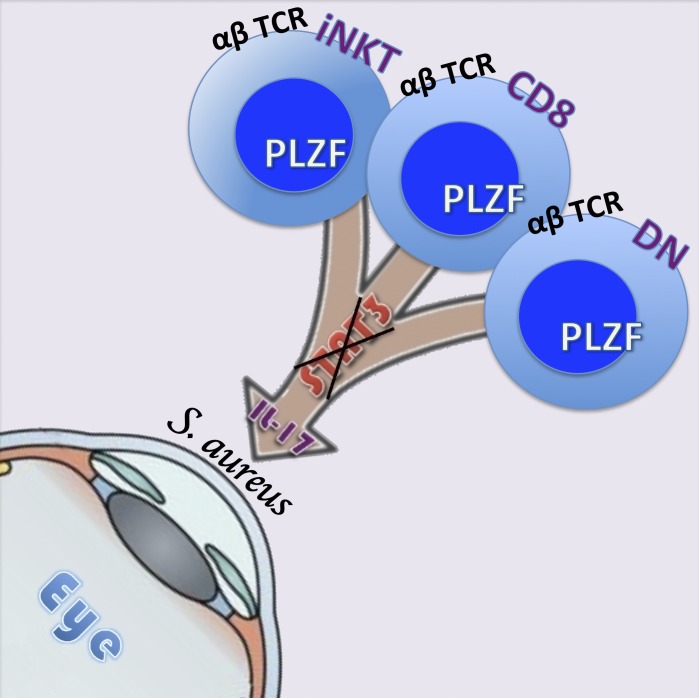
St. Leger et al. identify and examine innate-like αβ T cells that circumvent canonical STAT-3 phosphorylation to produce protective IL-17. These cells can exist in the ocular mucosa and protect the ocular surface from pathogenic Staphylococcus aureus infection.
Abstract
Appropriate regulation of IL-17 production in the host can mean the difference between effective control of pathogens and uncontrolled inflammation that causes tissue damage. Investigation of conventional CD4+ T cells (Th17 cells) has yielded invaluable insights into IL-17 function and its regulation. More recently, we and others reported production of IL-17 from innate αβ+ T cell populations, which was shown to occur primarily via IL-23R signaling through the transcription factor STAT-3. In our current study, we identify promyelocytic leukemia zinc finger (PLZF)–expressing iNKT, CD4−/CD8+, and CD4−/CD8− (DN) αβ+T cells, which produce IL-17 in response to TCR and IL-1 receptor ligation independently of STAT-3 signaling. Notably, this noncanonical pathway of IL-17 production may be important in mucosal defense and is by itself sufficient to control pathogenic Staphylococcus aureus infection at the ocular surface.
Graphical Abstract
Introduction
Adaptive IL-17–producing CD4+ T cells (Th17) are involved in host defense as well as autoimmunity. Studies of the mechanisms that control IL-17 production in Th17 cells have revealed that IL-6, IL-21, IL-23, TGF-β, and/or IL-1β, drive differentiation and production of IL-17 through the activation of STAT-3 and the master transcription factor RORγt (Ghoreschi et al., 2010).
Recently, attention has expanded to other populations of cells that produce IL-17, which include adaptive CD8+ T cells (Fletcher et al., 2010) as well as various innate T cells (Isailovic et al., 2015). Unlike adaptive Th17 cells that require priming and polarization for IL-17 production, innate IL-17–producing cells respond with quick and robust production of the cytokine (Sutton et al., 2009; Takatori et al., 2009; Myles et al., 2013). The ability to produce IL-17 rapidly was shown to be critical during early stages of infection with pathogens such as Staphylococcus aureus (Cho et al., 2010), Klebsiella pneumonia (Happel et al., 2003), Candida albicans (Gladiator et al., 2013; Conti et al., 2014), and Toxoplasma gondii (Passos et al., 2010). Cells that produce innate IL-17 include CD8+ T cells (Happel et al., 2003; Fletcher et al., 2010), γδ TCR+ cells, NK1.1− NKT cells (NKT17; Rachitskaya et al., 2008), mucosal-associated invariant T cells (MAIT cells; Dusseaux et al., 2011), CD4−CD8− T cells (Sherlock et al., 2012), natural Th17 cells (nTh17; Marks et al., 2009), lymphoid tissue inducer (LTi) cells, and type 3 innate lymphoid cells (ILC3s; Annunziato et al., 2015). Although different pathways to IL-17 induction have been described (Durant et al., 2010; Ghoreschi et al., 2011), all have reported a critical role for IL-23 and/or STAT-3, with therapeutic strategies to target IL-17 production now based largely around manipulation of these mediators.
In the current study, we report that IL-17 production by innate, promyelocytic leukemia zinc finger (PLZF)–expressing lymphocytes can be driven by TCR ligation and IL-1β, independently of both STAT-3 and IL-23 signaling, and has in vivo relevance. In particular, we examine three populations of T cells, CD44hi CD4–CD8+ T cells, CD44hi CD4–CD8– double-negative T cells (DNT), and iNKT cells, all of which appear to acquire effector function in the thymus, can use this pathway, and readily produce IL-17, even in mice genetically deficient in STAT-3. Most importantly, we show that in the presence of IL-1β, these cells produce sufficient levels of IL-17 to prevent the outgrowth of pathogenic S. aureus in the conjunctiva, demonstrating the relevance of the STAT-3–independent pathway of IL-17 production in mucosal infection.
Results and discussion
IL-17–producing T “memory” lymphocytes are present in mice deficient in IL-6, IL-21, and IL-23 signaling, which lack adaptive Th17 cells
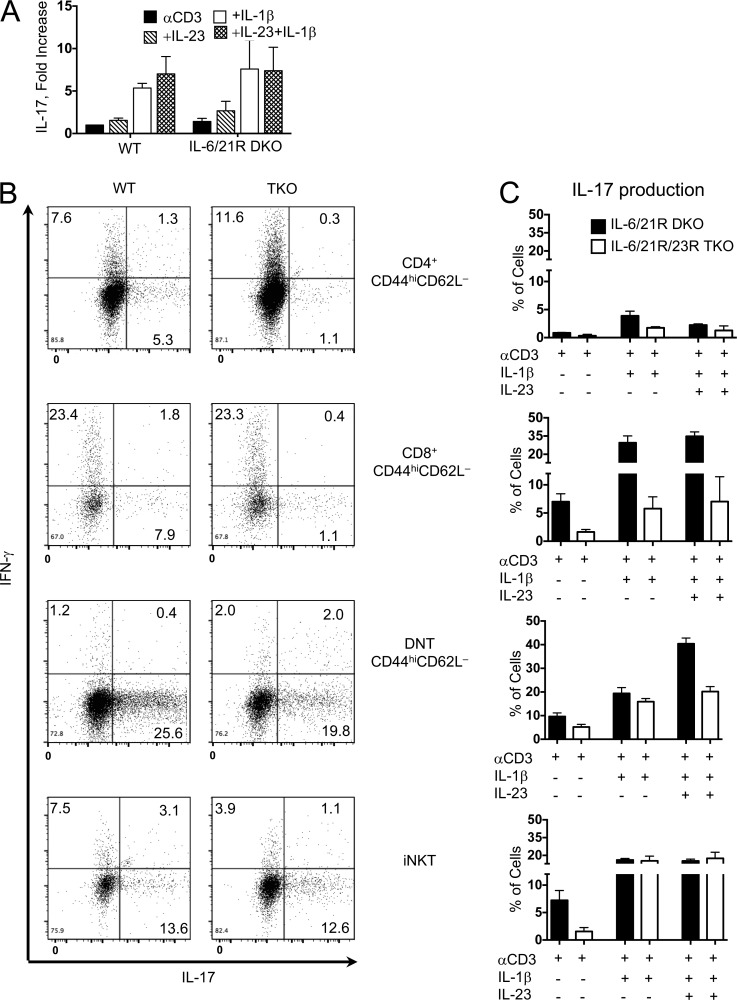
IL-1β provides a critical signal for both conventional Th17 and innate IL-17 responses (Chung et al., 2009; Ikeda et al., 2014). The downstream components of this pathway have not been clearly defined, but one possibility could be that IL-1β is inducing IL-17 through secondary mediators such as IL-6 and IL-21. To investigate this, we bred mice deficient in IL-6 and in IL-21 receptor (IL-6/21R double knockout [DKO]), which are thought to lack adaptive Th17 cells. To our surprise, αβ TCR+ cells with a memory phenotype (CD44hiCD62Llo), isolated from spleens and lymph nodes of these mice using NKT and γδ TCR+ exclusion gates (Fig. S1), exhibited robust IL-17 production after 72 h of stimulation with anti–CD3 (αCD3) and IL-1β (Fig. 1 A). IL-17 production after stimulation with IL-1β or IL-23 did not occur in the absence of CD3 stimulation (not depicted).
Figure 1.
IL-17–producing T “memory” lymphocytes are present in mice that lack adaptive Th17 cells. (A–C) CD44hiCD62Llow αβ T cells sorted from WT and IL-6/-21R DKO mice (A), WT and IL-6/-21R/-23R TKO (B), or DKO and TKO mice (C) and stimulated for 72 h with αCD3, IL-1β, and/or IL-23. Brefeldin A was added the last 6 h in culture. Cells were harvested, fixed, and permeabilized and stained for intracellular IL-17 and IFNγ. Flow dot plots represent cytokine production after stimulation between WT and TKO mice, and numbers represent frequencies of cytokine production. (C) Bars in graphs represent the mean frequency ± SEM of IL-17+ cells after culture. n = 3 (αCD3), 5 (αCD3+IL-1β), and 5 (αCD3+IL-1β+IL-23); each n consists of pooled cells from at least three mice.
We sought to identify the cell types within this population that produce IL-17 independently of both IL-6 and IL-21 signaling. To eliminate potential confounding effects of IL-23, which induces robust IL-17 production in innate cells as well as memory T cells, we additionally generated an IL-6/21R/23R triple-KO (TKO) mouse by crossing the IL-6/21R DKO mouse to the IL-23R GFP mouse, which is a functional IL-23 KO when bred to homozygosity (Awasthi et al., 2009). For both IL-6/21R DKO and IL-6/21R/23R TKO strains, we further fractionated the “Tmem” population into CD4+, CD8+, and DN αβ TCR+ cells (full scheme in Fig. S1) and stimulated them in vitro for 72 h with αCD3 and IL-1β. NKT17 cells, which we have previously shown to produce IL-17 independently of IL-6 and IL-21 (Rachitskaya et al., 2008), served as comparison and control.
As we expected, adaptive CD44hiCD4+ T cells from IL-6/21R DKO mice, which lack conventional Th17 cells, showed minimal IL-17 production after stimulation with αCD3 and IL-1β, and/or IL-23, and IL-17 was completely absent in CD4+ cells from IL-6/21R/23R TKO mice (Fig. 1, B and C, top). Although neither of these strains was expected to harbor conventional memory IL-17–producing cells, we found IL-17–producing cells within the memory phenotype CD8+ and DN αβ TCR+ cell populations of DKO and TKO mice (Fig. 1, B and C). Despite a reduction in IL-17 production when the functional IL-23R was also deleted, the memory-phenotype CD8+ and DNT cells maintained the ability to produce IL-17 (5% and 20%, respectively). Even iNKT cells, which were previously thought to require IL-23 for IL-17 production (Rachitskaya et al., 2008; Doisne et al., 2011), were unaffected by lack of IL-23R in the presence of IL-1β.
IL-17–producing memory-like αβ T cells in TKO mice express the innate-associated transcription factor PLZF and do not phosphorylate STAT-3
The data above suggested that the memory-phenotype CD8+ and DNT represent innate-like T cells rather than conventional adaptive lymphocyte populations and that they are able to produce IL-17 independently of the canonical signaling cascades previously described for adaptive memory (IL-6 and/or IL-21 dependent) and innate (IL-23 dependent) IL-17 production. We therefore set out to examine whether memory-phenotype CD8+ and DNT cells expressed PLZF, characteristic of innate lymphocytes (T and non-T; Savage et al., 2008), and whether STAT-3, used by all IL-17 induction pathways described thus far, is phosphorylated by stimuli that lead to IL-17 production in these cells.
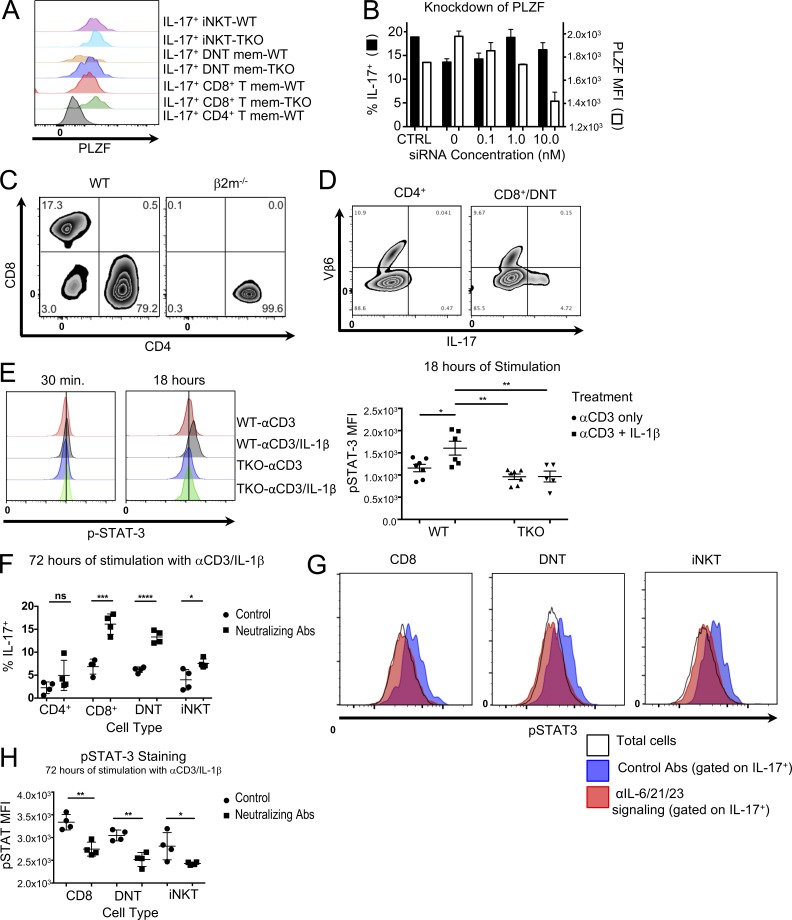
Sorted memory-phenotype cells from the lymphoid organs of WT and TKO mice were stimulated with αCD3/IL-1β for 72 h. IL-17–producing iNKT cells from both WT and TKO mice had increased expression of PLZF, as has already been described by others (Savage et al., 2008) and was absent, as expected, in conventional WT CD4+ memory T cells (Fig. 2 A). Notably, enhanced expression of PLZF was observed in IL-17-producing Tmem CD44hiCD8+ and DNT cells, supporting their classification as innate lymphocytes; however, PLZF was not a requirement of IL-17 production, as siRNA for PLZF reduced expression of the transcription factor but not IL-17 after stimulation (Fig. 2 B). The development of CD8+ and DNT innate-like cells appeared to require MHC class I and/or nonclassical MHC interactions, as they were not present in mice lacking β2-microglobulin (β2m; Fig. 2 C). Finally, they appeared to be distinct from MAIT cells, which also produce IL-17, express PLZF, and whose development is β2m dependent, in that they did not have the conserved TCR Vβ6/8 characteristic of MAIT cells (Le Bourhis et al., 2010; Fig. 2 D); however, definitive assessment of STAT-3 signaling in IL-17–producing MAIT cells will require the use of appropriate tetramer reagents.
Figure 2.
IL-17–producing memory-like αβ T cells in TKO mice express the innate-associated transcription factor PLZF and do not phosphorylate STAT-3. (A) CD44hiCD62Llow CD4+, CD8+, DNT, and iNKT cells were sorted, stimulated with IL-1β and/or αCD3 for 72 h, fixed, and permeabilized. Cells were then stained for intracellular IL-17, PLZF, and lineage-specific markers. The representative histogram (from two independent experiments) depicts the PLZF expression in IL-17+ cells from the specified populations. (B) CD44hi CD8+ and DNT cells from TKO mice were sorted and stimulated with CD3 and IL-1β in the presence of siRNA against PLZF for 72 h, and brefeldin A was added the last 6 h of culture. Cells were then stained for intracellular IL-17 and PLZF and analyzed by flow cytometry. Black bars represent IL-17 production, and white bars represent PLZF expression in the IL-17+ population. (C) CD4+ and CD8+ CD44hiCD62Llow αβ TCR+ γδ TCR− cells from pooled (n = 5) LNs of WT or β2m−/− mice. Data represent two independent experiments. (D) CD44hiCD62Llow CD4+ or CD8+/DNT cells were sorted as stated previously from IL-6/21R/23R TKO and cultured with IL-1β and αCD3 for 72 h. After stimulation, cells were stained for IL-17 and Vβ6. Data represent two individual experiments. (E) CD44hiCD62Llow CD8+ and DNT cells were isolated, combined, and cultured with IL-1β and/or αCD3 for 30 min and 18 h. After stimulation, cells were harvested, fixed, permeabilized, and stained for phospho–STAT-3. Symbols represent individual mice from two independent experiments. Bars represent mean ± SEM. MFI of pSTAT-3 in live cells. One-way ANOVA determined significance (*, P = 0.0295; ***, P < 0.0036). (F–H) WT CD44hiCD62Llow CD8+, DN, and iNKT cells were sorted as described previously. Each population was cultured with either IL-1β and αCD3 with or without αIL-6/αIL-6Rα/αIL-21/αIL-23p19/αIL-23R. After 72 h, cells were harvested, fixed, permeabilized, and stained for (F) intracellular IL-17 and (G and H) phospho–STAT-3. Symbols represent technical replicates from a single experiment that consisted of pooling cells from three mice. Bars represent the mean (E) IL-17% or (G) pSTAT-3 MFI ± SEM, and data are representative of two independent experiments. Student’s t test was used to determine significance (for F, *, P = 0.0274; ***, P = 0.0005; ****, P < 0.0001; for G, *, P = 0.0449; **, P < 0.0021).
The cytokines IL-23, IL-6, and IL-21 are all known to induce IL-17 production in CD4+ T cells by activation of STAT-3, which is considered to be a critical activating signal for the induction and maintenance of the Th17 lineage (Bettelli et al., 2006; Harris et al., 2007; Huber et al., 2009; Ghoreschi et al., 2010; El-Behi et al., 2011). To rule out the possibility that another cytokine might be circumventing conventional signaling, we examined whether IL-17–producing innate-like TKO cells still phosphorylate STAT-3. CD8+ and DNT innate-like cells isolated from TKO or WT mice were pooled within the genotype to obtain sufficient cells for analysis. The innate-like CD8+/DNT populations were then stimulated for 18 h with αCD3/IL-1β to ensure sufficient proliferation within the appropriate cell populations. We assessed STAT-3 phosphorylation by intracellular flow cytometry. Although stimulation with αCD3+IL-1β led to phosphorylation of STAT-3 in the innate-like cells from WT mice, no detectable phosphorylation occurred in cells from TKO mice (Fig. 2 E), suggesting the existence of an alternative pathway for IL-17 production that does not use STAT-3.
To address the possibility that this phenomenon was a compensatory mechanism unique to the TKO mice, we isolated CD8+, DNT, and iNKT cells from WT mice and stimulated them in vitro using neutralizing antibodies to the cytokines IL-6, IL-21, and IL-23 and their receptors. IL-17 production not only was maintained after 72 h in culture but also appeared to increase in the presence of the antibody cocktail (Fig. 2 F). Intracellular flow cytometry confirmed that STAT-3 was not being phosphorylated in these cells. (Fig. 2, G and H). Although the increase in IL-17 was not necessarily expected, it is conceivable that the pleotropic nature of IL-6 and IL-21 could regulate IL-17 production in cells that do not use STAT-3 signaling (Scheller et al., 2011; Croce et al., 2015).
Innate-like CD8+ and DN αβ T cells produce IL-17 independently of STAT-3 but require the transcription factor RORγt
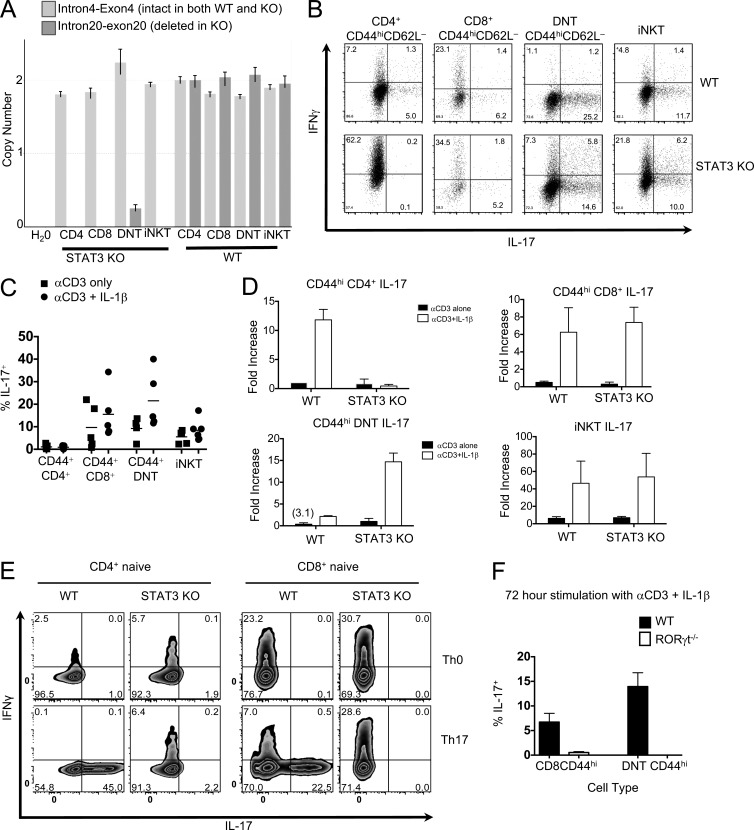
To directly examine whether STAT-3 is necessary for production of IL-17 by CD8+ and DNT cells, we used conditional KO CD4-Cre × Stat3fl/fl (STAT-3 KO) mice, in which all thymically derived T cells are rendered STAT-3 null during the CD4/CD8 double-positive stage in the thymus (Durant et al., 2010). First, we confirmed that STAT-3 is indeed absent in the innate-like cell populations of STAT-3 KO mice by quantitating STAT-3 genome copy number using RT-PCR (Fig. 3 A). Next, we examined whether innate-like cells that are genetically deficient in STAT-3 are able to produce IL-17 in response to TCR and IL-1 stimulation. Purified CD44hiCD4+ T cells, CD44hiCD8+ T cells, CD44hiDNT cells, and iNKT cells from STAT-3 KO mice and their WT littermates were stimulated with αCD3 alone or αCD3 plus IL-1β, and their cytokine production was compared using intracellular cytokine staining and ELISA of culture supernatants. (γδT cells, which do not undergo a CD4+ stage and hence are not rendered STAT-3 deficient in STAT-3 KO mice, were excluded, as shown in Fig. 1 A.) In contrast to CD44hiCD4+ T cells, all three STAT-3–deficient innate-like cell populations maintained IL-17 production in response to TCR ligation, and it was again strongly up-regulated by IL-1β (Fig. 3, B–D).
Figure 3.
Innate-like CD8+ and DN αβT cells produce IL-17 independently of STAT-3 but require the transcription factor RORγt. (A) DNA was isolated from at least 30,000 CD4+, CD8+, DN, and iNKT cells from CD4-Cre × Stat3fl/fl (STAT-3 KO) or Stat3fl/fl (WT) mice. Data shows copy number using TaqMan primers from ABI spanning intron4–exon4, (intact Stat3 region control, Mm00369932_cn) and intron20–exon20 (deleted region, Mm00369911_cn). (B–E) CD44hiCD62Llowαβ TCR+ γδ TCR− cells (B–D) or naive T cells (E) were sorted from WT or STAT-3 KO mice and stimulated for 72 h with IL-1β and/or αCD3. Brefeldin A was added the last 6 h in culture. After incubation, cells were harvested, fixed, permeabilized, and stained for intracellular cytokines, IL-17 and IFN-γ, for flow cytometric analysis. (B) Representative dot plots showing IL-17 and IFNγ production in T memory cells from WT (top) STAT-3 KO (bottom) mice. Frequencies of the cell populations are within the plots. (C) Bars represent the mean frequency of IL-17+ cells in T memory cells after culture. Symbols represent individual mice and data are representative of five independent experiments. (D) Bars represent the fold increase ± SEM in IL-17 protein in supernatants after culture. Background levels ranged from 10 to 50 pg/ml. Total n = 2; each n was a single experiment consisting of cells pooled from three mice. (E) Representative flow plots showing IL-17 and IFN-γ production in naive T cells after 72 h of culture using Th0 (top) or Th17 (bottom) skewing condition. Data are representative of at least four independent experiments. (F) CD44hiCD62Llowαβ TCR+ γδ TCR− cells from WT or RORγt−/− mice were sorted and stimulated with IL-1β and αCD3 for 72 h. Brefeldin A was added the last 6 h of culture. Cells were harvested, fixed, stained for intracellular IL-17, and analyzed by flow cytometry. Bars represent the mean frequency ± SEM of live cells that were IL-17+. Symbols represent technical replicates from cells pooled from four mice from a single experiment that was independently performed two times.
To further confirm that our innate-like CD8+ and DNT cells are not part of the conventional T memory compartment, we isolated naive CD4 and CD8 T cells from WT and STAT-3 KO mice and primed them in vitro under conventional IL-17–inducing conditions. In contrast to the (originally naive) WT T cells, neither the CD4+ nor the CD8+ T cells from STAT-3 KO mice gave rise to IL-17 production after 3 d of culture with IL-6, IL-23, and TGF-β (Fig. 3 E), supporting the notion that the populations we have defined are innate T cells that express memory markers rather than conventional memory T cells.
Finally, we wished to examine the RORγt dependence of IL-17 production by innate-like T cells. RORγt is known to play a role in the maintenance of innate IL-17–producing cells; however, its functional influence on innate IL-17 production is still unclear. Using RORγt−/− mice, we found that even under conditions of STAT-3 sufficiency, the innate-like CD8+ and DNT cells lacking RORγt failed to produce IL-17 after the 72 h with combined TCR and IL-1 stimulation (Fig. 3 F; iNKT cell numbers were too low to isolate for culture). These data show that despite deficiency of STAT-3, RORγt is required either for acquisition of function or for the development of IL-17–producing innate-like αβ T cells.
STAT-3–independent IL-17–producing innate-like αβT cells develop in the thymus
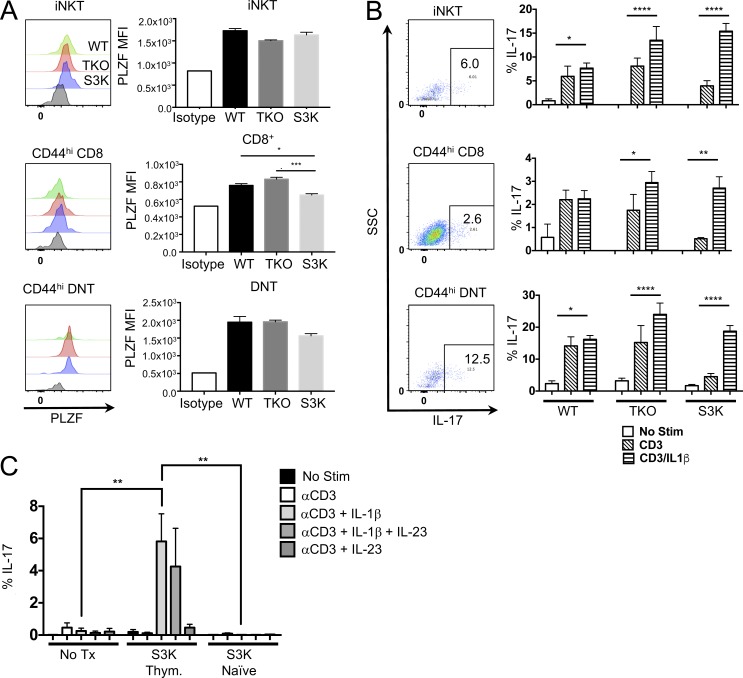
We asked whether these cells are thymically derived or develop in the periphery. Within the CD44hi population of thymocytes (gating strategy in Fig. S2), CD8, DN, and iNKT cells all expressed PLZF (Fig. 4 A) and produced IL-17 after stimulation with CD3 plus IL-1β (Fig. 4 B). We next asked whether peripheral naive STAT-3 KO cells could acquire this phenotype after expansion in a lymphopenic host as observed in a recent study investigating IFN-γ in CD44hi αβ T cells. After transfer of CD44hi CD8/DNT STAT-3 KO thymocytes or naive CD8/DNT STAT-3 KO cells into Rag2/Il2rg DKO, only the thymus-derived donor cells were able to produce IL-17. Naive peripheral donor cells acquired a memory phenotype (not depicted) and the ability to produce IFN-γ, similar to what was observed in spontaneous memory development in a different model (Kawabe et al., 2017). However in our study, memory cells derived from naive STAT-3 KO did not acquire the ability to produce IL-17 (Fig. 4 C). We conclude that CD44hi expression and effector function by innate-like CD8 and DN αβ T cells is acquired in the thymus rather than after activation in the periphery.
Figure 4.
STAT-3–independent IL-17–producing innate-like T cells exist in the thymus. (A and B) Single-cell suspensions of thymuses from 6-wk-old WT, TKO, or STAT-3 KO (S3K) mice were either stained for PLZF expression (A) or stimulated with CD3 and IL-1β for 72 h (B). Gating strategies are depicted in Fig. S2 A. Histograms represent the PLZF staining. Flow plots represent staining pattern in the thymus after stimulation with CD3 plus IL-1β. Bars in graphs represent the MFI of PLZF staining ± SEM (n = 3 for WT and TKO and n = 5 for S3K; *, P = 0.0130; ***, P = 0.0006). Data represent two independent experiments. (B) Flow plots represent the staining pattern after stimulation with either CD3 or CD3 and IL-1β (no stim samples yielded very few cells). Brefeldin A was added the last 6 h of culture, and IL-17 was assessed by flow cytometry. Bars represent the mean frequency ± SEM of cells that were IL-17+ (n = 3 for WT and TKO and n = 5 for S3K; *, P < 0.05; **, P < 0.01; ****, P < 0.0001). Statistical significance was determined using ANOVA. (C) 2 × 104 CD44hi CD8/DNT S3K thymocytes or 2 × 105 naive CD44hi CD8/DNT S3K cells were transferred i.v. into Rag2/Il2rg DKO mice. After 4 wk, splenic cells were isolated and stimulated with CD3 and IL-1β and/or IL-23 for 72 h. Brefeldin A was added the last 6 h of culture, and cells were stained for intracellular IL-17. Bars represent the mean frequency ± SEM of IL-17+ cells after culture (n = 3 for WT and S3K naive, n = 5 for S3K Thym.). Data represent two independent experiments. Statistical significance was determine by ANOVA (**, P = 0.002).
STAT-3–independent IL-17–producing innate-like αβ T cells are present in the mucosal tissues of the ocular surface and can protect from S. aureus infection
The following experiments primarily used TKO rather than STAT-3 KO mice, because in the latter, STAT-3 is not excised in γδ T cells, which can also produce IL-17 in the conjunctiva. As shown in Fig. 2 D, STAT-3 was not activated in TKO cells under conditions that lead to IL-17 production. This indicated that CD44hi CD8+ and DNT cells from TKO mice were a valid model to assess IL-17 production under STAT-3–insufficient conditions.
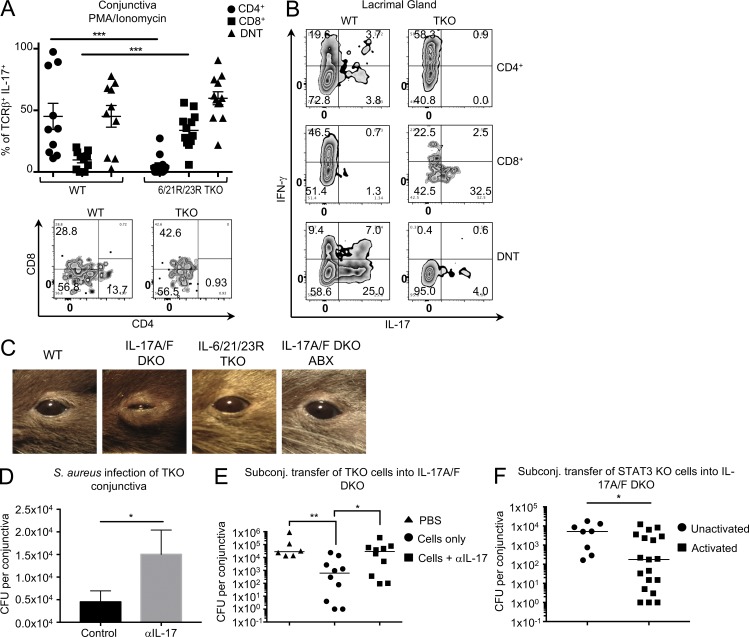
We next sought to identify whether TKO mice harbored CD44hi cells within ocular mucosa during steady-state conditions. We assessed the ability of CD44hi cells isolated from ocular mucosa of TKO and WT mice to produce IL-17 (a) directly ex vivo after a PMA/ionomycin pulse, as reflective of their status in situ, and (b) after culture of sorted populations with αCD3 plus IL-1β, as reflective of their potential after activation. Immunophenotyping combined with intracellular cytokine staining revealed a strikingly different pattern of IL-17 production in conjunctival cells from WT versus TKO mice. Under STAT-3 insufficiency, conjunctival CD4+ T cells do not produce IL-17 after PMA/ionomycin stimulation. In contrast, in the same conjunctiva there is an increased frequency of IL-17–producing CD8+ T cells (9.8% WT, 33.7% TKO) and a moderately increased frequency of IL-17–producing DNT cells (45.1% WT, 59.8% TKO; Fig. 5 A). These data suggest that rather than abolishing IL-17 production, STAT-3 insufficiency allows for the expansion of other cell populations that can produce IL-17. We next examined the response of purified cell subsets to activation. Because of insufficient numbers of cells in normal conjunctival tissue, we isolated cells from the lacrimal gland, which is also part of the ocular mucosal system. Highly purified CD4+, CD8+, and DNT cells from lacrimal gland tissue were subjected to αCD3/IL-1β stimulation. Again, IL-17 was produced by CD4+ T cells of WT, but not TKO mice (although TKO cells produced copious amounts of IFN-γ), and again, TKO-CD8+ T cells produced more IL-17 than WT controls (Fig. 5 B). We observed a dramatic difference in the pattern of cytokine production between the conjunctiva and lacrimal gland, suggesting that the lacrimal gland may lack a critical signal that drives this nonconventional IL-17 production and/or the tissue may harbor an unknown suppressive factor regulating the production of IL-17.
Figure 5.
STAT-3–independent IL-17–producing innate-like αβT cells are present in mucosal tissue of the ocular surface. (A and B) Conjunctiva (A) or lacrimal glands (B) were harvested from WT or TKO mice, treated with collagenase, and dispersed into single-cell suspensions. Cells were either pulsed for 4 h with PMA/ionomycin in the presence of brefeldin A (A) or stimulated with αCD3/IL-23/IL-1β for 72 h (B), and brefeldin A was added during the last 6 h of culture. Cells were then stained for TCRβ, CD4, and CD8, fixed and permeabilized, and stained for intracellular IL-17. (A) Each symbol represents an individual mouse, and bars represent the mean frequency ± SEM of TCRβ+ that produced IL-17. Zebra plots are gated on IL-17+ cells. Data are pooled from three independent experiments. Student’s t test determined significance (***, P < 0.0007; P = 0.1532 for DNT). (B) Zebra plots represent cytokine production from lacrimal gland cells from two independent experiments. (C) Representative pictures of mouse eyes from mice treated or not with antibiotics. Disease typically observed in 50% of IL-17A/F DKO mice. (D) TKO mice were given an i.p. injection of either PBS or 500 µg aIL-17 (DNAX). 2 d later, mice were anesthetized with a ketamine-xylazine mixture, given another i.p. injection of PBS or αIL-17, and conjunctivae were inoculated with a cotton swab soaked in S. aureus (USA300). 72 h later, conjunctivae were harvested, and S. aureus CFUs were calculated. Bars represent the mean ± SEM CFUs per conjunctiva. Statistical significance was determined using a Mann-Whitney U test (*, P = 0.133; n = 13 for control, n =16 for αIL-17). (E and F) S. aureus was topically applied on days −14 and −7. (E) On day 0, 10,000 activated CD8 and DN memory αβT cells from TKO mice were subconjunctivally transferred with or without 5 µg αIL-17. Mice were sacrificed, and conjunctiva was harvested on day 3. Statistical significance was determined using the Kruskal–Wallis test (*, P = 0.040; **, P = 0.009). (F) On day 0, 10,000 unactivated or activated CD8 and DN memory αβT cells from STAT-3 KO mice were subconjunctivally transferred. Mice were sacrificed and conjunctiva was harvested on day 3. Bars represent the median S. aureus CFU counts per conjunctiva. Statistical significance was determined using the Mann–Whitney U test (*, P = 0.015). Each point represents an individual conjunctiva pooled from two independent experiments.
Despite compelling evidence that populations of innate-like CD8+ and DN αβT cells could rapidly produce IL-17 independently of STAT3, it was unclear whether these cells, or indeed this pathway, might have relevance in vivo. We noted that IL-17A/IL-17F genetically deficient (A/F DKO) mice maintained in our animal facility spontaneously develop severe conjunctivitis accompanied by swelling of their eyelids. A bacterial nature of this condition was suggested by a published report that IL-17A/F DKO mice are prone to S. aureus infections of the lungs and nares (Ishigame et al., 2009). In line with this, we were able to culture S. aureus from the eyes of IL-17A/F DKO mice, and the inflammation was cleared by treatment with antibiotics (Fig. 5 C). Because eyes of TKO and STAT-3 KO mice housed under the same conditions remained healthy (Fig. 5 C and not depicted), we hypothesized that STAT-3 KO and TKO mice may harbor innate-like αβT cells in ocular tissue that produce protective IL-17 under STAT-3–insufficient conditions to prevent outgrowth of the bacteria.
We examined whether IL-17 was required for protection from ocular infection by ocularly challenging TKO mice that were treated with control or IL-17 neutralizing antibody with S. aureus. Indeed, IL-17 was required to limit S. aureus in ocular tissue as mice treated with αIL-17 threefold more CFUs compared with the control group (Fig. 5 D). Similarly, we next showed that preactivated CD44hi CD8/DNT cells subconjunctivally injected into IL-17A/F could protect mice from local S. aureus challenge (Fig. 5 E). Together, these data suggested that IL-17 produced from CD44hi cells under condition of STAT-3 insufficiently was sufficient to protect ocular mucosa. Finally, we asked if activated STAT-3 KO CD44hi CD8/DNT cells could protect the ocular surface from S. aureus challenge. Indeed, after activation in vitro and subconjunctival transfer into IL-17A/F DKO mice, these cells reduced S. aureus burden at the ocular surface compared with mice that received unactivated cells (Fig. 5 F), suggesting that cells can protect the ocular surface once activated.
In conclusion, although the requirement for STAT-3 signaling in inducible Th17 development has been clearly demonstrated, less attention has been paid to whether the same applies to naturally occurring IL-17–producing αβTCR+ populations. Although Tanaka et al. (2009) suggested that nTh17 cells develop independently of STAT-3, more recent studies suggest that this population still requires IL-23 signaling for IL-17 production (Conti et al., 2014), and neither study addressed the role of IL-1β. Indeed, a lack of appreciation for the role of IL-1β in IL-17 production led to its omission in early characterization studies (including our own), which instead focused solely on IL-6, IL-21, and IL-23. Our study provides clear evidence that IL-1β is sufficient to drive IL-17 production independently of both STAT-3 and IL-23 in PLZF-expressing iNKT, CD8+, and DNT αβTCR+ cells. Furthermore, nTh17 cells appear to have distinct migratory patterns compared with the cells described in our study. Whereas nTh17 cells appear to localize to airways and the oral cavity, they appear to be absent in the ocular mucosa of mice with STAT-3 insufficiency (TKO or STAT-3 KO), highlighting a potential role for STAT-3 in the migration pattern of nTh17 cells to ocular mucosa (Oh et al., 2011). Conversely, we observed an enhancement of innate-like CD8+ and DNT cells at the ocular surface in mice that cannot use STAT-3 signaling, suggesting that these cells may play a unique biologically relevant role at the ocular surface.
The expression of PLZF in the IL-17–producing cells described in this study confirms their innate-like nature (Savage et al., 2008; Constantinides et al., 2014) and may explain their memory phenotype (Kovalovsky et al., 2010); however, the requirement of PLZF for this phenotype has not been addressed. Our data demonstrate that IL-1 stimulation can induce rapid IL-17 production in a TCR-dependent manner by using signaling pathways distinct from conventional Th17 cells, which may include the usage of transcription factors, NF-κb and/or interferon regulatory factors (IRFs). Although our study does not address what is the TCR signal in vivo, we speculate that it could originate from the same microorganisms that induce IL-1β production. Notably, despite the evidence that implicates (adaptive) IL-17 as a pathogenic cytokine in ocular diseases (Chauhan et al., 2009; Suryawanshi et al., 2011; Zaidi et al., 2012), we show a beneficial role of innate IL-17 at the ocular surface and provide evidence that this IL-17 can be produced via a distinct pathway that does not necessarily depend on STAT-3. Importantly, even a small number STAT-3–deficient cells stimulated to produce IL-17 can rapidly and substantially reduce S. aureus within ocular mucosa. These data indicate for the first time that STAT-3–independent IL-17 production is sufficient to modulate immunity and prevent infection at the ocular surface, and potentially other sites, without the need for an adaptive Th17 response.
In the aggregate, our data support the interpretation that innate-like production of IL-17 through a noncanonical pathway that does not require IL-23 or activation of STAT-3 may have a physiological role in mucosal host defense. That said, our data do not exclude the possibility that when dysregulated this innate IL-17 could also be deleterious. It is of note that IL-17–producing CD8 and DNT cells have been identified in several human disease states (Blanco et al., 2005; He et al., 2006; Tzartos et al., 2008; Res et al., 2010; Sherlock et al., 2012; Huber et al., 2013). Future studies will hopefully aim to explore potential functional parallels with the murine IL-17–producing CD8+ and DNT cells we have described. Although beyond the scope of our current study, such information could be important for therapeutic approaches to T cell–mediated diseases, in which IL-17 production from such cells would be refractory to STAT-3 inhibitors currently under development. While this might lessen the effectiveness of therapy, it could also mitigate increased susceptibility to infections that can result from targeting cytokines that function in host defense, and promote a more favorable immunological balance.
Materials and methods
Mice
C57BL/6 (WT) and IL-6 KO mice were purchased from The Jackson Laboratory. RAG2/IL2rg DKO mice were purchased from Taconic Farms. IL-21R–deficient mice (Ozaki et al., 2002), IL-23R-GFP reporter mice (Awasthi et al., 2009), STAT-3–deficient mice (Lee et al., 2002), and IL-17A/F–deficient mice (Ogura et al., 2008; Ishigame et al., 2009) were generated and provided by the referenced collaborators. Crosses of these various strains were generated and maintained in the animal facilities of the National Institutes of Health (NIH) in Bethesda or Frederick, MD, in full compliance with institutional guidelines. Male and female mice aged 6 to 12 wk were used for analyses. Littermate controls were used for all experiments with STAT-3 KO mice. TKO mice were derived from the respective single KO mice and bred homozygously. Their controls were in-house WT mice. All studies were performed under specific pathogen–free (SPF) conditions in compliance with NIH institutional guidelines, under IACUC-approved protocols (NEI-581 and NIAID/LCID 8E).
Flow sorting and cell surface marker analysis
T cell populations were isolated from spleen and peripheral lymph nodes of naive animals by first enriching for T cells using CD19 and MHCII depletion using MACS separation. After the separation, cell suspensions were stained with fluorescent antibodies against surface antigens, αβ TCR, γδ TCR, CD62L, CD44, CD4, CD8, CD1d tetramer binding, NK1.1, and B220. After staining, CD44hi CD4+, CD44hi CD8+, CD44hi DN, and NKT cell populations were sorted on a FACS ARIA II (BD Biosciences; Fig. 1 A). Typical yields of CD4+, CD8+, DN, and NKT cells from a single mouse were ∼4 × 105, 105, 2 × 104, and 105, respectively. For experiments, typically up to three mice were pooled. All antibodies were purchased from BioLegend, eBioscience, or BD Biosciences and used interchangeably, with the exception of B220-V500 (BD Biosciences) and CD62L-eFluor 450 (eBioscience). APC- or PE-labeled CD1d tetramers loaded with the αGalCer analogue, PBS57, were made and provided by the NIH tetramer facility (Emory, GA). We performed pSTAT-3 staining 18 h after cell stimulation to assure that enough proliferation of target cells occurred to provide reliable detection of pSTAT-3.
Culture conditions
HL-1 medium supplemented as described previously (Rachitskaya et al., 2008) plus 4% heat-inactivated fetal calf serum (Hyclone) was used for all cell cultures. FACS-isolated T cell populations were plated between 2 and 5 × 104 cells per well. All cultures were stimulated using plates precoated with αCD3 (3–4 µg/ml) and where indicated, 10 ng/ml recombinant murine IL-23 or 20 ng/ml IL-1β was added (R&D Systems). For neutralization experiments, we used αIL-6 (LEAF purified clone MP5-20F3) and αIL-23p19 (LEAF purified clone MMp19B2) from BioLegend at a concentration of 10 µg/ml. In addition, we used αIL-6Rα, αIL-21R, and IL-23R from R&D Systems at a concentration of 10 µg/ml. Cultures typically were harvested after 72 h.
Detection of cytokine expression
Cytokine levels in culture supernatants were measured either by sandwich ELISA Duosets (R&D Systems) or bead array (Flowcytomix, eBioscience) per the manufacturer’s instructions. For intracellular cytokine analysis, brefeldin A (BD Biosciences) was added to culture wells 6 h before harvest. This was followed by fluorescent antibody staining for surface markers followed by fixation, permeabilization, and intracellular staining for IL-17 and IFN-γ. Samples were analyzed using a MACS Quant Analyzer and FlowJo software (Tree Star).
Antibiotic treatment
IL-17A/F DKO mice were given a cocktail of antibiotics in the drinking water for up to 6 wk. Antibiotic cocktail consisted of ampicillin (1g/liter), metronidazole (1g/liter), neomycin sulfate (1g/liter), and vancomycin (0.5g/liter). Only mice with discernible disease were given antibiotic water.
In vitro knockdown of PLZF
Three siRNA constructs targeting PLZF (Zbtb16 ID235320 Trilencer-27 Mouse siRNA, catalog SR41884) were purchased from OriGene. RNAs were reconstituted with OriGene buffer to give a concentration of 20 µM for a stock solution, and these were further diluted to 5 µM for the working solution. Working solutions were then diluted in DMEM to reach a final concentration of 10 nM, 1.0 nM, and 0.1 nM when added to 200 µl CD44hi CD8/DNT cells that were sorted from pooled cells of at least three TKO mice and stimulated with αCD3 and 20 ng/ml IL-1β.
Subconjunctival injection of cells
CD44hi CD8+ and CD44hi DNT cells were activated in vitro for 3 d using plate-bound αCD3 and 20 ng/ml IL-1β. After 3 d, cells were FACS sorted, resuspended in PBS at 104 cells/5 µl, and left on ice until transfer. Mice were anesthetized using a mix of ketamine and xylazine. After anesthesia, 5 µl cells or PBS was injected subconjunctivally using a 5-µl Hamilton syringe. 3 d after transfer, mice were sacrificed and assessed for bacterial burden and immune cell infiltrate. For inoculation experiments, only mice without discernible disease were used to ensure that prior colonization with S. aureus would not cloud results. For CFU quantification, tissue homogenates were assessed by an investigator that was blinded to the treatment groups.
Statistical analysis and data presentation
All experiments were repeated at least twice, and usually three or more times, with reproducible results. Graphs and data analysis were done using GraphPad Prism (GraphPad Software). Experimental procedures determined sample size to ensure adequate power.
Online supplemental material
Fig. S1 shows a gating strategy for isolating CD44hi CD4+, CD44hi CD8+, CD44hi DNT, and iNKT cells. Fig. S2 shows the gating strategy for Fig. 5.
Supplementary Material
Acknowledgments
The authors thank Drs. Warren Leonard (National Institutes of Health), Vijay K. Kuchroo (Harvard University/Brigham and Women’s), David Levy (New York University), and Yoichiro Iwakura (Tokyo University of Science) for mice used in this study. We thank Drs. Jacques Van Snick and Catherine Uyttenhoeve (Ludwig Institute for Cancer Research, Brussels, Belgium) for their gift of anti–IL-17A and anti-IL-17F antibodies. We thank the staff of the National Eye Institute Flow Cytometry Core for expert cell sorting and analysis.
This work was supported by the ntramural Research Programs of the National Eye Institute (project EY000184; R.R. Caspi) and extramural National Institutes of Health (grant K99EY025761; A.J. St. Leger).
The authors declare no competing financial interests.
Author contributions: A.J. St. Leger, A.M. Hansen, H. Karauzum, S.K. Datta, and R.R. Caspi designed the experiments. A.J. St. Leger, A.M. Hansen, H. Karauzum, R. Horai, C.-R. Yu, P. Silver, and R. Villasmil performed experiments. A.L., K.D. Mayer-Barber, C.-R. Yu, C. Egwuagu, and S.K. Datta contributed unique biological material and expertise and edited the manuscript. A.J. St. Leger, A.M. Hansen, and R.R. Caspi wrote the manuscript. R.R. Caspi supervised the study.
References
- Annunziato F., Romagnani C., and Romagnani S.. 2015. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 135:626–635. 10.1016/j.jaci.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Awasthi A., Riol-Blanco L., Jäger A., Korn T., Pot C., Galileos G., Bettelli E., Kuchroo V.K., and Oukka M.. 2009. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 182:5904–5908. 10.4049/jimmunol.0900732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., and Kuchroo V.K.. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Blanco P., Pitard V., Viallard J.F., Taupin J.L., Pellegrin J.L., and Moreau J.F.. 2005. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 52:201–211. 10.1002/art.20745 [DOI] [PubMed] [Google Scholar]
- Chauhan S.K., El Annan J., Ecoiffier T., Goyal S., Zhang Q., Saban D.R., and Dana R.. 2009. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 182:1247–1252. 10.4049/jimmunol.182.3.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.S., Pietras E.M., Garcia N.C., Ramos R.I., Farzam D.M., Monroe H.R., Magorien J.E., Blauvelt A., Kolls J.K., Cheung A.L., et al. . 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120:1762–1773. 10.1172/JCI40891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., and Dong C.. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587. 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides M.G., McDonald B.D., Verhoef P.A., and Bendelac A.. 2014. A committed precursor to innate lymphoid cells. Nature. 508:397–401. 10.1038/nature13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti H.R., Peterson A.C., Brane L., Huppler A.R., Hernández-Santos N., Whibley N., Garg A.V., Simpson-Abelson M.R., Gibson G.A., Mamo A.J., et al. . 2014. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J. Exp. Med. 211:2075–2084. 10.1084/jem.20130877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce M., Rigo V., and Ferrini S.. 2015. IL-21: a pleiotropic cytokine with potential applications in oncology. J. Immunol. Res. 2015:696578 10.1155/2015/696578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne J.M., Soulard V., Bécourt C., Amniai L., Henrot P., Havenar-Daughton C., Blanchet C., Zitvogel L., Ryffel B., Cavaillon J.M., et al. . 2011. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J. Immunol. 186:662–666. 10.4049/jimmunol.1002725 [DOI] [PubMed] [Google Scholar]
- Durant L., Watford W.T., Ramos H.L., Laurence A., Vahedi G., Wei L., Takahashi H., Sun H.W., Kanno Y., Powrie F., and O’Shea J.J.. 2010. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 32:605–615. 10.1016/j.immuni.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., and Lantz O.. 2011. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 117:1250–1259. 10.1182/blood-2010-08-303339 [DOI] [PubMed] [Google Scholar]
- El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., and Rostami A.. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12:568–575. 10.1038/ni.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J.M., Lalor S.J., Sweeney C.M., Tubridy N., and Mills K.H.. 2010. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162:1–11. 10.1111/j.1365-2249.2010.04143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., Yang X.P., Tato C.M., McGeachy M.J., Konkel J.E., Ramos H.L., Wei L., Davidson T.S., Bouladoux N., et al. . 2010. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 467:967–971. 10.1038/nature09447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., Yang X.P., Hirahara K., and O’Shea J.J.. 2011. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 32:395–401. 10.1016/j.it.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladiator A., Wangler N., Trautwein-Weidner K., and LeibundGut-Landmann S.. 2013. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 190:521–525. 10.4049/jimmunol.1202924 [DOI] [PubMed] [Google Scholar]
- Happel K.I., Zheng M., Young E., Quinton L.J., Lockhart E., Ramsay A.J., Shellito J.E., Schurr J.R., Bagby G.J., Nelson S., and Kolls J.K.. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432–4436. 10.4049/jimmunol.170.9.4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J., Grosso J.F., Yen H.R., Xin H., Kortylewski M., Albesiano E., Hipkiss E.L., Getnet D., Goldberg M.V., Maris C.H., et al. . 2007. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179:4313–4317. 10.4049/jimmunol.179.7.4313 [DOI] [PubMed] [Google Scholar]
- He D., Wu L., Kim H.K., Li H., Elmets C.A., and Xu H.. 2006. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 177:6852–6858. 10.4049/jimmunol.177.10.6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Heink S., Grothe H., Guralnik A., Reinhard K., Elflein K., Hünig T., Mittrücker H.W., Brüstle A., Kamradt T., and Lohoff M.. 2009. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 39:1716–1725. 10.1002/eji.200939412 [DOI] [PubMed] [Google Scholar]
- Huber M., Heink S., Pagenstecher A., Reinhard K., Ritter J., Visekruna A., Guralnik A., Bollig N., Jeltsch K., Heinemann C., et al. . 2013. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J. Clin. Invest. 123:247–260. 10.1172/JCI63681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S., Saijo S., Murayama M.A., Shimizu K., Akitsu A., and Iwakura Y.. 2014. Excess IL-1 signaling enhances the development of Th17 cells by downregulating TGF-β-induced Foxp3 expression. J. Immunol. 192:1449–1458. 10.4049/jimmunol.1300387 [DOI] [PubMed] [Google Scholar]
- Isailovic N., Daigo K., Mantovani A., and Selmi C.. 2015. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 60:1–11. 10.1016/j.jaut.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. . 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 30:108–119. 10.1016/j.immuni.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Kawabe T., Jankovic D., Kawabe S., Huang Y., Lee P.H., Yamane H., Zhu J., Sher A., Germain R.N., and Paul W.E.. 2017. Memory-phenotype CD4+ T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci. Immunol. 2:eaam9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D., Alonzo E.S., Uche O.U., Eidson M., Nichols K.E., and Sant’Angelo D.B.. 2010. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J. Immunol. 184:6746–6755. 10.4049/jimmunol.1000776 [DOI] [PubMed] [Google Scholar]
- Le Bourhis L., Martin E., Péguillet I., Guihot A., Froux N., Coré M., Lévy E., Dusseaux M., Meyssonnier V., Premel V., et al. . 2010. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11:701–708. 10.1038/ni.1890 [DOI] [PubMed] [Google Scholar]
- Lee C.K., Raz R., Gimeno R., Gertner R., Wistinghausen B., Takeshita K., DePinho R.A., and Levy D.E.. 2002. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 17:63–72. 10.1016/S1074-7613(02)00336-9 [DOI] [PubMed] [Google Scholar]
- Marks B.R., Nowyhed H.N., Choi J.Y., Poholek A.C., Odegard J.M., Flavell R.A., and Craft J.. 2009. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat. Immunol. 10:1125–1132. 10.1038/ni.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles I.A., Fontecilla N.M., Valdez P.A., Vithayathil P.J., Naik S., Belkaid Y., Ouyang W., and Datta S.K.. 2013. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat. Immunol. 14:804–811. 10.1038/ni.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H., Murakami M., Okuyama Y., Tsuruoka M., Kitabayashi C., Kanamoto M., Nishihara M., Iwakura Y., and Hirano T.. 2008. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 29:628–636. 10.1016/j.immuni.2008.07.018 [DOI] [PubMed] [Google Scholar]
- Oh H.M., Yu C.R., Lee Y., Chan C.C., Maminishkis A., and Egwuagu C.E.. 2011. Autoreactive memory CD4+ T lymphocytes that mediate chronic uveitis reside in the bone marrow through STAT3-dependent mechanisms. J. Immunol. 187:3338–3346. 10.4049/jimmunol.1004019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Feng C.G., Qi C.F., Cheng J., Sher A., Morse H.C. III, Liu C., Schwartzberg P.L., and Leonard W.J.. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science. 298:1630–1634. 10.1126/science.1077002 [DOI] [PubMed] [Google Scholar]
- Passos S.T., Silver J.S., O’Hara A.C., Sehy D., Stumhofer J.S., and Hunter C.A.. 2010. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J. Immunol. 184:1776–1783. 10.4049/jimmunol.0901843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachitskaya A.V., Hansen A.M., Horai R., Li Z., Villasmil R., Luger D., Nussenblatt R.B., and Caspi R.R.. 2008. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180:5167–5171. 10.4049/jimmunol.180.8.5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res P.C., Piskin G., de Boer O.J., van der Loos C.M., Teeling P., Bos J.D., and Teunissen M.B.. 2010. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 5:e14108 10.1371/journal.pone.0014108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage A.K., Constantinides M.G., Han J., Picard D., Martin E., Li B., Lantz O., and Bendelac A.. 2008. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 29:391–403. 10.1016/j.immuni.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., and Rose-John S.. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 1813:878–888. 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- Sherlock J.P., Joyce-Shaikh B., Turner S.P., Chao C.C., Sathe M., Grein J., Gorman D.M., Bowman E.P., McClanahan T.K., Yearley J.H., et al. . 2012. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat. Med. 18:1069–1076. 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- Suryawanshi A., Veiga-Parga T., Rajasagi N.K., Reddy P.B., Sehrawat S., Sharma S., and Rouse B.T.. 2011. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J. Immunol. 187:1919–1930. 10.4049/jimmunol.1100736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., and Mills K.H.. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 31:331–341. 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Takatori H., Kanno Y., Watford W.T., Tato C.M., Weiss G., Ivanov I.I., Littman D.R., and O’Shea J.J.. 2009. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35–41. 10.1084/jem.20072713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Yoshimoto T., Naka T., Nakae S., Iwakura Y., Cua D., and Kubo M.. 2009. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J. Immunol. 183:7523–7530. 10.4049/jimmunol.0803828 [DOI] [PubMed] [Google Scholar]
- Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., and Fugger L.. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172:146–155. 10.2353/ajpath.2008.070690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi T.S., Zaidi T., Pier G.B., and Priebe G.P.. 2012. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect. Immun. 80:3706–3712. 10.1128/IAI.00249-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.