Abstract
The relationship between stressful life events and the onset of disease is well documented. However, the role of psychological stress as a risk factor for life-threatening cerebrovascular insults such as stroke remains unspecified, but could explain individual variation in stroke outcome. To discover the mechanisms through which psychological stress may alter stroke outcome, we modeled the effects of chronic social intimidation and stress on ischemia-induced bcl-2 expression and early neuronal cell loss resulting from cerebral artery occlusion in mice (C57BL/6). The bcl-2 protooncogene promotes cell survival and protects against apoptosis and cellular necrosis in numerous neurodegenerative disorders, including stroke. In our study, male mice were chronically exposed to aggressive social stimuli before induction of a controlled, mild ischemic insult. Stressed mice expressed ≈70% less bcl-2 mRNA than unstressed mice after ischemia. In addition, social stress greatly exacerbated infarct in wild-type mice but not in transgenic mice that constitutively express increased neuronal bcl-2. Despite similar postischemic concentrations of corticosterone, the major stress hormone in mice, high corticosterone concentrations were significantly correlated with larger infarcts in wild-type mice but not bcl-2 transgenic mice. Thus, enhanced bcl-2 expression offsets the potentially deleterious consequences of high postischemic plasma corticosterone concentrations. Taken together, these data demonstrate that stressful prestroke social milieu strongly compromises an endogenous molecular mechanism of neuroprotection in injured brain and offer a new behavioral target for stroke therapy.
The relationship between stressful life events and the onset of disease is well documented (1), and among the general public it is commonly believed that stress is an important factor in the etiology of stroke (2). A few studies and case reports have provided support for a relationship between severe emotional stress and stroke (2–4). However, other reports have concluded that there is no effect of emotional factors on stroke incidence (5–8). These contrasting results likely reflect, among other factors, differences in criteria used for identification of stressors and clinical determination of stroke. Furthermore, it is difficult to distinguish between effects of stress on stroke incidence versus stroke outcome in clinical populations. This is particularly pertinent to small, so called silent strokes, which are underreported in the general population (9), but may be unmasked by factors that exacerbate their severity and clinical presentation.
Despite disagreement regarding the effects of prior exposure to stress on stroke incidence, several clinical and nonclinical animal studies have provided evidence suggesting that peri-ischemic concentrations of glucocorticoid hormones affect stroke outcome. These hormones, i.e., cortisol in humans and corticosterone in rats, increase during stressful events. In humans, post-stroke cortisol concentrations are predictive of stroke outcome; high cortisol is associated with increased morbidity and mortality (10–12). Furthermore, experimental manipulations in rats that increase blood corticosterone concentrations during or after cerebral ischemia also increase infarct volume (13), whereas surgical or pharmacological suppression of corticosterone concentrations is neuroprotective (14, 15). It has been proposed that rather than killing neurons directly, exposure to high concentrations of corticosteroids typically induces a physiological state that renders neurons more susceptible to subsequent neurologic insults (16). We hypothesized that suppressed bcl-2 expression may be one such mechanism through which stress compromises neuronal survival following stroke. The role of bcl-2 protooncogene in stroke outcome has been studied extensively. Increased bcl-2 expression promotes cell survival and protects against apoptosis and cellular necrosis in numerous neurodegenerative disorders (17). Under ischemic conditions, bcl-2 expression is selectively increased in the peri-infarct region (18–22). Alterations in bcl-2 expression by either genetic (23–30) or pharmacological (18, 31–33) manipulations ultimately affect tissue infarction; treatments that increase bcl-2 expression tend to be neuroprotective. The effects of environmental stress on bcl-2 expression in brain are unknown. However, ligand-induced activation of glucocorticoid receptors, which mediate many of the cellular changes associated with stress (34), results in suppressed bcl-2 expression and increased incidence of apoptosis in the hippocampus (35). These data suggest that a mechanism exists through which stress may alter bcl-2 expression in brain and ultimately exacerbate ischemic outcome.
The purpose of the present study was to evaluate altered bcl-2 expression as one of the mechanisms through which psychological stress may influence stroke outcome. We modeled the effects of chronic social intimidation and stress on ischemia-induced bcl-2 expression and early neuronal cell loss resulting from middle cerebral artery occlusion, which is a reproducible and reliable model of cerebral ischemia in humans (36, 37). Experiment 1 compared the effects of chronic social stress on stroke-induced bcl-2 expression. Experiment 2 tested the hypotheses that (i) exposure to social stress increases infarct volume and (ii) enhanced bcl-2 expression ameliorates stress-induced increases in infarct volume.
Methods
Animals.
This study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and the protocols were approved by the local Institutional Animal Care and Use Committees. Adult, sexually naive male mice (2–4 months old; 23–30 g) were individually housed, allowed ad libitum access to food and water, and maintained on a 14L:10D light-dark cycle. C57BL/6 mice used in the ribonuclease (RNase) protection assay were obtained from Charles River Breeding Laboratories. Transgenic mice overexpressing bcl-2 under control of neuron-specific enolase promoter and WT controls were bred at the Walter and Eliza Hall Institute of Medical Research (Australia) as described (18).
Social Stress.
Experimental animals were placed in the home cage of a large aggressive male mouse (>30 g). The animals were allowed to interact freely until they engaged in five antagonistic “bouts.” Then, a screen barrier was used to divide the cage in half and separate the two animals. The screen prevented additional physical interaction but allowed the animals to continue to make visual and auditory threats. The experimental animal remained in the cage for an additional 45 min. On the following 2 days, the experimental animal interacted freely with the aggressive male until one aggressive bout occurred, then the two animals were separated by the screen for an additional 45 min. At the end of each stress session, the experimental and stimulus animals were closely inspected before being returned to their home cages. None of the animals sustained wounds that required medical intervention. Control animals were handled and inspected daily in a fashion similar to the animals in the social stress group, but otherwise remained undisturbed in their home cages.
Experimental Stroke.
Transient focal cerebral ischemia was induced in male mice by middle cerebral artery occlusion as described (38). Briefly, the mice were anesthetized with 1–1.5% halothane in oxygen-enriched air. Unilateral middle cerebral artery occlusion was achieved by using the intraluminal filament insertion technique, which consisted of introducing a 6-0 nylon monofilament into the internal carotid artery, via the external carotid artery, to a point 6 mm distal to the internal carotid artery–pterygopalantine artery bifurcation. Once the filament was secured, the animals were allowed to emerge from anesthesia. After 60 min of ischemia, the animals were reanesthetized briefly, and reperfusion was initiated through withdrawal of the filament. This surgical protocol typically results in a core infarct limited to parietal cerebral cortex and caudate putamen. Body temperature was maintained at ≈37°C during surgery and recovery through the use of heat lamps and water pads. There were no significant group differences in body weight measured immediately before middle cerebral artery occlusion [F(3,20) = 0.69; P = 0.57].
RNase Protection Assay.
Radiolabel bcl-2 antisense riboprobe was transcribed from a 272-bp rat bcl-2 cDNA fragment, cloned into a plasmid vector (Bluescript, Stratagene) under control of T3 bacteriophage polymerase promoter to a specific activity of ≈108 cpm/μg. RNase protection was performed directly in tissue lysates as described (18). Briefly, 24 h after ischemia, the brains were rapidly removed and snap frozen in chilled isopentane. At the time of RNase protection assay, the brains were thawed, and each hemisphere homogenized in a commercial lysis solution (Ambion, Austin, TX) and incubated in excess bcl-2 and actin probes overnight. Unhybridized probes were removed by ribonuclease digestion. Hybridized probes were visualized via autoradiography following denaturing PAGE. Images of the autoradiographic bands were digitized, and optical densities of the bcl-2 bands were normalized to corresponding B-actin.
Determination of Stroke Volume.
Brains were removed and sectioned into five 2-mm-thick coronal sections. Sections were incubated for 10 min on each side in 2,3,5-triphenyltetrazolium maintained at 37°C. Following staining, the sections were fixed in 10% formalin for 24 h, then photographed. Images were analyzed (Inquiry; Loats Associates, Westminster, MD), and infarct size was expressed as a percentage of the contralateral hemisphere after correcting for edema (38). Eight animals, distributed across all experimental groups, were excluded from analysis because of unsuccessful induction of infarct.
Determination of Blood Corticosterone Concentrations.
Blood samples were collected via rapid cervical dislocation and decapitation 6–8 h into the light phase of the circadian cycle. The samples were centrifuged at 4°C for 10 min at 3,000 rpm. The serum was collected, then stored at −70°C. Corticosterone concentration was determined by using an I125 corticosterone kit (ICN). All samples were quantified in the same assay.
Experiment 1.
Stroke-induced bcl-2 expression was compared in animals subjected to chronic social stress (n = 8) versus unstressed controls (n = 8). The stress animals were exposed to social stress on three consecutive days, as described above. The last stress session occurred approximately 1 h before induction of cerebral ischemia. Following 24 h of reperfusion, the blood and brain tissue samples were collected. Corticosterone concentrations were measured by using an RIA kit, and bcl-2 expression was determined through RNase protection assay. The hormone and mRNA data were analyzed by using unpaired t tests. The relationship between corticosterone concentration and bcl-2 expression was analyzed by using Pearson Product Moment Correlation. Effects were considered statistically significant at P < 0.05.
Experiment 2.
To determine whether stress-induced suppression of postischemic bcl-2 expression was functionally significant, we compared infarct volumes of wild-type (WT) mice (stress n = 5, no stress n = 5) and bcl-2 transgenic mice (stress n = 6, no stress n = 5). The experimentally stressed animals were exposed to social stress on three consecutive days and then subjected to cerebral ischemia as described above. Following 24 h of reperfusion, the blood samples and brains were collected. Additional blood samples were collected from nonischemic WT and bcl-2 transgenic mice at baseline and 1 h after initiation of social stress. Infarct and hormone data were analyzed by using ANOVA followed by post hoc analysis using Fisher's test. The relationship between post-ischemic corticosterone concentration and infarct size was analyzed by using Pearson Product Moment Correlation. Effects were considered statistically significant at P < 0.05.
Results
Experiment 1.
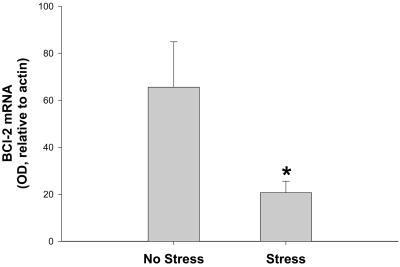
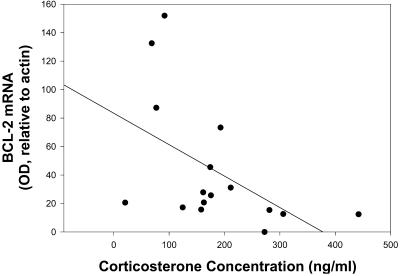
RNase protection assay was used to assess the effects of chronic social stress, induced through repeated exposure to a large aggressive male, on bcl-2 expression in WT mice subjected to transient focal ischemia. Bcl-2 mRNA levels in the ischemic hemisphere of the stressed mice were significantly lower (≈70%) than in the unstressed control mice (t = 2.3, P < 0.05; Fig. 1). There was no difference in bcl-2 mRNA levels (OD, relative to actin) between the stressed and unstressed groups in the nonischemic hemisphere (75 ± 8 versus 81 ± 10, respectively; P > 0.05). Serum corticosterone concentration was significantly elevated in stressed animals relative to unstressed controls (234 ± 36 ng/ml versus 131.5 ± 29 ng/ml, respectively; t = 2.18, P < 0.05). Furthermore, there was a significant correlation between serum corticosterone concentration and bcl-2 expression (correlation coefficient = −0.52; P < 0.05; Fig. 2).
Figure 1.
Stress alters postischemic expression of bcl-2 mRNA measured by RNase protection assay. Male mice exposed to social stress exhibit bcl-2 mRNA levels that are less than 31% of control levels in the ischemic hemisphere 24 h after transient experimental stroke. Data (n = 8/group) were analyzed by using an unpaired t test and are presented as mean ± SEM. An asterisk indicates statistical significance at P < 0.05.
Figure 2.
Postischemic corticosterone concentrations (ng/ml) and bcl-2 mRNA levels are significantly correlated in stressed (n = 8) and control (n = 8) mice. Bcl-2 mRNA is measured by RNase protection assay.
Experiment 2.
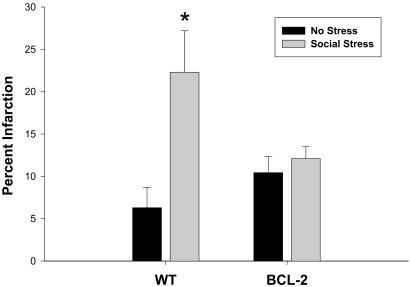
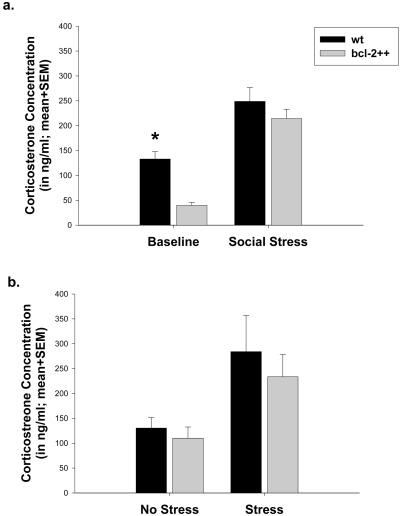
Based on the RNase protection assay data, we formulated the following hypothesis: if chronic stress exacerbates ischemic outcome by suppressing bcl-2 expression, then stressed WT mice will exhibit larger infarct volumes than unstressed WT mice, whereas transgenic mice that constitutively express high levels of neuronal bcl-2 will be protected against the effect of stress on infarct volume. Consistent with this hypothesis, WT mice that were exposed to social stress on three consecutive days before stroke exhibited infarct volumes that were approximately four times larger than unstressed WT mice, whereas infarct volumes did not differ significantly between stressed and unstressed bcl-2 transgenic mice (ANOVA; F = 5.2, P < 0.05; Fig. 3). The unmanipulated transgenic mice displayed lower baseline corticosterone concentrations than the WT mice, but the two genotypes exhibited similar corticosteroid responses to social stress (F = 23.06, P < 0.05; Fig. 4a). After 24 h of reperfusion, blood corticosterone concentrations were similar between genotypes of stressed and unstressed animals (F = 3.23, P < 0.05; Fig. 4b). However, postischemic corticosterone concentrations were correlated positively with infarct size in WT mice (correlation coefficient 0.67; P < 0.05; Fig. 5) but not in the bcl-2 transgenic mice (correlation coefficient 0.18; P > 0.05).
Figure 3.
Increased bcl-2 expression protects against stress-induced exacerbation of cerebral ischemia. Social stress increases infarct size in WT mice but not transgenic mice that express increased bcl-2. Data (n = 5–6/group) are presented as mean ± SEM. An asterisk indicates statistical significance at P < 0.05 compared with the other three experimental groups.
Figure 4.
(a) Blood corticosterone concentrations (ng/ml) are significantly lower in bcl-2 transgenic mice than WT controls at baseline but not 1 h after social stress. (b) Twenty-four hours after transient focal ischemia, blood corticosterone concentrations are similar between genotypes of stressed and unstressed animals. Data (n = 5–7/group) are presented as mean ± SEM. An asterisk indicates a statistical significance at P < 0.05 between WT and bcl-2++ mice at baseline.
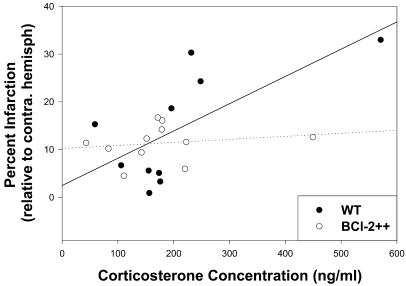
Figure 5.
Postischemic corticosterone concentrations (ng/ml) and percent infarct (relative to the contralateral hemisphere) are significantly correlated in WT mice (solid line) but not transgenic mice that constitutively express excess bcl-2 (dotted line). ●, WT data; ○, bcl-2 transgenic mice data. Data include both stressed and unstressed animals in each genotype.
Discussion
The results of this study provide evidence that extrinsic factors, such as chronic social intimidation and stress, can suppress postischemic bcl-2 expression in brain and exacerbate experimental stroke outcome. As expected, inducing social stress through the use of a resident-intruder paradigm resulted in increased corticosterone concentrations (Fig. 4a; refs. 39 and 40). In addition, postischemic corticosterone concentrations also were elevated in the previously stressed WT mice compared with controls (Fig. 4b). Infarct volumes in stressed WT mice were four times larger than in unstressed animals (Fig. 3) and were correlated with circulating corticosterone concentrations (Fig. 5). Our data corroborate clinical studies in which poststroke cortisol concentrations were predictive of stroke outcome (10–12). Furthermore, experimental manipulations that increase blood corticosterone concentrations during or after cerebral ischemia increase infarct volume in rats (13), whereas manipulations that decrease corticosterone concentrations are neuroprotective (14, 15). Thus, in both humans and rodents, elevated corticosteroid concentrations during cerebral ischemia or reperfusion are associated with exacerbated outcome measures.
Under a limited number of circumstances, stress has been shown to be directly responsible for compromising neurogenesis or neuronal survival in the hippocampal region of adult animals. For example, exposure to psychosocial stress (41, 42) or exogenous corticosteroids (43) suppresses neurogenesis in the dentate gyrus, a region of the hippocampus that is unusual because of its ability to produce new neurons in adulthood. Furthermore, animals who are maintained long-term on exogenous glucocorticoids in the upper physiological range exhibit selective neuronal loss (44). However, most corticosteroid-mediated neuronal damage is probably caused by increasing susceptibility to subsequent neurologic insults (16). We propose herein that down-regulation of bcl-2 expression may be one such mechanism through which stress compromises neuronal survival following experimental stroke.
In the present study, bcl-2 mRNA was ≈70% lower in the ischemic hemisphere of stressed animals compared with unstressed animals. Although it is clear that stress suppresses ischemic bcl-2 expression in a manner unlikely to benefit tissue survival after stroke, the mechanism requires further dissection. In these experiments, bcl-2 mRNA levels were similar in the nonischemic hemisphere of stressed and unstressed animals, suggesting that stress does not alter baseline bcl-2 expression. In unstressed animals, bcl-2 mRNA levels were also similar in ischemic and nonischemic hemispheres at 24 h. This was not unexpected, as bcl-2 expression is thought to be either increased after stroke or, at minimum, preferentially preserved in injured cells with restricted protein synthesis (18–22). The similar bcl-2 levels in infarcted and noninfarcted tissue of unstressed animals argues against the notion that decreased bcl-2 levels merely reflects a larger infarct relative to unstressed animals. The apparently similar mRNA levels in both hemispheres of unstressed animals may actually represent increased relative bcl-2 expression within a smaller number of surviving neurons. The negligible bcl-2 induction could be partly explained by our technique of measuring bcl-2 mRNA in whole hemisphere without distinction of high vs. low cellular or regional expression. In contrast, bcl-2 levels ipsilateral to the insult decreased relative to the nonischemic hemisphere in stressed animals. This fall to below baseline steady-state expression in the ischemic hemisphere suggests enhanced bcl-2 mRNA degradation. Although the precise mechanism will require further study, suppression of peri-ischemic bcl-2 in stressed brain likely has functional significance, as emphasized by the results from constitutive bcl-2 overexpressing mice.
In cerebral ischemia studies, manipulations that preserve or augment bcl-2 result in smaller infarcts (18, 25–27, 29–33), whereas manipulations that interfere with endogenous bcl-2 expression or enhance its degradation increase infarct size (24, 28). The present data suggest that preserved bcl-2 expression can provide a buffer against stress-induced exacerbation of ischemic injury. Despite corticosterone concentrations similar to those of the WT, stress did not exacerbate infarct volume in bcl-2 transgenics. Thus, in contrast to WT mice, serum corticosterone was not correlated with infarct volume in bcl-2 transgenic mice. Whether the apparent neuroprotection afforded by bcl-2 in the focal cerebral ischemia model is associated with preservation of functional capabilities remains to be determined. It is also important to determine how acute stress, which has been shown to induce bcl-2 expression in peripheral tissue (45), affects bcl-2 expression in the brain. There are other well defined examples of exposure to acute versus chronic stress having opposite physiological effects (46).
In conclusion, we have demonstrated that psychosocial stress is capable of compromising an endogenous molecular mechanism of neuroprotection in injured brain by down-regulation of bcl-2 expression. However, animals with increased neuronal bcl-2 expression are not affected by exposure to stress despite typical corticosteroid responses to the induction of social stress and ischemia. Through a mechanism similar to the one described here, stress may impact the course of other ischemic diseases, such as myocardial infarction, that are known to be sensitive to stress (47) and influenced by bcl-2 expression (48).
Acknowledgments
We thank Dr. Deborah Drazen for conducting the corticosterone assay, Dr. Randy Nelson and Staci Bilbo for critiquing the manuscript, and Ms. Noelle Shearer for assistance with manuscript preparation. This work was supported by grants from The Rockefeller Foundation and the National Institutes of Health.
Abbreviation
- WT
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Baum A, Posluszny D. Annu Rev Psychol. 1999;50:137–163. doi: 10.1146/annurev.psych.50.1.137. [DOI] [PubMed] [Google Scholar]
- 2.House A, Dennis M, Mogridge L, Hawton K, Warlow C. J Neurol Neurosurg Psychiatry. 1990;53:1024–1028. doi: 10.1136/jnnp.53.12.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harmsen P, Rosengren A, Tsipogianni A, Wilhelmsen L. Stroke. 1990;21:223–229. doi: 10.1161/01.str.21.2.223. [DOI] [PubMed] [Google Scholar]
- 4.Carasso R, Yehuda S, Ben-uriah Y. Int J Neurosci. 1981;14:223–225. doi: 10.3109/00207458108985837. [DOI] [PubMed] [Google Scholar]
- 5.Agewall S, Wikstrand J, Fagerberg B. Stroke. 1998;29:2329–2333. doi: 10.1161/01.str.29.11.2329. [DOI] [PubMed] [Google Scholar]
- 6.Macko R F, Ameriso S F, Barndt R, Clough W, Weiner J M, Fisher M. Stroke. 1996;27:1999–2004. doi: 10.1161/01.str.27.11.1999. [DOI] [PubMed] [Google Scholar]
- 7.Storey P. Adv Psychosom Med. 1985;13:71–84. doi: 10.1159/000411300. [DOI] [PubMed] [Google Scholar]
- 8.Gentry W D, Jenkins C D, Kaplan B H, Heyman A, Breslin M S, Gianturco D T. Heart Lung. 1979;8:1113–1116. [PubMed] [Google Scholar]
- 9.Kase C, Wolf P, Chodosh E, Zacker H, Kelly-Hayes M, Kannel W, D'Agostino R, Scampini L. Stroke. 1989;20:850–852. doi: 10.1161/01.str.20.7.850. [DOI] [PubMed] [Google Scholar]
- 10.Feibel J H, Hardy P M, Campbell R G, Goldstein R G, Goldstein M N, Joynt R J. JAMA. 1977;238:1374–1376. [PubMed] [Google Scholar]
- 11.Murros K, Fogelholm R, Kettunen S, Vuorela A L. J Neurol Sci. 1993;116:12–17. doi: 10.1016/0022-510x(93)90083-b. [DOI] [PubMed] [Google Scholar]
- 12.Olsson T. J Int Med. 1990;228:177–181. doi: 10.1111/j.1365-2796.1990.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 13.Koide T, Wieloch T W, Siesjo B K. J Cereb Blood Flow Metab. 1986;6:395–404. doi: 10.1038/jcbfm.1986.72. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky R M, Pulsinelli W A. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 15.Smith-Swintosky V L, Pettigrew L C, Sapolsky R M, Phares C, Craddock S D, Brooke S M, Mattson M P. J Cereb Blood Flow Metab. 1996;16:585–598. doi: 10.1097/00004647-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Sapolsky R M. J Neurosci. 1985;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergeron L, Yuan J. Curr Opin Neurobiol. 1998;8:55–63. doi: 10.1016/s0959-4388(98)80008-1. [DOI] [PubMed] [Google Scholar]
- 18.Alkayed, N. J., Goto, S., Sugo, N., Joh, H. D., Klaus, J., Crain, B. J., Bernard, O., Traystman, R. J. & Hurn, P. D. (2001) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 19.Ferrer L, Lopez E, Blanco R, Rivera R, Ballabriga J, Pozas E, Marti E. Exp Brain Res. 1998;121:167–173. doi: 10.1007/s002210050448. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita K, Matsuyama T, Kitagawa K, Matsumoto M, Yanagihara T, Sugita M. Neuroscience. 1998;83:439–448. doi: 10.1016/s0306-4522(97)00391-6. [DOI] [PubMed] [Google Scholar]
- 21.Honkaniemi J, Massa S M, Breckinridge M, Sharp F R. Mol Brain Res. 1996;42:79–88. doi: 10.1016/s0169-328x(96)00121-0. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Graham S H, Chan P H, Lan J, Zhou R L, Simon R P. NeuroReport. 1995;6:394–398. doi: 10.1097/00001756-199501000-00040. [DOI] [PubMed] [Google Scholar]
- 23.De Bilbao F, Guarin E, Nef P, Vallet P, Giannakopoulos P, Dubois-Dsauphin M. Eur J Neurosci. 2000;12:921–934. doi: 10.1046/j.1460-9568.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 24.Hata R, Gillardon F, Michaelidis T M, Hossmann K A. Metab Brain Dis. 1999;14:117–124. doi: 10.1023/a:1020709814456. [DOI] [PubMed] [Google Scholar]
- 25.Antonawich F J, Fereoff H J, Davis J N. Exp Neurol. 1999;156:130–137. doi: 10.1006/exnr.1998.7004. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence M S, McLaughlin J R, Sun G H, Ho D Y, McIntosh L, Kunis D M, Sapolsky R M, Steinberg G K. J Cereb Blood Flow Metab. 1997;17:740–744. doi: 10.1097/00004647-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Martinou J C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Simon R P, Nagayama T, Zhu R, Loeffert J E, Watkins S C, Graham S H. J Cereb Blood Flow Metab. 2000;20:1033–1039. doi: 10.1097/00004647-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. J Cereb Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Shimazaki K, Urabe M, Monahan J, Ozawa K, Kawai N. Gene Ther. 2000;7:1244–1249. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- 31.Dubal D B, Shughrue P J, Wilson M E, Merchenthaler I, Wise P M. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linnik M D, Zahos P, Geschwind M D, Federoff H J. Stroke. 1995;26:1670–1674. doi: 10.1161/01.str.26.9.1670. [DOI] [PubMed] [Google Scholar]
- 33.Schabitz W R, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S. Stroke. 2000;31:2212–2217. doi: 10.1161/01.str.31.9.2212. [DOI] [PubMed] [Google Scholar]
- 34.de Kloet E R, Vreugdenhil E, Oitzl M S, Joels M. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 35.Almeida O F X, Conde G L, Crochemore C, Demeneix B A, Fischer D, Hassan A H S, Meyer M, Holsboer F, Michaelidis T M. FASEB J. 2000;14:779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- 36.Tamura A, Graham D I, McCulloch J, Teasdale G M. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 37.Bederson J, Pitts L, Tsuji M, Nishimura M, Davis R, Bartkowski H. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 38.Hattori K, Lee H, Hurn P, Crain B, Traystman R, DeVries A. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- 39.File S E. Physiol Behav. 1984;33:345–347. doi: 10.1016/0031-9384(84)90152-5. [DOI] [PubMed] [Google Scholar]
- 40.Sgoifo A, de Boer S F, Haller J, Koolhaas J M. Physiol Behav. 1996;60:1403–1407. doi: 10.1016/s0031-9384(96)00229-6. [DOI] [PubMed] [Google Scholar]
- 41.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gould E, McEwen B S, Tanapat P, Galea L A, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cameron H A, Gould E. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 44.Sapolsky R M, Krey L C, McEwen B S. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konturek P, Brzozowski T, Konturek S, Pajdo R, Konturek J, Kwieciea S, Taut A, Hahn E. J Physiol Pharm. 1999;50:211–225. [PubMed] [Google Scholar]
- 46.McEwen B S. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 47.Scheuer D A, Mifflin S W. Am J Physiol. 1998;274:R470–R475. doi: 10.1152/ajpregu.1998.274.2.R470. [DOI] [PubMed] [Google Scholar]
- 48.Brocheriou V, Hagege A A, Oubenaissa A, Lambert M, Mallet V O, Duriez M, Wassef M, Kahn A, Menasche P, Gilgenkrantz H. J Gene Med. 2000;2:326–333. doi: 10.1002/1521-2254(200009/10)2:5<326::AID-JGM133>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]