Abstract
Emerging evidence has indicated that the perturbed expression of homocysteine (Hcy) may induce mitochondrial dysfunction and disturb bone metabolism. Sirtuin 1 (SIRT1) and AMP-activated protein kinase (AMPK) are two critical sensors that regulate mitochondrial biogenesis and have been recognized as therapeutic targets in osteoarthritis (OA). This study was designed to test whether Hcy caused pro-osteoarthritic changes through modulation of SIRT1 and AMPK. Our results showed that administration of Hcy reduced the SIRT1/AMPK/PGC-1α signaling in chondrocytes, leading to mitochondrial dysfunction as a result of increased oxidative stress and apoptosis. Moreover, we demonstrated that the expression of NF-κB, COX-2, IL-8, and MMP-13 were elevated subsequent to inhibition of SIRT1/AMPK/PGC-1α/PPAR-γ pathway by homocysteine, thereby causing detrimental effects on chondrocytes. In the animal model of diet-induced hyperhomocysteinemia (HHcy), we observed the similar findings that SIRT1/PGC-1α/PPAR-γ cascades were downregulated with elevated MMP-13 and COX-2. Taken together, data from the current study revealed that the reduced SIRT1 by Hcy may contribute to degradative cartilage process, which provided insight into the etiology of OA.
Keywords: SIRT1, AMPK, Osteoarthritis, Hyperhomocysteinemia, Chondrocytes
Highlights
-
•
Homocysteine-reduced SIRT1 leads to downregulation of phosphorylated AMPK and PGC-1α.
-
•
Homocysteine-inhibited SIRT1 results in mitochondrial dysfunction.
-
•
Homocysteine-induced oxidative stress is reversed by activation of SIRT1/AMPK/PGC-1α signaling.
-
•
Homocysteine-induced pro-apoptosis effect is rescued by overexpression of SIRT1/AMPK/PGC-1α cascades.
-
•
Expression levels of pro-inflammatory mediators and MMP13 are elevated subsequent to suppression of PPAR-γ by homocysteine.
1. Introduction
The implication of mitochondrial dysfunction in the pathogenesis of osteoarthritis (OA) has been well-documented [1], [2], [3]. However, the etiology of mitochondrial impairment in OA has not been fully understood. Hyperhomocysteinemia (HHcy) is a disorder of methionine metabolism, leading to different diseases in human. Recently, it has been indicated that the perturbed expression of homocysteine (Hcy), a sulfur-containing amino acid, may induce mitochondrial dysfunction and disturb bone metabolism [4]. Indeed, various studies have demonstrated that Hcy induces mitochondrial dysfunction through regulation of oxidative stress [5], [6]. The elevated expression of Hcy has been shown to alter the osteoclastogenesis via an increase in matrix metalloproteinases (MMPs) and oxidative stress-associated extracellular matrix degradation [7]. Also, Hcy may affect the properties of calcified cartilage as it decreases the chondrocyte-mediated mineralization [8]. Nevertheless, the detailed mechanism underlying the pathogenesis of OA through Hcy-induced mitochondrial dysfunction still remains to be elucidated.
Among various factors regulating bioenergy metabolism, sirtuin 1 (SIRT1) and AMP-activated protein kinase (AMPK) are two critical sensors that have been recognized as new therapeutic targets in OA [9]. SIRT1 and AMPK both regulate each other, and have been found to directly affect the activity of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) through deacetylation and phosphorylation, respectively [10]. PGC-1α has emerged as a master regulator of mitochondrial biogenesis and is decreased in OA cartilages [11]. Also, the aberrant SIRT1/AMPK/PGC-1α signaling was found in OA chondrocytes [11]. It has been shown that the diminished SIRT1 in OA [12] was critical for chondrocyte survival via downregulation of a potent pro-apoptotic protein, PTP1B [13], or suppression of cartilage-degrading enzymes through modulation of the NF-κB pathway [14], [15]. Given that Hcy is involved in mitochondrial dysfunction and downregulation of SIRT1/AMPK/PGC-1α may lead to OA, we sought to investigate whether elevation of Hcy caused mitochondrial dysfunction in cartilage via inhibition of SIRT1.
In this work, we examined whether SIRT1/AMPK/PGC-1α pathway was associated with the increased expression of Hcy in chondrocytes. Additionally, we evaluated the pro-apoptotic response and oxidative stress after confirmation of mitochondrial dysfunction. Moreover, we investigated the relationship between the aberrant SIRT1/AMPK/PGC-1α signaling and the expression of PPAR-γ as well as NF-κB in order to elucidate the contribution of Hcy in the increased expression of MMP13 and proinflammatory mediators, IL-8 and COX-2, in Hcy-treated chondrocytes.
2. Materials and method
2.1. Reagents
Trypsin-EDTA and Dulbecco's modified Eagle's medium (DMEM) were bought from Gibco (Grand Island, NY, USA). SIRT1720, AICAR, Apocynin, Diphenyleneiodonium (DPI), collagenase B, penicillin and streptomycin were all purchased from Sigma (St. Louis, USA). MitoSOX™ Red Mitochondrial Superoxide Indicator was obtained from Thermo Fisher Scientific (MA, USA). Anti-SIRT1 anti-PGC-1, anti-p-AMPK, anti-PPAR-γ, anti-Bcl-2, anti-COX-2, anti-Bax, anti-β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-conjugated anti-rabbit secondary antibodies were purchased from Transduction Laboratories (CA, USA). Antioxidant enzymes kits were obtained from Biovision (CA, USA).
2.2. Human chondrocytes isolation
30 patients diagnosed with knee OA over 5 years were recruited in this study. The study protocol was approved by the Ethics Committee of E-Da Hospital (EMRP-105-077), and each participant provided written informed consent. Cartilages of knee joint were obtained from OA subjects who underwent arthroplastic knee surgery. Cartilage samples were cut into small pieces and washed with PBS for three times. Cartilage fragments were digested with collagenase B in DMEM at 37 °C overnight on a shaker. The isolated chondrocytes were centrifuged and washed two times with PBS. Chondrocytes were cultured in MDEM with 10% FBS, 2 mM L-glutamine, 25 mM HEPES, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37 °C in a humidified atmosphere of 95% air and 5% CO2 [16].
2.3. Western blot analysis
RIPA (Millipore) was used to extract lysate. The proteins were transferred on to a PVDF membrane after separation by SDS/PAGE. The membranes were blocked for 1 h at 37 °C followed byincubation with primary antibodies overnight at 4 °C hybridization with HRP (horseradish peroxidase)-conjugated secondary antibodies were applied for 1 h. The intensities were quantified by densitometric analysis.
2.4. SIRT1 activity
SIRT1 deacetylase activity was investigated by using a SIRT1 Assay Kit (Abcam, ab156065) according to the manufacturer's instructions.
2.5. Measurement of mitochondrial membrane potential
The membrane-permeant JC-1 dye was used to explore the effect of quercetin on mitochondrial membrane potential (ΔΨm). JC-1 exists either as a green fluorescent monomer at depolarized membrane potentials or as a red fluorescent J-aggregate at hyperpolarized membrane potentials. After treating cells with Hcy for 24 h, cells were rinsed with DMEM and then loaded with JC-1 (5 μM). After a 30-min incubation at 37 °C, cells were examined by flow cytometry.
2.6. Biogenesis of mitochondrial membrane potential
Mitochondrial mass was tested using N-nonyl acridine orange (NAO) staining. Cells were stained with NAO (5 µM) in the dark for 30 mins at 37 °C, and cells were examined by flow cytometry. Real-time PCR was used to test cellular and mitochondrial DNA (mtDNA) content. The following DNA primers were designed to investigate mitochondrial complex II: sense primer 5′-CAAACCTACGCCAAAATCCA-3′ and antisense primer 5′-GAAATGAATGAGCCTACAGA-3′ and β-actin: sense primer 5′-AGGTCATCACTATTGGCAACGA-3′ and antisense primer 5′-CACTTCATGATGGAATTGAATGTAGTT-3′. PCR was performed using SYBR Green on an ABI 7000 sequence detection system (Applied Biosystems) according to the manufacturer's instructions.
2.7. Measurement of ROS production
ROS generation in chondrocytes was tested using MitoSOX. Cells in 96-well plates were incubated for 24 h with Hcy. After removing the medium from the wells, cells were incubated with 10 μM MitoSOX for 30 mins. The fluorescence intensity was calculated by flow cytometry.
2.8. ELISA
Cells were seeded in 24-well plates at 0.5 × 105 cells. After 2 days, cells were treated Hcy for 24 hrs. Cell supernatants were removed and assayed for IL-8, MMP-13 and COX-2 concentrations using an ELISA kit obtained from R&D Systems (Minneapolis, MN).
2.9. SOD activity assay
SOD activity in chondrocytes was investigated via an enzymatic assay using commercial kits (Biovision, K335) according to the manufacturer's instructions.
2.10. NF-κB activity assay
NF-κB activity was measured by an NF-κB p65 ActiveELISA kit (Imgenex Corp, San Diego, CA) according to the manufacturer's instructions. The absorbance at 405 nm was determined using a microplate reader (spectraMAX 340).
2.11. TUNEL assay
Apoptotic cells were analyzed by TUNEL assay (Roche-11684795910). After treatment with Hcy for 24 h, cells were rinsed twice in PBS before fixation for 30 min at room temperature with 4% paraformaldehyde. Next, cells were washed in PBS before incubation in the prepared solution (0.1% Triton X-100, 0.1% sodium citrate) for 5 min. Cells were then incubated with 100 μl TUNEL reaction mixture in a humidified atmosphere for 1 h at 37 °C in the dark, washed in PBS, and analyzed by flow cytometry.
2.12. Overexpression and cDNA transfection
Human SIRT1, AMPK, PGC-1α and PPAR-γ were obtained from Addgene (Cambridge, MA, USA). Human SIRT1, AMPK, PGC-1α and PPAR-γ were subcloned into p3XFLAG-Myc-CMV-26 expression vector. Transient transfections with 2 µg plasmid DNA were accomplished with Lipofectamine 2000 (Invitrogen) at 1: 3 ratio for 48 h. Transfection efficiencies had been confirmed by Western blotting assay.
2.13. Animals
A total of 12 C57BL mice (6 in each group) were used in this study. The animals were fed with or without 1% L-methionine (w/w) in water for 4 months to induce Hyperhomocysteinemia (HHcy). All procedures followed the guidelines of the care and use of laboratory animals and were approved by the animal center of the National Cheng Kung University in Tainan, Taiwan. The Hcy levels in the plasma were tested by ELISA assay (Biomatik, DW, USA) to confirm that HHcy was induced. Tissues from HHcy cartilage were collected for RNA expression test. RNAs were isolated using a RNA isolation kit. Reverse transcription was performed at 42 °C for 60 min, followed by incubation at 95 °C for 5 min. The reaction 20 mixture (20 μl of total volume) consisted of 2 μg of isolated total RNA, 1 mM dNTP, 1 unit/μl of recombinant RNasin ribonuclease inhibitor, 15 U/μg of avian myeloblastosis 22 virus (AMV) reverse transcriptase, 5× RT buffer, and 0.5 μg of oligo (dT)12 primer. The gene-specific primers used are listed in Table 1. Real-time PCR reactions were performed using the SYBR Green method in an ABI 7000 sequence detection system (Applied Biosystems, Foster City, CA) according to the manufacturer's guidelines. Primers were designed using the computer software Primer Express 2.0. The reactions were set by mixing 12.5 μl of the SYBR Green Master Mix with 1 μl of a solution containing 10 μM concentrations of both primers and 2 μl of cDNA solution. The Ct value was defined as the number of PCR cycles required for the fluorescence signal to exceed the detection threshold value. The relative amounts of mRNA for each gene were normalized based on the amount of the housekeeping gene β-actin.
Table 1.
The oligonucleotide sequences.
| Forward | Reverse | |
|---|---|---|
| SIRT1 | 5′-CGGATTAAAATTTGAGTTGTTTC-3′ | 5′-CCTTCCTCTTTATAACGAACGTA-3′ |
| PGC-1 | 5′-GCAACATGCTCAAGCCAAAC-3′ | 5′-TGCAGTTCCAGAGAGTTCCA-3′ |
| PPAR-γ | 5′-GCCTGTCTGTCGGGATGT-3′ | 5′-GGCTTCGTGGATTCTCTTG-3′ |
| MMP-13 | 5′-TCCCAGGAATTGGTGATAAAGTAGA-3′ | 5′-CTGGCATGACGCGAACAATA-3′ |
| COX-2 | 5′-CCCTTGGGTGTCAAAGGTAA-3′ | 5′-GCCCTCGCTTATGATCTGTC-3′ |
| β-actin | 5′-GAATTCTGGCCACGGCTGCTTCCAGCT-3′ | 5′-AAGCTTTTTCGTGGATGCCACAGGACT-3′ |
2.14. Statistical analyses
Results are expressed as the means ± SD. Statistical analyses were performed using one-way or two-way ANOVA, followed by Tukey's test as appropriate. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Homocysteine-reduced SIRT1 leads to downregulation of phosphorylated AMPK and PGC-1α
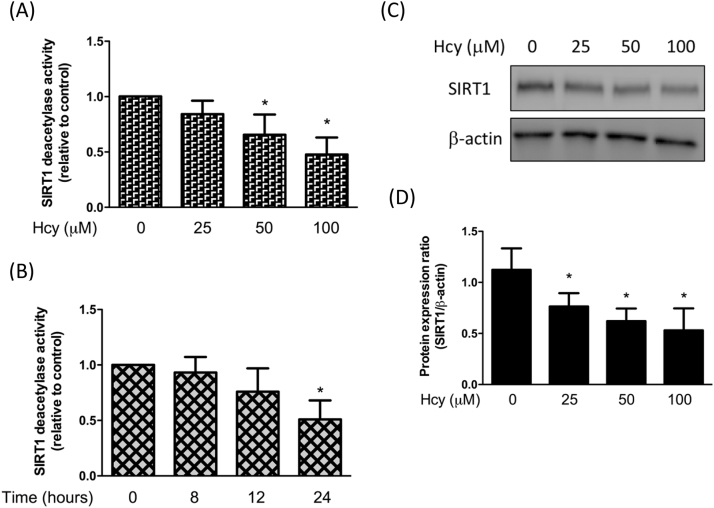
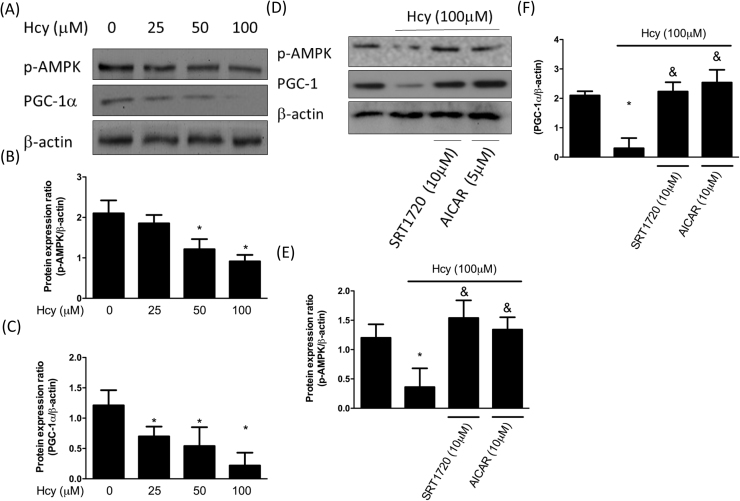
In order to investigate whether Hcy affected the deacetylase activity of SIRT1, we treated chondrocytes with various concentration of Hcy. Our result showed that Hcy dose-dependently reduced the activity of SIRT1 (Fig. 1A) and this effect was time-dependent (Fig. 1B). Also, the expression of SIRT1 was decreased by Hcy in a dose-dependent manner (Fig. 1C). SIRT1 and AMPK are two critical metabolic sensors that have been known to directly affect the activity of PGC-1α [10]. We found that the expression levels of phosphorylated AMPK and PGC-1α were both downregulated as the concentration of Hcy increased (Fig. 2A-C). However, treatment of chondrocytes with the SIRT1 activator, SRT1720, restored the phosphorylation of AMPK and PGC-1α, suggesting that SIRT1 induced the activation of AMPK/PGC-1α pathway (Fig. 2D-F). Additionally, a similar phenomenon in chondrocytes was observed in response to AICAR, an activator of AMPK (Fig. 2D-F). Altogether, these findings showed that the Hcy-inhibited SIRT1 could induce the phosphorylation of AMPK and PGC-1α.
Fig. 1.
Hcy mitigates SIRT1 expression in human chondrocytes. Human chondrocytes were treated with Hcy (100–25 μM) for 24 h (A) or treated with Hcy (100 μM) for 8, 12 or 24 h (B) followed by assessment of deacetylase activity using a commercial kit. The protein expression levels of SIRT1 (C) and quantification of the blot (D) in Hcy-stimulated chondrocytes were shown. (Data are presented as the mean ± SD of three different experiments. * p < 0.05 compared to control group).
Fig. 2.
Hcy represses AMPK and PGC-1α levels in human chondrocytes. Human chondrocytes were treated with Hcy (100–25 μM) for 24 h. The protein expression levels of AMPK and PGC-1α (A) and quantification of the blot (B,C) in Hcy-stimulated chondrocytes were shown. Human chondrocytes were treated with Hcy 100 μM for 24 h with SRT1720 or AICAR pretreatment. The protein expression levels of AMPK and PGC-1α (D) and quantification of the blot (E,F) in Hcy-stimulated chondrocytes were shown. (Data are presented as the mean ± SD of three different experiments. * p < 0.05 compared to control group; & p < 0.05 compared to Hcy-treated group).
3.2. Homocysteine-inhibited SIRT1 results in mitochondrial dysfunction
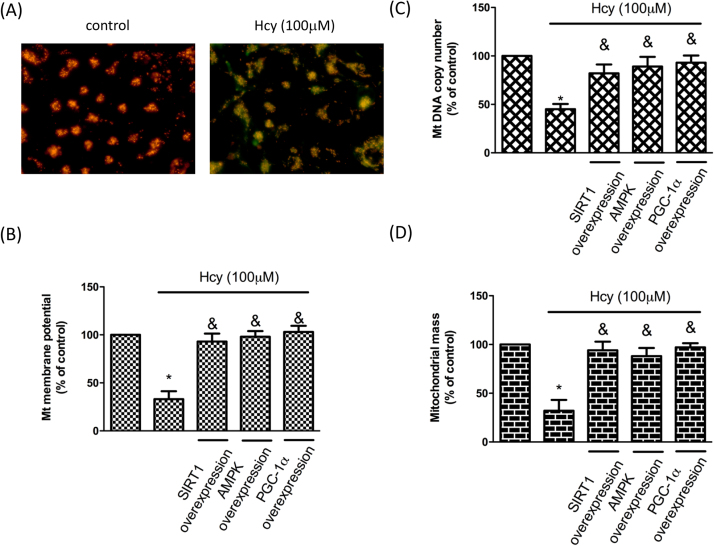
To evaluate whether Hcy-suppressed SIRT1 impaired the mitochondrial function, we first examined the mitochondria membrane potential as it related to the capacity of cells to generate ATP by oxidative phosphorylation [17] and OA chondrocytes have been found to show reduced membrane potential [1]. As shown in Fig. 3A and B, treatment of chondrocytes with Hcy significantly reduced the mitochondria membrane potential. Nevertheless, overexpression of SIRT1, AMPK or PGC-1α in chondrocytes reversed the membrane potential, indicating that SIRT1/AMPK/PGC-1α pathway involved in the Hcy-induced mitochondrial abnormity. On the other hand, it was reported that mitochondrial DNA (mtDNA) copy number was altered [18] and mtDNA content and mass were reduced [11] in OA chondrocytes. Our results showed that the mtDNA copy (Fig. 3C) and mitochondrial mass (Fig. 3D) were diminished in response to Hcy. Likewise, overexpression of SIRT1, AMPK or PGC-1α in chondrocytes eliminated the aberrant mtDNA copy and mitochondrial mass (Fig. 3C-D). These results suggested that Hcy-induced impairment of mitochondrial biogenesis was via the SIRT1/AMPK/PGC-1α pathway.
Fig. 3.
Hcy causes mitochondrial dysfunction in human chondrocytes. Cells were treated with Hcy (100 μM) for 24 h. cDNAs, including SIRT1, AMPK and PGC-1 were transfected 48 h before Hcy stimulation. (A) ΔΨm was inspected with the signal from JC-1 fluorescence, as described previously. No treatment (right); Hct-treated cells (left). (B) Results were quantified by flow cytometry. (C) The mitochondrial copy number and (D) mitochondrial mass were tested to investigate the influence of Hcy on mitochondrial biogenesis. (Data are presented as the mean ± SD of three different experiments. *p < 0.05 compared with untreated control cells. & p < 0.05 compared to Hcy-treated group).
3.3. Homocysteine-induced oxidative stress is reversed by activation of SIRT1/AMPK/PGC-1α signaling
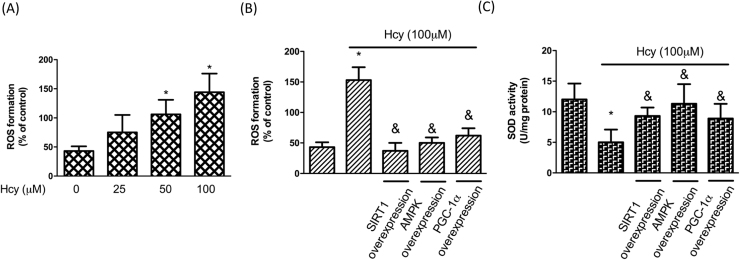
It is well-known that mitochondria consume most of the cellular oxygen and produce reactive oxygen species (ROS) as by-products. Various studies have shown that downregulation of superoxide dismutase 2 (SOD2) and upregulation of ROS following mitochondrial dysfunction contribute to the pathogenesis of OA [1], [19], [20]. In addition to mitochondrial biogenesis, we also examined the ROS production and found that ROS increased markedly after Hcy treatment whereas overexpressed SIRT1, AMPK or PGC-1α ameliorated this effect (Fig. 4A). Besides, the reduced expression of SOD2 in response to Hcy was also restored by overexpression of SIRT1, AMPK or PGC-1α (Fig. 4B). Accordingly, we demonstrated that the increased oxidative stress by Hcy in chondrocytes was mediated by SIRT1/AMPK/PGC-1α signaling pathway.
Fig. 4.
Hcy induces oxidative stress in human chondrocytes. Cells were treated with Hcy (100 μM) for 24 h. cDNAs of SIRT1, AMPK and PGC-1 were transfected for 48 h before Hcy stimulation. (B, C) (A) Fluorescent intensity of cells was measured using a fluorescence microplate reader to examine ROS concentrations. (B) Fluorescence distribution of MitoSOX is expressed as a percentage of increased intensity to quantify ROS levels in cells with Hcy. (C) SOD activity was tested using a SOD activity kit. (Data are presented as the mean ± SD of three different experiments. *p < 0.05 compared with untreated control cells. & p < 0.05 compared to Hcy-treated group).
3.4. Homocysteine-induced pro-apoptosis effect is rescued by overexpression of SIRT1/AMPK/PGC-1α cascades
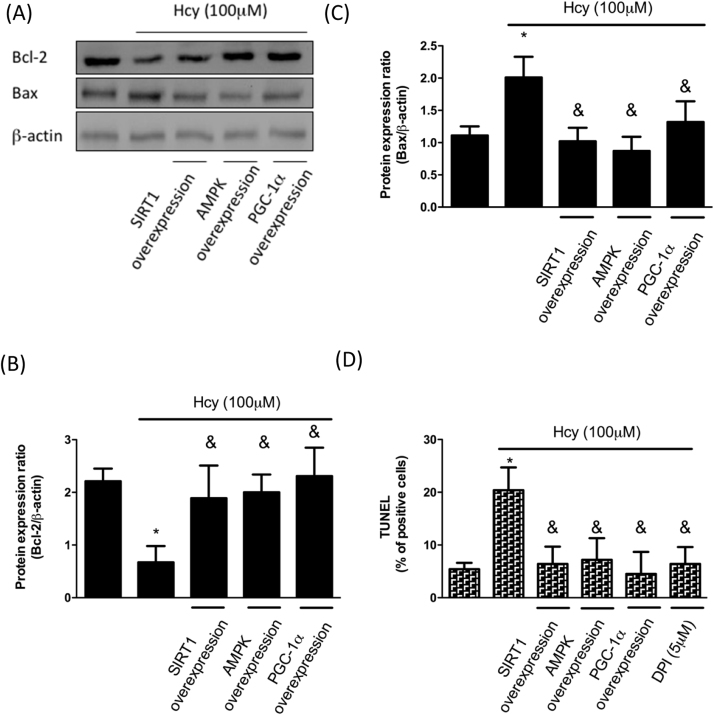
It was reported that OA cartilage contains a high number of apoptotic chondrocytes and the increased expression of caspase-3 and -7 as well as the reduced Bcl-2 were observed following NO induction [1]. Bcl-2, an anti-apoptotic protein, prevents mitochondrial permeabilization and block cytochrome c release. As shown in Fig. 5A and B, the expression of Bcl-2 was downregulated in Hcy-treated chondrocytes, but it was reverted by overexpression of SIRT1, AMPK or PGC-1α. On the other hand, the expression of Bax exhibited the opposite pattern of change (Fig. 5A and C). These results showed that treatment of Hcy initiated the pro-apoptosis response in chondrocytes. Therefore, we performed the TUNEL assay to confirm our finding. There was a higher percentage of positive cells following Hcy treatment, while upregulation of SIRT1, AMPK or PGC-1α prevented the increased apoptotic rate (Fig. 5D). DPI has been commonly used to reduce ROS generation, and our data revealed that DPI incubation repressed the elevated percentage of apoptotic cells (Fig. 5D), indicating that Hcy-induced oxidative stress was responsible for the pro-apoptosis effect and it was coordinated by a network involving SIRT1, AMPK, and PGC-1α.
Fig. 5.
Hcy increases apoptosis in human chondrocytes. Cells were treated with Hcy (100 μM) for 24 h. For overexpression, cDNAs of SIRT1, AMPK and PGC-1 were transfected for 48 h before Hcy stimulation. (A-C) Representative Western blots and quantification data showing that Hcy treatment upregulated pro-apoptotic protein (Bax) and downregulated anti-apoptotic (Bcl-2) protein as a result of inhibition of SIRT1/AMPK/PGC-1α. (D) In DPI intervention group, DPI was pretreated for 1 h before Hcy stimulation. A TUNEL assay was used for investigation of apoptosis. (Data are presented as the mean±SD of three different experiments. *p < 0.05 compared with untreated control cells. & p < 0.005 compared to Hcy-treated group).
3.5. Expression levels of pro-inflammatory mediators and MMP13 are elevated subsequent to suppression of PPAR-γ by homocysteine
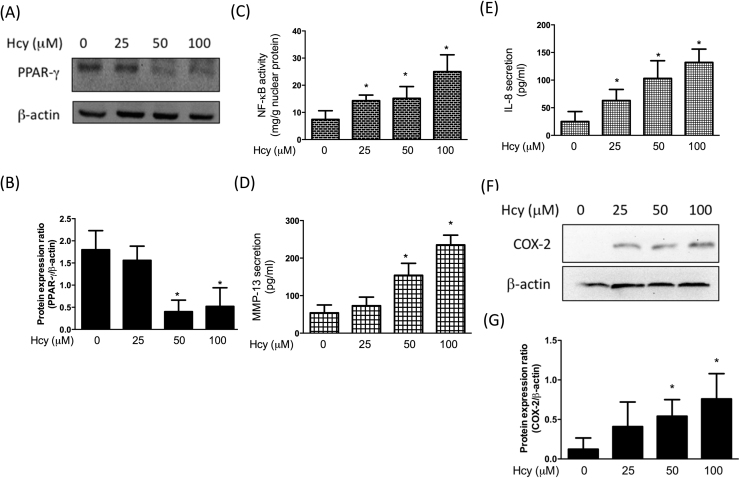
In our previous study, we have found that mitigation of chondroprotective PPAR-γ resulted in matrix metalloproteinases (MMP)-13 upregulation in OA chondrocytes [21]. Furthermore, we demonstrated that upregulation of two important mediators of OA pathogenesis, cyclooxygenase (COX)-2 and IL-8, was associated with NF-κB activation [21]. In an effort to determine whether Hcy triggered inflammation and MMP-13 in human chondrocytes, we assessed the expression of these factors after addition of Hcy. As expected, we observed that PPAR-γ was downregulated (Fig. 6A and B) along with increased NF-κB (Fig. 6C), MMP-13 (Fig. 6D), IL-8 (Fig. 6E) in Hcy-treated cells. In addition, Hcy stimulation also elevated intracellular COX-2 expression levels using Western blotting assay (Fig. 6F). These data indicated that the impaired PPAR-γ expression in Hcy-stimulated chondrocytes may lead to increased MMP-13 and be associated with NF-κB, IL-8, and COX-2.
Fig. 6.
Hcy induces inflammation in human chondrocytes. Cells were treated with Hcy (100 μM) for 24 h. (A-B) Representative Western blots and quantification data showing that Hcy treatment downregulated PPAR-γ expression. (C) NF-κB activity was tested using a NF-κB activity kit. (D-E) MMP-13 and IL-8 levels were tested by ELISA assay. (F, G) COX-2 levels were tested by Western blotting assay. (Data are presented as the mean ± SD of three different experiments. *p < 0.05 compared with untreated control cells. & p < 0.05 compared to Hcy-treated group).
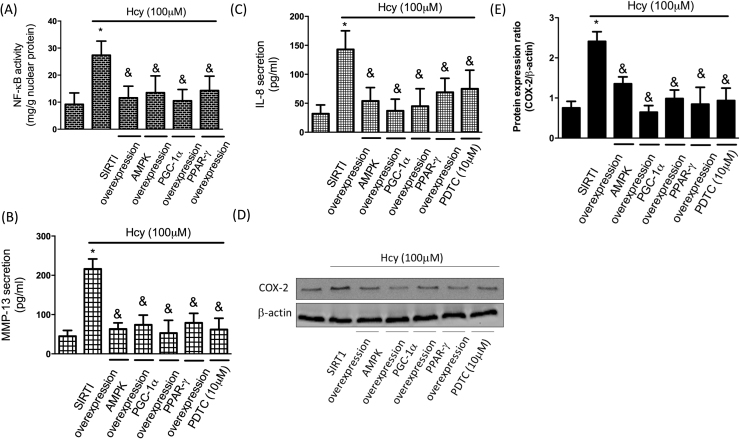
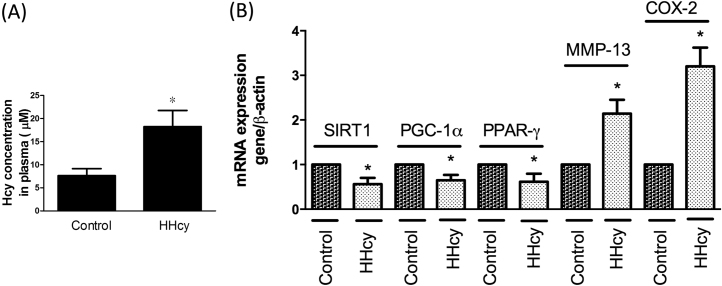
To clarify whether SIRT1/AMPK/PGC-1α pathway was implicated in the augmented expression of pro-inflammatory mediators and MMP-13 following Hcy addition, we examined their expression levels after overexpression of SIRT1/AMPK/PGC-1α. We found that upregulation of SIRT1, AMPK, PGC-1α, or PPAR-γ mitigated Hcy-caused NF-κB activation (Fig. 7A), suggesting this pathway was involved in this inflammatory response. To ascertain that NF-κB contributed to the MMP-13 upregulation and inflammatory mediators’ secretion, we employed an inhibitor of NF-κB, pyrrolidine dithiocarbamate (PDTC), in the Hcy-treated chondrocytes. As anticipated, suppression of NF-κB prevented the increase in MMP-13 (Fig. 7B), IL-8 (Fig. 7C). Moreover, inhibition of NF-κB or up-regulation of SIRT1, AMPK, PGC-1α, PPAR-γ reduced intracellular COX-2 expression (Fig. 7D). Furthermore, we conducted the animal study to support our hypothesis. In Fig. 8A, the plasma Hcy levels were elevated after methionine feeding, suggesting that the animal model of HHcy was successfully induced. In addition, we confirmed these findings using real-time PCR and showed that SIRT1/PGC-1α/PPAR-γ were indeed downregulated, whereas MMP-13 and COX-2 were upregulated in chondrocytes from HHcy animals (Fig. 8B). Our results demonstrated that the elevated expression of MMP-13, COX-2, and IL-8 by the increased NF-κB was modulated by the SIRT1/AMPK/PGC-1α/PPAR-γ signaling.
Fig. 7.
Hcy causes inflammation in human chondrocytes by modulation of SIRT1/AMPK/PGC-1/PPAR-γ/NF-κB pathway Cells were treated with Hcy (100 μM) for 24 h. In PDTC intervention group, PDTC was pretreated for 1 h before Hcy stimulation. (A) NF-κB activity was tested using a NF-κB activity kit. (B, C) MMP-13 and IL-8 levels were tested by ELISA assay. (D, F) COX-2 levels were tested by Western blotting assay. (Data are presented as the mean± SD of three different experiments. *p 0 < 0.05 compared with untreated control cells. & p < 0.05 compared to Hcy-treated group).
Fig. 8.
Expression levels of SIRT1, PGC-1, PPAR-γ, MMP-13, COX-2 in HHcy animals. A total of 12 C57BL mice (6 in each group) were used in this study. The animals were fed with or without 1% L-methionine (w/w) in water for 4 months to induce HHcy. (A) The plasma Hcy levels in the control and HHCy animals. (B) Tissues from the control and the HHcy cartilage were collected for RNA expression test. SIRT1, PGC-1, PPAR-γ, MMP-13, COX-2 were tested by real-time PCR assay. (Data are presented as the mean ± SD of three different experiments. *p < 0.05 compared with untreated control.).
4. Discussion
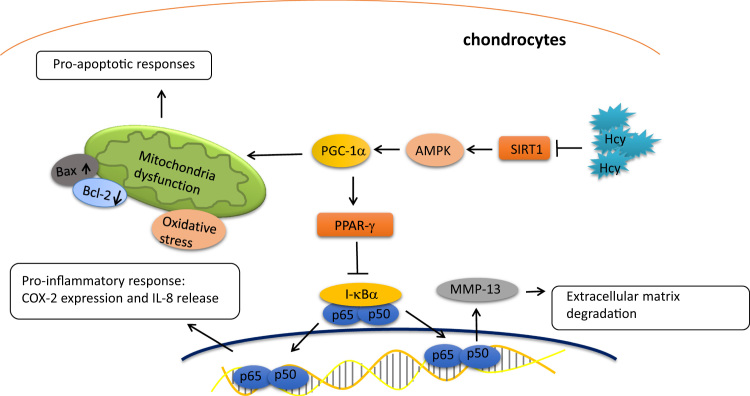
Hcy is a naturally occurring amino acid derived from methionine metabolism. Dysregulation of methyl group metabolism or homocysteine imbalance have emerged as critical contributing factors in a number of pathological conditions [22]. In fact, various studies have revealed that the abnormality of Hcy was associated with several orthopedic disorders, such as osteoporosis [23] and fractures [24]. Hcy in bone was bound to collagen of the extracellular matrix, resulting in reduced bone strength in diet-induced hyperhomocysteinemia [25]. It has been known that Hcy treatment caused matrix disorganization and impaired cartilage calcification [8] as well as augmented osteoclast activity through increased generation of intracellular ROS [26]. In the current study, we found that Hcy induced numerous detrimental changes in chondrocytes that may lead to osteoarthritis (OA), including mitochondrial dysfunction, apoptosis, oxidative stress accumulation, increased expression of COX-2, IL-8, and MMP-13. Besides, we demonstrated that these effects were due to Hcy-induced downregulation of SIRT1 (Fig. 9).
Fig. 9.
Schematic diagram of the major findings in this study.
The pathogenesis of OA has been attributed to dysregulation of mitochondrial biogenesis [1], [2], [3], and it is well-known that SIRT1 and its substrate, PGC-1α, regulate aspects of energy metabolism through the mitochondria [17]. Accumulating evidence has revealed the significance of SIRT1 in contributing mitochondria dysfunction and thereafter OA progression. It has been proven that SIRT1 enzymatic activity was required for cartilage homeostasis [20] and SIRT1 knockout mice exhibited an altered cartilage phenotype with elevated apoptosis and MMP-13 expression, leading to a potential increase of cartilage breakdown [19]. Another study showed that SIRT1+ chondrocytes decreased during aging, and loss of SIRT1 in chondrocytes also led to increase in MMP-13, apoptotic markers, and NF-κB, resulting in the accelerated development of OA [18]. Apart from MMP-13, apoptosis is another feature of OA cartilage [27], [28], and chondrocyte apoptosis was found to be positively associated with the degree of cartilage damage [29]. The role of SIRT1 in the regulation of apoptosis in chondrocyte was investigated by Takayama et al. [30]. Their data showed that inhibition of SIRT1 increased the percentage of TUNEL-positive cells and the cleaved PARP accompanying with elevated Bax and decreased Bcl expression. It is known that Bcl-2 family regulate mitochondrial membrane permeability and activate caspase proteases to initiate apoptosis [31]. Takayama et al. demonstrated that SIRT1 was essential to orchestrate the mitochondria-related apoptotic signals. SIRT1 also has been shown to repress a potent pro-apoptotic protein, PTP1B, which was elevated in OA cartilage [21]. In line with these studies, our results revealed that Hcy-reduced SIRT1 elicited mitochondrial dysfunction (Fig. 3) and increased apoptosis (Fig. 5) in chondrocytes. Furthermore, we demonstrated that this impairment was mediated by SIRT1/AMPK/PGC-1α pathway.
In OA chondrocytes, the deficient mitochondrial biogenesis is present along with reduced expression of SIRT-1, AMPK PGC-1α [1]. Our results revealed that Hcy dose-dependently inhibited the expression of SIRT-1/AMPK/PGC-1α signaling in chondrocytes and reversal of this pathway successfully rescued the mitochondrial dysfunction, suggesting that elevation of Hcy may be a risk factor that leads to OA. In addition to the increased apoptosis, we also showed that Hcy induced excessive oxidative stress as a result of mitochondrial abnormality. Various studies have indicated that oxidative stress made chondrocytes more susceptible to external damage and prone to senescence [32], [33]. Various studies have indicated that the expression of SIRT1 was tightly associated with oxidative stress resistance. Pfluger et al. have shown that SIRT1 induced antioxidant proteins SOD2 and Nrf1 via stimulation of PGC1α [34]. Another study demonstrated that SIRT1 increased SOD2, whereas knockdown of SIRT1 elevated the ROS levels in muscle cells [35]. In associated with these findings, we showed that reduced SIRT1 by Hcy led to downregulation of SOD2 and increased ROS formation via AMPK/PGC-1α signaling.
Meanwhile, work from Pfluger et al. showed that SIRT1 lowered the activation of pro-inflammatory cytokines via down-modulation of NF-κB activity [34]. In fact, a variety of studies have indicated that SIRT1 reduced inflammatory cytokines and the associated response in chondrocytes. Upregulation of SIRT1 has been demonstrated to inhibit TNF-α-induced COX-2, MMP-13 and NF-κB activation [36]. Overexpression of SIRT1 decreased the IL-1β-induced upregulation of MMP- 13 and acetylation of NF-κB p65 [14]. In accordance with these findings, we demonstrated that insufficient SIRT1 resulted in elevated expression of pro-inflammatory cytokines, COX-2 and IL-8, as well as NF-κB. As our previous study has shown that upregulation COX-2 and IL-8 was associated with NF-κB activation in OA chondrocytes [21], the results of this study suggested that Hcy-reduced SIRT1 may be implicated in OA pathogenesis. Another finding in our previous work was that the repressed PPAR-γ resulted in MMP-13 upregulation in OA chondrocytes [21]. Loss of cartilage matrix is accompanied by an increase in MMP-13, which is responsible for degradation of type II collagen. It has been shown that overexpression of SIRT1 during IL-1β challenge impeded the MMP-13 gene expression in chondrocytes [22]. In the present study, we found that elevation of Hcy may be another cause for upregulated MMP-13. Collectively, our data suggested that Hcy-reduced SIRT1 elevated the pro-inflammatory cytokines, NF-κB, and MMP-13, which may lead to initiation and progression of OA.
In this study, we demonstrated that downregulation of SIRT1/AMPK/PGC-1α as a result of the elevated Hcy in chondrocytes gave rise to mitochondrial dysfunction with the increased pro-apoptotic response and oxidative stress. Moreover, the aberrant SIRT1/AMPK/PGC-1α signaling caused the diminished PPAR-γ along with increased NF-κB, thereby increasing the expression of MMP13 and pro-inflammatory IL-8 and COX-2 in Hcy-treated chondrocytes. Overall, we showed that Hcy was another contributing factor that induced impaired mitochondrial function and caused an increase in ROS production and apoptosis in chondrocytes. Our findings also revealed that the reduced SIRT1 by Hcy could possibly contribute to degradative cartilage process and provide insight into the etiology of OA.
Acknowledgments
This study was supported by grants from National Cheng Kung University and E-Da Hospital (NCKUEDA10613). E-Da Hospital (EDCHP106002). This study was also supported by grants from the Ministry of Science and Technology (MOST 105-2311-B-006-008 and 106-2314-B-006-023).
Acknowledgments
Conflict of interest
None.
Contributor Information
Pei-Ling Hsieh, Email: akinosha@hotmail.com.
Kun-Ling Tsai, Email: kunlingtsai@mail.ncku.edu.tw.
References
- 1.Blanco F.J., Lopez-Armada M.J., Maneiro E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion. 2004;4(5–6):715–728. doi: 10.1016/j.mito.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Maneiro E., Martin M.A., de Andres M.C., Lopez-Armada M.J., Fernandez-Sueiro J.L., del Hoyo P., Galdo F., Arenas J., Blanco F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48(3):700–708. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- 3.Blanco F.J., Rego I., Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011;7(3):161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 4.Kalani A., Kamat P.K., Voor M.J., Tyagi S.C., Tyagi N. Mitochondrial epigenetics in bone remodeling during hyperhomocysteinemia. Mol. Cell. Biochem. 2014;395(1–2):89–98. doi: 10.1007/s11010-014-2114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharjee N., Borah A. Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson's disease. Neurochem. Int. 2016;101:48–55. doi: 10.1016/j.neuint.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Chen S., Dong Z., Zhao Y., Sai N., Wang X., Liu H., Huang G., Zhang X. Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci. Rep. 2017;7(1):6932. doi: 10.1038/s41598-017-07112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vacek T.P., Kalani A., Voor M.J., Tyagi S.C., Tyagi N. The role of homocysteine in bone remodeling. Clin. Chem. Lab. Med. 2013;51(3):579–590. doi: 10.1515/cclm-2012-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M., Yamauchi M., Srisawasdi S., Stiner D., Doty S., Paschalis E.P., Boskey A.L. Homocysteine decreases chondrocyte-mediated matrix mineralization in differentiating chick limb-bud mesenchymal cell micro-mass cultures. Bone. 2001;28(4):387–398. doi: 10.1016/s8756-3282(01)00409-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu-Bryan R. Inflammation and intracellular metabolism: new targets in OA. Osteoarthr. Cartil. 2015;23(11):1835–1842. doi: 10.1016/j.joca.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canto C., Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Zhao X., Lotz M., Terkeltaub R., Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor gamma coactivator 1alpha. Arthritis Rheumatol. 2015;67(8):2141–2153. doi: 10.1002/art.39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita N., Matsushita T., Ishida K., Kubo S., Matsumoto T., Takayama K., Kurosaka M., Kuroda R. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 2011;29(4):511–515. doi: 10.1002/jor.21284. [DOI] [PubMed] [Google Scholar]
- 13.Gagarina V., Gabay O., Dvir-Ginzberg M., Lee E.J., Brady J.K., Quon M.J., Hall D.J. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62(5):1383–1392. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita T., Sasaki H., Takayama K., Ishida K., Matsumoto T., Kubo S., Matsuzaki T., Nishida K., Kurosaka M., Kuroda R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 2013;31(4):531–537. doi: 10.1002/jor.22268. [DOI] [PubMed] [Google Scholar]
- 15.Elayyan J., Lee E.J., Gabay O., Smith C.A., Qiq O., Reich E., Mobasheri A., Henrotin Y., Kimber S.J., Dvir-Ginzberg M. LEF1-mediated MMP13 gene expression is repressed by SIRT1 in human chondrocytes. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2017;31(7):3116–3125. doi: 10.1096/fj.201601253R. [DOI] [PubMed] [Google Scholar]
- 16.Yui N., Yoshioka H., Fujiya H., Musha H., Beppu M., Karasawa R., Yudoh K. The DNA repair enzyme apurinic/apyrimidinic endonuclease (Apex nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis. Int. J. Mol. Sci. 2014;15(9):14921–14934. doi: 10.3390/ijms150914921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson L.V., Walsh M.L., Bockus B.J., Chen L.B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 1981;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang H., Liu X., Shen L., Li F., Liu Y., Chi H., Miao H., Lu J., Bai Y. Role of mtDNA haplogroups in the prevalence of knee osteoarthritis in a southern Chinese population. Int. J. Mol. Sci. 2014;15(2):2646–2659. doi: 10.3390/ijms15022646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavriilidis C., Miwa S., von Zglinicki T., Taylor R.W., Young D.A. Mitochondrial dysfunction in osteoarthritis is associated with down-regulation of superoxide dismutase 2. Arthritis Rheum. 2013;65(2):378–387. doi: 10.1002/art.37782. [DOI] [PubMed] [Google Scholar]
- 20.Scott J.L., Gabrielides C., Davidson R.K., Swingler T.E., Clark I.M., Wallis G.A., Boot-Handford R.P., Kirkwood T.B., Taylor R.W., Young D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010;69(8):1502–1510. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma C.H., Wu C.H., Jou I.M., Tu Y.K., Hung C.H., Hsieh P.L., Tsai K.L. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox Biol. 2018;14:72–81. doi: 10.1016/j.redox.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schalinske K.L., Smazal A.L. Homocysteine imbalance: a pathological metabolic marker. Adv. Nutr. 2012;3(6):755–762. doi: 10.3945/an.112.002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Meurs J.B., Dhonukshe-Rutten R.A., Pluijm S.M., van der Klift M., de Jonge R., Lindemans J., de Groot L.C., Hofman A., Witteman J.C., van Leeuwen J.P., Breteler M.M., Lips P., Pols H.A., Uitterlinden A.G. Homocysteine levels and the risk of osteoporotic fracture. N. Engl. J. Med. 2004;350(20):2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 24.Yang J., Hu X., Zhang Q., Cao H., Wang J., Liu B. Homocysteine level and risk of fracture: a meta-analysis and systematic review. Bone. 2012;51(3):376–382. doi: 10.1016/j.bone.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann M., Tami A., Wildemann B., Wolny M., Wagner A., Schorr H., Taban-Shomal O., Umanskaya N., Ross S., Garcia P., Hubner U., Herrmann W. Hyperhomocysteinemia induces a tissue specific accumulation of homocysteine in bone by collagen binding and adversely affects bone. Bone. 2009;44(3):467–475. doi: 10.1016/j.bone.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 26.Koh J.M., Lee Y.S., Kim Y.S., Kim D.J., Kim H.H., Park J.Y., Lee K.U., Kim G.S. Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 2006;21(7):1003–1011. doi: 10.1359/jbmr.060406. [DOI] [PubMed] [Google Scholar]
- 27.Heraud F., Heraud A., Harmand M.F. Apoptosis in normal and osteoarthritic human articular cartilage. Ann. Rheum. Dis. 2000;59(12):959–965. doi: 10.1136/ard.59.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharif M., Whitehouse A., Sharman P., Perry M., Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004;50(2):507–515. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- 29.Thomas C.M., Fuller C.J., Whittles C.E., Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthr. Cartil. 2007;15(1):27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Takayama K., Ishida K., Matsushita T., Fujita N., Hayashi S., Sasaki K., Tei K., Kubo S., Matsumoto T., Fujioka H., Kurosaka M., Kuroda R. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60(9):2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 31.Wang C., Youle R.J. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yudoh K., Nguyen v T., Nakamura H., Hongo-Masuko K., Kato T., Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. Ther. 2005;7(2):R380–R391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlo M.D., Jr., Loeser R.F. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 34.Pfluger P.T., Herranz D., Velasco-Miguel S., Serrano M., Tschop M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanno M., Kuno A., Yano T., Miura T., Hisahara S., Ishikawa S., Shimamoto K., Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010;285(11):8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon M.H., Jeong J.K., Lee Y.J., Seol J.W., Jackson C.J., Park S.Y. SIRT1, a class III histone deacetylase, regulates TNF-alpha-induced inflammation in human chondrocytes. Osteoarthr. Cartil. 2013;21(3):470–480. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]