Ríos relates the history of Ca2+-induced Ca2+ release and how its contribution to skeletal muscle physiology was determined.
Abstract
Ryanodine-sensitive intracellular Ca2+ channels (RyRs) open upon binding Ca2+ at cytosolic-facing sites. This results in concerted, self-reinforcing opening of RyRs clustered in specialized regions on the membranes of Ca2+ storage organelles (endoplasmic reticulum and sarcoplasmic reticulum), a process that produces Ca2+-induced Ca2+ release (CICR). The process is optimized to achieve large but brief and localized increases in cytosolic Ca2+ concentration, a feature now believed to be critical for encoding the multiplicity of signals conveyed by this ion. In this paper, I trace the path of research that led to a consensus on the physiological significance of CICR in skeletal muscle, beginning with its discovery. I focus on the approaches that were developed to quantify the contribution of CICR to the Ca2+ increase that results in contraction, as opposed to the flux activated directly by membrane depolarization (depolarization-induced Ca2+ release [DICR]). Although the emerging consensus is that CICR plays an important role alongside DICR in most taxa, its contribution in most mammalian muscles appears to be limited to embryogenesis. Finally, I survey the relevance of CICR, confirmed or plausible, to pathogenesis as well as the multiple questions about activation of release channels that remain unanswered after 50 years.
Introduction
CICR is a process that occurs in many cells and tissues whereby an increase of [Ca2+] in the cytosol causes a further increase as Ca2+ is released from intracellular stores. It is the consequence at the cellular level of the ability of intracellular Ca2+ channels to be “activated by Ca2+”, that is, to open when cytosolic [Ca2+] rises above a threshold level.
General descriptions of Ca2+-dependent processes in biology usually start by stating that Ca2+ is a ubiquitous cellular messenger, if not the most ubiquitous one. It is also recognized that the existence, and often coexistence, of multiple “signals” conveyed by Ca2+ is made possible by their encoding in specific temporal sequences and spatial scales. The encoding relies crucially on the ability of cells to increase cytosolic [Ca2+] to values two or three orders of magnitude above resting levels, producing events that may be quite localized (of submicron spatial spread at peak amplitude) and temporally brief, events for which Ca2+ sparks (Cheng and Lederer, 2008) are a paradigm.
Two properties of intracellular Ca2+ channels make these key features possible: the arrangement of the channels in clusters and the control of their gating by Ca2+ itself. Thus appointed, RyR channels can open concertedly to produce large local transients. Ca2+-induced channel opening is therefore a defining property at the core of Ca2+ signaling.
Like many aspects of Ca2+ signaling, CICR was discovered and first studied in detail in skeletal muscle (Endo et al., 1970; Ford and Podolsky, 1970). As befits a fundamental process, it is now known to occur in many tissues and serve multiple purposes. In addition to the original discovery, and perhaps because of that head start, the studies of CICR in skeletal muscle went on to provide additional findings and advances, which later extended to other cells and tissues.
Shortly after the discovery of CICR in skeletal muscle, a similar process was demonstrated in cardiac muscle (Fabiato and Fabiato, 1972; Fabiato et al., 1972). CICR was later shown to be the sole mechanism of activation of Ca2+ release in the heart; consequently, the studies in cardiac muscle now vastly surpass, in volume and detail, what has been done in skeletal muscle.
By comparison, the relevance of CICR to skeletal muscle has always been controversial. This article reviews the research and the contested evidence for its physiological role. The studies are reviewed in a loose chronological sequence. Because the methods evolved from largely biophysical techniques in the 1970s and 1980s to studies of structure and heterologous expression in live animals in the last decade of the twentieth century, the chronological approach allows separate consideration of the different methodologies. This article omits much to dwell on works that, in my view, provided unique mechanistic insights.
CICR in the heart has been reviewed often, with standout articles by Bers (2001) and Cheng and Lederer (2008). For this reason, the studies of cardiac muscle are examined only in cases in which the advances were first made there or where differences between the tissues provide additional insight. Direct precedents for skeletal muscle include an authoritative article by Endo (2009) and an informative examination by Murayama and Kurebayashi (2011) of the differential involvement of two isoforms of the ryanodine-sensitive ion channel, RyR, in CICR. Recently, Meissner (2017) reviewed structure–function relationships in the RyR, and Franzini-Armstrong (2018) sketched the history of the excitation–contraction (EC) coupling field.
Before the discovery of CICR
The initial observations of CICR occurred in the conceptual hotbed generated by the discovery of the role of Ca2+ in the control of interactions between myofilament proteins.1 This precedent required the introduction of Ca2+ buffers as essential tools that would later be applied for various purposes in the study of CICR. In brief, Bozler (1954) first reported the relaxing effect of Ca2+ buffers on permeabilized muscle, noting their similarity with the effects of the “relaxing factor” (Marsh, 1951; Bendall, 1953) later identified with the SR. The essential role of Ca2+, which justified the effect, was established by Weber (1959) and Setsuro Ebashi, who was a postdoc at that time in the Kumagai laboratory of the University of Tokyo. In a study done in 1958 that was initially confusing for its authors, Ebashi et al. (1960) found that EDTA was much less relaxing than a buffer of similar calcium affinity then called GEDTA. Later, Ebashi (1960) explained this as being the result of the occupancy of EDTA by Mg2+. Thus introduced, GEDTA, or EGTA as it was later named, became essential in studies that required setting Ca2+ at low values in the presence of much greater Mg2+.
Discovery
The first demonstrations of CICR were communicated nearly simultaneously by Endo et al. (1970) working at the University of Tokyo and by Ford and Podolsky (1970) in the laboratories of the National Institutes of Health in Maryland. As narrated by Makoto Endo, their finding was the result of serendipity and scholarly duty. As reluctant Department of Pharmacology members who were really interested in physiology, Endo and starting postdoc Yasuo Ogawa were tasked to prepare something to present, “as a show of fidelity,” for the 1966 meeting of the Japanese Pharmacological Society. Looking for something easy to do, they set out to confirm, in the more physiological setting of the skinned frog muscle fiber, the observation by Weber (1968) and Weber and Herz (1968), communicated preliminarily in 1965, that caffeine caused Ca2+ release from isolated SR. Surprisingly, they found that low caffeine induced a large contraction, which recurred at intervals of minutes. The contractions were, in fact, propagated waves of shortening moving along the fiber, which suggested a self-sustained process that could only be ascribed to activation by Ca2+.
Meanwhile, in Maryland, Ford and Podolsky reached the same conclusion by directly applying high [Ca2+] solutions to skinned frog muscle fibers.2 The two lines of work intersected in 1968 as back-to-back talks given by Ford and Endo at the 24th International Congress of Pharmacology held in Washington, D.C. After both presentations, Podolsky stood up to declare his “pleasure that the same discovery was done simultaneously on both sides of the Pacific Ocean” (as told by Ogawa and Endo).
The dominant mechanism of activation of Ca2+ release in skeletal muscle was later shown to be a conformational signal (Schneider and Chandler, 1973) induced in the L-type Ca2+ channel (CaV1.1 or dihydropyridine receptor [DHPR]) by depolarization of the t tubule membrane, in a process dubbed DICR (depolarization-induced Ca2+ release; Ríos and Brum, 1987; Tanabe et al., 1988; Nakai et al., 1996). In part as a consequence of the establishment of this mechanism as primary, the relevance of CICR to skeletal muscle was controversial from the start, with one of its discoverers as the main questioner (Endo, 2009).
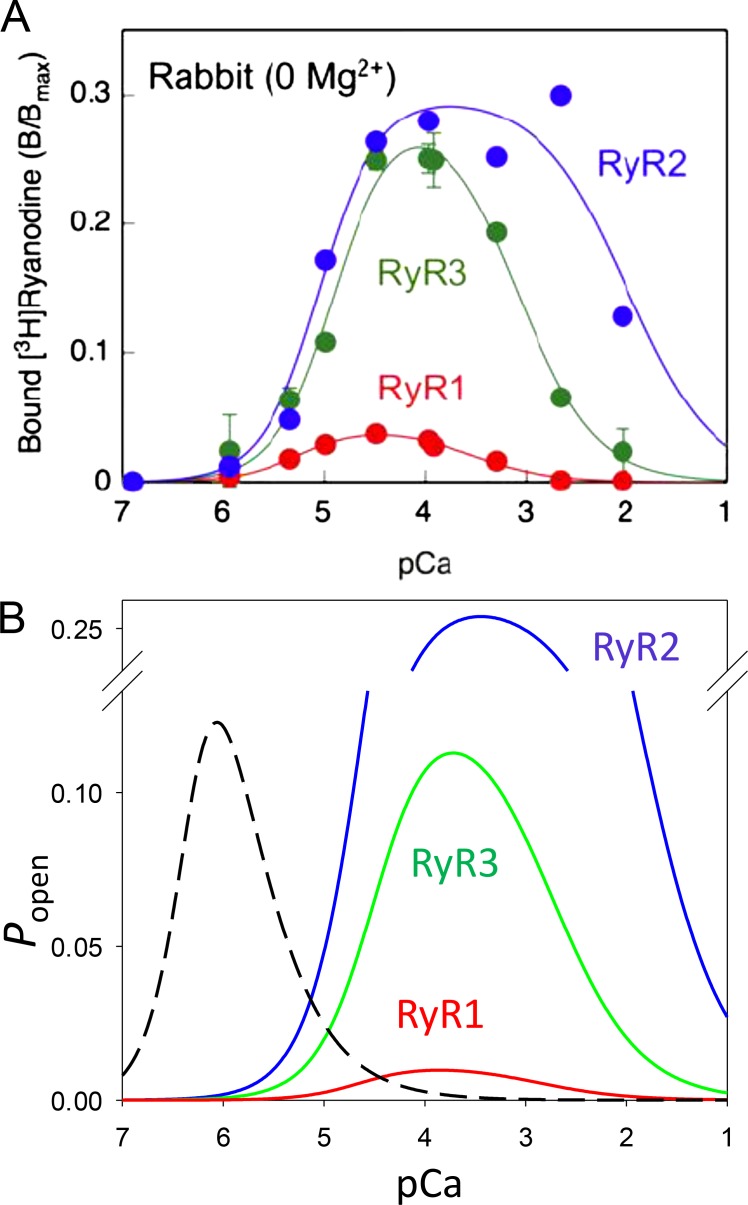
The RyR in subcellular preparations
Since the identification of the SR Ca2+ release channels, their Ca2+-dependent properties have been defined in subcellular preparations. As reviewed by Murayama and Kurebayashi (2011), a similar dependence of Popen (or other measures of channel openness) versus [Ca2+] is found in skinned frog myofibers, SR vesicles, and purified channels in bilayers. In simplified systems, including vesicles and purified proteins, [3H]ryanodine binding can provide a proportional measure of channel openness.3 Under standard near-physiological conditions, Popen increases with [Ca2+] in the micromolar range and decreases at greater concentrations. The biphasic dependence is conventionally explained by the existence of an excitatory binding site (“A” in the nomenclature of Laver et al., 1997) and an inhibitory (“I”) site of much lower affinity. The fraction of activated channels (which in a statistical ensemble equals Popen) is
| (1) |
and represent the fractional occupancies of the A and I sites. The observed inhibitory effects of Mg2+ are well described, assuming that it binds to both A and I sites; the occupancy of I by Mg2+ or Ca2+ is inhibitory, whereas that of A by Mg2+ inhibits by competition with Ca2+. in Eq. 1 adopts values between 0 and 1, representing the effects on the channel of factors (say, ATP) that alter its Popen without affecting its Ca2+ or Mg2+ sensitivity. The site occupancy is approximately described by a conventional “Hill” equation,
| (2) |
where index i represents A or I. Fig. 1 A shows experimental values and/or fits with Eq. 1 for all isoforms of the channel in the absence or presence of Mg2+.
Figure 1.
The basic properties revealed by bilayer reconstitution cannot be simply extrapolated to the living cell because the channels are sensitive to several ligands, including proteins, small peptides, and ions, as well as chemical modification (Fill and Copello, 2002; Meissner, 2017). Additionally, the independence of sites A and I implicit in Eq. 1 ignores dynamic aspects of control that determine the response in vivo. A demonstration of these complexities is in the study by Sánchez et al. (2003), which defined the [Ca2+] dependence of Ca2+ release from cardiac SR vesicles by stopped-flow mixing. Their results still show a biphasic dependence of the rate of Ca2+ release on [Ca2+] (Fig. 1 B, dashed line), but with shifts to lower values in the effective KDs of both activation and inactivation by nearly two orders of magnitude. Activation by submicromolar cytosolic Ca2+ was confirmed much later in permeabilized frog myofibers (Figueroa et al., 2012).
Many ligands other than Ca2+ favor the open state of the RyR channels. Three have been intensively studied and used as tools: ryanodine, because its fractional binding is used as a measure of the channel’s Popen; ATP, because it operates as an essential cofactor that must be present for CICR to work; and caffeine, the paradigmatic promoter of activation by Ca2+. In recent years, with the advent of direct electron detection cryo–electron microscopy (EM; Li et al., 2013), the structure of RyRs 1 and 2 have been determined at near-atomic resolution (Efremov et al., 2015; Yan et al., 2015; Zalk et al., 2015). The improvements in resolution allowed advances in understanding the structural underpinnings of function, including the location of the binding sites for all four agonists. The sites of Ca2+, ATP, and caffeine binding are close together. The structural changes associated with their actions are consistent with a synergistic effect, whereby binding of Ca2+ alone (or ATP and caffeine alone) puts the channel in a “primed” state, which progresses to opening when all agonists (presumably ATP and Ca2+ in the physiological situation) are bound (des Georges et al., 2016). Meissner (2017) critically reviewed this evidence.
The central role of CICR in cardiac muscle
The demonstration of CICR in heart muscle was largely done by A. Fabiato and coworkers, who developed methods to exchange solutions rapidly around myocytes with plasma membrane removed. Their series of papers showed that ICa, the membrane Ca2+ current underlying an action potential, is capable of inducing Ca2+ release (Fabiato, 1985a); that the CICR response is rapid and sufficient to activate contraction (Fabiato, 1981); that Ca2+ release and Ca2+ removal occur via separate SR pathways (Fabiato, 1985b); and that Ca2+ release is inactivated by Ca2+ itself (Fabiato, 1985b). The work provided quantification of the dependencies of both activation and inactivation on [Ca2+] and an initial evaluation of the effects of Mg2+, H+, and calmodulin (Fabiato, 1983). The results included evidence of activation of Ca2+ release by SR Ca2+ overload (Fabiato, 1985c, 1992) and advanced the case for a unique Ca2+ release pathway with multiple agonists: a single Ca2+ release channel. These experiments also pioneered a variety of optical approaches to monitor cytosolic Ca2+ transients and other cellular variables putatively associated with Ca2+ release (Fabiato and Fabiato, 1979; Fabiato, 1982, 1985d).
This prodigious series of papers used mainly skinned cardiomyocytes. Contemporary work on intact cells and bundles (Cannell et al., 1987; Beuckelmann and Wier, 1988; Niggli and Lederer, 1990a) was largely consistent with Fabiato’s conclusions and established that CICR constitutes the sole mechanism of activation of Ca2+ release for cardiac EC coupling (Lederer et al., 1989). This consensus is ultimately based on two observations. One is that activation of Ca2+ release decays at high Vm, reflecting the decrease in the trigger current ICa (Näbauer et al., 1989). The other is that, in cardiac muscle, the t- or plasma membrane–SR junctions lack the strict stoichiometry and spatial overlap between CaVs and RyRs thought to be necessary for DICR in skeletal muscle (Block et al., 1988; Tanabe et al., 1990).
The “calcium paradox of control”4
The establishment of CICR as chief activation mechanism for Ca2+ release in cardiac muscle brought with it a mechanistic problem, first recognized for the heart. Because Ca2+ released from the SR adds to the initial trigger of Ca2+ entering the cytosol via ICa, the release response should self-reinforce and reach its maximum in all-or-none fashion. Ca2+ release, instead, is graded with depolarization. More dramatically, Ca2+ release can be cut short during an action potential by changes in voltage that suppress the trigger ICa. Michael Stern derived constraints for any model that would successfully reproduce this “paradox of control.” In a classic paper, (Stern, 1992a) he first achieved the improbable feat of analyzing by linearization an intrinsically nonlinear system (the cell endowed with CICR). To do it, he used to his advantage the paradox itself—the fact that release is graded, capable of infinitesimal increments in spite of its feedback by CICR—and hence had a linear range of responses. This analysis ruled out the possibility of a common pool system in which trigger and released Ca2+ share the same compartment, stressing instead the mechanistic significance of locally inhomogeneous [Ca2+]cyto.5
Ca2+ sparks solve the paradox of control
The interest in local Ca2+ gradients generated by the analyses of Stern and the earlier introduction of digital fluorescence Ca2+ imaging (Williams et al., 1985; Wier et al., 1987) overlapped in time with the biological application of the first laser-scanning confocal microscopes (Amos et al., 1987). Confocal microscopy was first used in muscle to image immunofluorescence (Somlyo et al., 1988). Confocal Ca2+ imaging was possible after the development of high dynamic range fluorescent indicators with fluorescein or rhodamine chromophores (Minta et al., 1989). Confocal imaging of fluo-3 fluorescence was applied simultaneously in 1990 for resolving Ca2+ transients in the heart (Niggli and Lederer, 1990b), neurons (Hernández-Cruz et al., 1990), and glia (Cornell-Bell et al., 1990).
The tired “paradigm-changing” qualifier is still appropriate for the discovery of Ca2+ sparks that ensued (Cheng et al., 1993). Like other major advances, this one resulted from the convergence of a strong group of researchers in a field in technical and conceptual movement. It took place in the laboratory of Jon Lederer, who was pioneering the application of confocal microscopy to Ca2+ imaging. As recalled by Mark Cannell, a visiting scholar at the time, he and then-student Heping Cheng contributed enhancements to the commercial scanner in use, precise synchronization to stimulus generators, custom-made improvements in signal/noise and sensitivity, original image analysis, and the will to increase laser power with total disregard for cell survival. They found a discrete substructure of Ca2+ release in cardiac myocytes, composed by spatially small and temporally brief events immediately dubbed “sparks” (thought of as “sparktifacts” by some, including the editors that rejected the manuscript in Nature). These events appeared to be functionally independent (they composed the cellular transient by addition, with individual properties relatively independent of the presence of other sparks). Sparks thus confirmed Stern’s conclusion that CICR excludes large common pools and focused mechanistic thinking on the virtues of local pools, or, as they were seductively called, micro- and nanodomains of elevated Ca2+cyto (Llinás et al., 1992; Ríos and Stern, 1997; Fedchyshyn and Wang, 2005).
The discovery of Ca2+ sparks “solved” the paradox of control in a general sense. As discussed by Cannell et al. (1995), sparks reconciled the tendency of a CICR mechanism to saturate (it could do so locally) while remaining graded at a cell-wide level. It did not solve the question of mechanism, however, as there was no agreement on even the most basic aspects of Ca2+ sparks, including whether they emanate from single or multiple open channels (more on this in the section named Ca2+ sparks affirm CICR in skeletal muscle).
CICR in skeletal muscle probed “biophysically”
In 1987, Sidney Fleischer’s group clarified the biochemical nature of the Ca2+ release channels of the SR (Inui et al., 1987), identifying them both with the ryanodine receptors of heavy SR fractions and the so-called “feet” described by Clara Franzini-Armstrong (1970) in triadic structures. Also in 1987, Gustavo Brum and I identified the receptors of Ca channel blockers present in the t tubules (DHPRs, later named CaV1.1) as the voltage sensors for EC coupling; meanwhile, the laboratory of Shosaku Numa determined their primary sequence, noting similarities with the voltage-sensitive Na channel, now NaV1.4 (Tanabe et al., 1987). These advances took the focus of research away from the possible roles of CICR, as they strengthened the consensus for a conformational switch (in the DHPR–RyR connection) that translates action potential depolarization to opening of the Ca2+ release pathway (Schneider and Chandler, 1973).
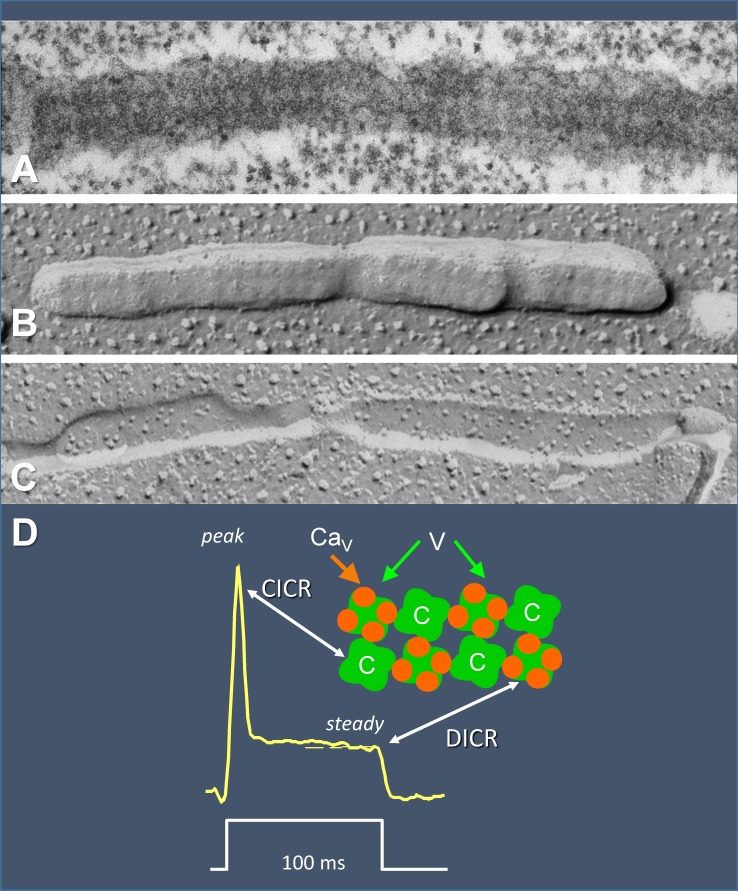
But almost at the same time, Franzini-Armstrong’s group revealed the peculiar geometric alignment of voltage sensors and Ca2+ release channels, which systematically skip every other channel in a checkered double row (Fig. 2; Block et al., 1988). To make functional sense of this arrangement, Ríos and Pizarro (1988) proposed that the RyR channels that lack overlapping voltage sensors (Fig. 2 D, parts labeled C) are operated by CICR.
Figure 2.
The relative placement of RyRs and CaVs in muscle. (A) Thin section showing double rows of “feet” (RyRs). (B) Freeze fracture of SR membrane, again showing feet. (C) Freeze fracture of t tubule membrane. (D) Interpretation by Block et al. (1988) and proposed correspondence between components of release flux and contributions by either V channels (linked to sensors and therefore assumed to engage in DICR) or C channels (assumed to activate by CICR and rapidly undergo CDI). A–C, previously unpublished, are a gift from C. Franzini-Armstrong. D recasts drawings by Ríos and Pizarro (1988).
Although there was no specific functional evidence in favor of this proposal, it also justified kinetic aspects of the waveform of global (i.e., cell averaged) SR Ca2+ release flux, derived a few years earlier from the cytosolic Ca2+ transients of frog twitch muscle (Baylor et al., 1983; Melzer et al., 1984, 1987). As shown in Fig. 2 D, the waveform elicited by a depolarizing step starts with a peak, followed by a lower, nearly steady plateau. Ríos and Pizarro (1988) proposed that the depolarization first opens the V channels and the ensuing local increase in [Ca2+]cyto causes the C channels to open. Although the activity of C channels was envisioned as short lived and terminated by inactivation (justifying the flux peak), the proposal included that V channels are kept open by the voltage sensors for the duration of the pulse, thus explaining the plateau. This hybrid model was neither formulated quantitatively nor tested experimentally until the following decade (see sections named Ca2+ signals of mammalian muscle and The couplons of skeletal and cardiac muscle). Although eventually proven wrong in many respects, the model was a useful stepping stone in the emerging theory.
Starting with Tanabe et al. (1988), Kurt Beam and collaborators confirmed and defined in increasing detail the control of Ca2+ release by DHPRs; their approach was the expression of CaVs, wild type or mutated, in primary myotubes derived from pups with a recessive mutation (i.e., dysgenic; Powell and Fambrough, 1973) that causes the deletion of CaV1.1. This illuminating approach also demonstrated crucial functional differences between the skeletal CaV1.1 and the cardiac CaV1.2, which render the latter unable to activate the RyR in the characteristically Ca2+-independent DICR manner, although still capable of activating it via CICR (Tanabe et al., 1990). Combined with EM of the dysgenic myotubes expressing heterologous DNA, the approach demonstrated the ability of CaV1.1 (but not CaV1.2) to recreate the junctional tetrads of particles present in the t-tubular membrane of wild-type myofibers (Takekura et al., 1994). Since 1994, the availability of mice engineered for the deletion of RyR1 (Takeshima et al., 1994) was used to demonstrate reciprocal functional effects between RyR1 and CaV1.1 (Nakai et al., 1996). This feature was evidence that the interaction involves mechanical contact, that the presence of RyR1 is required for the formation of CaV1.1 tetrads (Takekura et al., 1995), and that these interactions are specific for the skeletal isoforms (RyR1 and CaV1.1).
These newly understood functional and structural interactions fostered a binary view whereby DICR, a conformationally mediated mechanism, and CICR, a “chemical” transduction, present respectively in skeletal and cardiac muscle and are mutually exclusive. The demonstration of Ca2+ sparks in skeletal muscle came to disrupt this consensus.
Ca2+ sparks affirm CICR in skeletal muscle
The discovery of Ca2+ sparks in cardiac myocytes rapidly led to their description in frog skeletal (Tsugorka et al., 1995) and arterial smooth muscle (Nelson et al., 1995). An essential contribution by Klein et al. (1996) showed that Ca2+ sparks of skeletal muscle can be elicited by elevated [Ca2+] in the cytosol, as well as caffeine, a promoter of CICR. Reporting that Vm-evoked events increased their amplitude in a quantized manner (consistent with equal contributions by a variable, small number of channels) as Vm was incremented, Klein et al. (1996) proposed, in agreement with Ríos and Pizarro (1988), that the increase is caused by CICR-mediated recruitment of C by V channels (Fig. 2 D), which grows as more V channels open within sparks.6
The number of channels contributing to a Ca2+ spark is mechanistically informative; the nearly simultaneous opening of multiple channels requires concerted gating, and Ca2+ appears as a likely mediator. The calculation of this number was done by “forward” modeling, which assumes the individual channel flux and calculates how many channels would be needed to build the measured Ca2+ spark (Jiang et al., 1999), or by awkwardly named “backward” calculations, which solve the inverse problem of determining the Ca2+ flux that caused the measured spark (Ríos et al., 1999). The various calculations led to estimates between 1 and 60 channels. Based on modeling comparisons, Chandler et al. (2003) provided a way of reconciling the wide range of estimates, inferring that sparks involve many more channels in permeabilized myofibers than in intact ones. This conclusion, however, has not been confirmed by direct comparisons on the same setup. Other evidence that multiple channels contribute to a spark include the observation that full sparks are much greater and spatially complex than events induced by imperatoxin, ryanodine, and bastadin 10, toxins that open channels to a known fraction of full conductance (Schneider, 1999; González et al., 2000a). Likewise, large differences were found between sparks and “embers” (i.e., long-lasting events, presumably reflecting the opening of single channels, which may precede or follow sparks; see additional discussion below; González et al., 2000b).
The distribution of measured spark amplitudes was also scrutinized to unveil mechanisms. The apparent quantization and presence of local maxima (modes) in this distribution (Klein et al., 1996; Shirokova and Ríos, 1997) were first naively interpreted as reflecting a stereotypical activation of multiple channels (which implies CICR). This interpretation requires that the distribution of Ca2+ sparks remains quantized after imaging by the microscope. This condition does not hold in general; four papers advanced the theory of confocal image acquisition later in the decade, proving that the observed (imaged) distributions cannot retain modes present in the actual (true) distributions. Pratusevich and Balke (1996) predicted that modes would disappear upon confocal sampling. Izu et al. (1998) and Cheng et al. (1999) independently showed that sparks of fixed amplitude α, when occurring at random distances from the focal plane of a confocal imaging system, will give rise to an observed distribution of amplitudes a of density f(a) = k/a (where k is constant). This relationship, valid for a ≤ α, implies that the apparent amplitude distribution will be monotonically decaying, regardless of the true amplitudes, single or multiple, of the imaged events. Ríos et al. (2001) generalized the study to find that (under reasonable assumptions) the observed distribution f(a) and the true distribution g(a) are related by
| (3) |
where k is a constant.
This theory allowed the back calculation of g(a) starting from the observed f(a). Thus corrected, the amplitude distribution had a mode in most cases (González et al., 2000a). In the same fibers, much smaller events could be elicited, which in cardiac muscle were dubbed “quarks” (Lipp and Niggli, 1996) and in skeletal muscle appeared as embers after sparks evoked by agonist drugs (González et al., 2000a).
The demonstration of modes in the distribution of amplitudes, together with the presence of much smaller embers and quarks, confirmed that sparks are caused by the opening of multiple channels. Moreover, modes in g(a) were accompanied by modes in the distribution of rise times. The implications are profound; as discussed by Bridge et al. (1999), Cannell and Soeller (1999), and González et al. (2000b), modes in rise times rule out Markovian channels gating reversibly, i.e., with rate constants depending on the present state only and satisfying microscopic reversibility (Colquhoun and Hawkes, 1995). Later work demonstrated that neither cardiac Ca2+ sparks (Wang et al., 2002) nor spontaneous events of Ca2+ release in frog skeletal muscle (Rengifo et al., 2002) could be caused by channels gating reversibly. The conclusion from these studies was that sparks result from multiple channels gating concertedly, which requires a synchronization mechanism, and irreversibly, which requires an energy source. CICR appeared as the obvious concerting mechanism; the source of energy is presumably the SR-to-cytosol Ca2+ gradient, which couples to the gates via CICR.
In spite of the consensus that Ca2+ sparks are a manifestation of CICR, whether sparks (and CICR) were relevant to skeletal EC coupling remained in dispute. Examination of the global, cell-wide Ca2+ transients provided some answers.
Ca2+ signals of mammalian muscle
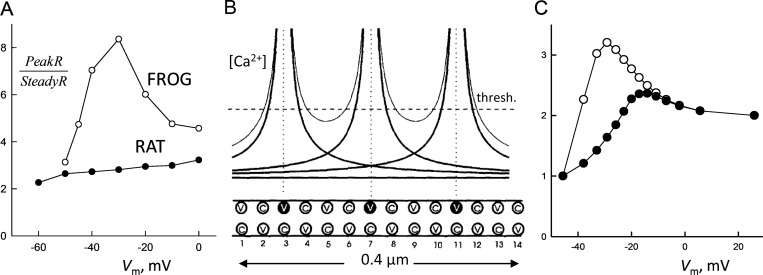
The early studies of Ca2+ sparks overlapped in time with the extension to mammals (Delbono and Stefani, 1993; Garcia and Schneider, 1993) of techniques developed on frog muscle to determine flux of Ca2+ release. Using these techniques, Shirokova et al. (1996) found a striking difference between muscles of amphibians and rodents. As shown in Fig. 3 A, in the frog, the dependence of the peak amplitude of Ca2+ flux (P) with applied voltage Vm differs sharply from that of the steady level (S) reached after the peak. Consequently, the ratio P/S rises as Vm increases, reaches a maximum at about −40 mV, and decays at higher Vm. In contrast, P(Vm) is nearly proportional to S(Vm) in the rodents, so that P/S only rises slowly throughout the voltage range. The simple calculation of Fig. 3 (B and C), a hybrid of DICR and CICR whereby the local Ca2+ domains generated around open V channels add up to activate intercalated C channels, explained without added assumptions the peculiar dependence of P/S on Vm observed in frogs.
Figure 3.
Voltage dependence of the ratio of release flux measures. Flux measures P (peak) and S (steady) are defined in Fig. 2. (A) Measured ratios P/S in frog and rat muscle. (B) A model in which the peak component is attributed to flux through C channels activated by Ca2+ domains near open V channels. The graphs illustrate components provided by three open V channels (thick lines) and their sum (thin lines). C channels open when [Ca2+] goes above a threshold level (dashed line). (C) The model accounted qualitatively for the modal dependence P/S (Vm) in frog muscle (open circles). A simple change in parameters that made the C channels less excitable did not fully account for the qualitative characteristics of the dependence in the rat (filled circles). Details in Shirokova et al. (1996).
The calculation of Shirokova et al. (1996) included drastic simplifications, as it assumed static concentration profiles and ignored any contribution of C channels to the local [Ca2+]cyto. It reproduced, however, the essential nonlinearity of the system at low Vm (as arising from an additive interaction of concentrations facing a binary activation threshold). It also explained the decay of P/S at higher Vm, as the CICR contribution stops increasing when all C channels are open.
The observations revealed a major difference between taxa. The flat P/S (Vm) of rodent muscle, which according to the model reflects an absence of CICR, together with evidence of a greater density of RyRs (relative to CaVs) in amphibians (Bers and Stiffel, 1993; Margreth et al., 1993; Anderson et al., 1994), suggested that the amphibians’ CICR is carried via their excess RyRs. This proposal matched emerging evidence of the presence of two RyR isoforms in amphibian (Lai et al., 1992) and avian muscle (Airey et al., 1990). These isoforms, named α and β, are respectively orthologues of mammalian skeletal RyR1 and of RyR3, which is expressed in neurons and other cells but not in most skeletal muscles (Conti et al., 1996; Ottini et al., 1996). In subcellular preparations, α and β activated respectively by DICR and CICR (Ivanenko et al., 1995; Kashiyama et al., 2010). The obvious expectation was that frogs’ CICR be carried by their β isoform.
Shirokova et al. (1996) also reported that peak release flux is greater in frogs than in mammals under comparable conditions. This difference is consistent with the excess channels and the putative presence of CICR in amphibians. At a Biophysical Society meeting, Elizabeth Stephenson stood by Shirokova’s poster and suggested a compelling teleological explanation of these differences. She noted that although frog muscles have one triad per sarcomere, located near the Z disk, mammals have two, placed much closer to the target for the released Ca2+ (the region of overlap between thick and thin filaments). Given the nonlinear relationship between distance and time in diffusion processes, the dual triads of mammals should allow for a tighter control of contraction, with less released Ca2+ and no need for the intrinsically explosive CICR mechanism.
The couplons of skeletal and cardiac muscle
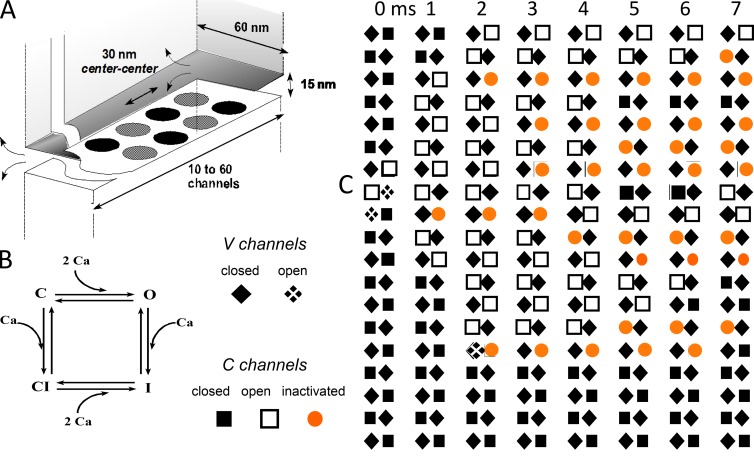
The inconsistencies in the hybrid DICR/CICR calculation by Shirokova et al. (1996) mentioned in the previous section were removed by Stern et al. (1997), who computed all channel interactions dynamically using the Monte Carlo simulation and the more appropriate geometry shown in Fig. 4. The simulations derived global Ca2+ flux by averaging the stochastic release events produced by sets of RyRs and their controlling CaVs. The number of RyRs in these sets was chosen to match the size of t–SR junctions. CaVs activated alternate RyRs (V type) according to allosteric interaction rules established earlier by comparing release activation and voltage sensor charge movements (Ríos et al., 1993). The Monte Carlo runs produced realizations, Ca2+ release events leading to local Ca2+ transients. Fig. 4 includes a typical realization in which activation, started at two V channels, propagates by CICR along the set of RyRs.
Figure 4.
Self-consistent simulation of a hybrid DICR–CICR model of Ca2+ release. (A) Geometry. Ca2+ is released via alternating V and C RyRs in double rows. Local [Ca2+] is determined by Ca2+ diffusing inside a junctional gap and surrounding wide cytosol. (B) A diagram depicts the model for activation and inactivation of C channels. V channels depend on Vm according to a quantitative allosteric scheme (Ríos et al., 1993). They are assumed not to inactivate. (C) Successive snapshots of the array of channels in one Monte Carlo realization. An event started at 0 ms with voltage activation of two channels progresses via CICR along the array. The Ca2+ transient associated with this event has spatial and temporal properties of a Ca2+ spark. Note that it was sufficient with the inactivation of one channel ahead of the activation wave to stop its downward progression. From Stern et al. (1997).
The local events produced were thus passable copies of experimental sparks. Their averages reproduced features of the cell-wide records, including the observed mode in P/S (Vm). The success of the model endorsed the coexistence of DICR and CICR and Stern’s conclusion that control is local.
The simulations also showed that, under reasonable parameter values, it is sufficient with inactivation of one C channel to interrupt propagation (in Fig. 4, a single inactivated channel prevented propagation to the lower third of the set). This fragility implies that activation in one side of a triad can hardly propagate to the other side. The release channels and voltage sensors on one side of a triad thus behave as a functional unit, which was named “couplon.” The definition was immediately extended to cardiac muscle (Stern et al., 1999), where the interaction between CaVs and RyRs was modeled as having been purely caused by CICR, although the RyR–RyR interaction could be mediated by both CICR and conformational signaling.
That RyR2 channels may interact allosterically was contemplated often in later work but never supported by hard evidence. Recently, studies of cardiac couplons showing that the clusters of RyRs have fewer elements, with numbers and geometric arrangement more variable than originally thought (Baddeley et al., 2009; Soeller and Baddeley, 2013; Asghari et al., 2014), have made conformational RyR2–RyR2 interaction less attractive. However, the cryo-EM studies of Cabra et al. (2016) have showed that one of the configurations in which RyR2s mutually interact in vitro involve an apparent overlap, consistent with an intricate contact that might support allosteric interactions if present in vivo.
Additionally, progress in biochemical and imaging studies demonstrated the presence of many smaller molecules that bind to RyRs and CaVs. Initially, the attention was placed on the SR membrane protein triadin (Kim et al., 1990) and the luminal protein calsequestrin (MacLennan and Wong, 1971); a long and growing number of proteins are now known to interact with CaVs, RyRs, or both. The couplon definition was accordingly generalized to include them (Ríos et al., 2015). Whether and how altering any of these proteins modifies the couplon function are the subject of active research (Rebbeck et al., 2014). This line of work was punctuated recently by the demonstration of an essential role of the adapter protein Stac3 in the activation of RyR1 by voltage (Horstick et al., 2013; Nelson et al., 2013; Polster et al., 2016) and the enormous enhancement that it produces in the expression of heterologous CaV1.1 in Xenopus laevis oocytes (Wu et al., 2018).
Direct tests of a physiological role of CICR in skeletal muscle
The evidence for CICR operation in skeletal muscle reviewed so far is largely indirect. The many experiments done to more directly test and quantify the putative physiological role of CICR will be reviewed in two groups: those involving the introduction of a Ca2+ buffer inside cells, realized largely in the 1980s and 1990s, and those using molecular manipulations, developed after the year 2000.
Probing CICR with Ca2+ buffers
The first use of a cation buffer to probe muscle contraction was communicated by Emil Bozler in this journal, in 1954. Bozler used EDTA to remove Ca2+ from membrane-permeabilized muscle. The more selective buffer EGTA (Weber and Winicur, 1961) later allowed for setting [Mg2+] higher to probe the roles of Ca2+ and Mg2+ in controlling the interactions of actin and myosin (Herz et al., 1969). EGTA was first applied intracellularly by Portzehl et al. (1964), who used the contractile response to solutions of different [Ca2+] injected into muscle cells to establish that resting [Ca2+]cyto is <200 nM. EGTA was again used as monitor, this time of released Ca2+, in a method called EGTA–phenol red (Pape et al., 1995) based on the near stoichiometric displacement of H+ by Ca2+ as it binds to EGTA. That deprotonation of EGTA must precede or accompany Ca2+ binding both slows the reaction kinetics and makes it pH dependent; these inconveniences led to the development of BAPTA (Tsien, 1980) and its use (Marty and Neher, 1985) as a much faster Ca2+ chelator.
Conversely, Ca2+ monitors proved useful as buffers. In an early application, Kovacs et al. (1983) calculated the cytosolic concentration of endogenous Ca2+ buffers from the changes in the decay kinetics of Ca2+ transients induced by known concentrations of the absorption indicator Antipyrylazo III; their work also called attention to the inescapable perturbation of Ca2+ transients by its monitors.
Baylor and Hollingworth (1988) pioneered the use of the buffer properties of monitors to probe mechanism. Large quantities of Fura-2 in frog myofibers changed the dye signals in ways consistent with an increase in action potential–induced Ca2+ release, seen as resulting from a reduction of the inactivation of Ca2+ release by Ca2+ (Ca2+-dependent inactivation [CDI]). This affirmation of CDI indirectly negates a major role of CICR. Also weighing against this role was the reported absence of effects of the specific CICR inhibitors procaine and adenine (Endo, 1985) and the anemic rates of Ca2+ release evoked by Ca2+ either in skinned frog fibers (Murayama et al., 2000) or in vesicular SR fractions of rabbit muscle (Meissner et al., 1986).
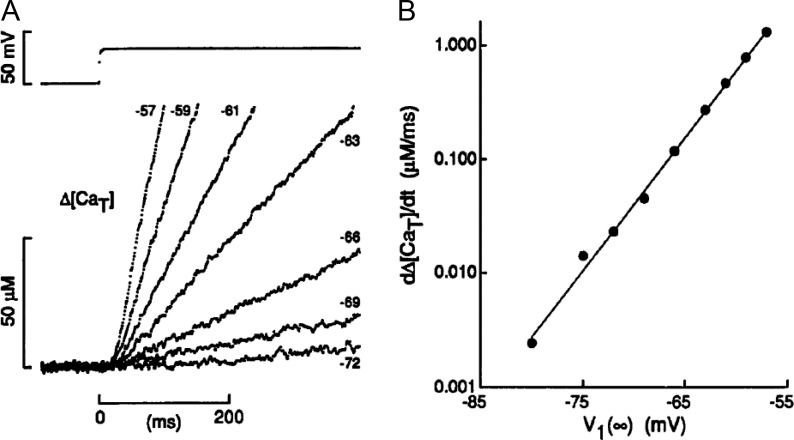
Additional arguments against the physiological operation of CICR came from the laboratory of W.K. Chandler. Pape et al. (1995) used the EGTA–phenol red technique to accurately define the Vm dependence of release flux at Vm near the resting potential. Fig. 5 shows superimposed changes in total cytosolic Ca2+ concentration upon long-lasting depolarization at the Vm values listed. In B, the rate of change of [CaT] is shown to vary exponentially with Vm, a dependence consistent with activation by voltage, exclusive of other mechanisms (Almers, 1978). In experimental observations (Klein et al., 1996; Lipp and Niggli, 1996), CICR manifests as a nonlinear additive component to DICR. The observed exponential dependence is therefore indicative of exclusive control of release by a voltage sensor in the negative range of voltages tested. Pape et al. (2002) would later show how to reconcile these results with the operation of CICR (also see Fig. 7).
Figure 5.
Ca2+ release near the resting potential. (A) Evolution of total released calcium [CaT] upon application of pulses (top) at Vm near the resting potential. (B) Log of the slope of plots in A versus applied voltage. The slope (3.7 mV)−1 corresponds to an effective sensing charge of 6.7 e. At Vm greater than −55 mV, the slope was reported to diminish. From Pape et al. (1995).
Figure 7.
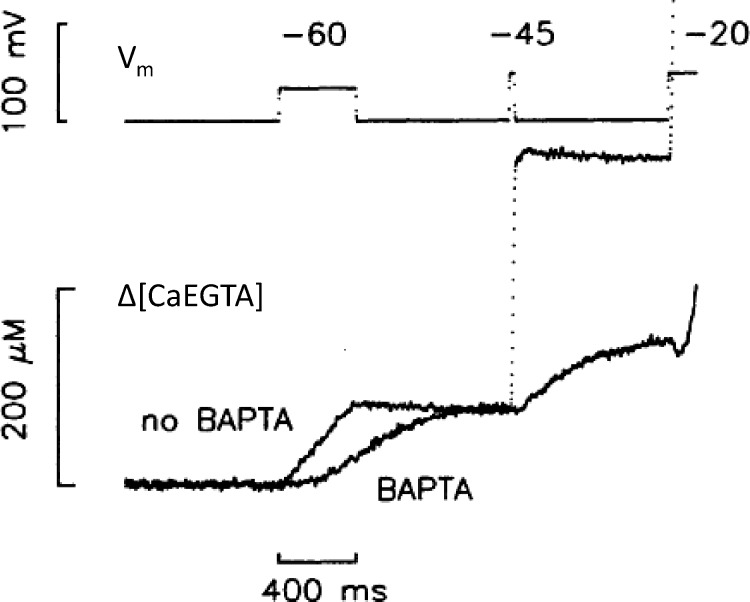
The effect of BAPTA on Vm-elicited flux is strongly dependent on the applied voltage. Δ[CaEGTA] measures total Ca released. The difference in amount at −60 mV is nil (BAPTA delays the transfer of released Ca2+ to EGTA). It is maximal at intermediate voltages (−45 mV in this case). This evidence of Vm dependence of a putative CICR component is consistent with the modal Vm dependence of P/S illustrated with Fig. 3. From Pape et al. (2002).
Calcium concentration near arrays of open channels
The articles by Pape et al. (1995, 1998) constituted milestones in the analytical description of [Ca2+]cyto near open Ca2+ channels. In earlier attempts, Neher (1986) and Stern (1992b) derived an expression for the steady increase in concentration versus distance r from the mouth of a point source (channel) of flux ϕ in an isotropic medium containing a buffer at high concentration (say, 20 mM EGTA):
| (4) |
In this expression, the first factor represents the distribution in the absence of buffer. The effect of the buffer is to “smear” the distribution by an exponential of space constant λ, determined by the ion’s diffusion constant, D, and its rate of binding to the buffer (which is the inverse of the mean time required by the buffer to complex Ca2+: ).
| (5) |
The approach used by Stern (1992b) removed the high buffer simplification (and showed that it was good in most cases of interest). Pape et al. (1995) then found an expression for the concentration profile at times t after channel opening, which turned out to be equal to the steady-state profile (Eq. 4) multiplied by a function F of time and space that starts at value 0 at t = 0 and tends to 1 as time increases:
| (6) |
Under the buffering conditions of interest and at distances of <0.5 µm, F is very close to 1 (and the steady solutions are therefore appropriate).
One Ca2+ flux waveform leads to two opposite conclusions
Using the tools above, Pape et al. (1998) described analytically [Ca2+](r) near a set of active Ca2+ release channels with couplon geometry, immersed in a medium with realistic buffers. They compared the effect of the presence of buffers, which reduce Δ[Ca2+]cyto more effectively at greater distances from the open channel, with that of SR depletion, which affects the unitary Ca2+ current and therefore reduces Δ[Ca2+]cyto evenly at all distances. As total SR Ca content ([Ca]T, SR) was reduced, the fraction of total SR Ca released by an action potential increased greatly, suggesting reduction of CDI. With one exception,7 the experiments failed to reveal the effects predicted if CICR was operative. Application of Eqs. 5 and 6 located the inhibitory site at <22 nm from the open channel; that is, on the same RyR or the nearest neighbor in the couplon.
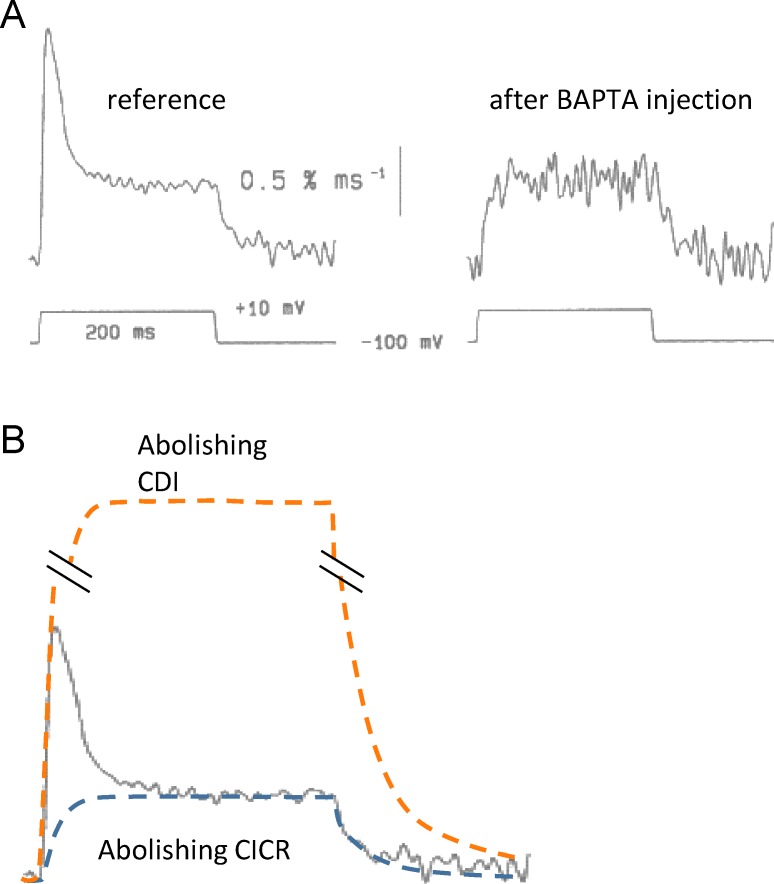
Surprisingly, M.F. Schneider and colleagues reached the opposite conclusion from a similar approach. Injection in frog myofibers of BAPTA, together with Fura-2 (which served as monitor of both the injection of BAPTA and the change in cytosolic Ca2+), altered calculated Ca2+ release flux in ways consistent with inhibition of CICR (Jacquemond et al., 1991; Csernoch et al., 1993). After injection of the buffers, the waveform of Ca2+ release flux lost its peak (Fig. 6 A). As illustrated in Fig. 6 B, two interpretations of the blunting effect were possible: in the hypothesis of a hybrid activation mechanism, the loss of the peak could simply reflect loss of the CICR component; alternatively, if the buffers just prevent CDI, a loss of peak will also ensue, caused by loss of the decay that follows the peak.
Figure 6.
The effect of a fast Ca2+ buffer on calculated Ca2+ release flux. (A) Flux calculated by Jacquemond et al. (1991) from cytosolic Ca2+ transients measured in frog myofibers before and after injection of BAPTA. (B) Reference record from A with superimposed lines depicting predictions of the records that would result if BAPTA abolished inactivation (orange) or activation by Ca2+ (blue). The calculations of flux by Jacquemond et al. (1991) are consistent with abolition of CICR.
The disparate interpretations of the loss or blunting of flux peak emerged from different methods to evaluate the flux magnitude, which resulted in strikingly different scalings of a similar waveform (Fig. 6 B, blue and red traces). Although the disagreement was never truly solved, Pizarro and Ríos (2004) tried to reconcile the conclusions by applying both methods (the removal analysis of Melzer et al., 1987, used in the Schneider laboratory, and the EGTA–phenol red method of Chandler and colleagues) simultaneously to the same frog cells. Their conclusion essentially split the difference, finding buffer-induced reductions in both CICR and CDI.
In a further stab at reconciliation, Pape et al. (2002) recorded Ca2+ flux in frog myofibers under voltage clamp while curtailing the increase in [Ca2+]cyto near open channels, either by BAPTA in the cytosol or depleting [Ca]T, SR. Their novel idea was that this change should reduce the putative CICR contribution in a Vm-dependent manner, much in the same way as P/S depends on Vm and for the reasons proposed in Fig. 3. The result (Fig. 7) was that BAPTA, entering the cytosol from cut fiber ends, markedly reduced the Ca2+ released at intermediate Vm, although at −60 mV the amount remained the same (the kinetic difference shown is expected, as Ca2+ must transit to EGTA from the faster reacting BAPTA). The selective effect at −45 mV is consistent with both the Vm dependence of P/S (Fig. 3 A) and the results in Fig. 5, suggesting that release at −60 mV reflects DICR and, at higher Vm, recruits an extra CICR component.
On balance, the application of intracellular buffers suggested but did not demonstrate a significant contribution of CICR to Ca2+ transients for EC coupling in skeletal muscle. Although Ca2+ sparks were believed to involve CICR, fully establishing their mechanism and their contribution to physiological signals required other approaches.
Probing CICR with heterologous expression
As described earlier in this article, this approach started with the expression of CaV1.1 and RyRs in primary myotubes. Consequently, it was not useful at first to establish the physiological role of CICR in adult muscle. Two advances at the turn of the century changed this situation. First, Felder and Franzini-Armstrong (2002) noted parajunctional feet (PJFs; RyRs located outside the t–SR junctions). PJFs were found in muscles containing both isoforms 1 and 3 (including frog fast-twitch and fish swim muscles), and in RyR1-only muscles, feet appeared exclusively in a double row at the junction (junctional feet [JFs]), in interaction with CaVs. Felder and Franzini-Armstrong proposed that PJFs are of isoform 3. Based on the different clustering patterns of JFs and PJFs, they also surmised that JFs are exclusively of isoform 1.8
This evidence forced revision of the models of Shirokova et al. (1996) and Stern et al. (1997) because the assumed double row geometry was wrong for the (frog) cells that originate the simulated phenomena. Instead, the geometry was adequate for fast-twitch mammalian muscle, which has no PJFs. The simulation of events was wrong there as well, as no sparks were observed in mammalian muscle under stimuli that would cause them in the frog (Shirokova et al., 1998; Csernoch et al., 2004).
Evidence of different properties of RyR1 and RyR3 was gathered in myotubes from RyR (1 or 3)-null mouse embryos (Conklin et al., 2000) and by expressing either isoform (Ward et al., 2000, 2001) in a dyspedic myogenic cell line devoid of RyRs (Moore et al., 1998). Although both isoforms generated spontaneous spark-like events, RyR3 did it better, but failed to activate by membrane depolarization.
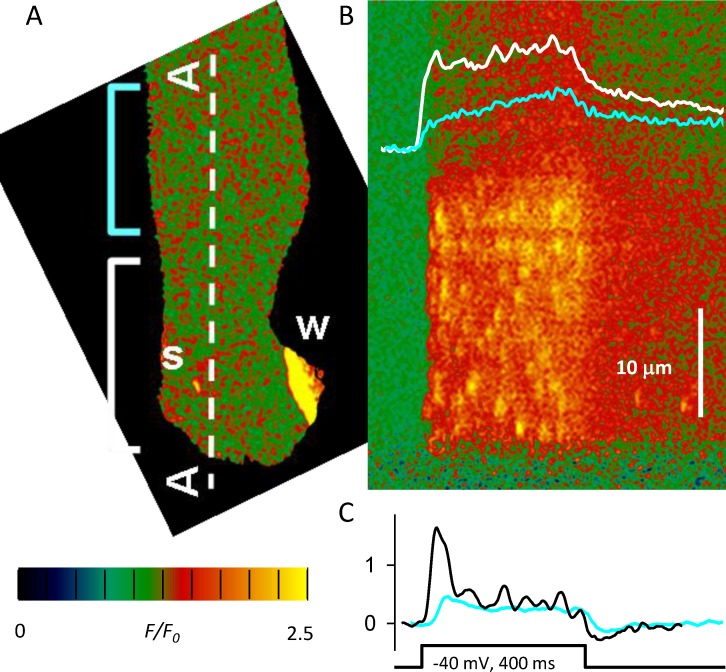
After these advances and using a technique to express heterologous proteins in muscles of adult mice optimized by DiFranco et al. (2006), two groups endeavored to express RyR3 in adult murine muscles, with striking results. Pouvreau et al. (2007) found that the expression caused sparks to appear, both spontaneously and under depolarization, in myofibers initially devoid of events. As shown in Fig. 8 A, the Vm-elicited sparks appeared in segments of the myofiber located near perinuclear regions actively synthesizing the foreign protein, where spontaneous events were frequent. This segmental expression permitted a comparison with nearby segments that were silent, i.e., with no evidence of exogenous activity. The sparks activated by voltage (Fig. 8 B) contributed to a peak of Ca2+ release (Fig. 8 C) much greater than that present in the silent regions. Using the same approach, Legrand et al. (2008) similarly found segmental expression, spontaneous activity confined to areas of high RyR3 expression, and a more prominent peak of Ca2+ release, but did not detect Vm activation of sparks. This difference notwithstanding, the experiments established RyR3 as a source of Ca2+ release not directly activated by voltage. Additionally, the RyR3-dependent modification of the global Vm-induced Ca2+ flux waveform and the observation of Vm-stimulated spark-like events completed the demonstration of a hybrid DICR–CICR mechanism with separate RyR1 and RyR3 components.
Figure 8.
Expression of RyR3 in a myofiber from adult mouse. (A) Isolated myofiber held at resting potential under patch clamp. Fluo-4 reveals abortive Ca waves originating at a swollen nucleus (W). Simultaneously, spontaneous sparks (s) appear randomly, exclusively in the fiber segment within the white bracket. (B) Confocal scan along line A–A in A. An applied pulse of −40 mV elicits a response that includes sparks in the segment within the white bracket, but is devoid of sparks in the adjacent segment (cyan bracket). The Ca2+ transient is greater in the segment with sparks (white trace). (C) The calculated Ca2+ release flux includes a peak in the sparking region (black trace) that is not present in the sparkless area (cyan). Modified from Pouvreau et al. (2007).
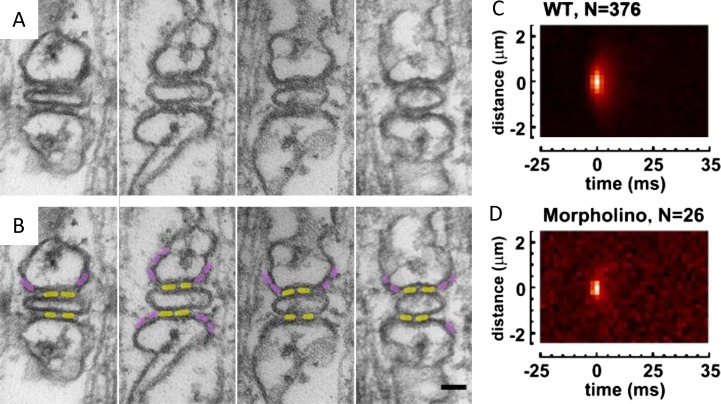
In spite of these advances, doubts remained regarding the nature of the Ca2+ sparks and their contribution to physiological Ca2+ release. Two groups set out to address these questions directly. Our laboratory took advantage of a novel dual confocal scanner (Zeiss), which scans simultaneously with two separately focused lasers. We used one scanner to deliver a local Ca2+ bolus at selectable locations via photorelease of caged Ca2+ while monitoring the response with the other (Figueroa et al., 2012). Two-photon excitation of a novel high efficiency “cage” resulted in a bolus (named SLIC, for synthetic local increase in calcium) that was adjustable to mimic the spatial size and duration of a Ca2+ spark. The SLIC was delivered outside the fibers (at 2 µm from the plasma membrane, permeabilized by saponin) both to avoid direct stimulation by the uncaging irradiation and to allow quantitative measurement of stimulus [Ca2+] separate from the response. With frog muscle, SLICs consistently stimulated Ca2+ release. The stimulus [Ca2+] level measured at the cell boundary could be as low as 180 nM and still elicit a response. In contrast, mouse myofibers did not produce measurable release even with the most intense SLICs (8 µM at contact point) and with the inhibitory Mg2+ set at unphysiologically low concentrations. In view of this low threshold [Ca2+], we concluded that CICR occurs during normal EC coupling in frog muscle and does not work in mammals under physiological conditions.
The question was also addressed directly by EM imaging and functional probing of zebrafish “morphant” larvae, which were injected at the one-cell stage with a morpholino that impedes expression of RyR3 (Perni et al., 2015). Wild-type muscle showed normal JF and PJF arrays (Fig. 9, A and B) as well as frog muscle–like Ca2+ sparks. Morphant muscle instead had an 80-fold lower frequency of sparks, which were smaller (Fig. 9, C and D). Most importantly, PJF were nearly absent in morphants. These observations establish that RyR3 are parajunctional and generate sparks by CICR. As to the physiological relevance of CICR, the work was inconclusive because it did not find deficits in the swimming behavior of morphant larvae.
Figure 9.
Junctional and parajunctional feet in muscle of young zebrafish embryo. Triads shown in transversal section. (A) EM images. (B) Interpretive colorization. Parajunctional feet, marked purple, disappear upon injection of morpholinos (not depicted in this figure). (C) Average of reference sparks. (D) Average of events in morphant larvae, which are scarce and smaller. Modified from Perni et al. (2015). Bar, 50 nM.
The failure of RyR1 to engage in CICR is surprising, as in bilayers RyR1 and RyR3 open at about the same [Ca2+] (Fig. 1; also see section named The RyR in subcellular preparations). This discrepancy has been addressed; working with vesicular fractions, Murayama and Ogawa (2001) found that the binding of ryanodine, as a measure of channel activation, was 20 times greater for the β than the α isoform (Fig. 1). This result suggests that the ability for CICR of α/RyR1 is inhibited in the SR vesicles by interactions lost in the process of molecular isolation.9
Shirokova et al. (1999) reported a striking segregation of Ca2+ events in primary mouse myotubes. In response to depolarization, myotubes produced Ca2+ release devoid of sparks, typical of the adult. Sparks only occurred in areas unresponsive to depolarization. This segregation occurred in both wild-type and RyR3-null myotubes, hence proving that RyR1 are capable of both Ca2+ sparks and sparkless function. Consistent with this observation, Chun et al. (2003) reported that both RyR1 and 3 can generate sparks in embryonic and early postnatal myofibers. Later, it was found that sparks occur only where t tubules are absent (Zhou et al., 2006; Brown et al., 2007). The repression of spontaneous events at t tubules was associated with the presence of CaV1.1, as it did not occur in cells cultured from CaV1.1-less muscular-dysgenic (mdg) mice (Zhou et al., 2006) or in regions with disorganized t tubules of adult myofibers undergoing dedifferentiation in culture (Brown et al., 2007). These observations confirmed that the intrinsic sensitivity of RyR1 for activation by Ca2+ is prevented when the channels join in the DICR-capable couplon.
CICR in disease
Although CICR does not operate in most mammalian muscles under physiological conditions, its operation under altered or diseased situations is now evident. Kirsch et al. (2001) described conditions to systematically observe local events in muscle of mice and rats. These were dubbed elementary Ca2+ release events (ECRE) because, unlike sparks, they appeared in a variety of spatial shapes and time courses. Because large ECRE require Ca2+ release through multiple channels, they should, in principle, involve CICR. The conditions for their production included mechanically removing or chemically permeabilizing the plasma membrane, an observation consistent with the inhibition by CaVs proposed earlier to explain the absence of CICR in rodent muscle. Later, Wang et al. (2005) showed that mechanical stress on the plasma membrane (applied via osmotic changes) elicits ECRE and demonstrated an increased susceptibility to their induction by either osmotic changes or fatiguing exercise in a mouse model of Duchenne muscular dystrophy (mdx). This propensity, attributed to reactive oxygen species (Martins et al., 2008) or destabilization of triadic junction by loss of dystrophin (Teichmann et al., 2008), suggested roles of sparks or ECRE in other diseases.
Thus, the operation of CICR was sought in models of malignant hyperthermia (MH), a condition characterized by life-threatening hypermetabolic events mediated by uncontrolled Ca2+ release in skeletal muscle and typically associated with gain-of-function mutations in RyR1. Given that hypersensitivity to CICR-promoting caffeine is an MH diagnostic criterion, an enhancement of CICR was expected in MH. This expectation was affirmed by the observation of increased Ca2+ sensitivity to activation in isolated human RyRs with MH-linked mutation G2434R (Richter et al., 1997). The outcomes in living cells, however, were largely negative. In a technically heroic comparison of voltage-clamped fiber segments biopsied from 18 patients with either positive (MHS) or negative (MHN) diagnosis (Struk et al., 1998), the Vm-elicited Ca2+ release was kinetically similar, differing only for greater amplitude in MHS cells. Likewise, Manno et al. (2013) found neither ECRE nor responses to SLICs in mouse muscle bearing the Y522S mutation, a significant negative finding, as this animal models a human mutation that causes an MH of florid phenotype, including lesions interpreted as resulting from local CICR. The altered response was instead consistent with a reduced susceptibility of RyRs to CDI.
In an unexpected turn, Apostol et al. (2009) showed that ECRE would not be elicited by osmotic changes if mouse myofibers were held depolarized, which inactivates CaV1.1. The observation thus indicated an agonist role of CaV1.1, consistent with Apostol’s finding of fewer ECRE in myogenic cell lines devoid of CaV1.1. Collectively, these studies identified t-tubular membrane deformation as the main determinant of ECRE and evinced that they may result from altered interactions involving multiple couplon members.
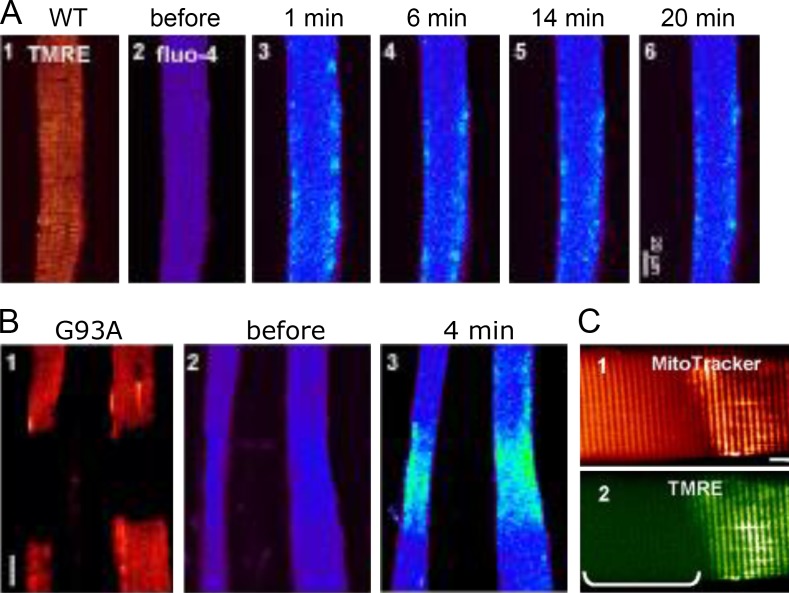
The agonist role of CaV1.1 suggests that ECRE might just be an abnormal version of DICR. Another disease, however, provided clear evidence of the involvement of CICR in ECRE. On myofibers of a mouse model of amyotrophic lateral sclerosis, Zhou et al. (2010) found segments where mitochondria either lost function or disappeared altogether (Fig. 10). In these regions, osmotically elicited ECRE evolved to widespread Ca2+ release that stopped sharply at the edge of the failing segment (Fig. 10 B). The observations demonstrated that buffering by mitochondria is crucial for the stable patterning of muscle Ca2+ signals. They also helped assert in general that CICR is involved in ECRE.10
Figure 10.
CICR in amyotrophic lateral sclerosis. (A) Single myofiber from a reference mouse. (B) Myofibers from a mouse constitutively expressing an amyotrophic lateral sclerosis–linked SOD1 mutation. Absence of tetramethyl rhodamine ethyl ester (TMRE) fluorescence marks segments lacking mitochondrial transmembrane potential. Cytosolic Fluo-4 reveals ECRE in response to osmotic stress. In the mutant, ECRE evolve to global Ca2+ release, which stops at the edge of the damaged segment. (C) MitoTracker staining indicates that mitochondria are present in the damaged regions. Eventually, the lesions progress to mitochondrial destruction. Modified from Zhou et al. (2010).
Collectively, these studies identify local abnormal Ca2+ release as a potential contributor to the pathogenic processes linked to mutations in various proteins of the triad. In most cases, however, CICR does not appear to contribute to the altered Ca2+ release.
Conclusions and questions
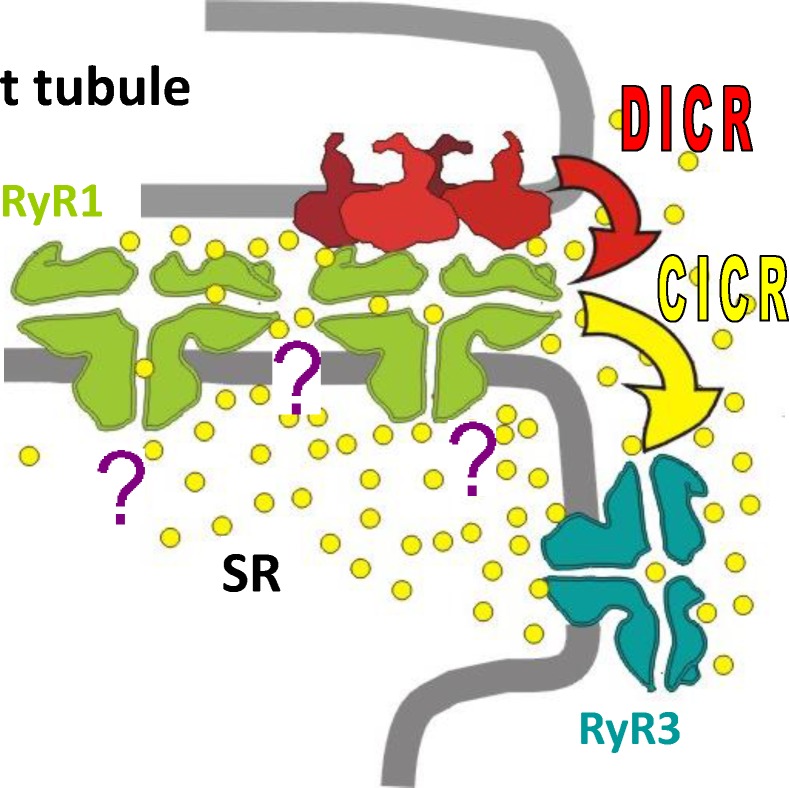
The laboriously reached consensus, illustrated by Fig. 11, is that CICR contributes to EC coupling in skeletal muscle, where it results in the production of Ca2+ sparks. Normally, the contribution is limited to muscles containing RyR isoform 3, including many tested muscles of birds, fish, and amphibians. In these muscles, CICR contributes to the fast rise in cell-wide flux and is rapidly terminated by CDI. In most mammalian muscles, CICR is nil under physiological conditions; the waveform of Ca2+ flux elicited by step depolarization still shows a peak, however, which is explained by rapid CDI. Most muscles of nonmammalian taxa have one triad per sarcomere; in these, a CICR amplification of DICR flux may be needed to ensure adequate binding of Ca2+ to its target sites on troponin, located hundreds of nanometers away from the release sites. The stages of evolution leading to mammals apparently found more liabilities than advantages in CICR, resulting instead in the adoption of a design comprising two exclusively Vm-controlled triads per sarcomere. CICR is present, however, in developing muscle, where it appears limited to regions devoid of t tubules. CICR-mediated local events, ECRE, also appear in adult mammalian muscle under special conditions, including after removal of plasmalemma and dedifferentiation as well as mechanical stress and some diseases. ECRE should be contemplated as a pathogenic mechanism in myopathies that exhibit local cellular damage.
Figure 11.
Consensus and questions. RyR1 or α, located exclusively at t–SR junctions, are activated conformationally by CaV1.1 (DICR). RyR3 or β, absent in most mammalian muscles, are located parajunctionally and activated by Ca2+ (CICR). Question marks represent the unknown role of uncoupled junctional RyR1 (C channels), the unconfirmed possibility of allosteric RyR–RyR interactions, and the evolving quest on RyR control from within the SR. Modified from Pouvreau et al. (2007).
The processes that result in the stoichiometric arrangement of CaVs and RyRs remain mysterious, as do two consequences of this arrangement (Fig. 11, question marks). They are the interdiction of CICR for RyR1 channels (which, in isolation, are sensitive to Ca2+) and the role of C channels (which could be allosteric “slaves” of their coupled neighbors, occasional contributors via highly restricted Ca2+ activation, or reserves for replacement or passive spacers). More questions emerge when the often contradictory indications of control of Ca2+ release inside cardiac muscle SR are translated to skeletal muscle (Fig. 11; Sobie et al., 2017).
Acknowledgments
I would like to thank Drs. Nagomi Kurebayashi, Takashi Murayama, Paulina Donoso, Cecilia Hidalgo, Clara Franzini-Armstrong, Stefano Perni, Paul Pape, Martin F. Schneider, Sandrine Pouvreau, Jingsong Zhou, Natalia Shirokova, Leandro Royer, Jianxun Yi, Michael D. Stern, Gonzalo Pizarro, Jesús García Martínez, Gustavo Brum, and Gerhard Meissner for allowing the use of their figures or unpublished images in this work. I am also grateful to Drs. Makoto Endo, Yasuo Ogawa, Clara Franzini-Armstrong, and Mark B. Cannell for sharing their recollections. Carolina Figueroa, Carlo Manno, Paul Pape, and Eshwar Tammineni made insightful suggestions.
This work was supported by the National Institute of General Medical Sciences (grant R01GM111254) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant R01AR071381).
The author declares no competing financial interests.
Olaf S. Andersen served as editor.
Footnotes
Hill (1949) had envisioned Ca2+ as a hypothetical activator in his argument for the inability of diffusion to effect the internal spread of activation in muscle.
Ford and Podolsky also obtained contractures upon applying changes in the anion composition of the bathing solution, a result that was incorrectly interpreted for a long time as a consequence of SR membrane depolarization and only clarified two decades later by Sue Donaldson in our department at Rush University (Donaldson, 1985; Donaldson et al., 1989).
Channel activity is determined using a concentration of [3H]ryanodine below the KD of its reaction with RyR, using a [RyR] that is less than [ryanodine]. Under those conditions, Popen is usually proportional to RyR-bound [3H]ryanodine (Meissner, G., personal communication).
The term “calcium paradox” is also used in cardiac physiology to refer to the damage caused by perfusion of isolated hearts with normal saline after exposure to Ca2+-free media (Boink et al., 1976).
Stern’s efforts to build a local model of Ca2+ release control were not equally successful but still defined concepts (calcium synapse, cluster bomb, and stochastic attrition) that became ingrained in all the mechanistic thinking that ensued.
Klein et al. (1996) also provided two simple technical insights that helped “sparkologists”: the use of a confocal aperture (pinhole size) >1 Airy disk (theoretically ideal for resolution) to maximize sensitivity and the advantage of scanning planes close to the cell surface to minimize scattering. The study of [Mg2+] dependence by Schneider’s group (Lacampagne et al., 1998) additionally defined conditions for the collection of large numbers of sparks. In a later paper, Klein et al. (1997) amended their initial conclusion, demonstrating that the frequency but not the size of sparks increased with depolarization.
A single item of evidence in favor of CICR emerged in these experiments: Ca2+ release flux decreased by about 50% upon increasing the concentration of Fura-2 in the cytosol from 5–6 to 7–8 mM (Jong et al., 1993). This decrease, however, could just reflect a deleterious effect of the dye.
An implicit assumption was that feet are homotetramers (i.e., that isoforms 1 and 3 do not combine in tetramers). This guess was upheld by the experiments of Xiao et al. (2002), which established that RyR2 can instead combine with RyR1 and RyR3.
The possibility of comparing signals in segments with and without functional mitochondria within the same cell was later used by Yi et al. (2011) for the first quantitative estimation of the flux of Ca2+ removal by mitochondria in EC coupling of skeletal muscle.
References
- Airey J.A., Beck C.F., Murakami K., Tanksley S.J., Deerinck T.J., Ellisman M.H., and Sutko J.L.. 1990. Identification and localization of two triad junctional foot protein isoforms in mature avian fast twitch skeletal muscle. J. Biol. Chem. 265:14187–14194. [PubMed] [Google Scholar]
- Almers W. 1978. Gating currents and charge movements in excitable membranes. Rev. Physiol. Biochem. Pharmacol. 82:96–190. 10.1007/BFb0030498 [DOI] [PubMed] [Google Scholar]
- Amos W.B., White J.G., and Fordham M.. 1987. Use of confocal imaging in the study of biological structures. Appl. Opt. 26:3239–3243. 10.1364/AO.26.003239 [DOI] [PubMed] [Google Scholar]
- Anderson K., Cohn A.H., and Meissner G.. 1994. High-affinity [3H]PN200-110 and [3H]ryanodine binding to rabbit and frog skeletal muscle. Am. J. Physiol. 266:C462–C466. 10.1152/ajpcell.1994.266.2.C462 [DOI] [PubMed] [Google Scholar]
- Apostol S., Ursu D., Lehmann-Horn F., and Melzer W.. 2009. Local calcium signals induced by hyper-osmotic stress in mammalian skeletal muscle cells. J. Muscle Res. Cell Motil. 30:97–109. 10.1007/s10974-009-9179-8 [DOI] [PubMed] [Google Scholar]
- Asghari P., Scriven D.R.L., Sanatani S., Gandhi S.K., Campbell A.I.M., and Moore E.D.W.. 2014. Nonuniform and variable arrangements of ryanodine receptors within mammalian ventricular couplons. Circ. Res. 115:252–262. 10.1161/CIRCRESAHA.115.303897 [DOI] [PubMed] [Google Scholar]
- Baddeley D., Jayasinghe I.D., Lam L., Rossberger S., Cannell M.B., and Soeller C.. 2009. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc. Natl. Acad. Sci. USA. 106:22275–22280. 10.1073/pnas.0908971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S.M., and Hollingworth S.. 1988. Fura-2 calcium transients in frog skeletal muscle fibres. J. Physiol. 403:151–192. 10.1113/jphysiol.1988.sp017244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S.M., Chandler W.K., and Marshall M.W.. 1983. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J. Physiol. 344:625–666. 10.1113/jphysiol.1983.sp014959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall J.R. 1953. Further observations on a factor (The ‘Marsh’ factor) effecting relaxation of ATP-shortened muscle-fibre models and the effect of Ca and Mg ions upon it. J. Physiol. 121:232–254. 10.1113/jphysiol.1953.sp004944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Springer, New York: 427 pp. 10.1007/978-94-010-0658-3 [DOI] [Google Scholar]
- Bers D.M., and Stiffel V.M.. 1993. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am. J. Physiol. 264:C1587–C1593. 10.1152/ajpcell.1993.264.6.C1587 [DOI] [PubMed] [Google Scholar]
- Beuckelmann D.J., and Wier W.G.. 1988. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J. Physiol. 405:233–255. 10.1113/jphysiol.1988.sp017331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B.A., Imagawa T., Campbell K.P., and Franzini-Armstrong C.. 1988. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 107:2587–2600. 10.1083/jcb.107.6.2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boink A.B., Ruigrok T.J., Maas A.H., and Zimmerman A.N.. 1976. Calcium paradox: changes in high-energy phosphate compounds of isolated perfused rat hearts. Recent Adv. Stud. Cardiac Struct. Metab. 11:559–564. [PubMed] [Google Scholar]
- Bozler E. 1954. Relaxation in extracted muscle fibers. J. Gen. Physiol. 38:149–159. 10.1085/jgp.38.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J.H., Ershler P.R., and Cannell M.B.. 1999. Properties of Ca2+ sparks evoked by action potentials in mouse ventricular myocytes. J. Physiol. 518:469–478. 10.1111/j.1469-7793.1999.0469p.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.D., Rodney G.G., Hernández-Ochoa E., Ward C.W., and Schneider M.F.. 2007. Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 292:C1156–C1166. 10.1152/ajpcell.00397.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabra V., Murayama T., and Samsó M.. 2016. Ultrastructural Analysis of Self-Associated RyR2s. Biophys. J. 110:2651–2662. 10.1016/j.bpj.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., and Soeller C.. 1999. Mechanisms underlying calcium sparks in cardiac muscle. J. Gen. Physiol. 113:373–376. 10.1085/jgp.113.3.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., Berlin J.R., and Lederer W.J.. 1987. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 238:1419–1423. 10.1126/science.2446391 [DOI] [PubMed] [Google Scholar]
- Cannell M.B., Cheng H., and Lederer W.J.. 1995. The control of calcium release in heart muscle. Science. 268:1045–1049. 10.1126/science.7754384 [DOI] [PubMed] [Google Scholar]
- Chandler W.K., Hollingworth S., and Baylor S.M.. 2003. Simulation of calcium sparks in cut skeletal muscle fibers of the frog. J. Gen. Physiol. 121:311–324. 10.1085/jgp.200308787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., and Lederer W.J.. 2008. Calcium sparks. Physiol. Rev. 88:1491–1545. 10.1152/physrev.00030.2007 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J., and Cannell M.B.. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744. 10.1126/science.8235594 [DOI] [PubMed] [Google Scholar]
- Cheng H., Song L.S., Shirokova N., González A., Lakatta E.G., Ríos E., and Stern M.D.. 1999. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys. J. 76:606–617. 10.1016/S0006-3495(99)77229-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun L.G., Ward C.W., and Schneider M.F.. 2003. Ca2+ sparks are initiated by Ca2+ entry in embryonic mouse skeletal muscle and decrease in frequency postnatally. Am. J. Physiol. Cell Physiol. 285:C686–C697. 10.1152/ajpcell.00072.2003 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., and Hawkes A.G.. 1995. The principles of the stochastic interpretation of ion channel mechanisms. In Single-Channel Recording. Sakmann B. and Neher E., editors. Plenum Press, New York: 397–482. 10.1007/978-1-4419-1229-9_18 [DOI] [Google Scholar]
- Conklin M.W., Ahern C.A., Vallejo P., Sorrentino V., Takeshima H., and Coronado R.. 2000. Comparison of Ca(2+) sparks produced independently by two ryanodine receptor isoforms (type 1 or type 3). Biophys. J. 78:1777–1785. 10.1016/S0006-3495(00)76728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti A., Gorza L., and Sorrentino V.. 1996. Differential distribution of ryanodine receptor type 3 (RyR3) gene product in mammalian skeletal muscles. Biochem. J. 316:19–23. 10.1042/bj3160019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell A.H., Finkbeiner S.M., Cooper M.S., and Smith S.J.. 1990. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 247:470–473. 10.1126/science.1967852 [DOI] [PubMed] [Google Scholar]
- Csernoch L., Jacquemond V., and Schneider M.F.. 1993. Microinjection of strong calcium buffers suppresses the peak of calcium release during depolarization in frog skeletal muscle fibers. J. Gen. Physiol. 101:297–333. 10.1085/jgp.101.2.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Zhou J., Stern M.D., Brum G., and Ríos E.. 2004. The elementary events of Ca2+ release elicited by membrane depolarization in mammalian muscle. J. Physiol. 557:43–58. 10.1113/jphysiol.2003.059154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O., and Stefani E.. 1993. Calcium transients in single mammalian skeletal muscle fibres. J. Physiol. 463:689–707. 10.1113/jphysiol.1993.sp019617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Georges A., Clarke O.B., Zalk R., Yuan Q., Condon K.J., Grassucci R.A., Hendrickson W.A., Marks A.R., and Frank J.. 2016. Structural basis for gating and activation of RyR1. Cell. 167:145–157.e17. 10.1016/j.cell.2016.08.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco M., Neco P., Capote J., Meera P., and Vergara J.L.. 2006. Quantitative evaluation of mammalian skeletal muscle as a heterologous protein expression system. Protein Expr. Purif. 47:281–288. 10.1016/j.pep.2005.10.018 [DOI] [PubMed] [Google Scholar]
- Donaldson S.K. 1985. Peeled mammalian skeletal muscle fibers. Possible stimulation of Ca2+ release via a transverse tubule-sarcoplasmic reticulum mechanism. J. Gen. Physiol. 86:501–525. 10.1085/jgp.86.4.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S.K., Gallant E.M., and Huetteman D.A.. 1989. Skeletal muscle excitation-contraction coupling. I. Transverse tubule control of peeled fiber Ca2+-induced Ca2+ release in normal and malignant hyperthermic muscles. Pflugers Arch. 414:15–23. 10.1007/BF00585621 [DOI] [PubMed] [Google Scholar]
- Ebashi S. 1960. Calcium binding and relaxation in the actomyosin system. J. Biochem. 48:150–151. [Google Scholar]
- Ebashi S., Ebashi F., and Fujie Y.. 1960. THE EFFECT OF EDTA AND ITS ANALOGUES ON GLYCERINATED MUSCLE FIBERS AND MYOSIN ADENOSINETRIPHOSPHATASE*. J. Biochem. 47:54–59. 10.1093/oxfordjournals.jbchem.a127039 [DOI] [Google Scholar]
- Efremov R.G., Leitner A., Aebersold R., and Raunser S.. 2015. Architecture and conformational switch mechanism of the ryanodine receptor. Nature. 517:39–43. 10.1038/nature13916 [DOI] [PubMed] [Google Scholar]
- Endo M. 1985. Calcium release from sarcoplasmic reticulum. In Structure and Function of Sarcoplasmic Reticulum. Fleischer S., editor. Academic Press, Orlando, FL: 181–230. 10.1016/B978-0-12-260380-8.50037-3 [DOI] [Google Scholar]
- Endo M. 2009. Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 89:1153–1176. 10.1152/physrev.00040.2008 [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., and Ogawa Y.. 1970. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 228:34–36. 10.1038/228034a0 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1981. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J. Gen. Physiol. 78:457–497. 10.1085/jgp.78.5.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. 1982. Fluorescence and differential light absorption recordings with calcium probes and potential-sensitive dyes in skinned cardiac cells. Can. J. Physiol. Pharmacol. 60:556–567. 10.1139/y82-075 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1983. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 245:C1–C14. 10.1152/ajpcell.1983.245.1.C1 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1985a Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 85:291–320. 10.1085/jgp.85.2.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. 1985b Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 85:247–289. 10.1085/jgp.85.2.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. 1985c Spontaneous versus triggered contractions of “calcium-tolerant” cardiac cells from the adult rat ventricle. Basic Res. Cardiol. 80(Suppl 2):83–87. [PubMed] [Google Scholar]
- Fabiato A. 1985d Use of aequorin for the appraisal of the hypothesis of the release of calcium from the sarcoplasmic reticulum induced by a change of pH in skinned cardiac cells. Cell Calcium. 6:95–108. 10.1016/0143-4160(85)90037-5 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1992. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv. Exp. Med. Biol. 311:245–262. 10.1007/978-1-4615-3362-7_18 [DOI] [PubMed] [Google Scholar]
- Fabiato A., and Fabiato F.. 1972. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ. Res. 31:293–307. 10.1161/01.RES.31.3.293 [DOI] [PubMed] [Google Scholar]
- Fabiato A., and Fabiato F.. 1979. Use of chlorotetracycline fluorescence to demonstrate Ca2+-induced release of Ca2+ from the sarcoplasmic reticulum of skinned cardiac cells. Nature. 281:146–148. 10.1038/281146a0 [DOI] [PubMed] [Google Scholar]
- Fabiato F., Fabiato A., and Sonnenblick E.H.. 1972. [Regenerative calcium liberation by the sarcoplasmic reticulum on skeletal and cardiac muscle cells]. J. Physiol. (Paris). 65(Suppl 1):1–: 118A.. [PubMed] [Google Scholar]
- Fedchyshyn M.J., and Wang L.-Y.. 2005. Developmental transformation of the release modality at the calyx of Held synapse. J. Neurosci. 25:4131–4140. 10.1523/JNEUROSCI.0350-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder E., and Franzini-Armstrong C.. 2002. Type 3 ryanodine receptors of skeletal muscle are segregated in a parajunctional position. Proc. Natl. Acad. Sci. USA. 99:1695–1700. 10.1073/pnas.032657599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa L., Shkryl V.M., Zhou J., Manno C., Momotake A., Brum G., Blatter L.A., Ellis-Davies G.C.R., and Ríos E.. 2012. Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle. J. Physiol. 590:1389–1411. 10.1113/jphysiol.2011.225854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., and Copello J.A.. 2002. Ryanodine receptor calcium release channels. Physiol. Rev. 82:893–922. 10.1152/physrev.00013.2002 [DOI] [PubMed] [Google Scholar]
- Ford L.E., and Podolsky R.J.. 1970. Regenerative calcium release within muscle cells. Science. 167:58–59. 10.1126/science.167.3914.58 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. 1970. STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J. Cell Biol. 47:488–499. 10.1083/jcb.47.2.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., and Schneider M.F.. 1993. Calcium transients and calcium release in rat fast-twitch skeletal muscle fibres. J. Physiol. 463:709–728. 10.1113/jphysiol.1993.sp019618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Kirsch W.G., Shirokova N., Pizarro G., Brum G., Pessah I.N., Stern M.D., Cheng H., and Ríos E.. 2000a Involvement of multiple intracellular release channels in calcium sparks of skeletal muscle. Proc. Natl. Acad. Sci. USA. 97:4380–4385. 10.1073/pnas.070056497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Kirsch W.G., Shirokova N., Pizarro G., Stern M.D., and Ríos E.. 2000b The spark and its ember: separately gated local components of Ca(2+) release in skeletal muscle. J. Gen. Physiol. 115:139–158. 10.1085/jgp.115.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., and Adams P.R.. 1990. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 247:858–862. 10.1126/science.2154851 [DOI] [PubMed] [Google Scholar]
- Herz R., Weber A., and Reiss I.. 1969. The role of magnesium in the relaxation of myofibrils. Biochemistry. 8:2266–2271. 10.1021/bi00834a005 [DOI] [PubMed] [Google Scholar]
- Hill A.V. 1949. The abrupt transition from rest to activity in muscle. Proc. R. Soc. Lond. B Biol. Sci. 136:399–420. 10.1098/rspb.1949.0033 [DOI] [PubMed] [Google Scholar]
- Horstick E.J., Linsley J.W., Dowling J.J., Hauser M.A., McDonald K.K., Ashley-Koch A., Saint-Amant L., Satish A., Cui W.W., Zhou W., et al. . 2013. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 4:1952 10.1038/ncomms2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M., Saito A., and Fleischer S.. 1987. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J. Biol. Chem. 262:1740–1747. [PubMed] [Google Scholar]
- Ivanenko A., McKemy D.D., Kenyon J.L., Airey J.A., and Sutko J.L.. 1995. Embryonic chicken skeletal muscle cells fail to develop normal excitation-contraction coupling in the absence of the alpha ryanodine receptor. Implications for a two-ryanodine receptor system. J. Biol. Chem. 270:4220–4223. 10.1074/jbc.270.9.4220 [DOI] [PubMed] [Google Scholar]
- Izu L.T., Wier W.G., and Balke C.W.. 1998. Theoretical analysis of the Ca2+ spark amplitude distribution. Biophys. J. 75:1144–1162. 10.1016/S0006-3495(98)74034-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V., Csernoch L., Klein M.G., and Schneider M.F.. 1991. Voltage-gated and calcium-gated calcium release during depolarization of skeletal muscle fibers. Biophys. J. 60:867–873. 10.1016/S0006-3495(91)82120-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.H., Klein M.G., and Schneider M.F.. 1999. Numerical simulation of Ca2+ “sparks” in skeletal muscle. Biophys. J. 77:2333–2357. 10.1016/S0006-3495(99)77072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong D.S., Pape P.C., Chandler W.K., and Baylor S.M.. 1993. Reduction of calcium inactivation of sarcoplasmic reticulum calcium release by fura-2 in voltage-clamped cut twitch fibers from frog muscle. J. Gen. Physiol. 102:333–370. 10.1085/jgp.102.2.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiyama T., Murayama T., Suzuki E., Allen P.D., and Ogawa Y.. 2010. Frog alpha- and beta-ryanodine receptors provide distinct intracellular Ca2+ signals in a myogenic cell line. PLoS One. 5:e11526 10.1371/journal.pone.0011526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.C., Caswell A.H., Talvenheimo J.A., and Brandt N.R.. 1990. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry. 29:9281–9289. 10.1021/bi00491a025 [DOI] [PubMed] [Google Scholar]
- Kirsch W.G., Uttenweiler D., and Fink R.H.. 2001. Spark- and ember-like elementary Ca2+ release events in skinned fibres of adult mammalian skeletal muscle. J. Physiol. 537:379–389. 10.1111/j.1469-7793.2001.00379.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M.G., Cheng H., Santana L.F., Jiang Y.H., Lederer W.J., and Schneider M.F.. 1996. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 379:455–458. 10.1038/379455a0 [DOI] [PubMed] [Google Scholar]
- Klein M.G., Lacampagne A., and Schneider M.F.. 1997. Voltage dependence of the pattern and frequency of discrete Ca2+ release events after brief repriming in frog skeletal muscle. Proc. Natl. Acad. Sci. USA. 94:11061–11066. 10.1073/pnas.94.20.11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., and Schneider M.F.. 1983. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J. Physiol. 343:161–196. 10.1113/jphysiol.1983.sp014887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacampagne A., Klein M.G., and Schneider M.F.. 1998. Modulation of the frequency of spontaneous sarcoplasmic reticulum Ca2+ release events (Ca2+ sparks) by myoplasmic [Mg2+] in frog skeletal muscle. J. Gen. Physiol. 111:207–224. 10.1085/jgp.111.2.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F.A., Liu Q.Y., Xu L., el-Hashem A., Kramarcy N.R., Sealock R., and Meissner G.. 1992. Amphibian ryanodine receptor isoforms are related to those of mammalian skeletal or cardiac muscle. Am. J. Physiol. 263:C365–C372. 10.1152/ajpcell.1992.263.2.C365 [DOI] [PubMed] [Google Scholar]
- Laver D.R., Baynes T.M., and Dulhunty A.F.. 1997. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J. Membr. Biol. 156:213–229. 10.1007/s002329900202 [DOI] [PubMed] [Google Scholar]
- Lederer W.J., Cannell M.B., Cohen N.M., and Berlin J.R.. 1989. Excitation-contraction coupling in heart muscle. Mol. Cell. Biochem. 89:115–119. 10.1007/BF00220762 [DOI] [PubMed] [Google Scholar]
- Legrand C., Giacomello E., Berthier C., Allard B., Sorrentino V., and Jacquemond V.. 2008. Spontaneous and voltage-activated Ca2+ release in adult mouse skeletal muscle fibres expressing the type 3 ryanodine receptor. J. Physiol. 586:441–457. 10.1113/jphysiol.2007.145862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Mooney P., Zheng S., Booth C.R., Braunfeld M.B., Gubbens S., Agard D.A., and Cheng Y.. 2013. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods. 10:584–590. 10.1038/nmeth.2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., and Niggli E.. 1996. Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes. J. Physiol. 492:31–38. 10.1113/jphysiol.1996.sp021286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., and Silver R.B.. 1992. Presynaptic calcium concentration microdomains and transmitter release. J. Physiol. Paris. 86:135–138. 10.1016/S0928-4257(05)80018-X [DOI] [PubMed] [Google Scholar]
- MacLennan D.H., and Wong P.T.. 1971. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 68:1231–1235. 10.1073/pnas.68.6.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C., Figueroa L., Royer L., Pouvreau S., Lee C.-S., Volpe P., Nori A., Zhou J., Meissner G., Hamilton S.L., and Ríos E.. 2013. Altered Ca2+ concentration, permeability and buffering in the myofibre Ca2+ store of a mouse model of malignant hyperthermia. J. Physiol. 591:4439–4457. 10.1113/jphysiol.2013.259572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margreth A., Damiani E., and Tobaldin G.. 1993. Ratio of dihydropyridine to ryanodine receptors in mammalian and frog twitch muscles in relation to the mechanical hypothesis of excitation-contraction coupling. Biochem. Biophys. Res. Commun. 197:1303–1311. 10.1006/bbrc.1993.2619 [DOI] [PubMed] [Google Scholar]
- Marsh B.B. 1951. A factor modifying muscle fibre synaeresis. Nature. 167:1065–1066. 10.1038/1671065a0 [DOI] [PubMed] [Google Scholar]
- Martins A.S., Shkryl V.M., Nowycky M.C., and Shirokova N.. 2008. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J. Physiol. 586:197–210. 10.1113/jphysiol.2007.146571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., and Neher E.. 1985. Potassium channels in cultured bovine adrenal chromaffin cells. J. Physiol. 367:117–141. 10.1113/jphysiol.1985.sp015817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. 2017. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 149:1065–1089. 10.1085/jgp.201711878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Darling E., and Eveleth J.. 1986. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 25:236–244. 10.1021/bi00349a033 [DOI] [PubMed] [Google Scholar]
- Melzer W., Rios E., and Schneider M.F.. 1984. Time course of calcium release and removal in skeletal muscle fibers. Biophys. J. 45:637–641. 10.1016/S0006-3495(84)84203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Rios E., and Schneider M.F.. 1987. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys. J. 51:849–863. 10.1016/S0006-3495(87)83413-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J.P., and Tsien R.Y.. 1989. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 264:8171–8178. [PubMed] [Google Scholar]
- Moore R.A., Nguyen H., Galceran J., Pessah I.N., and Allen P.D.. 1998. A transgenic myogenic cell line lacking ryanodine receptor protein for homologous expression studies: reconstitution of Ry1R protein and function. J. Cell Biol. 140:843–851. 10.1083/jcb.140.4.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T., and Kurebayashi N.. 2011. Two ryanodine receptor isoforms in nonmammalian vertebrate skeletal muscle: possible roles in excitation-contraction coupling and other processes. Prog. Biophys. Mol. Biol. 105:134–144. 10.1016/j.pbiomolbio.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Murayama T., and Ogawa Y.. 2001. Selectively suppressed Ca2+-induced Ca2+ release activity of α-ryanodine receptor (α-RyR) in frog skeletal muscle sarcoplasmic reticulum: potential distinct modes in Ca2+ release between α- and β-RyR. J. Biol. Chem. 276:2953–2960. 10.1074/jbc.M005809200 [DOI] [PubMed] [Google Scholar]
- Murayama T., Kurebayashi N., and Ogawa Y.. 2000. Role of Mg(2+) in Ca(2+)-induced Ca(2+) release through ryanodine receptors of frog skeletal muscle: modulations by adenine nucleotides and caffeine. Biophys. J. 78:1810–1824. 10.1016/S0006-3495(00)76731-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., and Morad M.. 1989. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 244:800–803. 10.1126/science.2543067 [DOI] [PubMed] [Google Scholar]
- Nakai J., Dirksen R.T., Nguyen H.T., Pessah I.N., Beam K.G., and Allen P.D.. 1996. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 380:72–75. 10.1038/380072a0 [DOI] [PubMed] [Google Scholar]
- Neher E. 1986. Concentration profiles of intracellular Ca2+ in the presence of diffusible chelator. In Calcium Electrogenesis and Neural Functioning. Heinemann U., Klee M., Neher E., and Singer W., editors. Springer-Verlag, Berlin, Heidelberg: 80–96. 10.1007/978-3-642-70744-5_8 [DOI] [Google Scholar]
- Nelson B.R., Wu F., Liu Y., Anderson D.M., McAnally J., Lin W., Cannon S.C., Bassel-Duby R., and Olson E.N.. 2013. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA. 110:11881–11886. 10.1073/pnas.1310571110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.T., Cheng H., Rubart M., Santana L.F., Bonev A.D., Knot H.J., and Lederer W.J.. 1995. Relaxation of arterial smooth muscle by calcium sparks. Science. 270:633–637. 10.1126/science.270.5236.633 [DOI] [PubMed] [Google Scholar]
- Niggli E., and Lederer W.J.. 1990a Voltage-independent calcium release in heart muscle. Science. 250:565–568. 10.1126/science.2173135 [DOI] [PubMed] [Google Scholar]
- Niggli E., and Lederer W.J.. 1990b Real-time confocal microscopy and calcium measurements in heart muscle cells: towards the development of a fluorescence microscope with high temporal and spatial resolution. Cell Calcium. 11:121–130. 10.1016/0143-4160(90)90065-3 [DOI] [PubMed] [Google Scholar]
- Ottini L., Marziali G., Conti A., Charlesworth A., and Sorrentino V.. 1996. Alpha and beta isoforms of ryanodine receptor from chicken skeletal muscle are the homologues of mammalian RyR1 and RyR3. Biochem. J. 315:207–216. 10.1042/bj3150207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape P.C., Jong D.S., and Chandler W.K.. 1995. Calcium release and its voltage dependence in frog cut muscle fibers equilibrated with 20 mM EGTA. J. Gen. Physiol. 106:259–336. 10.1085/jgp.106.2.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape P.C., Jong D.S., and Chandler W.K.. 1998. Effects of partial sarcoplasmic reticulum calcium depletion on calcium release in frog cut muscle fibers equilibrated with 20 mM EGTA. J. Gen. Physiol. 112:263–295. 10.1085/jgp.112.3.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape P.C., Fénelon K., and Carrier N.. 2002. Extra activation component of calcium release in frog muscle fibres. J. Physiol. 542:867–886. 10.1113/jphysiol.2002.017095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perni S., Marsden K.C., Escobar M., Hollingworth S., Baylor S.M., and Franzini-Armstrong C.. 2015. Structural and functional properties of ryanodine receptor type 3 in zebrafish tail muscle. J. Gen. Physiol. 145:253 10.1085/jgp.20141130302112015c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro G., and Ríos E.. 2004. How source content determines intracellular Ca2+ release kinetics. Simultaneous measurement of [Ca2+] transients and [H+] displacement in skeletal muscle. J. Gen. Physiol. 124:239–258. 10.1085/jgp.200409071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster A., Nelson B.R., Olson E.N., and Beam K.G.. 2016. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. Proc. Natl. Acad. Sci. USA. 113:10986–10991. 10.1073/pnas.1612441113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portzehl H., Caldwell P.C., and Rueegg J.C.. 1964. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim. Biophys. Acta. 79:581–591. [DOI] [PubMed] [Google Scholar]
- Pouvreau S., Royer L., Yi J., Brum G., Meissner G., Ríos E., and Zhou J.. 2007. Ca(2+) sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle. Proc. Natl. Acad. Sci. USA. 104:5235–5240. 10.1073/pnas.0700748104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.A., and Fambrough D.M.. 1973. Electrical properties of normal and dysgenic mouse skeletal muscle in culture. J. Cell. Physiol. 82:21–38. 10.1002/jcp.1040820104 [DOI] [PubMed] [Google Scholar]
- Pratusevich V.R., and Balke C.W.. 1996. Factors shaping the confocal image of the calcium spark in cardiac muscle cells. Biophys. J. 71:2942–2957. 10.1016/S0006-3495(96)79525-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck R.T., Karunasekara Y., Board P.G., Beard N.A., Casarotto M.G., and Dulhunty A.F.. 2014. Skeletal muscle excitation-contraction coupling: who are the dancing partners? Int. J. Biochem. Cell Biol. 48:28–38. 10.1016/j.biocel.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Rengifo J., Rosales R., González A., Cheng H., Stern M.D., and Ríos E.. 2002. Intracellular Ca(2+) release as irreversible Markov process. Biophys. J. 83:2511–2521. 10.1016/S0006-3495(02)75262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M., Schleithoff L., Deufel T., Lehmann-Horn F., and Herrmann-Frank A.. 1997. Functional characterization of a distinct ryanodine receptor mutation in human malignant hyperthermia-susceptible muscle. J. Biol. Chem. 272:5256–5260. 10.1074/jbc.272.8.5256 [DOI] [PubMed] [Google Scholar]
- Ríos E., and Brum G.. 1987. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 325:717–720. 10.1038/325717a0 [DOI] [PubMed] [Google Scholar]
- Ríos E., and Pizarro G.. 1988. Voltage Sensors and Calcium Channels of Excitation-Contraction Coupling. Physiology (Bethesda). 3:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., and Stern M.D.. 1997. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu. Rev. Biophys. Biomol. Struct. 26:47–82. 10.1146/annurev.biophys.26.1.47 [DOI] [PubMed] [Google Scholar]
- Ríos E., Karhanek M., Ma J., and González A.. 1993. An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 102:449–481. 10.1085/jgp.102.3.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Stern M.D., González A., Pizarro G., and Shirokova N.. 1999. Calcium release flux underlying Ca2+ sparks of frog skeletal muscle. J. Gen. Physiol. 114:31–48. 10.1085/jgp.114.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Shirokova N., Kirsch W.G., Pizarro G., Stern M.D., Cheng H., and González A.. 2001. A preferred amplitude of calcium sparks in skeletal muscle. Biophys. J. 80:169–183. 10.1016/S0006-3495(01)76005-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Figueroa L., Manno C., Kraeva N., and Riazi S.. 2015. The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle. J. Gen. Physiol. 145:459–474. 10.1085/jgp.201411321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez G., Hidalgo C., and Donoso P.. 2003. Kinetic studies of calcium-induced calcium release in cardiac sarcoplasmic reticulum vesicles. Biophys. J. 84:2319–2330. 10.1016/S0006-3495(03)75037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.F. 1999. Ca2+ sparks in frog skeletal muscle: generation by one, some, or many SR Ca2+ release channels? J. Gen. Physiol. 113:365–372. 10.1085/jgp.113.3.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.F., and Chandler W.K.. 1973. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 242:244–246. 10.1038/242244a0 [DOI] [PubMed] [Google Scholar]
- Shirokova N., and Ríos E.. 1997. Small event Ca2+ release: a probable precursor of Ca2+ sparks in frog skeletal muscle. J. Physiol. 502:3–11. 10.1111/j.1469-7793.1997.003bl.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N., García J., Pizarro G., and Ríos E.. 1996. Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J. Gen. Physiol. 107:1–18. 10.1085/jgp.107.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N., García J., and Ríos E.. 1998. Local calcium release in mammalian skeletal muscle. J. Physiol. 512:377–384. 10.1111/j.1469-7793.1998.377be.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N., Shirokov R., Rossi D., González A., Kirsch W.G., García J., Sorrentino V., and Ríos E.. 1999. Spatially segregated control of Ca2+ release in developing skeletal muscle of mice. J. Physiol. 521:483–495. 10.1111/j.1469-7793.1999.00483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E.A., Williams G.S.B., and Lederer W.J.. 2017. Ambiguous interactions between diastolic and SR Ca2+in the regulation of cardiac Ca2+release. J. Gen. Physiol. 149:847–855. 10.1085/jgp.201711814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C., and Baddeley D.. 2013. Super-resolution imaging of EC coupling protein distribution in the heart. J. Mol. Cell. Cardiol. 58:32–40. 10.1016/j.yjmcc.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Somlyo A.V., Broderick R., Shuman H., Buhle E.L. Jr., and Somlyo A.P.. 1988. Atrial-specific granules in situ have high calcium content, are acidic, and maintain anion gradients. Proc. Natl. Acad. Sci. USA. 85:6222–6226. 10.1073/pnas.85.16.6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D. 1992a Theory of excitation-contraction coupling in cardiac muscle. Biophys. J. 63:497–517. 10.1016/S0006-3495(92)81615-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D. 1992b Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 13:183–192. 10.1016/0143-4160(92)90046-U [DOI] [PubMed] [Google Scholar]
- Stern M.D., Pizarro G., and Ríos E.. 1997. Local control model of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 110:415–440. 10.1085/jgp.110.4.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., Song L.S., Cheng H., Sham J.S., Yang H.T., Boheler K.R., and Ríos E.. 1999. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J. Gen. Physiol. 113:469–489. 10.1085/jgp.113.3.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struk A., Lehmann-Horn F., and Melzer W.. 1998. Voltage-dependent calcium release in human malignant hyperthermia muscle fibers. Biophys. J. 75:2402–2410. 10.1016/S0006-3495(98)77684-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekura H., Bennett L., Tanabe T., Beam K.G., and Franzini-Armstrong C.. 1994. Restoration of junctional tetrads in dysgenic myotubes by dihydropyridine receptor cDNA. Biophys. J. 67:793–803. 10.1016/S0006-3495(94)80539-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekura H., Nishi M., Noda T., Takeshima H., and Franzini-Armstrong C.. 1995. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc. Natl. Acad. Sci. USA. 92:3381–3385. 10.1073/pnas.92.8.3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Iino M., Takekura H., Nishi M., Kuno J., Minowa O., Takano H., and Noda T.. 1994. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 369:556–559. 10.1038/369556a0 [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., and Numa S.. 1987. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 328:313–318. 10.1038/328313a0 [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K.G., Powell J.A., and Numa S.. 1988. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 336:134–139. 10.1038/336134a0 [DOI] [PubMed] [Google Scholar]
- Tanabe T., Mikami A., Numa S., and Beam K.G.. 1990. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 344:451–453. 10.1038/344451a0 [DOI] [PubMed] [Google Scholar]
- Teichmann M.D.H., Wegner F.V., Fink R.H.A., Chamberlain J.S., Launikonis B.S., Martinac B., and Friedrich O.. 2008. Inhibitory control over Ca(2+) sparks via mechanosensitive channels is disrupted in dystrophin deficient muscle but restored by mini-dystrophin expression. PLoS One. 3:e3644 10.1371/journal.pone.0003644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R.Y. 1980. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 19:2396–2404. 10.1021/bi00552a018 [DOI] [PubMed] [Google Scholar]
- Tsugorka A., Ríos E., and Blatter L.A.. 1995. Imaging elementary events of calcium release in skeletal muscle cells. Science. 269:1723–1726. 10.1126/science.7569901 [DOI] [PubMed] [Google Scholar]
- Wang S.-Q., Song L.-S., Xu L., Meissner G., Lakatta E.G., Ríos E., Stern M.D., and Cheng H.. 2002. Thermodynamically irreversible gating of ryanodine receptors in situ revealed by stereotyped duration of release in Ca(2+) sparks. Biophys. J. 83:242–251. 10.1016/S0006-3495(02)75165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Weisleder N., Collet C., Zhou J., Chu Y., Hirata Y., Zhao X., Pan Z., Brotto M., Cheng H., and Ma J.. 2005. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 7:525–530. 10.1038/ncb1254 [DOI] [PubMed] [Google Scholar]
- Ward C.W., Schneider M.F., Castillo D., Protasi F., Wang Y., Chen S.R., and Allen P.D.. 2000. Expression of ryanodine receptor RyR3 produces Ca2+ sparks in dyspedic myotubes. J. Physiol. 525:91–103. 10.1111/j.1469-7793.2000.t01-2-00091.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C.W., Protasi F., Castillo D., Wang Y., Chen S.R., Pessah I.N., Allen P.D., and Schneider M.F.. 2001. Type 1 and type 3 ryanodine receptors generate different Ca(2+) release event activity in both intact and permeabilized myotubes. Biophys. J. 81:3216–3230. 10.1016/S0006-3495(01)75957-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. 1959. On the role of calcium in the activity of adenosine 5′-triphosphate hydrolysis by actomyosin. J. Biol. Chem. 234:2764–2769. [PubMed] [Google Scholar]
- Weber A. 1968. The mechanism of the action of caffeine on sarcoplasmic reticulum. J. Gen. Physiol. 52:760–772. 10.1085/jgp.52.5.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., and Herz R.. 1968. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J. Gen. Physiol. 52:750–759. 10.1085/jgp.52.5.750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., and Winicur S.. 1961. The role of calcium in the superprecipitation of actomyosin. J. Biol. Chem. 236:3198–3202. [PubMed] [Google Scholar]
- Wier W.G., Cannell M.B., Berlin J.R., Marban E., and Lederer W.J.. 1987. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 235:325–328. 10.1126/science.3798114 [DOI] [PubMed] [Google Scholar]
- Williams D.A., Fogarty K.E., Tsien R.Y., and Fay F.S.. 1985. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 318:558–561. 10.1038/318558a0 [DOI] [PubMed] [Google Scholar]
- Wu F., Quiñonez M., DiFranco M., and Cannon S.C.. 2018. Stac3 enhances expression of human CaV1.1 inXenopusoocytes and reveals gating pore currents in HypoPP mutant channels. J. Gen. Physiol.:jgp.201711962 10.1085/jgp.201711962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B., Masumiya H., Jiang D., Wang R., Sei Y., Zhang L., Murayama T., Ogawa Y., Lai F.A., Wagenknecht T., and Chen S.R.. 2002. Isoform-dependent formation of heteromeric Ca2+ release channels (ryanodine receptors). J. Biol. Chem. 277:41778–41785. 10.1074/jbc.M208210200 [DOI] [PubMed] [Google Scholar]
- Yan Z., Bai X., Yan C., Wu J., Li Z., Xie T., Peng W., Yin C., Li X., Scheres S.H.W., et al. . 2015. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 517:50–55. 10.1038/nature14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J., Ma C., Li Y., Weisleder N., Ríos E., Ma J., and Zhou J.. 2011. Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (E-C) coupling. J. Biol. Chem. 286:32436–32443. 10.1074/jbc.M110.217711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalk R., Clarke O.B., des Georges A., Grassucci R.A., Reiken S., Mancia F., Hendrickson W.A., Frank J., and Marks A.R.. 2015. Structure of a mammalian ryanodine receptor. Nature. 517:44–49. 10.1038/nature13950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yi J., Royer L., Launikonis B.S., González A., García J., and Ríos E.. 2006. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am. J. Physiol. Cell Physiol. 290:C539–C553. 10.1152/ajpcell.00592.2004 [DOI] [PubMed] [Google Scholar]
- Zhou J., Yi J., Fu R., Liu E., Siddique T., Ríos E., and Deng H.-X.. 2010. Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J. Biol. Chem. 285:705–712. 10.1074/jbc.M109.041319 [DOI] [PMC free article] [PubMed] [Google Scholar]