In this issue of JEM, Malireddi et al. demonstrate that macrophage-specific loss of TAK1 causes spontaneous NLRP3 inflammasome activation, driven by unregulated TNF secretion and signaling. This has implications for therapeutically targeting TAK1, enhancing its potential function as an anticancer drug treatment.
Abstract
In this issue of JEM, Malireddi et al. (https://doi.org/10.1084/jem.20171922) demonstrate that macrophage-specific loss of TAK1 causes spontaneous NLRP3 inflammasome activation, driven by unregulated TNF secretion and signaling. This has implications for therapeutically targeting TAK1, enhancing its potential function as an anticancer drug treatment.

Insight from Matthew Stephen Mangan and Eicke Latz
Inflammation and cell death are two essential, dichotic elements in the response to pathogenic organisms or tissue injury, critical for initiating an immune response and preventing establishment of a replicative niche for intracellular pathogens. However, if unregulated, both can cause or contribute to disease conditions, particularly those caused by autoinflammation Therefore, understanding how they are controlled is critical for the development of new therapeutics. Transforming growth factor 1 activating kinase (TAK1) is an apical kinase governing activation of both of these pathways. It is pivotal in regulating activation of Receptor-interacting protein (RIP) kinase 1 following stimulation of TNFR1, as inhibition or loss of TAK1 initiates RIP1-mediated apoptosis or RIP1–RIP3-mediated necrosis (Guo et al., 2016). When present, TAK1 is considered an important component of proinflammatory signaling through activation of NF-κB downstream of TNFR1 or TLR receptor activation, where it acts downstream of ubiquitinated RIP1 or TRAF6, respectively (Mihaly et al., 2014). Given its central role in these fundamental inflammatory pathways, TAK1 inhibitors have attracted significant attention as potential therapeutics for triggering cell death in cancer therapy or limiting inflammatory signaling in autoinflammatory disorders (Sakurai, 2012). Paradoxically, when tested in animal models, these inhibitors have caused autoinflammatory disorders, though the mechanism through which this occurs was not understood.
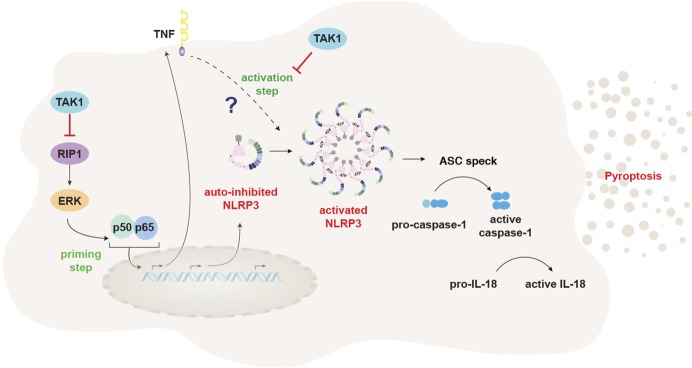
In this issue, Malireddi and colleagues used a myeloid-specific TAK1 KO mouse to shed new light on a novel, antiinflammatory function of TAK1 in macrophages that may explain, at first sight, the paradoxical inflammatory findings. As previously demonstrated in other cell models, they found that TAK1 deletion or inhibition resulted in spontaneous death of the TAK1-deficient macrophages, mediated by enhanced RIP1 and RIP3 signaling (Malireddi et al., 2018). Strikingly, however, these cells also underwent spontaneous NLRP3 inflammasome activation in the absence of any exogenous signals. This was surprising, as inflammasome activation is heavily regulated because of their high inflammatory potential. This is particularly true for NLRP3, which requires a minimum of two signals for activation: a priming signal that increases transcription of NLRP3, followed by a second stimulus that activates NLRP3, triggering assembly of the inflammasome, release of proinflammatory cytokines, including IL-1β, and pyroptosis, a caspase-1–dependent form of inflammatory cell death (Próchnicki et al., 2016). It is therefore particularly remarkable that TAK1 deficiency removes the requirements for both an exogenous priming signal, occurring through RIP1-mediated unregulated TNFα secretion, and the second activating signal through deregulation of TNFR1 signaling, enabling NLRP3 activation (see figure). This posits TAK1 as an essential regulator of NLRP3 activation in macrophages, contrasting to the initial hypothesis of TAK1 as a proinflammatory molecule.
TAK1 restrains both NLRP3 priming and activation. TAK1 activity restricts NLRP3 priming by limiting spontaneous activation of RIP1, preventing activation of NF-κB and subsequent release of TNFα. It then restrains TNFα/TNFR1-driven NLRP3 activation through an unknown mechanism.
It is interesting to note that in spite of ongoing NLRP3-driven inflammation, the TAK1 myeloid–deficient mice were not reported to display signs of autoinflammatory diseases, but rather loss of myeloid cells and an increase in neutrophils. This was RIP1 dependent, presumably because RIP1 caused both constitutive TNFα secretion and TNFR1-dependent myeloid cell death. However, it is unclear how aberrant NLRP3 activation contributes to this loss of myeloid cells and whether TAK1-driven NLRP3 activation in myeloid cells is sufficient to cause autoinflammatory diseases. Further research focusing on the TAK1-deficient mouse also lacking NLRP3 should provide further information.
This study once again defines NLRP3 as a key inflammatory mediator downstream of the RIP1–RIP3 cell death axis. RIP1 and RIP3 are essential for execution of cell death when apoptosis or inflammatory cascades are inhibited, which occurs most commonly during infection with an intracellular pathogen. RIP1, when activated through TNFR1 under appropriate circumstances, recruits caspase-8 and triggers apoptosis (Guo et al., 2016). If caspase-8 is inhibited, RIP3 is activated, leading to MLKL activation and necroptosis (Guo et al., 2016). NLRP3 activation is profoundly integrated with RIP3-dependent cell death: RIP3/RIP1-driven caspase-8 activation downstream of TNFR1 or TLR-mediated TRIF activation (Lawlor et al., 2015; Gaidt et al., 2016), as well as RIP3-driven MLKL activation, triggers NLRP3 in the event of caspase-8 inhibition (Conos et al., 2017). Malireddi et al. (2018) demonstrate yet another intersection between the RIP1–RIP3 signaling axis and NLRP3 activation, as in the absence of TAK1, RIP1, through its kinase activity, can mediate NLRP3 activation by driving TNF secretion. Notably, activation of NLRP3 in this case was alternative to apoptotic and necroptotic death, contrasting to previous findings where it was subsequent to them. This suggests that NLRP3 senses dysregulated TNFR1 signaling, contributing to the inflammatory reaction, but does not directly sense cell death.
A major unresolved question from this study is how the absence of TAK1 downstream of TNFR1 leads to NLRP3 activation. It was surprising that RIP3 had no involvement in activation of NLRP3 in this context given that it has previously been demonstrated to activate NLRP3 through MLKL activation (Lawlor et al., 2015). However, in spite of RIP3-MLKL–mediated death occurring subsequent to TAK1 inhibition, neither was involved in NLRP3 activation. This suggests that TAK1 activity may be required in this context to mediate NLRP3 activation. There is precedence for this, as TAK1 has been previously implicated in activation of NLRP3 by lysosomal damage and exposure to hypotonic solutions (Compan et al., 2012; Okada et al., 2014). RIP1 may also play a role in activation of NLRP3, but this is unlikely as it signals upstream of TAK1. Investigation of this would also need to be determined by adding exogenous TNF in Tnf−/− macrophages in the presence of a TAK1 inhibitor and presence or absence of a RIP1 inhibitor. A third possibility is that inhibition of the NF-κB signaling pathway downstream of TAK1 in the TNFR1 signaling cascade causes NLRP3 activation. It has been previously shown that deletion of IKKβ, an important component of the NF-κB signaling pathway, leads to IL-1β secretion in a caspase-1–dependent manner (Greten et al., 2007), similar to the paradoxical inflammatory effect observed on TAK1 deletion. IKKβ activation occurs distally to TAK1, and so it might explain the autoactivation phenotype seen in the TAK1 KO. Further research understanding the requirements of NLRP3 activation downstream of TAK1 would increase our understanding of how the TAK1–IKKβ axis regulates NLRP3 activation. This could be determined by activating TLR signaling in TNF/TAK1 double-deficient macrophages to determine whether the response phenocopies that seen in the IKKβ-deficient macrophages.
Overall, this study consolidates TAK1 inhibitors as attractive target for therapeutics in cancer therapy, as it is central to multiple cell death pathways. The involvement of NLRP3-driven inflammation adds extra impetus to such a treatment, as it has been shown that NLRP3 activation in various tissue environments can activate an NK cell response, which could contribute to clearance of the tumor (Dagenais and Saleh, 2016; van den Boorn et al., 2016). Thus, the dual function of a TAK1 inhibitor to cause cancer cell death while simultaneously activating NLRP3 makes it a potentially powerful an anticancer agent, albeit with the provision that such a drug would have to be properly dosed to avoid undesired autoinflammatory responses and prevent depletion of the myeloid compartment. These results also suggest that TAK1 might be a poor target for antiinflammatory therapies, as its inhibition, at least in macrophages, would drive rather than inhibit inflammation.
References
- Compan V., et al. Immunity. 2012 doi: 10.1016/j.immuni.2012.06.013. [DOI] [Google Scholar]
- Conos S.A., et al. Proc. Natl. Acad. Sci. USA. 2017 doi: 10.1073/pnas.1613305114. [DOI] [Google Scholar]
- Dagenais M., and Saleh M. OncoImmunology. 2016 doi: 10.1080/2162402X.2015.1129484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt M.M., et al. Immunity. 2016 doi: 10.1016/j.immuni.2016.01.012. [DOI] [Google Scholar]
- Greten F.R., et al. Cell. 2007 doi: 10.1016/j.cell.2007.07.009. [DOI] [Google Scholar]
- Guo X., et al. Cell Death Dis. 2016 doi: 10.1038/cddis.2016.294. [DOI] [Google Scholar]
- Lawlor K.E., et al. Nat. Commun. 2015 doi: 10.1038/ncomms7282. [DOI] [Google Scholar]
- Malireddi R.K.S., et al. J. Exp. Med. 2018 doi: 10.1084/jem.20171922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly S.R., et al. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., et al. J. Biol. Chem. 2014 doi: 10.1074/jbc.M114.579961. [DOI] [Google Scholar]
- Próchnicki T., et al. F1000 Res. 2016 doi: 10.12688/f1000research.8614.1. [DOI] [Google Scholar]
- Sakurai H. Trends Pharmacol. Sci. 2012 doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- van den Boorn J.G., et al. Immunity. 2016 doi: 10.1016/j.immuni.2016.05.008. [DOI] [Google Scholar]