Abstract
Background
β-arrestins have been shown to play a critical role in the progression of diabetic nephropathy. Nevertheless, the potential mechanism of β-arrestins on the regulation of podocyte apoptosis has rarely been discussed. This study aimed to elucidate the regulation of β-arrestin 1/2 on podocyte apoptosis through the Wnt/β-catenin pathway.
Material/Methods
This study structured β-arrestin 1/2 down-regulated and up-regulated expression by plasmid transfection. The protein levels were detected with Western blotting, and mRNA expression was detected with RT-qPCR. The apoptotic cells were measured by flow cytometry.
Results
β-arrestin 1/2 expression levels of podocytes were up-regulated in high-glucose-induced podocytes. β-arrestin 1/2 overexpression inhibited the expression of nephrin and podocin protein. Up-regulated β-arrestin 1/2 promoted podocyte apoptosis and p53 pathway by increasing Bax, cleaved caspase-3, and p-p53 levels in high-glucose-induced podocytes. Flow cytometry showed that the apoptotic cells were markedly higher in the β-arrestin 1/2 up-regulated group compared with the scramble group. Expression of β-catenin was increased in the β-arrestin 1/2 up-regulated group, which indicated that the Wnt/β-catenin pathway was activated. Wnt/β-catenin pathway inhibitor (Dkk1) distinctly suppressed the apoptosis induced by β-arrestin 1/2 overexpression and high glucose.
Conclusions
These results provide a molecular pathomechanism of β-arrestin 1/2 and Wnt/β-catenin pathway on podocyte apoptosis and provide new ideas for the treatment of diabetic nephropathy, which paves the way for the future study of diabetic nephropathy and podocytes.
MeSH Keywords: Apoptosis, Diabetic Nephropathies, Podocytes, Wnt Signaling Pathway
Background
Diabetic nephropathy (DN), as a severe complication of diabetes, has become a lethal cause of end-stage renal disease, with undefined etiology and limited treatment options [1,2]. Previous studies have shown that the pathological characteristics of DN are glomerular basement membrane thickening, extracellular matrix accumulation, glomerular sclerosis, renal tubular atrophy, and renal interstitial fibrosis [3,4]. Moreover, studies in vivo have provided evidence that podocytes are functionally and structurally injured in the early stage. Podocytes are terminal differentiation of glomerular epithelial cells attached on the outside of the GBM and are an important part of glomerular filtration units. Podocytes-related proteins (nephrin and podocin) are important proteins of the slit diaphragm, with anti-apoptotic signaling properties, which are down-regulated by hyperglycemia [5]. Proteinuria is a terminal consequence of injury of podocytes. The loss of nephrin and podocin lead to foot process effacement of podocytes and increased proteinuria. Wnt/β-catenin signaling is activated in glomerular podocytes in a wide variety of proteinuric kidney diseases [6]. The signaling transduction cascade controls many physiological activities and underlies a wide range of kidney pathologies. Studies have confirmed a role for Wnt/β-catenin signaling in mediating podocyte dysfunction and proteinuria [7].
The arrestin family includes 2 classes: the visual arrestins (arrestin 1/4) are located in photoreceptor cells, and the non-visual arrestins (arrestin 2/3, also named β-arrestins 1/2) are distributed ubiquitously [8,9]. The sequences are highly conserved between different species, and β-arrestins 1/2 proteins are very similar [10]. β-arrestins 1/2 (Arrbs) were originally identified as negative adaptors of G protein-coupled receptors (GPCRs) [11]. GPCRs are a large membrane proteins family involved in a variety of physiological functions, including neurotransmission, secretion, and immunity [12,13]. GPCR activation is facilitated by many of extracellular ligands [14].
There is scant literature on the potential mechanism of β-arrestins on the regulation of podocyte apoptosis. The present study used comprehensive detection technologies to elucidate the role of β-arrestin 1/2 on podocyte apoptosis through Wnt/β-catenin signaling pathway and explored new therapeutic targets for diabetic nephropathy.
Material and Methods
Conditionally immortalized mouse podocytes culture
Conditionally immortalized mouse podocytes were purchased from the Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences (Cell Resource Center of Peking Union Medical College, Beijing, China). The cell line was located in glomerular H-2Kb-tsA58 transgenic mice with SV40-T antigen. The cells were cultured in RPMI 1640 medium containing 10% FBS and 70 U/ml penicillin-streptomycin (Gibco, Grand Island, NY, USA). Then, 50 U/ml recombinant mouse γ-interferon was added to induce the promoter and synthesis, conditionally immortalizing tsA58 T antigen. The cells were digested by trypsin and filtered by sieve 5 days later. The undifferentiated cells were replated in DMEM culture medium at permissive conditions containing 10% FBS, 10 U/ml γ-interferon, 100 U/ml penicillin, 100 μg/ml streptomycin, 33°C, and 5% CO2. After 6 generations, the cells were induced to differentiate at nonpermissive conditions containing 10% FBS, 10 U/ml γ-interferon, 100 U/ml penicillin, 100 μg/ml streptomycin, 37°C, and 5% CO2 without γ-interferon.
β-arrestin 1/2 down-regulated and up-regulated expression
Lipofectamine plus (Invitrogen, Carlsbad, CA, USA) was applied to transfect podocytes. The cells were planked with lower 90% cell density in the normal culture medium without antibiotic. Lipofectamine plus 1 ug was diluted by 50 ul serum-free RPMI 1640 and incubated at room temperature for 5 min. Plasmids were mixed with the solution and incubated for 30 min at room temperature. Afterwards, the mixture was incubated in 12-well plates for 24 h (37°C, 5% CO2). There was no need to eliminate the reagents after transfection. shRNA-β-arrestins-1 (Biosune, Shanghai, China) sequences were: forward, 5′-AAAGC CTTCTGTGCT GAGAAC-3′. shRNA-β-arrestins-2 (Biosune, Shanghai, China) sequences were: forward, 5′-AAGGACCGGAAAGTGTTCGTG-3′. β-arrestins 1/2 overexpression plasmid was transfected to upregulate the β-arrestins 1/2 expression. The grouping includes normal control group, scramble group, shRNA-β-arrestins 1/2 group, and β-arrestins 1/2 overexpression group.
Western blotting analysis
Podocytes were split with RIPA lysis solution (100 ul, including 1 mmol/L PMSF). The liquid was transferred into a clean EP tube by Eppendorf and split on ice for 15 min. Then, the EP tube was centrifuged 12 000 rpm for 10 min. Primary antibodies (1: 1000, Abcam, Cambridge, MA, USA) were diluted to test the β-arrestins-1 and β-arrestins-2. β-actin acted as the internal reference. Blocking buffer was prepared with TBST and 5% fat-free milk. The PVDF membrane was blocked in blocking buffer for 1 h on a shaking table at room temperature. Primary antibodies were diluted by 2% fat-free milk. The PVDF membrane was incubated in the diluted primary antibodies at 4°C overnight. The PVDF membrane was washed with TBST 3 times for 10 min. Then, the PVDF membrane was diluted in secondary antibody (Abcam, USA, rabbit, 1: 1000) at 4°C overnight. After that, the PVDF membrane was washed again with TBST 3 times for 10 min. The PVDF membrane was displayed by use of the Gel Doc XR+ imaging system (Bio-Rad, Hercules, CA, USA).
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from the nutrient solution with Trizol reagent (Invitrogen, Carlsbad, CA, USA). The RNA concentration was identified by ultraviolet spectrophotometer. The OD value at 260–280 nm was measured and the appropriate value was 1.8–2.0, which demonstrated that the purity and concentration of RNA samples were up to standard. The reverse transcription reaction total system was 20 μl (containing 1000 ng RNA). The preliminary primers were synthesized (Sangon biotech, Shanghai, China). The PCR primer pairs were: β-arrestins-1: forward: 5′-ACCTTTGAGATCCCGCCAAA-3′, reverse: 5′-CAGGGGCATACTGAACCTTC-3′; β-arrestins-2: forward: 5′-GGAGTAGACTTTGAGATTCGAGC-3′, reverse: 5′-CTTTCTGAT GATAAGCCGCACA-3′. β-actin, forward: 5′-GGCTGTATTCCCCT CCATCG-3′, reverse: 5′-CCAGTTGGTAACAATGCCATGT-3′. The PCR reaction condition was: pre-denaturation was at 95°C for 5 min and following by at 95°C for 30 s, 59°C for 30 s, 72°C for 30 s, and a final elongation step at 72°C for 10 min. The amplification was 40 cycles. The internal reference was β-actin and the relative expression levels were expressed relative to it, which was calculated with the 2−ΔΔCt method.
Flow cytometry
Nutrient solution was added into EP tubes and centrifuged at 1000 rpm for 10 min. After discarding the supernatant, the precipitate was washed in PBS 3 times. Then, the cells were added with slight pancreatin and digested in an incubator at 37°C for 10 min. Centrifugation as above one was carried 1 more time. The cell sediment was suspended with 250 μl buffer and filtered by 200-mesh nylon net. We added 5 μl FITC into a flow tube and incubated it 10 min. Next, 10 μl PI and 400 μl buffer were added. Finally, the apoptosis was detected by flow cytometry (Becton, Dickinson, and Company, NY, USA).
Statistical analysis
Data are expressed as a mean ± standard deviation. Experiments were carried out at least in triplicate. The differences between and within multiple groups were analyzed by one-way ANOVA or Student’s t test. P<0.05 was considered to be statistically significant.
Results
The expression of β-arrestin 1/2 in podocytes
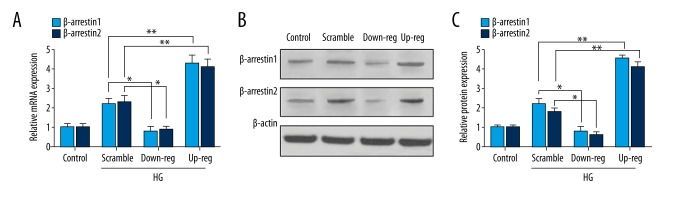
Stable down-regulated and up-regulated expression of β-arrestin 1/2 was established by plasmid transfection. The protein levels were detected with Western blotting and expression of mRNA was detected with RT-qPCR. Figure 1A shows the mRNA expression of β-arrestin 1/2 in each group. Figure 1B and 1C shows β-arrestin 1/2 protein expression. The above results illustrate that lipofectamine transfection successfully built an overexpression or lower-expression model.
Figure 1.
Plasmid transfection accommodated the expression of β-arrestin 1/2. (A) mRNA expression was detected by RT-qPCR. (B) The Western blotting of β-arrestin 1/2 protein. (C) β-arrestin 1/2 protein relative quantitative. Data represent means ±SD. * P<0.05, ** P<0.01 compared with scramble group. Down-reg represents down-regulation, up-reg represents up-regulation, HG represents high glucose.
β-arrestin 1/2 promote podocyte apoptosis and p53 pathway
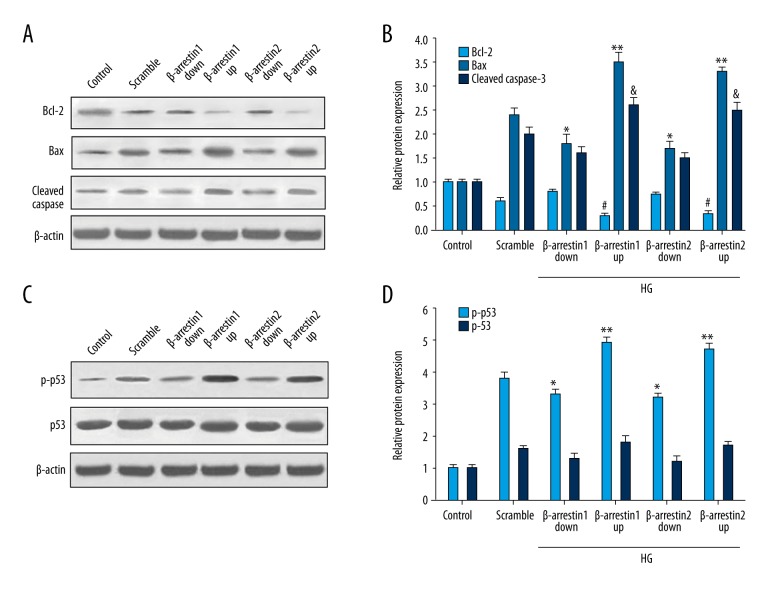
To delineate the molecular mechanism of β-arrestin 1/2 in podocyte apoptosis, we detected the Bcl-2, Bax, cleaved Caspase-3, total p53 protein, and phosphorylation of p53 (p-p53) levels. Figure 2A and 2B show the Bax protein level was increased in the scramble and up-regulated groups. Inversely, Bcl-2 level was inhibited by up-regulated expression of β-arrestin 1/2. Figure 2C and 2D show that p-p53 level induced by high glucose increased obviously, especially in the β-arrestin 1/2 up-regulated group. These results indicate that β-arrestin 1/2 promotes podocyte apoptosis and p53 pathway.
Figure 2.
Effect of β-arrestin 1/2 on apoptosis and p53 pathway. (A) Western blotting analysis of Bcl-2, Bax, and cleaved casapse-3 protein levels in different groups. (B) Quantitative protein levels of Bcl-2, Bax, and cleaved casapse-3. (C) Western blotting analysis of p-p53 and total p53 protein levels in different groups. (D) Quantitative protein levels of p-p53 and p53. Data represent means ±SD. *#& P<0.05, ** P<0.01 compared with scramble group.
The podocin and nephrin expression of podocytes
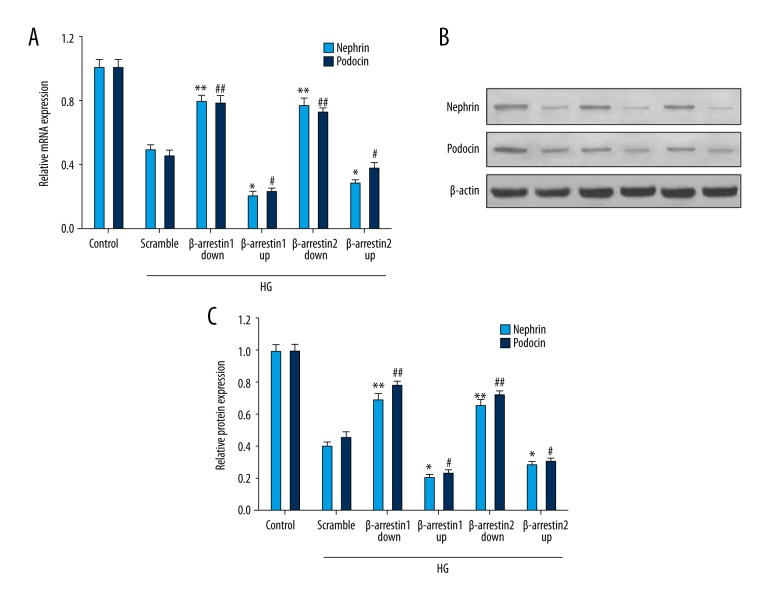
Nephrin and podocin existed on slit diaphragms of podocytes. Research has shown that nephrin and podocin protein levels are decreased in a high-glucose environment. Our study explored the interaction of β-arrestins and nephrin and podocin. Figure 3A shows the mRNA expression of nephrin and podocin. Figure 3B shows the protein expression of nephrin and podocin. Our study indicates that β-arrestin 1/2 overexpression suppresses the expression of nephrin and podocin.
Figure 3.
Expression of nephrin and podocin was detected by Western blotting and RT-qPCR. (A) Expression of nephrin and podocin mRNA was detected by RT-qPCR. (B) Western blotting of nephrin and podocin protein. (C) The nephrin and podocin protein relative quantities. #* P<0.05, ##** P<0.01 compared with scramble group. β-arrestin 1/2 down/up represents β-arrestin 1/2 down-regulated/up-regulated expression.
β-arrestin 1/2 activates the Wnt/β-catenin pathway
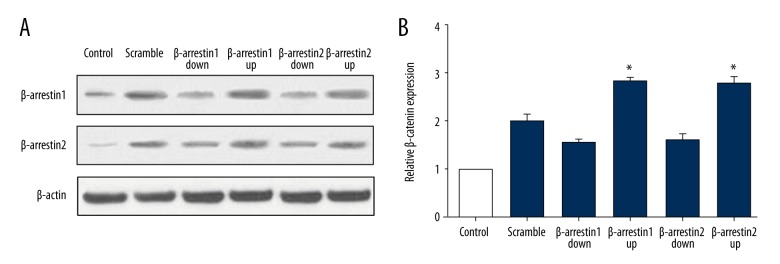
β-catenin grew in quantity when the Wnt/β-catenin pathway was activated. The protein level of β-catenin was detected by Western blotting. Figure 4A and 4B show the β-catenin protein level in podocytes. The accumulated β-catenin protein in each group revealed that the Wnt/β-catenin pathway was observably activated in the β-arrestin 1/2 up-regulated group compared with scramble and down-regulated group.
Figure 4.
β-catenin expression level in β-arrestin 1/2 up-regulated and down-regulated group. (A) Western blotting analysis of β-catenin protein levels in different groups. (B) Quantitative protein levels of β-catenin. Data represent means ±SD. *P<0.05 compared with scramble group.
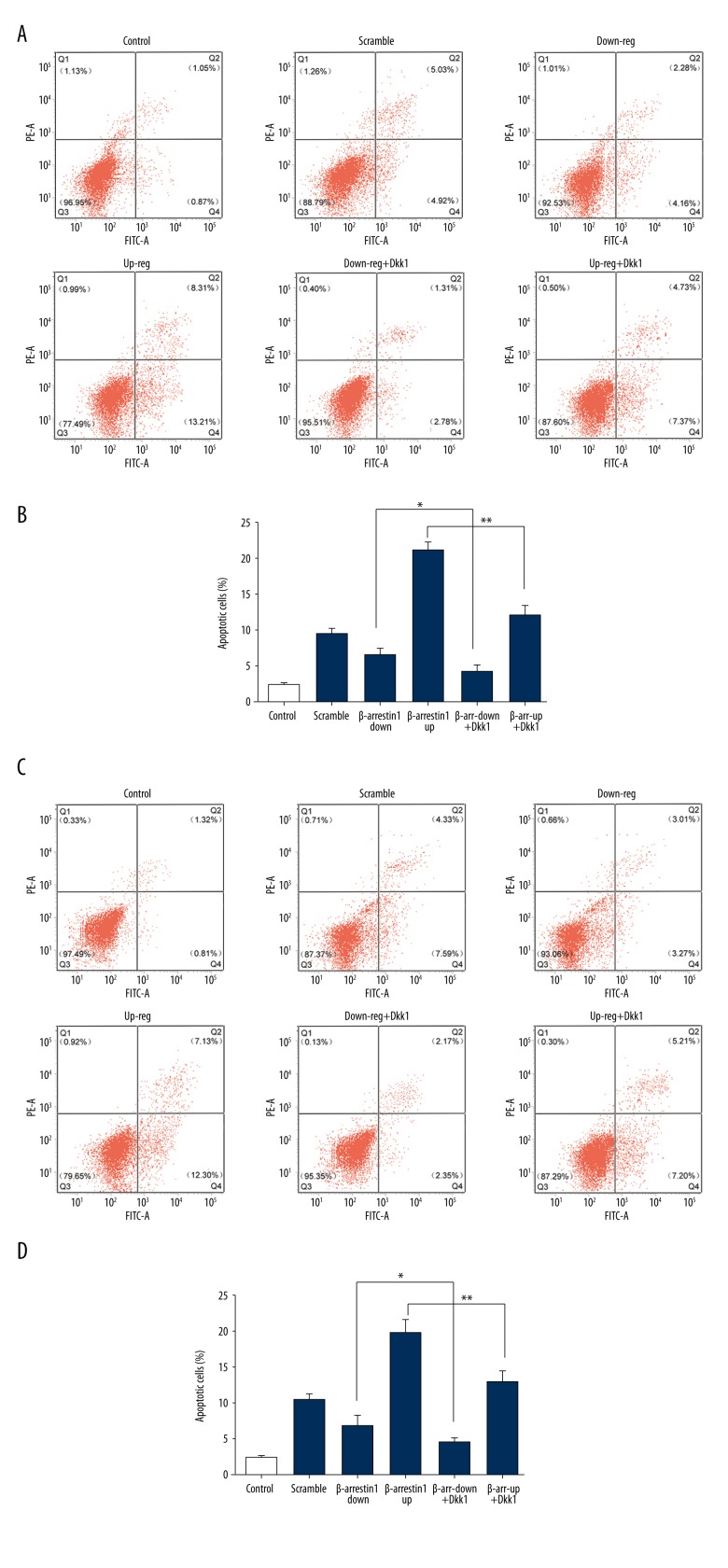
β-arrestin 1/2 aggravate podocyte apoptosis detected by flow cytometry
To examine the role of β-arrestin 1/2 in podocyte apoptosis through Wnt/β-catenin pathway, apoptotic cells were examined by flow cytometry according to the manufacturer’s instructions (BD Biosciences, USA). Figure 5A and 5B show apoptotic cells in the β-arrestin 1 group, and Figure 5C and 5D show apoptotic cells in the β-arrestin 2 group. The results show that high-glucose-induced podocytes exhibited a significant increase in apoptotic cells as compared with normal-glucose-induced podocytes. The apoptosis was ameliorated as the inhibitor of the Wnt/β-catenin pathway (Dkk1) was applied. Furthermore, these above data suggest that β-arrestin 1/2 aggravates high-glucose-induced podocytes apoptosis through the Wnt/β-catenin pathway.
Figure 5.
Flow cytometry was used to detect the effect of β-arrestin 1/2 on apoptosis. (A) Podocytes were stained with FITV A/PE-A for flow cytometry analysis. Apoptotic cells in β-arrestin 1 group were divided into 2 stages (early apoptotic and late apoptotic cells). (B) The total apoptosis rates in the β-arrestin 1 group were examined by flow cytometry, including the early and late apoptosis rates. (C) Apoptotic cells in the β-arrestin 2 group were detected by FITV A/PE-A staining. (D) The total apoptosis rates in β-arrestin 2 group. Data represent means ±SD. * P<0.05, ** P<0.01 compared with scramble group. β-a-1/2-down/up+Dkk1 represents β-arrestin 1/2-down-regulated/up-regulated + Dkk1.
Discussion
β-arrestins are multifaceted proteins which play a critical role in inhibiting GPCR signaling by inducing its desensitization and internalization, and activating signaling pathways [15]. There has been evidence that β-arrestins participate in the pathological development of DN [16–18]. Podocytes, a key component of nephrons, are susceptible to various maladaptive or catastrophic responses such as glomerular injuries and hyperglycemia. Research on podocytes has been a focus of therapeutic options for DN [19]. Furthermore, podocytes, which are highly specialized and terminally differentiated, are critical a component of the kidney glomerular filtration barrier.
In our study, overexpression or underexpression models were built successfully by lipofectamine transfection. The expression of β-arrestin-1 and β-arrestin-2 were up-regulated in podocytes induced by high glucose (Figure 1). To study the apoptotic effect of β-arrestin 1/2 on podocytes, our study detected the levels of Bcl-2, Bax, p53, and p-p53. The results indicated that β-arrestin 1/2 promote podocyte apoptosis and p53 pathway (Figure 2). In the present study, we found that up-regulated β-arrestin 1/2 clearly promotes podocyte apoptosis. The underlining mechanisms need to explored in further experiments. Nephrin and podocin are components of glomerular slit diaphragm and single transmembrane spanning receptor, which play an important role in maintaining normal glomerular filtration function. In a first step, we were able to demonstrate expression of β-arrestin 1/2 in podocytes. To further investigate the possible interaction of β-arrestin 1/2 with nephrin and podocin, we detected the expression of nephrin and podocin. The above-mentioned expression was suppressed by β-arrestin 1/2 overexpression (Figure 3). The interaction of β-arrestin 1/2 and nephrin and podocin imply that there are deeper bonds within them [20]. One of the capable mechanism is that up-regulated β-arrestin 1/2 activates the Wnt/β-catenin signaling pathway and impairs the podocytes.
β-catenin, is a key transcriptional regulator essential for kidney development, whose activity is largely silenced in mature stage [21]. The Wnt/β-catenin signaling transduction cascade controls many physiological activities and underlies a wide range of kidney pathologies. Wnt/β-catenin signaling is activated in glomerular podocytes in a wide variety of proteinuric kidney diseases [22,23]. In our study, the expression of β-catenin was increased observably in the β-arrestin 1/2 up-regulated group (Figure 4). The elevation of β-catenin concentration indicates that the canonical Wnt/β-catenin pathway is activated. Accordingly, the apoptosis was ameliorated when the inhibitor of the Wnt/β-catenin pathway (Dkk1) was applied (Figure 5). Furthermore, the data imply that β-arrestin 1/2 aggravate high-glucose-induced podocytes apoptosis through the Wnt/β-catenin pathway.
A series of studies have proved that activation of the Wnt/β-catenin signaling pathway is closely correlated to fibrosis of liver, lung, and kidney. Tan et al. (2013) [24] identified a signaling cascade by which β-catenin takes part in podocyte injury with endothelin antagonism. Quack et al. (2006) [25] indicated that β-arrestin2 induces nephrin endocytosis and attenuates nephrin signaling, as well as impairing slit diaphragm integrity. Bryja et al. (2007) [26] have reported that beta-arrestin is a necessary component for Wnt/beta-catenin signaling, linking Dvl and axin, and open a vast array of signaling avenues and possibilities for cross-talk with other beta-arrestin-dependent signaling pathways. Zhou et al. (2015) [27] state that Wnt/β-catenin controls the expression of several key mediators, including Snail1 and matrix metalloproteinase, and targeted inhibition of Wnt/β-catenin signaling by a variety of approaches preserves podocyte integrity, reduces proteinuria, and ameliorates kidney damage. Zhou et al. (2015) [28] reported that β-catenin specifically targets WT1 for ubiquitin-mediated degradation, leading to podocyte dedifferentiation and mesenchymal transition.
Conclusions
Our present study investigated the interaction mechanism between β-arrestin 1/2 and downstream protein of the Wnt/β-catenin signaling pathway. In further research, we will investigate the potential intrinsic mechanism. This study demonstrates a significant effect of β-arrestin 1/2 on apoptosis of podocytes through the Wnt/β-catenin signaling pathway, which should be explored in greater depth.
Acknowledgement
We thank the Basic Medical Research Center of Tianjin Medical University for excellent technical assistance and Dr. Zhou for careful reading and helpful criticism of the manuscript.
Footnotes
Source of support: Departmental sources
References
- 1.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16:S30–33. doi: 10.1681/asn.2004110970. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Lei T. Rs12976445 polymorphism is associated with risk of diabetic nephropathy through modulating expression of MicroRNA-125 and interleukin-6R. Med Sci Monit. 2015;21:3490–97. doi: 10.12659/MSM.894987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–73. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 4.Tervaert TWC, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–63. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 5.Wolf G, Chen SD, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease – Podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–34. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Lin CL, Wang JY, Huang YT, et al. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006;17:2812–20. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- 8.Kabeya Y, Mizushima N, Uero T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–28. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lymperopoulos A, Bathgate A. Arrestins in the cardiovascular system. Prog Mol Biol Transl Sci. 2013;118:297–334. doi: 10.1016/B978-0-12-394440-5.00012-7. [DOI] [PubMed] [Google Scholar]
- 10.Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–36. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 11.Venkatakrishnan AJ, Deupi X, Lebon G, et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature. 2016;536:484–87. doi: 10.1038/nature19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 13.Munro S, Thomas KL, Abushaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 14.Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Park EC, Lee H, et al. beta-Arrestin 1 mediates non-canonical Wnt pathway to regulate convergent extension movements. Biochem Biophys Res Commun. 2013;435:182–87. doi: 10.1016/j.bbrc.2013.04.088. [DOI] [PubMed] [Google Scholar]
- 16.Buelli S, Rosano L, Gagliardini E, et al. beta-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol. 2014;25:523–33. doi: 10.1681/ASN.2013040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lymperopoulos A. Beta-arrestin biased agonism/antagonism at cardiovascular 7 transmembrane-spanning receptors. Curr Pharm Des. 2012;18:192–98. doi: 10.2174/138161212799040475. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Li QX, Wang XJ, et al. beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis. 2016;7:e2183. doi: 10.1038/cddis.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu X, Zhang C, Fan Q, et al. Inhibiting MicroRNA-503 and microRNA-181d with losartan ameliorates diabetic nephropathy in KKAy mice. Med Sci Monit. 2016;22:3902–9. doi: 10.12659/MSM.900938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quack I, Woznowski M, Potthoff SA, et al. PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J Biol Chem. 2011;286:12959–70. doi: 10.1074/jbc.M110.204024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Luo W, Sun Y, et al. Wnt/beta-catenin signaling mediated-UCH-L1 expression in podocytes of diabetic nephropathy. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091404. pii: E1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu BL, Chen YP, Cheng H, et al. The protective effects of curcumin on obesity-related glomerulopathy are associated with inhibition of Wnt/beta-catenin signaling activation in podocytes. Evid Based Complement Alternat Med. 2015;2015:827472. doi: 10.1155/2015/827472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Wang X, Nie F, et al. miR-135 family members mediate podocyte injury through the activation of Wnt/beta-catenin signaling. Int J Mol Med. 2015;36:669–77. doi: 10.3892/ijmm.2015.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan RJ, Liu Y. Arrestin(g) podocyte injury with endothelin antagonism. J Am Soc Nephrol. 2014;25:423–25. doi: 10.1681/ASN.2013111230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quack I, Rump LC, Gerke P, et al. beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci USA. 2006;103:14110–15. doi: 10.1073/pnas.0602587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryja V, Gradl D, Schambony A, et al. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:6690–95. doi: 10.1073/pnas.0611356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Liu Y. Wnt/beta-catenin signaling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015;11:535–45. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Li Y, He W, et al. Mutual antagonism of Wilms’ tumor 1 and beta-catenin dictates podocyte health and disease. J Am Soc Nephrol. 2015;26:677–91. doi: 10.1681/ASN.2013101067. [DOI] [PMC free article] [PubMed] [Google Scholar]