Abstract
Cisplatin is a major chemotherapeutic drug for solid tumors whereas it may lead to severe nephrotoxicity. Despite decades of efforts, effective therapies remain largely lacking for this disease. In the current research, we investigated the therapeutic effect of hydrogen polysulfide, a novel hydrogen sulfide (H2S) derived signaling molecule, in cisplatin nephrotoxicity and the mechanisms involved. Our results showed that polysulfide donor Na2S4 ameliorated cisplatin-caused renal toxicity in vitro and in vivo through suppressing intracellular reactive oxygen species (ROS) generation and downstream mitogen-activated protein kinases (MAPKs) activation. Additionally, polysulfide may inhibit ROS production by simultaneously lessening the activation of NADPH oxidase and inducing nucleus translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) in RPT cells. Interestingly, polysulfide possesses anti-cancer activity and is able to add on more anti-cancer effect to cisplatin in non-small cell lung cancer (NSCLC) cell lines. Moreover, we observed that the number of sulfur atoms in polysulfide well reflected the efficacy of these molecules not only in cell protection but also cancer inhibition which may serve as a guide for further development of polysulfide donors for pharmaceutical usage. Taken together, our study suggests that polysulfide may be a novel and promising therapeutic agent to prevent cisplatin-induced nephrotoxicity.
Keywords: Polysulfide, Hydrogen sulfide, Cisplatin nephrotoxicity, Reactive oxygen species, NADPH oxidase, Nuclear factor erythroid 2-related factor 2
Graphical abstract
Highlights
-
•
Polysulfide ameliorates cisplatin-caused renal toxicity in vitro and in vivo.
-
•
Polysulfide suppresses cisplatin-induced ROS generation in renal cells.
-
•
Polysulfide inhibits cisplatin-induced activation of NADPH oxidase.
-
•
Polysulfide induces nucleus translocation of Nrf2.
1. Introduction
Cisplatin is a widely used chemotherapeutic drug for solid tumors arising from multiple organs such as head and neck, testicular, cervical, ovaries, lung and bladder; however, clinical studies have revealed that cisplatin usage is accompanied with severe adverse effects including nephrotoxicity, ototoxicity and neurotoxicity [1]. Among these side effects, cisplatin-induced neurotoxicity is most severe and prevalent as evidence shows that over 30% of patients show symptoms of acute kidney injury (AKI) following the administration of cisplatin [2].
Cisplatin nephrotoxicity is characterized with massive renal proximal tubular (RPT) cell death, consisting of both necrosis and apoptosis [3]. As a result, renal insufficiency begins as manifested by increases of serum creatinine and blood urea nitrogen levels several days after the administration of cisplatin, along with a reduction of serum magnesium and potassium levels [4]. Oxidative stress has long been recognized as an important factor contributing to cisplatin-induced RPT cell death [5]. Numerous studies have observed the massive production of reactive oxygen species (ROS) upon cisplatin treatment in cultured renal tubular cells, kidney slices, and in vivo animals [6], [7]. Further studies have suggested that cisplatin-induced activation of NADPH oxidase contributes to the pathophysiology as pharmacological inhibition of NADPH oxidase protects renal cells in cultured proximal tubule cells and in vivo animals [1], [8], [9], [10]. On the other hand, whether cisplatin stimulates the production of mitochondrial ROS remains controversial in RPT cells [11], [12].
Polysulfide is a category of chemical compounds comprising chains of sulfur atoms. In mammalian system, polysulfide can be generated from hydrogen sulfide (H2S), an endogenous gasotransmitter, as described in the following equation: 2nH2S + 1/2 (2n-1)O2 → H2S2n + (2n-1)H2S in the presence of oxygen [13]. Interestingly, besides directly derived from H2S, Kimura and others [14] demonstrated that they are also generated by H2S producing enzyme 3-mercaptopyruvate sulfurtransferase (3MST), implying its possible physiological importance. Although the biological functions of polysulfide are not fully acknowledged, existing evidence shows that polysulfide may possess various biological effects similar to H2S. For example, Nagai et al. [15] found that polysulfide was able to activate TRPV channels more potently than H2S does. Subsequently, Oosumi and others [16] determined that Cysteine 422 and Cysteine 622 in the TRPV 1 channel were sensitive to polysulfide. The anti-oxidant effect of polysulfide was also studied recently. Koike and colleagues [17] reported that polysulfide exhibited protective effects against cytotoxicity caused by oxidative stress in neuroblastomaSH-SY5Y cells. They also showed that polysulfide may activate the translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) into nucleus by dimerizing Kelch-like ECH-associated protein 1 (keap1) and as a result facilitate the expression of anti-oxidant genes [17]. Therefore, we hypothesized that polysulfide may prevent cisplatin nephrotoxicity by attenuating ROS generation. Besides, the effect of polysulfide on the anti-cancer activity of cisplatin was also examined in non-small cell lung cancer cell (NSCLCC) lines.
2. Materials and methods
2.1. Reagents and antibodies
N-acetyl-cysteine (NAC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), 2,7-dichlorofluorescein diacetate (DCFH-DA), Hoechst 33342, propidium iodide (PI) were purchased from Sigma-Aldrich (St Louis, MO. USA). Polysulfide donors including Na2S2, Na2S3 and Na2S4 were obtained from Dojindo Molecular Technologies Dojindo (Kumamoto, Japan). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), streptomycin/penicillin and trypsin were obtained from Hyclone Laboratories (South Logan, UT.USA). The RIPA buffer was purchased from ThermoFisher Scientific Inc (Waltham, MA. USA). The Bradford colorimetric protein assay kit (Rockford, IL. USA) was used for protein quantification. The antibody for p-p47phox was from ThermoFisher Scientific Inc (Waltham, MA. USA). All the other antibodies were obtained from Cell Signaling Technology (Beverly, MA. USA). All other chemical reagents were purchased from Sigma-Aldrich (St. Louis,MO) unless otherwise stated.
2.2. Cell culture
The porcine RPT cell line namely LLC-PK1 was purchased from ATCC (Rockville, MA. USA) and cultured in DMEM supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 1% penicillin (100 U)/streptomycin (100 mg/mL) in a humidified atmosphere of 95% air and 5% CO2 at 37 ℃. The monolayer cells were deprived from FBS for about 18 h prior to experiments.
2.3. MTT assay
The cell viability was tested by MTT reduction assay as previously described [18]. For MTT reduction assay, 0.5 mg/mL MTT was incubated with treated cells for 4 h after which the formazon crystals were dissolved with DMSO. The absorbance was measured at 570 nm with a Varioskan Flash microplate reader (Waltham, MA. USA).
2.4. Hoechst 33342/propidium iodide staining
Hoechst 33342/propidium iodide (PI) staining was performed in 96 well plates. After treatment, the cells were incubated with phenol red free DMEM containing with 5 µg/mL Hoechst 33342 and 15 µg/mL PI for 15 min at 37 °C. The images were taken in Cytation 3 imaging reader (BioTek, VT. USA).
2.5. Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) assay was performed with a commercial kit from Sigma-Aldrich (St Louis, MO. USA) according to the manufacturer's instruction. Briefly, 50 µL medium after treatment was mixed with LDH detection reagents. After incubation, the absorbance was measured at 450 nm.
2.6. Western blot assay
Renal cortical tissue and cell samples were lysed with RIPA buffer containing phosphatase and protease inhibitors. The protein content was measured using BCA colorimetric protein kit. Equal amount of protein were separated with 12% SDS-PAGE and transferred onto a PVDF membrane. After blocking with 10% nonfat milk, the membranes were incubated with primary antibody overnight with mild shake at 4 °C. Then the membrane was washed for 3 times with TBST buffer followed by 1 h incubation with horseradish peroxidase-conjugated secondary antibody. The immunoblots were visualized with ECL Western blotting substrate. Protein bands were normalized with non-phosphorylated form of proteins or β-actin.
2.7. NADPH oxidase activity assay
The activity of NADPH oxidase was measured as described previously [19]. Briefly, LLC-PK1 cells were washed with ice-cold PBS and then homogenized in KH2PO4 buffer (20 mM, pH 7.1) containing 1 mM EGTA and protease inhibitors. After centrifugation (800g, 10 min, 4 ℃), 50 µL homogenates were added to 150 µL of 50 mM phosphate buffer (pH 7.0), 1 mM EGTA, 150 mM sucrose, 50 µM lucigenin, and 100 µM NADPH in the presence or absence of 200 µM apocynin. Photon emission from lucigenin was measured every 30 s for 5 min in a luminometer. No NADPH oxidase activity was measured in the presence of 200 µM apocynin which was subtracted from the corresponding value in the absence of apocynin. The data were converted to relative light unites/min/mg of protein. NADPH oxidase activity of control cells was arbitrarily set at 100%. The protein content was measured using BCA colorimetric protein kit.
2.8. Nuclear protein extraction
The nuclear protein was extracted with NE-PER Nuclear and Cytoplasmic Extraction Reagents (ThermoFisher) according to the manufacture's instruction. Briefly, ice-cold CER I and CER II were used to extract the cytoplamsic proteins. Subsequently, NER reagent was employed to extract the nuclear proteins. The ratio of CER I: CER II: NER was maintained at 200:11:100 µL respectively during the experiment.
2.9. Intracellular ROS measurement
The intracellular ROS was measured by a fluorescence dye CM-H2DCFDA in 96-well plate. After treatment, cells were washed with PBS and then incubated with 100 μL of DCFH-DA (10 µM, dissolved in phenol red-free DMEM) for 30 min at 37 ℃. The fluorescence intensity was detected with excitation and emission wavelengths of 485 nm and 535 nm in a Varioskan Flash microplate reader (Waltham, MA. USA).
2.10. Measurement of plasma creatinine and blood urea nitrogen
The kits used for the measurement of plasma creatinine and blood urea nitrogen (BUN) were obtained from BioAssay Systems (Hayward, CA. USA). The experiments were performed according to the manufacturer's instruction. The levels of plasma creatinine and BUN were normalized with control groups.
2.11. TUNEL staining
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling assay (TUNEL assay) was performed using an in situ cell death detection kit (Roche, Penzberg. Germany) according to the manufacturer's instructions. Briefly, tissue taken from kidneys was fixed and embedded in paraffin and 4-μm sections were prepared. After dewax and rehydrate, sections were stained with terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling solution and incubated at 37 °C for 1 h, washed twice with phosphate-buffered saline, and mounted with Prolong Gold antifade solution containing DAPI. The amount of DNA fragmentation was visualized using a confocal laser scanning fluorescence microscope (Leica, Heidelberg, Germany). Nuclear counterstaining (blue) was performed using DAPI. Positive and negative controls were treated with 0.1 mg/mL pancreatic DNase I or labeling respectively. The number of TUNEL positive nuclei was determined by examining six randomly selected microscopic fields from each experimental group. The staining was analyzed in a blinded fashion.
2.12. Morphology analysis
The kidney tissues obtained from rats were sliced into tissue pieces, and immersed in 4% paraformaldehyde overnight. Fixed tissues were then dehydrated, cleared, and embedded in paraffin wax. The tissues were cut into 4 µm sections and stained with hematoxylin and eosin (H&E) for morphology analysis. Sections were examined by microscopy and images were acquired using Olympus software.
2.13. Animal models
All protocols employing animals were conducted in accordance with the principles and guidance of Institutional Animals Care and Use Committee at National of University of Singapore. The therapeutic effects of Na2S4 were evaluated in rat model of cisplatin nephrotoxicity. Specifically, total 28 SD rats (200–220 g) were employed in the study. The rats were divided into 4 groups including control group (n=6), cisplatin group (n=8), Na2S4 + cisplatin group (n=8), Na2S4 group (n=6). Na2S4 (5.6 mg/kg, in saline) were administrated by i.p. injection every 24 h for a total of 7 times. Cisplatin (7 mg/kg) was given by a single intraperitoneal (i.p.) injection 30 min after the second administration of Na2S4. Rats from control group and cisplatin group were given with saline instead of Na2S4. All the rats were sacrificed 24 h after the last dose of Na2S4. Serum and kidney were collected when the animals were sacrificed.
2.14. Statistical analysis
All data were presented as mean ± SEM of at least three independent biological replicates with duplicate technical replicates. The number of independent assays in each experiment is described in each figure legend. Statistical analysis was performed using one-way analysis of variance (ANOVA). Differences with a p value less than 0.05 were considered to be statistically significant.
3. Results
3.1. Polysulfide attenuates cisplatin-induced RPT cell death
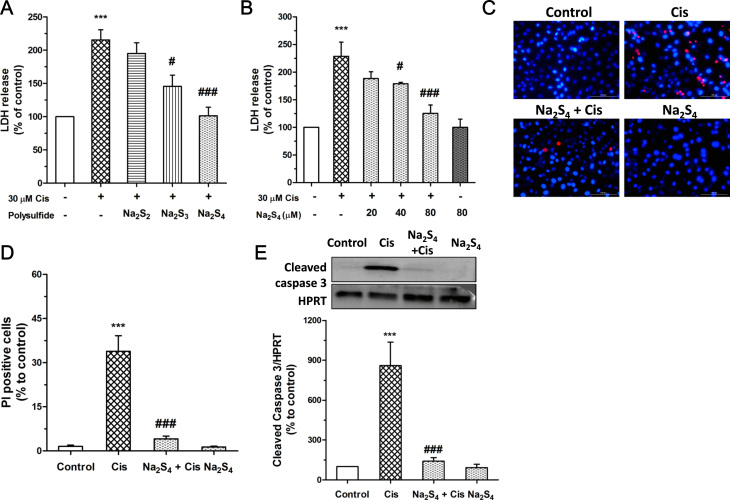
The protective effect of polysulfide was first examined within RPT cells. Our data showed that at a concentration of 80 µM sodium tetrasulfide (Na2S4) almost completely abolished cisplatin-caused LDH release from RPT cells which was slightly reduced by sodium trisulfide (Na2S3) and hardly influenced by sodium disulfide (Na2S2) (Fig. 1a). Therefore, Na2S4 was used as a polysulfide donor in the following studies. As shown in Fig. 1b, Na2S4 alleviated cisplatin-induced LDH release from RPT cell at a concentration dependent manner. Na2S4, at 80 µM, also largely reduced cisplatin-caused increase of PI positive cell numbers (Fig. 1c, d) and cleavage of caspase 3 (Fig. 1e). These results indicate the protective effect of polysulfide in cisplatin-induced RPT cell death.
Fig. 1.
Polysulfide attenuated cisplatin-induced RPT cell death. (A) Effects of polysulfide donors (80 µM, pretreatment for 30 min) on cisplatin (30 µM, 24 h) induced LDH release (n=4).(B) Effect of Na2S4 (pretreatment for 30 min) on cisplatin (30 µM, 24 h) induced LDH release (n=4). (C-D) Hoechst/PI staining assay showed that Na2S4 (80 µM, pretreatment for 30 min) attenuated cisplatin (30 µM, 24 h) induced RPT cell death (n=6). (E) Effect of polysulfide (80 µM, pretreatment for 30 min) on cisplatin-induced cleavage of caspase 3 (30 µM, 24 h) (n=4). ***p<0.001 versus control group;#p<0.05, ###p<0.001 versuscisplatin group. HPRT: Hypoxanthine Guanine Phosphoribosyltransferase.
3.2. Polysulfide suppresses cisplatin-induced ROS generation and MAPKs activation
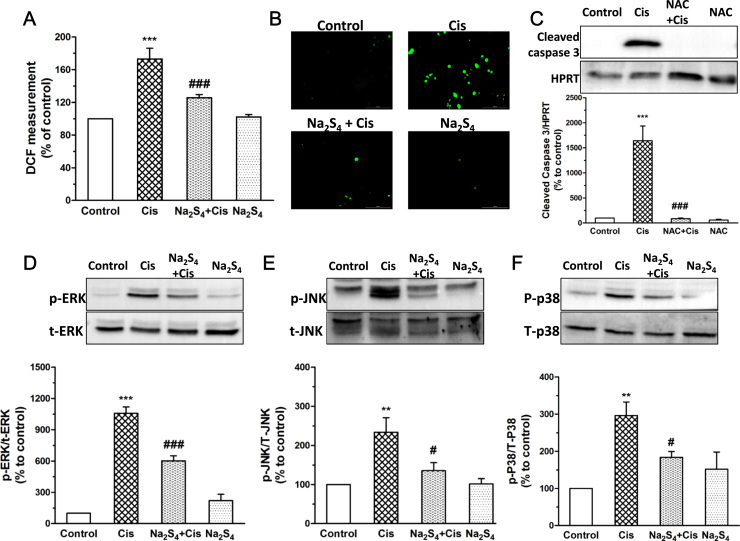
Upon cisplatin treatment, massive production of intracellular ROS was observed which was obviously suppressed by the pretreatment of Na2S4 (Fig. 2a, b). Importantly, elimination of ROS by N-acetyl-cysteine (NAC), an antioxidant, almost completely blocked cisplatin-caused cleavage of caspase 3 (Fig. 2c), indicating that the protective effect of polysulfide is at least partially due to the suppression of intracellular ROS production. We further measurement the involvement of MAPKs since cisplatin-mediated ROS activates MAPKs which subsequently contribute to RPT cell death [7]. Our results showed that cisplatin led to the phosphorylation of MAPKs of all types which were abolished by Na2S4 (Fig. 2d, e, f). These data suggest that the protective effect of polysulfide involves its suppressive effect on intracellular ROS generation and MAPKs activation.
Fig. 2.
Polysulfide suppressed cisplatin-induced ROS production and MAPKs activation in RPT cells. (A)Na2S4 (80 µM, pretreatment for 30 min) on cisplatin-induced intracellular ROS generation detected by plate reader (n=4) and (B) fluorescence microscope (Representative image from three independent experiments). (C) Effect of NAC (5 mM, pretreatment for 30 min) on cisplatin-induced cleavage of caspase 3 in RPT cells (n=6). (D-F) Effects of Na2S4 (80 µM, pretreatment for 30 min) on cisplatin (30 µM, 12 h) induced phosphorylation of ERK, JNK, p38 (n=4). **p<0.01, and ***p<0.001 versus control group; #p<0.05 and ###p<0.001 versus cisplatin group.
3.3. Polysulfide inhibits cisplatin-induced NADPH oxidase activation
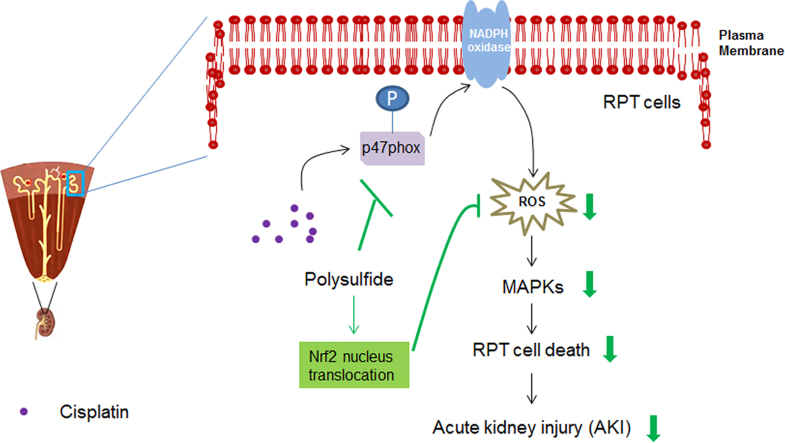
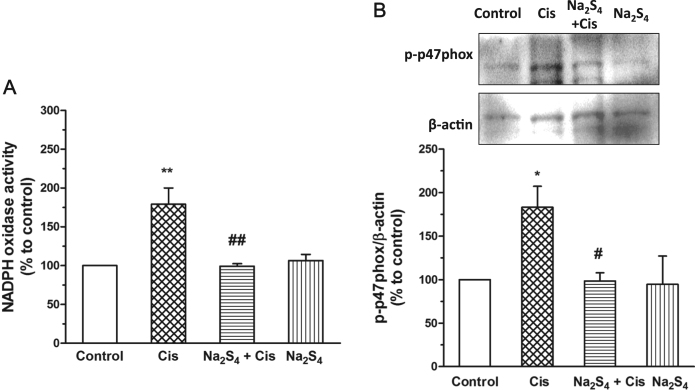
The effect of Na2S4 on the activity of NADPH oxidase was examined thereafter. The results showed that Na2S4 significantly inhibited cisplatin-induced activation of NADPH oxidase (Fig. 3a). We also detected whether Na2S4 influenced cisplatin-mediated phosphorylation of p47phox, a prerequisite for NADPH oxidase activation [20]. As shown in Fig. 3b, the phosphorylation of p47phox was suppressed by the pretreatment of Na2S4. These results imply that polysulfide may inhibit cisplatin-induced NADPH oxidase activation by suppressing the phosphorylation of p47phox.
Fig. 3.
Polysulfide inhibited cisplatin-induced NADPH oxidase activation. (A) Na2S4 (80 µM, pretreatment for 30 min) suppressed cisplatin (30 µM, 12 h) induced NADPH activity (n=3). (B) Na2S4 (80 µM, pretreatment for 30 min) suppressed cisplatin (30 µM, 8 h)-induced phosphorylation of p47phox (n=4). *p<0.05, and **p<0.01 versus control group; #p<0.05 and ##p<0.01 versus cisplatin group.
3.4. Polysulfide induces nucleus translocation of Nrf2
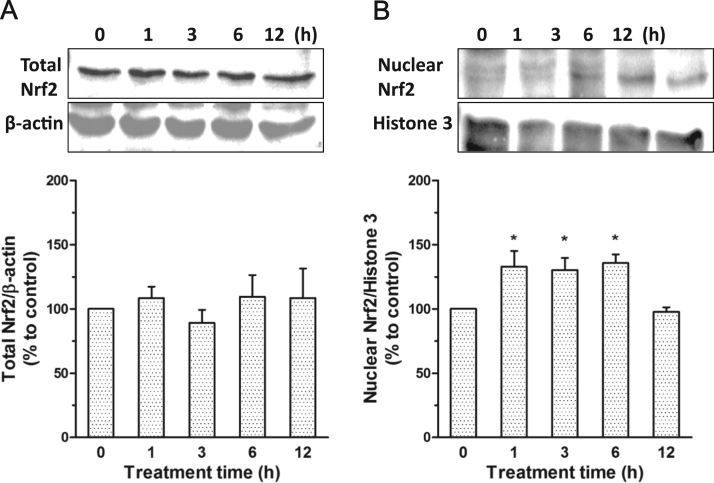
Previous studies showed the involvement of Keap1/Nrf2 pathway in the anti-oxidant effect of polysulfide [17]. The possible participation of the pathway was therefore investigated. The results showed that polysulfide led to the accumulation of Nrf2 in nucleus without causing obvious changes on the total expression Nrf2 (Fig. 4a, b). The nucleus translocation of Nrf2 is able to enhance the anti-oxidant capacity of cells [21] and therefore affords protective effects against cisplatin-induced injuries.
Fig. 4.
Polysulfide induced nucleus translocation of Nrf2. (A) Effect of Na2S4 (80 µM) on total expression level of Nrf2 (n=5). (B)Effect of Na2S4 (80 µM) on the nucleus level of Nrf2 (n=6).*p<0.05 versus time 0.
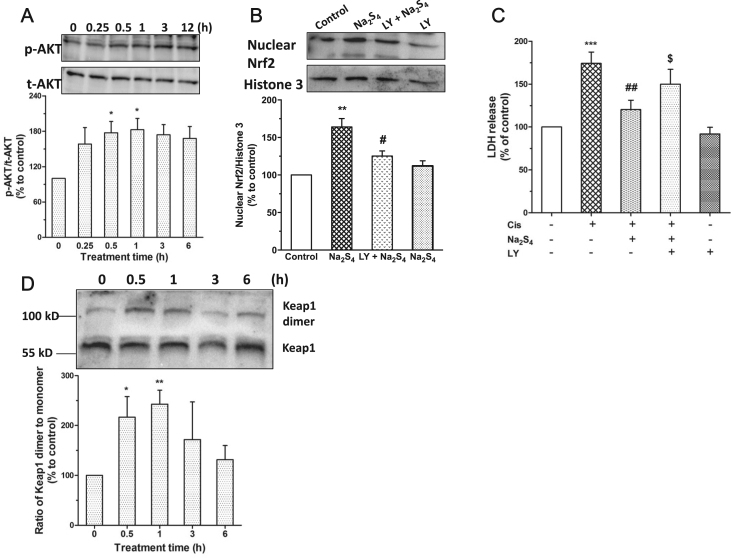
3.5. Polysulfide mediated nucleus translocation of Nrf2 involves AKT activation and Keap1 dimmerization
Subsequently, we examined the possible mechanisms underlying polysulfide mediated nucleus translocation of Nrf2. Our data showed that polysulfide led to the phosphorylation of AKT in RPT cells (Fig. 5a). Importantly, pharmacological inhibition of AKT activation not only attenuated nucleus translocation of Nrf2 but also lessened the protective effect of polysulfide (Fig. 5b,c). Moreover, we also observed that polysulfide caused the dimmerization of Keap1 (Fig. 5d), which may induce the release of Nrf2 and subsequent nucleus translocation [17].
Fig. 5.
Polysulfide mediated nucleus translocation of Nrf2 involved AKT and Keap1 dimmerization. (A) Effect of Na2S4 (80 µM) on the phosphorylation of AKT (n=5). Effect of LY (AKT inhibitor LY294002, 15 µM, pretreatment for 15 min) on Na2S4 (80 µM, 1 h) mediated nucleus translocation of Nrf2 (B; n=4) and cell protection (C; n=5). (D) Effect of Na2S4 on the dimmerization of Keap1 (n=5). *p<0.05, **p<0.01 versus time 0 for A&D. **p<0.01, ***p<0.001 versus control; #p<0.05, ##p<0.01 versus cisplatin; $p<0.05 versus cisplatin + Na2S4 for B&C.
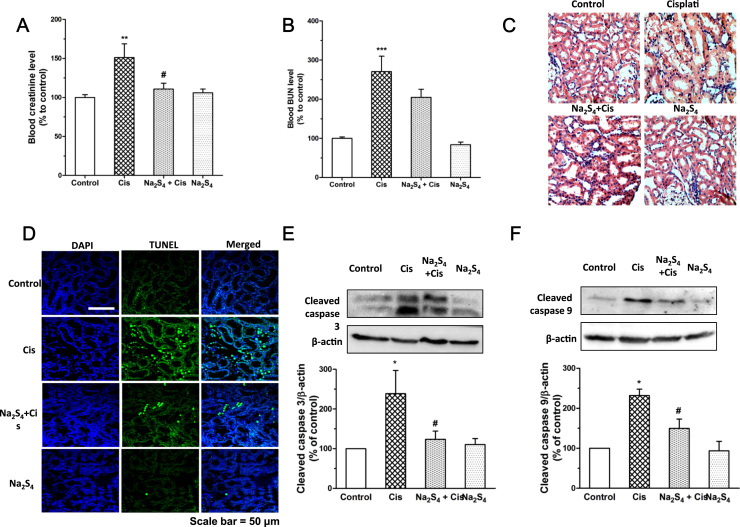
3.6. Polysulfide mitigates cisplatin-induced renal dysfunction and renal apoptosis
Furthermore, the therapeutic effects of polysulfide against cisplatin nephrotoxicity were evaluated in rats. As indicated in Fig. 6a, b, the upsurge of blood creatinine and BUN led by cisplatin was diminished with the treatment of polysulfide. In agreement with the renal functional result, histological analysis of renal cortical region showed severe injury in cisplatin treatment group (Fig. 6c). In the presence of polysulfide, less tubules lost brush border and cell lysis (Fig. 6c). We also evaluated the effect of polysulfide on cisplatin-induced apoptosis in the renal cortex by TUNEL staining. As shown in Fig. 6d, cisplatin treatment led to massive apoptosis in renal cortex with loss of tubular structure. In contrast, administration of polysulfide significantly reduced the apoptosis of proximal tubule cells and reserved the tubular structure in this region. In line with this result, the levels of cleaved caspase 9 and 3 are also significantly mitigated by polysulfide (Fig. 6e, f). These results suggest that polysulfide alleviates cisplatin-induced renal dysfunction and renal cortical apoptosis.
Fig. 6.
Polysulfide ameliorated cisplatin-induced renal dysfunction and apoptosis. Effects of Na2S4 on cisplatin-induced increase of plasma creatinine (A; n=6–8) and BUN (B; n=6–8). (C) Representative images of H&E staining of kidney tissues. (D) Representative images of TUNEL staining of kidney tissues. (E-F) Effects of Na2S4 on cisplatin-induced cleavage of caspase 3 (n=5) and 9 (n=5) in renal cortical tissues.*p<0.05, **p<0.01 and ***p<0.001 versus control group; #p<0.05 versus cisplatin group.
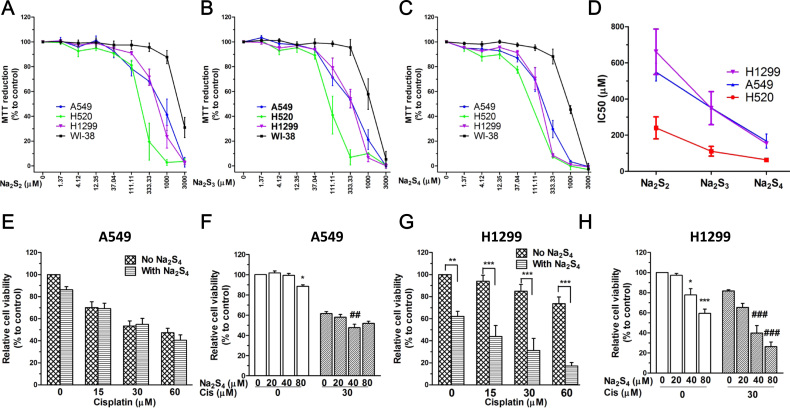
3.7. Polysulfide inhibits cell growth in non-small cell lung cancer cell lines
We then measured whether polysulfide donors (Na2S2, Na2S3 and Na2S4) affect the cell viability of cancerous cells with a panel of non-small cell lung cancer (NSCLC) cell lines. The results showed that polysulfide donors significantly inhibited cancer cell growth after 24 h without affecting that of non-cancerous lung fibroblast cells like WI-38 (Fig. 7a, b, c). Interestingly, their IC50 values indicate that the more sulfur atoms polysulfide contains the higher anti-cancer effect it may induce (Fig. 7d). These results indicate that polysulfide possesses anti-cancer activity in NSCLC cell lines.
Fig. 7.
Polysulfide did not compromise the anti-cancer activity of cisplatin in NSCLC cell lines. (A-C) Effect of Na2S4 on the cell viability of NSCLC cell lines and WI-38 after treatment for 24 h (n=4). (D) IC50 value of Na2S4 for the inhibition of NSCLC cell viability (n=4). Effect of Na2S4 (80 µM, pretreatment for 30 min) on the anti-cancer activity of cisplatin (24 h) in A549 cells (E-F; n=3) and H1299 cells (G-H; n=3).**p<0.01 and ***p<.001 for G; *p<0.05 and ***p<0.001 versus non-treated group for F&H; ##p<0.01 and ###p<0.001 versus cisplatin alone group for F&H.
3.8. Polysulfide does not compromise the anti-cancer activity of cisplatin in NSCLC cell lines
Subsequently, the effect of Na2S4 on the anti-cancer activity of cisplatin was evaluated in NSCLC cell lines A549 and H1299. The results showed that Na2S4 did not exhibit any significant influence on cisplatin-induced reduction of cell viability in A549 cells (Fig. 7e, f). Interestingly, it added more anti-cancer activity to cisplatin in H1299 (Fig. 7g, h). These data suggest that polysulfide does not compromise the anti-cancer activity of cisplatin at least in NSCLC cell lines.
4. Discussion
Despite being a well-known and powerful anti-proliferative drug, cisplatin usage is accompanied with moderate-to-severe adverse effects. Cisplatin-induced nephrotoxicity stands as the main factor limiting its clinical application due to its prevalence and severity. This urges us to search a possible agent that can prevent cisplatin nephrotoxicity without affecting its anti-cancer activity.
Polysulfide is a category of chemical compounds comprising chains of sulfur atoms. There are two main classes of polysulfide reported namely anions and organic polysulfide [22]. Anions have the general formula Sn2- which is the chemical basis of hydrogen polysulfide H2Sn and sodium polysulfide Na2Sn. Organic polysulfide, such as garlic derived diallyl disulfides (DADS) and diallyltrisulfides (DATS), usually possess a formula of RSnR where R is either an alkyl or aryl group [23]. In this study, we have employed sodium polysulfide Na2Sn as donors because they solely provide Sn2- in aqueous solutions and therefore more closely mimic 3MST produced hydrogen polysulfide as reported previously [24].
RPT cell injuries lead to subsequent nephrotoxicity upon cisplatin treatment [25]; therefore, the protective effect of polysulfide was first demonstrated within RPT cells. Notably, when comparing the cell protection mediated by different polysulfide donors such as Na2S2, Na2S3 and Na2S4, we found that the more sulfur atoms polysulfide contains the higher protective effect it may induce. Moreover, a similar result was later obtained in the context of cancer inhibition. Interestingly, Benavides et al. [26] found that the vasoactivity of garlic derived organic polysulfide was reflected by the tethering sulfur atoms. All the evidence may indicate the number of sulfur atoms in these molecules may determine its biological activity. This could be due to the differences of their releasing capacity of H2S [26] and/or reactivity with biomolecules (i.e. persulfidation) [27]. No matter how, it is reasonable to speculate that polysulfide donors containing over 4 sulfur atoms may possess stronger renal protective effect which warrants further studies in the future.
We then investigated the possible mechanisms underlying the protective effect of polysulfide. The axis of intracellular ROS and MAPKs plays a critical role in cisplatin-induced RPT cell injuries [25]. We found that polysulfide largely attenuated cisplatin-induced production of intracellular ROS. As a result, the subsequent activation of MAPKs was suppressed upon the supplementation of polysulfide. Though whether cisplatin stimulates the production of mitochondrial ROS remains controversial in RPT cells [11], [12], extensive evidence suggests a definitive role of NADPH oxidase [1], [8], [9], [10]. Our data displayed a suppressive effect of polysulfide on cisplatin-induced activation of NADPH oxidase probably by inhibiting p47phox phosphorylation which is a critical event for the assembly of NADPH oxidase [28], [29]. However, how polysulfide inhibits the phosphorylation of p47 (i.e. persulfidation) remains to be determined in the future.
A previous study by Koike et al. [17] showed that polysulfide induced the translocation of Nrf2 into nucleus and exhibited anti-oxidative effect in SH-SY5Y cells. We found a similar effect of polysulfide on Nrf2 nucleus translocation in RPT cells. Moreover, this effect can be at least partially ascribed to polysulfide mediated AKT activation. We also observed the dimerization of Keap1 which controls the release and nucleus translocation of Nrf2 [30]. Although it remains unclear whether this dimerization contribute to the subsequent effect of polysulfide, it is worth mentioning that H2S-mediated persulfidation of cysteine-151 on Keap1 indeed contributes to the release and nucleus translocation of Nrf2 [31].
The protective effect of polysulfide was further demonstrated in the animal models. We have used a similar dose of H2S donor NaHS (5.6 mg/kg) that elicited protective effects in cisplatin nephrotoxicity [32], as polysulfide tends to produce biological effect at a higher potency than H2S based on previous studies [15], [16], [33], [34], [35] and our result on RPT cells (data not shown). The renal protective effect of polysulfide was manifested by reduction of blood creatinine and cleavage of caspase 3 and 9 in renal cortical tissue. Since the efficacy of polysulfide donors with various numbers of sulfur atoms was not compared in animals, whether sulfur numbers in polysulfide correlate to the in vivo efficacy remains elusive and may be determined in the future.
When applying as a renal protective agent to treat cisplatin-induced nephrotoxicity, it is necessary to clarify whether the substance influences the anti-cancer activity of cisplatin. Therefore, the anti-cancer activity of polysulfide donors and combination with cisplatin were examined. Like garlic derived organic polysulfide [36], these inorganic polysulfide donors (Na2S2, Na2S3 and Na2S4) also exhibit selective cancer cell killing effect in NSCLC cell lines. Notably, combination of polysulfide donor Na2S4 and cisplatin resulted in stronger anti-cancer effect in NSCLC cell lines like H1299 and A549 which may also rule out the possible reaction between sulfur containing polysulfide and cisplatin [37] in our experimental settings. However, experiments are still needed to show whether polysulfide would influence the chemotherapeutic efficacy of cisplatin in tumor-bearing animal models in the future.
Of note, the presence of endogenous H2Sn was demonstrated recently by Koike et al. [38], suggesting that endogenous H2Sn may be a physiological molecule alongside H2S. This also implies that alternation of endogenous H2Sn may occur, which might contribute to pathogenesis of diseases like H2S does [39], [40], [41], [42], [43]. The demonstration of this hypothesis requires the accurate measurement of endogenous H2Snin vitro and in vivo. H2Sn measurement in biological samples can also aid to validate the pharmacological efficacy of this class of molecule. However, the methods remain to be established to accurately measure the concentration of endogenous polysulfide. In the abovementioned work [38], Koike et al. showed that the concentration of H2S2 is 20 nmol/g protein in mouse brain while that of H2S3 is below the detection limit. Within the method, even the standard Na2S3 and Na2S4 exhibit several peaks in detection, making them distinguishable in biological samples. Therefore, an accurate and specific detection method is of high demand for the measurement of endogenous H2Sn.
In conclusion, the current study provided a comprehensive understanding about the therapeutic potential of polysulfide in cisplatin nephrotoxicity and possible mechanisms involved. Our results indicate that polysulfide donor Na2S4 ameliorated cisplatin-caused renal toxicity probably by suppressing the activation of NADPH oxidase and inducting nucleus translocation of Nrf2. Moreover, polysulfide not only ameliorated cisplatin-caused renal injury but also added on more anti-cancer effect to cisplatin in NSCLC cancer cell lines. Based on the current study, polysulfide may be a novel and promising therapeutic agent for the treatment of cisplatin-induced nephrotoxicity and/or serve as an adjuvant with cisplatin for cancer therapy.
Acknowledgements
This work was supported by Ministry of Education of Singapore Tier 2 Research grant (MOE2017-T2-2-029) and NMRC (CIRG/1363/2013 and CIRG1432/2015).
Acknowledgments
Conflict of interest
None of the authors declare any financial or other conflicts of interest.
References
- 1.Lippert B. John Wiley & Sons; 1999. Cisplatin: Chemistry and Biochemistry of A Leading Anticancer Drug. [Google Scholar]
- 2.Ries F., Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am. J. Kidney Dis. 1986;8(5):368–379. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 3.Ramesh G., Reeves W.B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Ren. Physiol. 2003;285(4):F610–F618. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- 4.Miller R.P. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baliga R. Oxidant mechanisms in toxic acute renal failure. Drug Metab. Rev. 1999;31(4):971–997. doi: 10.1081/dmr-100101947. [DOI] [PubMed] [Google Scholar]
- 6.I. Arany, R.L. Safirstein. Cisplatin nephrotoxicity. In: Seminars in nephrology, 2003. Elsevier. [DOI] [PubMed]
- 7.Pabla N. Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J. Clin. Investig. 2011;121(7):2709. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashed L.A., Hashem R.M., Soliman H.M. Oxytocin inhibits NADPH oxidase and P38 MAPK in cisplatin-induced nephrotoxicity. Biomed. Pharmacother. 2011;65(7):474–480. doi: 10.1016/j.biopha.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Trujillo J. Superoxide anion production and expression ofgp91(phox) and p47(phox) are increased in glomeruli and proximal tubules of cisplatin-treated rats. J. Biochem. Mol. Toxicol. 2015;29(4):149–156. doi: 10.1002/jbt.21679. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y. Pharmacological inhibition of NADPH oxidase protects against cisplatin induced nephrotoxicity in mice by two step mechanism. Food Chem. Toxicol. 2015;83:251–260. doi: 10.1016/j.fct.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Kruidering M. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J. Pharmacol. Exp. Ther. 1997;280(2):638–649. [PubMed] [Google Scholar]
- 12.Takahashi A. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 2012;180(2):517–525. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Nagy P., Winterbourn C.C. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem. Res. Toxicol. 2010;23(10):1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 14.Kimura Y. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci. Rep. 2015;5 doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Y. Nagai, et al., Polysulfides induce calcium waves in rat hippocampal astrocytes. J. Pharmacol. Sci. Jpn. Pharmacological. Soc. Editorial. Off., Kantohya Bldg Gokomachi-ebisugawa Nakagyo-ku, Kyoto, 604, Japan, 2006.
- 16.Oosumi K. Polysulfide activates TRP channels and increases intracellular Ca2+ in astrocytes. Neurosci. Res. 2010;68:e109. [Google Scholar]
- 17.Koike S. Polysulfide exerts a protective effect against cytotoxicity caused by t‐buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett. 2013;587(21):3548–3555. doi: 10.1016/j.febslet.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y. HNO suppresses LPS-induced inflammation in BV-2 microglial cells via inhibition of NF-κB and p38 MAPK pathways. Pharmacol. Res. 2016;111:885–895. doi: 10.1016/j.phrs.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Abid M.R. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J. Biol. Chem. 2007;282(48):35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 20.A. Amanso, A.N. Lyle, K.K. Griendling, NADPH oxidases and measurement of reactive oxygen species. Hypertension: Methods and Protocols, 2017, p. 219–232. [DOI] [PubMed]
- 21.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol. Sci. 2013;34(6):340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Toland W.G. Oxidation of organic compounds with aqueous sulfate. J. Am. Chem. Soc. 1960;82(8):1911–1916. [Google Scholar]
- 23.Bradley J.M., Organ C.L., Lefer D.J. Garlic-derived organic polysulfides and myocardial protection. J. Nutr. 2016;146(2):403S–409S. doi: 10.3945/jn.114.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura Y. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci. Rep. 2015;5:14774. doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 26.Benavides G.A. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. 2007;104(46):17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.H. Kimura, Signaling of hydrogen sulfide and polysulfides, Mary Ann Liebert, Inc., 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA, 2015.
- 28.Brandes R.P., Weissmann N., Schröder K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 2010;49(5):687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 29.Griendling K.K., Sorescu D., Ushio-Fukai M. Nad (p) h Oxidase. Circ. Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 30.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 31.Yang G. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013;18(15):1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 32.Ahangarpour A. Hydrogen sulfide ameliorates the kidney dysfunction and damage in cisplatin-induced nephrotoxicity in rat. Vet. Res. Forum: Int. Q. J. 2014 [PMC free article] [PubMed] [Google Scholar]
- 33.Shigetomi E. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive d-serine release. J. Neurosci. 2013;33(24):10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyamoto R. Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci. Rep. 2017;7 doi: 10.1038/srep45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 2017 doi: 10.1016/j.bcp.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Miltonprabu S., Sumedha N., Senthilraja P. Diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int. Immunopharmacol. 2017;50:107–120. doi: 10.1016/j.intimp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann T., Chval Z., Burda J.V. Cisplatin interaction with cysteine and methionine in aqueous solution: computational DFT/PCM study. J. Phys. Chem. B. 2009;113(10):3139–3150. doi: 10.1021/jp807645x. [DOI] [PubMed] [Google Scholar]
- 38.Koike S. Analysis of endogenous H2S and H2Sn in mouse brain by high-performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radic. Biol. Med. 2017;113:355–362. doi: 10.1016/j.freeradbiomed.2017.10.346. [DOI] [PubMed] [Google Scholar]
- 39.Bos E.M. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 2013;24(5):759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao X., Bian J.-S. The role of hydrogen sulfide in renal system. Front. Pharmacol. 2016;7 doi: 10.3389/fphar.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao X. A new hope for a devastating disease: hydrogen sulfide in Parkinson's disease. Mol. Neurobiol. 2017 doi: 10.1007/s12035-017-0617-0. [DOI] [PubMed] [Google Scholar]
- 42.Hosgood S., Nicholson M. Hydrogen sulphide ameliorates ischaemia–reperfusion injury in an experimental model of non‐heart‐beating donor kidney transplantation. Br. J. Surg. 2010;97(2):202–209. doi: 10.1002/bjs.6856. [DOI] [PubMed] [Google Scholar]
- 43.Cao X. Renal protective effect of hydrogen sulfide in cisplatin-induced nephrotoxicity. Antioxid. Redox Signal. 2018 doi: 10.1089/ars.2017.7157. (ja) [DOI] [PubMed] [Google Scholar]