkaline phosphatase may be required [13].
5 Treatment
Gram-positive rods are not often considered in antimicrobial susceptibility studies, and very few large-scale or controlled clinical studies with these organisms are available. Clinical and Laboratory Standards Institute (CLSI) standardized disk susceptibility testing data are not available for Gram-positive bacilli. One study showed excellent correlations between the results of microdilution and disk susceptibility testing of corynebacteria when the CLSI streptococcal criteria for erythromycin and vancomycin and the Listeria criteria for penicillin were used [[22], [23]]. Another study found few discrepancies for Corynebacterium jeikeium when interpretative criteria for enterococci were applied [24].
For species intrinsically resistant to multiple antimicrobials such as C. jeikeium, vancomycin or teicoplanin might be the first and only choice of antimicrobial therapy [[9], [25], [26]]. In 1995, Soriano et al. showed multiple antimicrobial resistances in several species of the Corynebacterium family including C.jeikeium, C. urealyticum, C. xerosis, and C. minutissimum. Vancomycin was the most active antimicrobial against none-spore-forming gram positive bacilli and Corynebacterium species including C. jeikeium. Resistance to beta-lactam antimicrobials including ampicillin, cephalothin, cefuroxime, and imipenem was quite common among strains of Corynebacterium jeikeium and Corynebacterium urealyticum. Clindamycin was less active than the macrolides against Corynebacterium species, but resistance to erythromycin and azithromycin was very frequent among C. jeikeium. Although Ciprofloxacin was active against many isolates of none-spore-forming gram positive bacilli, resistance was common among strains of C. jeikeium. Only a few strains of C. jeikeium were resistant to doxycycline or Fusidic acid (i.e., at concentrations greater than 4 g/ml). C. jeikeium was also resistant to gentamicin, optochin, and fosfomycin, but nitrofurantoin was active against a few strains of C. jeikeium and C. urealyticum [27]. Their findings were compatible with previous studies [[7], [28], [29]]. Although high-level resistance to daptomycin has not been described in corynebacteria so far [[30], [31]], daptomycin’s activity against C. jeikeium is known to be inferior to its activity against staphylococci. The presence of intrinsic factors might be contributing to lower susceptibility of C. jeikeium to daptomycin[32]. There is also one reported case of a daptomycin resistant C. jeikeium in a previously treated neutropenic patient [6].
One of the important issues we need to consider while selecting the antimicrobial regimen for C.jeikeium is the genomospecies. Reports have shown that genomospecies 2 is distinguishable from the other three genomspecies due to resistance to gentamicin, tetracycline, and trimethoprim-sulfamethoxazole[33]. It is, however, difficult to distinguish the other genomospecies based on the antimicrobial resistance. There are no consistent antimicrobial resistance differences between all three genomspecies including 1, 3, and 4 [33].
Therapeutic plans in catheter-related bacteremia can differ based on several factors including specific causative organisms and the decision to remove or “salvage” a catheter. Wang et al. [34] retrospectively reviewed 53 cases of C. jeikeium bacteremia in bone marrow transplant recipients who had a Hickman catheter without signs of local catheter site infection. The results showed that salvage of catheter with vancomycin therapy is successful in most patients (93%) and only a minority (7%) of patients with attempted catheter salvage had recurrent bacteremia. The median time to negative blood cultures was similar in both groups (i.e., two days). The authors’ findings were in contrast to previous study which showed higher mortality in patients with retained catheters [35], possibly related to bacterial slime production interfering antimicrobial penetration into the site of the infection [4]. Wang et al. concluded that both primary and recurrent catheter-related C. jeikeium bacteremia may be successfully treated with vancomycin without removal of the catheter in the absence of local infection. They recommended catheter removal to be considered if C. jeikeium bacteremia persists beyond three days.
6 Conclusion
Nondiphtherial Corynebacteria (diphtheroids) are gram positive rods which are increasingly recognized as pathogenic organisms causing different human infections. C. jeikeium is the most well-known pathogen of this group in hospital and intensive care settings which can cause different forms of infectious disease including endocarditis. There are many different factors that can increase the risk of C. jeikeium endocarditis in various patients. According to our article review summarized in Table 1, five out of ten patients (50%) had valve replacement prior to infective endocarditis due to C. jeikeium. All these five patients (100%), suffered infective endocarditis on the same prosthetic valve. This is consistent with the study that showed majority of C. jeikeium endocarditis occurs in patients with prosthetic valves [7]. Similar to our patient, two out of ten patients (20%) had a history of renal insufficiency on hemodialysis and had an indwelling catheter. This can be correlated with the study that showed increased frequency of C. jeikeium infection in patients who are immunocompromised or have in-place catheter insertion [32]. Like our patient, all ten cases, except case numbers 4 and 10, were treated with vancomycin alone or as a combined therapy (see Table 1). Seven out of eight patients who were treated with vancomycin recovered, which supports the importance of vancomycin treatment in patients who suffer from C. jeikeium endocarditis. In multiple antimicrobial resistant species like C. jeikeium, vancomycin or teicoplanin are considered as the first choice of treatment [[9], [25], [26]].
References
- 1.Vanbosterhaut B., Surmont I., Vandeven J., Wauters G., Vandepitte J. Corynebacterium jeikeium (Group JK diphtheroids) endocarditis. Diagn Microbiol Infect Dis. 1989;12:265–268. doi: 10.1016/0732-8893(89)90025-4. [DOI] [PubMed] [Google Scholar]
- 2.David C.A., Horowitz M.D., Burke G.W., 3rd Aortic valve endocarditis in a liver transplant recipient–successful management by aortic valve replacement. Reprinted from Transplantation. 1992;63(June (6)):1366–1367. [PubMed] [Google Scholar]
- 3.Ross M.J., Sakoulas G., Manning W.J., Cohn W.E., Lisbon A. Corynebacterium jeikeium native valve endocarditis following femoral access for coronary angiography. Clin Infect Dis. 2001;32(7):e120–e121. doi: 10.1086/319592. [DOI] [PubMed] [Google Scholar]
- 4.Mookadam F., Cikes M., Baddour L.M., Tleyjeh I.M., Mookadam M. Corynebacterium jeikeium endocarditis: a systematic overview spanning four decades. Eur J Clin Microbiol Infect Dis. 2006;25:349–353. doi: 10.1007/s10096-006-0145-8. [DOI] [PubMed] [Google Scholar]
- 5.Bechara C., Gousseff M., Passeron A., Podglajen I., Day N., Pouchot J. Corynebacterium jeikeium pacemaker infection associated with antineutrophil cytoplasmic antibodies: a single positive blood culture could be sufficient for diagnosis. J Med Microbiol. 2011;60(February (Pt 2)):249–251. doi: 10.1099/jmm.0.023283-0. [DOI] [PubMed] [Google Scholar]
- 6.Lappa A., Donfrancesco S., Picozzi P., Vitozzi T., Marrapodi A., Menichetti A. Treatment with daptomycin for Corynebacterium jeikeium left-sided prosthetic valve endocarditis. Minerva Anestesiol. 2012;78(June (6)):729–732. [PubMed] [Google Scholar]
- 7.Lipsky B.A., Goldberger A.C., Tompkins L.S., Plorde J.J. Infections caused by nondiphtheria Corynebacteria. Rev Infect Dis. 1982;4:1220–1235. doi: 10.1093/clinids/4.6.1220. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y.W., von Graevenitz A., Waddington M.G., Hopkins M.K., Smith D.H., Li H. Identification of coryneform bacterial isolates by ribosomal DNA sequence analysis. J Clin Microbiol. 2000;38:1676–1678. doi: 10.1128/jcm.38.4.1676-1678.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funke G., von Graevenitz A., Clarridge J.E., III, Bernard K.A. Clinical microbiology of coryneform bacteria. Clin Microbiol Revision. 1997;10:125–159. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson W.D., Kaye D. Serious infections caused by diphtheroids. Annu N Y Acad Sci. 1970;174:568–576. doi: 10.1111/j.1749-6632.1970.tb45582.x. [DOI] [PubMed] [Google Scholar]
- 11.Hande K.R., Witebsky F.G., Brown M.S., Schulman C.B., Anderson S.E., Jr, Levine A.S. Sepsis with a new species of Corynebacterium. Annu Intern Med. 1976;85(4):423–426. doi: 10.7326/0003-4819-85-4-423. [DOI] [PubMed] [Google Scholar]
- 12.Pearson T.A., Braine H.G., Rathbun H.K. Corynebacterium sepsis in oncology patients. Predisposing factors, diagnosis, and treatment. JAMA. 1977;238(16):1737–1740. [PubMed] [Google Scholar]
- 13.Riley P.S., Hollis D.G., Utter G.B., Weaver R.E., Baker C.N. Characterization and identification of 95 diphtheroid (group JK) cultures isolated from clinical specimens. J Clin Microbiol. 1979;9(March (3)):418–424. doi: 10.1128/jcm.9.3.418-424.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackman P.J.H., Pitcher D.G., Pelczynska S., Borman P. Classification of corynebacteria associated with endocarditis (group JK) as Corynebacterium jeikeium sp. Syst Appl Microbiol. 1987;9(February (1–2)):83–90. [Google Scholar]
- 15.van der Lelie H., Leverstein-van Hall M., Mertens M., van Zaanen H.C.T., vanOers R.H.J., Thomas B.L.M. Corynebacterium CDC Group JK (Corynebacterium jeikeium) sepsis in haematological patients: a report of three cases and a systematic literature review. Scand J Infect Dis. 1995;27:581–584. doi: 10.3109/00365549509047071. [DOI] [PubMed] [Google Scholar]
- 16.Bernard K.A., Funke G., editors. Bergey's Manual of Systemic Bacteriology. 2nd ed. Springer; New York: 2012. Genus; pp. 245–289. [Google Scholar]
- 17.Bernard K. The genus. J Clin Microbiol. 2012;50:3152–3158. doi: 10.1128/JCM.00796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funke G., Renaud F.N., Freney J., Riegel P. Multicenter evaluation of the updated and extended API (RAPID) Coryne database 2.0. J Clin Microbiol. 1997;35:3122–3126. doi: 10.1128/jcm.35.12.3122-3126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khamis A., Raoult D., La Scola B. rpoB gene sequencing for identification of Corynebacterium species. J Clin Microbiol. 2004;42:3925–3931. doi: 10.1128/JCM.42.9.3925-3931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salipante S.J., Sengupta D.J., Cummings L.A., Robinson A., Kurosawa K., Hoogestraat D.R. Whole genome sequencing indicates Corynebacterium jeikeium comprises 4 separate genomospecies and identifies a dominantgenomospecies among clinical isolates. Int. J. Med. Microbiol. 2014;304(November (8)):1001–1010. doi: 10.1016/j.ijmm.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soto A., Zapardiel J., Soriano F. Evaluation of API Coryne system for identifying coryneform bacteria. J Clin Pathol. 1994;47:756–759. doi: 10.1136/jcp.47.8.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards . National Committee for Clinical Laboratory Standards; Villanova, PA: 1993. Performance standards for antimicrobial disk susceptibility tests. M2-A5. [Google Scholar]
- 23.Weiss K., Laverdiere M., Rivest K. Comparison of antimicrobial susceptibilities of Corynebacterium species by broth microdilution and disk diffusion methods. Antimicrob Agents Chemother. 1996:930–933. doi: 10.1128/aac.40.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traub W.H., Geipel U., Leonhard B., Bauer D. Antibiotic susceptibility testing (agar disk diffusion and agar dilution) of clinical isolates of Corynebacterium jeikeium. Chemotherapy. 1998;44(4):230–237. doi: 10.1159/000007119. [DOI] [PubMed] [Google Scholar]
- 25.Lagrou K., Verhaegen J., Janssens M., Wauters G., Verbist L. Prospective study of catalase- positive coryneform organisms in clinical specimens: identification, clinical relevance, and antibiotic susceptibility. Diagn Microbiol Infect Dis. 1998;30(1):7–15. doi: 10.1016/s0732-8893(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 26.Funke G., Puenter V., von Graevenitz A. Antimicrobial susceptibility patterns of some recently established coryneform bacteria. Antimicrob Agents Chemother. 1996;40(December (12)):2874–2878. doi: 10.1128/aac.40.12.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soriano Francisco, Zapardiel Javier. Eva nieto antimicrobial susceptibilities of Corynebacterium species and other non-Spore-Forming gram- positive bacilli to 18 antimicrobial agents. Antimicrob Agents Chemother. 1995;39(1):208–214. doi: 10.1128/aac.39.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill V.J., Manning C., Lamson M., Woltering P., Pizzo P.A. Antibiotic-resistant group JK bacteria in hospitals. J Clin Microbiol. 1981;13:472–477. doi: 10.1128/jcm.13.3.472-477.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippon A., Bimet F. In vitro susceptibility of Corynebacterium group D2 and Corynebacterium jeikeium to twelve antibiotics. Eur J Clin Microbiol Infect Dis Infect Dis. 1990;9:892–895. doi: 10.1007/BF01967505. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein E.J., Citron D.M., Merriam C.V., Warren Y.A., Tyrrell K.L., Fernandez H.T. In vitro activities of the new semisynthetic glycopeptide telavancin (TD-6424), vancomycin, daptomycin, linezolid, and four comparator agents against anaerobic gram-positive species and Coryne- bacterium spp. Antimicrob Agents Chemother. 2004;48:2149–2152. doi: 10.1128/AAC.48.6.2149-2152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streit J.M., Jones R.N., Sader H.S. Daptomycin activity and spectrum: a worldwide sample of 6737 clinical Gram-positive organisms. J Antimicrob Chemother. 2004;53:669–674. doi: 10.1093/jac/dkh143. [DOI] [PubMed] [Google Scholar]
- 32.Van der Auwera P. Ex vivo study of serum bactericidal titers and killing rates of daptomycin (LY146032) combined or not combined with amikacin compared with those of vancomycin. Antimicrob Agents Chemother. 1989;33:1783–1790. doi: 10.1128/aac.33.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoen C., Unzicker C., Stuhler G., Elias J., Einsele H., Grigoleit G.U. Life-threatening infection caused by daptomycin-resistant. J Clin Microbiol. 2009;47(May (7)):2328–2331. doi: 10.1128/JCM.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C.C., Mattson D., Wald A. Nature Publishing Group; 2001. Corynebacterium jeikeium bacteremia in bone marrow transplant patients with Hickman catheters; pp. 0268–3369/01. [DOI] [PubMed] [Google Scholar]
- 35.Soriano F., Rodriguez-Tudela J.L., Fernandez-Roblas R., Aguado J.M., San- tamaria M. Skin colonization by Corynebacterium groups D2 and JK in hospitalized patients. J Clin Microbiol. 1988;26:1878–1880. doi: 10.1128/jcm.26.9.1878-1880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]