Abstract
Oral squamous cell carcinoma (OSCC) is the seventh most common malignancy and the ninth most frequent cause of cancer death in Europe. Within Europe, Hungary has one of the highest rates of OSCC incidence and mortality. Thus, there is an urgent need to improve early detection. Saliva, as a readily available body fluid, became an increasingly important substance for the detection of biomarkers for many diseases. Different research groups have identified salivary biomarkers specific for OSCC for different countries. In this study, saliva samples of Hungarian patients with OSCC were studied to discover disease‐specific and perhaps region‐specific biomarkers. LC‐mass spectrometry (MS)/MS analysis on a linear ion trap‐Orbitrap mass spectrometer was used for qualitative and quantitative salivary protein profiling. More than 500 proteins were identified from saliva by shotgun proteomics. The up‐ and downregulated proteins in the saliva of patients with OSCC highlighted the importance of protein–protein interaction networks involving the immune system and proteolysis in disease development. Two potential biomarkers from our shotgun analysis and a third candidate reported earlier by a Taiwanese group were further examined by ELISA on a larger reference set of samples. Resistin, a biomarker reported in Taiwan but not validated in our study, highlights the necessity of application of standardized analysis methods in different ethnic or geographical populations to identify biomarkers with sufficient specificity and sensitivity.
Keywords: biomarker, ELISA, oral squamous cell carcinoma, proteomics analysis
Abbreviations
- MS
mass spectrometry
- OSCC
oral squamous cell carcinoma
- UPLC
ultraperformance liquid chromatography
The oral cavity is the most frequent site of head and neck cancers, developing predominantly as oral cavity squamous cell carcinomas (OSCCs) in the upper aerodigestive epithelium 1, 2. The three major recognized risk factors of OSCC are tobacco consumption, alcohol consumption, and poor oral hygiene 3, 4, 5. OSCC mortality rates reflect the different consumption patterns of alcohol and tobacco in European countries 6. Annually, more than 300 000 new patients are diagnosed with OSCC worldwide. The disease is associated with poor prognosis and high mortality mainly due to late diagnosis because of the lack of reliable early diagnostic markers 7. Mortality rate from OSCC is about 10‐fold higher for men than for women. However, female OSCC incidence increased dramatically in the last decade. In addition, a rising tendency was observed in younger patient cohorts 8. In contrast to other European countries where the mortality rates of OSCC started to decline, unfavorable incidence and mortality figures remained exceedingly high in Hungary since the 1970s representing a major public health challenge 9.
Development of cancer diagnostic tools with sufficiently high sensitivity and specificity is required to enable early detection of OSCC 10. Recent treatment strategies of patients with OSCC are based on traditional stage‐predicting indices and histological grading 11. Unfortunately, these predictors are relatively subjective and unreliable because tumors with the same staging and grading may respond to therapy differently. Thus, improving the diagnostic methods is required. A potential way of improving our diagnostic tools is to perform in‐depth salivary analyses to discover and to assess biochemical and immunological markers in the saliva for early oral cancer diagnosis 12, 13. Biomarkers identified in the last decades in biological fluids can be linked to carcinogenesis and may serve as prognostic factors and saliva is a new clinical biomarker source that can be easily collected by noninvasive means 14, 15, 16, 17, 18. As there is direct contact between saliva and the oral lesion(s), disease‐related concentration changes of saliva ingredients may provide as good or better clues than serum samples 19. More than 3700 salivary proteins have been identified by several research groups 20, 21. Many proteins were declared potential salivary biomarkers of OSCC in different countries 22, 23, 24. In this study, we present a two‐stage approach for the discovery of candidate OSCC‐specific salivary biomarkers in the Hungarian population. LC‐mass spectrometry (MS)/MS analysis using ultraperformance liquid chromatography (UPLC) coupled to a linear ion trap‐Orbitrap hybrid tandem mass spectrometer was applied for qualitative and quantitative salivary protein profiling. Selected proteins, based on the shotgun analysis of a few randomly selected samples, were further investigated by ELISA on a reference set of samples.
Materials and methods
Patients and saliva collection
Donor enrollment, sample collection, and processing conformed to the principles of the Helsinki Declaration. Ethical approval was obtained from the University of Debrecen Ethics Committee (No. 3385‐2011), and all subjects provided written informed consent. Clinical examinations were performed by dental surgeons from the Faculty of Dentistry, University of Debrecen. Adult patients (> 18 years) with histology‐proven OSCC were recruited into the study. Saliva samples were collected before starting any antitumor therapy. Age‐matched controls (MCTL) were consecutive patients and young controls (YCTL) were medical students admitted to the Faculty of Dentistry for regular dental checkup. Exclusion criteria included children (≤ 18 years), pregnancy and breast‐feeding, diabetes mellitus, human papillomavirus infection, human immunodeficiency virus infection, autoimmune and immunodeficiency disorders, and cancer other than OSCC.
Unstimulated saliva samples were collected from 43 donors between 9 a.m. and 11 a.m. at the Faculty of Dentistry, University of Debrecen (collection between May 9, 2013, and February 29, 2016). The test set contained three randomly selected samples from patients with OSCC and controls for proteomics analysis, whereas the reference set contained samples from 20 patients with OSCC (mean age: 57 years), six YCTL (mean age: 24.5 years), and 11 MCTL (mean age: 59 years) for biomarker verification. Saliva samples were kept on ice during collection and were filtered using Millipore SLSV025LS 5‐μm‐pore‐size syringe filters (Merck, Billerica, MA, USA). The filtered saliva was aliquoted and immediately placed at −70 °C until further use.
Sample preparation for mass spectrometry
Filtered saliva was dried in SpeedVac and redissolved in 25 mm pH 8.5 ammonium bicarbonate buffer. Total protein concentration of salivary samples was measured using the Bradford method 25. Following denaturation with 8 m urea, all samples were reduced with 10 mm dithiothreitol (Bio‐Rad, Hercules, CA, USA) in ammonium bicarbonate buffer. Then, samples were alkylated with 20 mm iodoacetamide (Bio‐Rad) in ammonium bicarbonate buffer and diluted with 25 mm ammonium bicarbonate (Sigma, St. Louis, MO, USA) to reduce the urea concentration to 1 m. Each sample was digested by MS‐grade modified trypsin (AB Sciex, Framingham, MA, USA) in 1 : 25 enzyme‐to‐protein ratio (w/w) at 37 °C overnight. The digested samples were dried in SpeedVac and redissolved in 0.1% formic acid. The digests were desalted on Pierce C18 Tips (Thermo Scientific, West Palm Beach, FL, USA), and the eluates were dried and stored at −70 °C until MS analysis.
Mass spectrometry analysis
Tryptic digests representing 2 μg total protein were analyzed by LC‐MS/MS using a Waters nanoACQUITY UPLC Online coupled to a linear ion trap‐Orbitrap hybrid tandem mass spectrometer (Orbitrap Elite; Thermo Scientific) operating in positive ion mode. After trapping at 3% B (Waters Symmetry C18 180 μm × 20 mm column, 5 μm particle size, 100 Å pore size; flow rate: 10 μL·min−1), peptides were fractionated using a linear gradient of 3–40% B in 100 min (Waters BEH C18 75 μm × 250 mm column, 1.7 μm particle size, 300 Å pore size; solvent A: 0.1% formic acid/water; solvent B: 0.1% formic acid/5% dimethyl sulfoxide/acetonitrile; flow rate: 400 nL·min−1). Data acquisition was carried out in a data‐dependent fashion, and the 10 most abundant, multiply charged ions were selected from each MS survey (m/z: 380–1600; resolution: 60 000, acquired in profile mode) for MS/MS analyses. CID analyses were performed in the linear ion trap (normalized collision energy: 35). Dynamic exclusion was enabled (exclusion time: 30 s).
Protein identification
Peak lists generated from the MS/MS data by the ‘pava’ software 26 were searched against the human subset of the UniProt database (downloaded on June 10, 2014; 136 245 target sequences concatenated with a randomized sequence for each entry) using the proteinprospector search engine (v.5.10.9.). Search parameters: enzyme: trypsin with maximum 1 missed cleavage site; fixed modification: carbamidomethyl (Cys); variable modifications: acetylation (protein N terminus), oxidation (Met), and pyroglutamic acid formation (N‐terminal Gln) allowing up to two variable modifications per peptide; and mass accuracy: 5 p.p.m. and 0.6 Da for precursor and fragment ions (both monoisotopic), respectively. The following acceptance criteria were applied: score > 22 and 15, and E‐value < 0.01 and 0.05 for protein and peptide identifications, respectively. The false‐positive rates of the identified proteins and peptides were < 1%. Relative abundance of individual proteins was estimated by spectral counting: The number of identifications per protein (PSMs) was normalized to the total number of identifications, and then, these relative spectral counts were compared across the different samples.
Functional analyses were performed in the case of proteins with at least three unique peptide identifications. For the calculation of the OSCC/control ratio, the proteins which were identified with at least three unique peptides in at least two of three samples in either the control or the OSCC group were considered.
Validation of the candidate biomarkers using ELISA
All saliva samples from patients with OSCC and controls were analyzed in duplicate with quantitative ELISA. The ELISA kit for heparin cofactor 2 (Cat. number: LS‐F13221) was purchased from LifeSpan Biosciences (Seattle, WA, USA), for resistin (Cat. number: KHP0051) from Thermo Fisher Scientific (West Palm Beach, FL, USA), and for complement C5 (Cat. number: ab125963) from Abcam (Branford, CT, USA). The concentration of the studied proteins in saliva was measured by the sandwich ELISA method according to the instruction provided by the vendor of each kit. Absorbance was measured at 450 nm, and concentrations were calculated based on the recorded 7‐point calibration curves.
First, the variation coefficient of the parallel measurements was calculated and those data having more than 25 CV % value were excluded from statistical analysis.
Bioinformatics
The cluster analysis was carried out with Cluster 3.0 (http://cluster2.software.informer.com/) using the c clustering library version 1.52, and the heat map was created with java treeview version 1.1.6r4 27.
The protein–protein interaction network of salivary proteins was generated using string version 10.5 28, 29 applying default settings and medium stringency. After the generation of networks, the enriched gene ontology (GO) terms provided by the software were also examined.
The statistical analysis of ELISA data was performed using the Mann–Whitney U‐test and the two‐sample t‐test to compare the protein concentrations between groups. The data were considered significantly different where the P value was < 0.05.
Results and Discussion
Demographic and clinical characteristics of patients with OSCC
Among the included 17 patients, 13 were males and 4 females between the age of 44 and 73 years. The tumor developed in the tongue (T) in six cases and in the floor of the mouth (F) in four cases, and in three cases, it was detected in the gingival (G) region. In four cases, the tumor development showed multiple localization, and in two patients, the tumor developed in the T and either in the F or in the G region, while in another two patients, the tumor development was detected in the T, in the F, and also in the G region. Eight patients were discovered in early tumor development stage (stage I: 5; and stage II: 3), and nine patients were diagnosed with advanced tumors (stage III: 4; and stage IV: 5). There were six well‐differentiated (W), seven moderately differentiated (M), and four poorly differentiated (P) OSCC samples (Table 1).
Table 1.
Demographic and clinical characteristics of patients with OSCC. In the case of each patient, the gender, age, tumor localization, TNM classification, tumor stage, and stage of differentiation are given. M is for male and F for female. Regarding tumor localization, T is for tongue, G is for gingiva, and F is for floor of the mouth. The W is for well‐differentiated, M is for moderately differentiated, and P is for poorly differentiated tumors
| Patient code | Gender | Age (year) | Tumor localization | TNM classification | Tumor stage | Stage of differentiation |
|---|---|---|---|---|---|---|
| 1 | M | 73 | T | T2N1M0 | III | W |
| 2 | F | 69 | G | T4N0M0 | IV | W |
| 3 | F | 67 | F | T4N2M0 | IV | W |
| 4 | M | 52 | T; G; F | T4N1M0 | IV | M |
| 5 | M | 57 | T | T3N0M0 | III | W |
| 6 | F | 59 | T | T1N0M0 | I | W |
| 7 | M | 67 | F | T1N0M0 | I | W |
| 8 | F | 50 | T | T2N0M0 | II | M |
| 9 | M | 52 | T; G | T2N2M0 | IV | M |
| 10 | M | 48 | T | T1N0M0 | I | M |
| 11 | M | 64 | T | T2N0M0 | II | P |
| 12 | M | 44 | G | T4N1M0 | IV | M |
| 13 | M | 44 | T; F | T3N0M0 | III | M |
| 14 | M | 60 | F | T2N0M0 | II | M |
| 15 | M | 49 | T; G; F | T3N1M0 | III | P |
| 16 | M | 47 | G | T1N0M0 | I | P |
| 17 | M | 64 | F | T1N0M0 | I | P |
Shotgun proteomics analysis of saliva samples
Three randomly selected samples from patients with OSCC and matched controls, respectively, were subjected to shotgun proteomics analysis. More than 500 proteins were identified from salivary samples. For protein quantification, spectral counting was used and the ratios of OSCC : CTL protein quantities have been determined. Detailed information of the identified proteins is presented in Table S1.
The proteins with at least three unique sequences and with at least twofold change value (OSCC/CTL ratio < 0.5 or > 2) were subjected to further examination. A cluster analysis was carried out, and a heat map was generated to visualize the changes in protein amount in CTL and OSCC samples (Fig. 1). Based on cluster analysis, the protein levels can discriminate the OSCC group from the CTL group. Proteins were classified as salivary proteins or proteins being present in saliva under normal conditions and as acute‐phase proteins (Table 2). For protein classification, the UniProt and Sys‐BodyFluid databases were used; the latter contains more than 10 000 proteins of different body fluid proteomes 30. In addition, some proteins were classified as salivary proteins based on the literature data 21, 31, 32, 33, 34, 35.
Figure 1.
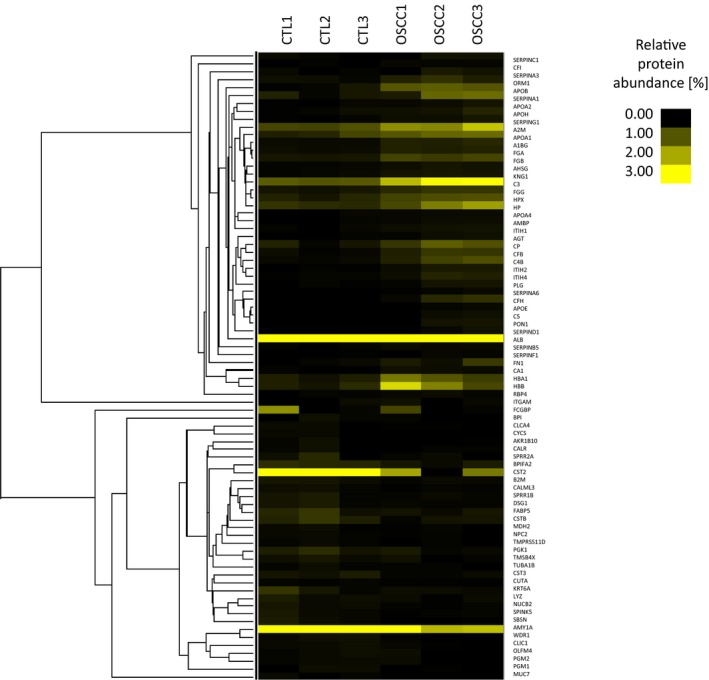
Cluster analysis and heat map of proteins identified in the CTL and OSCC groups. The relative peptide count (%), characteristic of each sample, is shown.
Table 2.
List of proteins with at least twofold change between OSCC and CTL groups. The UniProt protein ID, the protein name, and function are presented. The representative identification and quantification data, the number (#) of unique peptides, the sequence coverage (%Cov), and the OSCC/CTL ratio are given in each case. Classification indicating salivary (S) or acute‐phase (A) proteins is presented. The type of sample from patients with OSCC where the protein was identified is also listed. NI denotes proteins not identified in OSCC yet
| Protein ID | Protein name | # Unique peptide | %Cov | OSCC/CTL ratio | Classification | Function | Type of OSCC sample |
|---|---|---|---|---|---|---|---|
| O60218 | Aldo‐keto reductase family 1 member B10 | 5 | 17 | 0.10 | S | Metabolic enzyme | Salivaa 23 |
| P02763 | Alpha‐1‐acid glycoprotein 1 | 8 | 37 | 3.14 | AS | Immune response, transport | Saliva 16 |
| P01011 | Alpha‐1‐antichymotrypsin | 12 | 31 | 3.29 | AS | Protease inhibitor | NI |
| P01009 | Alpha‐1‐antitrypsin | 25 | 62 | 3.70 | S | Protease inhibitor | Saliva 36 |
| P04217 | Alpha‐1B‐glycoprotein | 12 | 39 | 3.25 | S | Immune response | Saliva 16 |
| P02765 | Alpha‐2‐HS‐glycoprotein | 7 | 26 | 2.70 | AS | Protease inhibitor, immune response, transport | NI |
| P01023 | Alpha‐2‐macroglobulin | 54 | 51 | 2.16 | S | Protease inhibitor | NI |
| P04745 | Alpha‐amylase 1 | 42 | 83 | 0.21 | S | Metabolic enzyme | Saliva 14 |
| P01019 | Angiotensinogen | 7 | 18 | 8.50 | AS | Renin–angiotensin system | NI |
| P01008 | Antithrombin III | 7 | 22 | 2.08 | AS | Protease inhibitor, blood coagulation | NI |
| P02647 | Apolipoprotein A‐I | 24 | 69 | 2.14 | S | Lipid metabolism | Saliva 23 |
| P02652 | Apolipoprotein A‐II | 7 | 67 | 3.85 | S | Lipid metabolism | Saliva 23 |
| P06727 | Apolipoprotein A‐IV | 5 | 16 | 3.55 | S | Lipid metabolism | Saliva 23 |
| P04114 | Apolipoprotein B‐100 | 42 | 13 | 8.12 | S | Lipid metabolism | NI |
| P02649 | APOE | 4 | 18 | Only in OSCC | S | Lipid metabolism | Saliva 23 |
| P17213 | Bactericidal permeability‐increasing protein | 4 | 12 | 0.24 | S | Immune response | NI |
| P02749 | Beta‐2‐glycoprotein 1 | 12 | 44 | 3.02 | S | Lipid metabolism, blood coagulation | Saliva 22 |
| P61769 | Beta‐2‐microglobulin | 5 | 57 | 0.46 | S | Immune response | NI |
| Q96DR5 | BPI fold‐containing family A member 2 | 11 | 41 | 0.49 | S | Immune response, defense | NI |
| Q14CN2 | Calcium‐activated chloride channel regulator 4 | 5 | 8 | 0.23 | S | Transport | NI |
| P27482 | Calmodulin‐like protein 3 | 6 | 64 | 0.37 | S | Metal binding, chaperone | NI |
| P27797 | Calreticulin | 4 | 19 | 0.37 | S | Chaperone | NI |
| P00915 | Carbonic anhydrase 1 | 6 | 34 | 8.55 | S | Metabolic enzyme, acid–base balance | Saliva 22 |
| P00450 | Ceruloplasmin | 27 | 37 | 3.65 | AS | Metal binding | Blood 39 |
| O00299 | Chloride intracellular channel protein 1 | 7 | 34 | 0.31 | S | Transport, cell cycle regulation | NI |
| P01024 | Complement C3 | 84 | 61 | 2.77 | AS | Immune response | Saliva 36 |
| P0C0L5 | Complement C4‐B | 32 | 25 | 6.69 | AS | Immune response | Saliva 36 |
| P01031 | Complement C5 | 7 | 5 | Only in OSCC | AS | Immune response | NI |
| B4E1Z4 | CFB | 22 | 22 | 5.44 | AS | Immune response | Saliva 36 |
| P08603 | CFH | 21 | 22 | Only in OSCC | AS | Immune response | NI |
| P05156 | Complement factor I | 3 | 7 | 6.42 | AS | Immune response | NI |
| P22528 | Cornifin‐B | 6 | 79 | 0.45 | S | Cornification | NI |
| P08185 | Corticosteroid‐binding globulin | 4 | 15 | Only in OSCC | AS | Protease inhibitor | Saliva 23 |
| P04080 | Cystatin‐B | 6 | 86 | 0.39 | S | Protease inhibitor | NI |
| P01034 | Cystatin‐C | 7 | 43 | 0.33 | S | Protease inhibitor | NI |
| P09228 | Cystatin‐SA | 13 | 69 | 0.35 | S | Protease inhibitor | Saliva 14 |
| P99999 | Cytochrome c | 4 | 32 | 0.00 | A | Electron transport chain, apoptosis | Tissue 53 |
| Q02413 | Desmoglein‐1 | 8 | 12 | 0.40 | S | Desmosome component | NI |
| P61916 | Epididymal secretory protein E1 | 4 | 33 | 0.30 | S | Lipid metabolism, cholesterol transport | NI |
| Q01469 | Fatty acid‐binding protein, epidermal | 12 | 79 | 0.49 | S | Lipid metabolism | Salivaa 23 |
| P02671 | Fibrinogen alpha chain | 11 | 13 | 2.67 | AS | Blood coagulation | Blood 40 |
| P02675 | Fibrinogen beta chain | 20 | 49 | 2.91 | AS | Blood coagulation | Blood 40 |
| P02679 | Fibrinogen gamma chain | 18 | 48 | 2.43 | AS | Blood coagulation | Blood 40 |
| B7ZLE5 | FN1 protein | 24 | 17 | 5.73 | S | Cell adhesion | Tissue 42 |
| P00738 | Haptoglobin | 29 | 67 | 2.61 | AS | Heme binding | Blood 38 |
| P69905 | Hemoglobin subunit alpha | 11 | 92 | 3.37 | S | Oxygen transport | Salivaa 23 |
| P68871 | Hemoglobin subunit beta | 17 | 94 | 4.41 | S | Oxygen transport | Salivaa 23 |
| P02790 | Hemopexin | 20 | 52 | 2.41 | AS | Heme binding | Saliva 16, 22 |
| P05546 | Heparin cofactor 2 | 8 | 17 | Only in OSCC | A | Blood coagulation | Saliva 23 |
| Q9Y6R7 | IgGFc‐binding protein | 52 | 17 | 0.49 | S | Immune response | NI |
| P11215 | Integrin alpha‐M | 6 | 9 | 2.01 | S | Immune response | Tissue 41 |
| P19827 | Inter‐alpha‐trypsin inhibitor heavy chain H1 | 8 | 14 | 3.76 | S | Protease inhibitor | Saliva 23 |
| P19823 | Inter‐alpha‐trypsin inhibitor heavy chain H2 | 10 | 18 | 11.23 | S | Protease inhibitor | Saliva 23 |
| Q14624 | Inter‐alpha‐trypsin inhibitor heavy chain H4 | 13 | 22 | 5.33 | S | Protease inhibitor | Saliva 23 |
| P02538 | Keratin, type II cytoskeletal 6A | 21 | 39 | 0.44 | S | Cytoskeleton | NI |
| P01042 | Kininogen‐1 | 11 | 18 | 2.89 | S | Protease inhibitor, blood coagulation | Saliva 23 |
| P61626 | Lysozyme C | 7 | 54 | 0.47 | S | Host defense | NI |
| P40926 | Malate dehydrogenase, mitochondrial | 4 | 17 | 0.37 | S | Metabolic enzyme | NI |
| Q8TAX7 | Mucin‐7 | 4 | 12 | 0.00 | S | Host defense | NI |
| P80303 | Nucleobindin‐2 | 8 | 26 | 0.32 | S | Metal binding | Saliva 23 |
| Q6UX06 | Olfactomedin‐4 | 7 | 20 | 0.47 | S | Cell adhesion | NI |
| P36871 | Phosphoglucomutase‐1 | 6 | 13 | 0.08 | S | Metabolic enzyme | NI |
| Q96G03 | Phosphoglucomutase‐2 | 5 | 11 | 0.40 | S | Metabolic enzyme | NI |
| P00558 | Phosphoglycerate kinase 1 | 10 | 33 | 0.44 | S | Metabolic enzyme | Saliva 22 |
| P36955 | Pigment epithelium‐derived factor | 4 | 12 | 7.17 | S | Tumor development, angiogenesis | NI |
| P05155 | Plasma protease C1 inhibitor | 8 | 21 | 5.95 | S | Protease inhibitor, blood coagulation | NI |
| P00747 | Plasminogen | 9 | 17 | 5.11 | AS | Blood coagulation | NI |
| P02760 | Protein AMBP | 6 | 23 | 5.41 | S | Protease inhibitor, host defense | Saliva 23 |
| O60888 | Protein CutA | 3 | 33 | 0.43 | S | Metal binding, enzyme binding | NI |
| P02753 | Retinol‐binding protein 4 | 5 | 24 | 2.54 | S | Protease inhibitor, host defense | Blood 38 |
| Q9NQ38 | Serine protease inhibitor Kazal‐type 5 | 11 | 13 | 0.32 | S | Lipid metabolism | NI |
| P36952 | Serpin B5 | 4 | 14 | 2.63 | S | Tumor suppressor | Blood 12 |
| P02768 | Serum albumin | 71 | 84 | 2.53 | S | Transport | Saliva 22 |
| P27169 | Serum paraoxonase/arylesterase 1 | 9 | 37 | Only in OSCC | A | Detoxification | Saliva 23 |
| P35326 | Small proline‐rich protein 2A | 6 | 79 | 0.29 | S | Cornification | Salivaa 23 |
| Q6UWP8 | Suprabasin | 12 | 33 | 0.05 | S | Cell proliferation | NI |
| P62328 | Thymosin beta‐4 | 5 | 64 | 0.42 | S | Actin binding, cell proliferation | NI |
| O60235 | Transmembrane protease serine 11D | 3 | 10 | 0.20 | S | Protease, host defense | NI |
| P68363 | Tubulin alpha‐1B chain | 4 | 12 | 0.40 | S | Microtubule component | Salivaa 23 |
| O75083 | WD repeat‐containing protein 1 | 7 | 19 | 0.13 | S | Cell migration | NI |
Indicates that not the protein itself, but another close family member of it was already found in OSCC.
Two proteins, cytochrome c and mucin‐7, were only present in the CTL samples, and six proteins, complement factor H (CFH) and C5 (C5), corticosteroid‐binding globulin (SERPINA6), heparin cofactor 2 (SERPIND1), apolipoprotein E (APOE), and serum paraoxonase/arylesterase 1 (PON1), were only present in the OSCC samples (Table 3).
Table 3.
Proteins identified only in the OSCC or CTL group
| Protein IDa | Protein name | Gene name | Function | Presence | Reference to previous studies |
|---|---|---|---|---|---|
| P02649 | APOE | APOE | Lipid metabolism | Only OSCC | Identified in saliva of patients with OSCC 23 |
| P01031 | Complement C5 | C5 | Innate immune response, complement component | Only OSCC | Not identified in cancer yet |
| P08603 | CFH | CFH | Innate immune response, complement component | Only OSCC | Identified in other forms of cancer but not in OSCC 49, 50 |
| P08185 | Corticosteroid‐binding globulin | SERPINA6 | Protease inhibitor | Only OSCC | Identified in saliva of patients with OSCC 23 |
| P05546 | Heparin cofactor 2 | SERPIND1 | Blood coagulation | Only OSCC | Identified in saliva of patients with OSCC 23 |
| P27169 | Serum paraoxonase/arylesterase 1 | PON1 | Detoxification | Only OSCC | Identified in saliva of patients with OSCC 23 |
| P99999 | Cytochrome c | CYCS | Electron transport chain, its release from mitochondria initiates apoptosis | Only Ctrl | Its release was inhibited in OSCC 53 |
| Q8TAX7 | Mucin‐7 | MUC7 | Antibacterial activity, host defense | Only Ctrl | Not identified in cancer yet |
Based on http://www.uniprot.org/.
Functional analysis of salivary proteins
It was observed that the level of some proteins such as apolipoproteins, components of the complement system, proteinases, proteinase inhibitors, components of the coagulation cascade is upregulated. This might indicate a change in proteolysis most probably associated with the interrelated coagulation cascade‐complement activation processes. At the same time, the level of proteins having role in metabolism and host defense was downregulated showing extensive cancer‐related changes (Table 2). For a more detailed functional analysis of the differentially expressed proteins, GO analysis was performed; the Biological Process, Molecular Function, and Cellular Localization according to GO (http://www.geneontology.org/) were examined. First, the network of differentially expressed proteins was generated using string version 10.5 28, 29, followed by GO enrichment analysis provided by String. The network of downregulated proteins contained 35 proteins (nodes) and 27 possible protein–protein interactions analyzed at medium stringency (Fig. 2A). No biological function was enriched in the downregulated proteins in this loosely connected network (Fig. 2B); however, seven of 35 downregulated proteins are metabolic enzymes participating mainly in carbohydrate metabolism and 10 of 35 proteins have a role in defense. The upregulated 45 proteins show a highly interconnected protein–protein interaction network with 400 interactions analyzed at medium stringency (Fig. 2C). The enriched functions indicate active regulatory mechanisms implicating the immune system, lipid metabolism, plasminogen activation, antioxidant activity, and inhibition of enzymatic activities (Fig. 2D). Regarding localization of up‐ or downregulated proteins, all are mainly extracellular proteins according to GO (Fig. 2B,D), but a part of the upregulated proteins originate from lipid particles or platelet alpha‐granules indicating the presence of a possibly cancer‐induced complex process involving systemic mechanisms.
Figure 2.
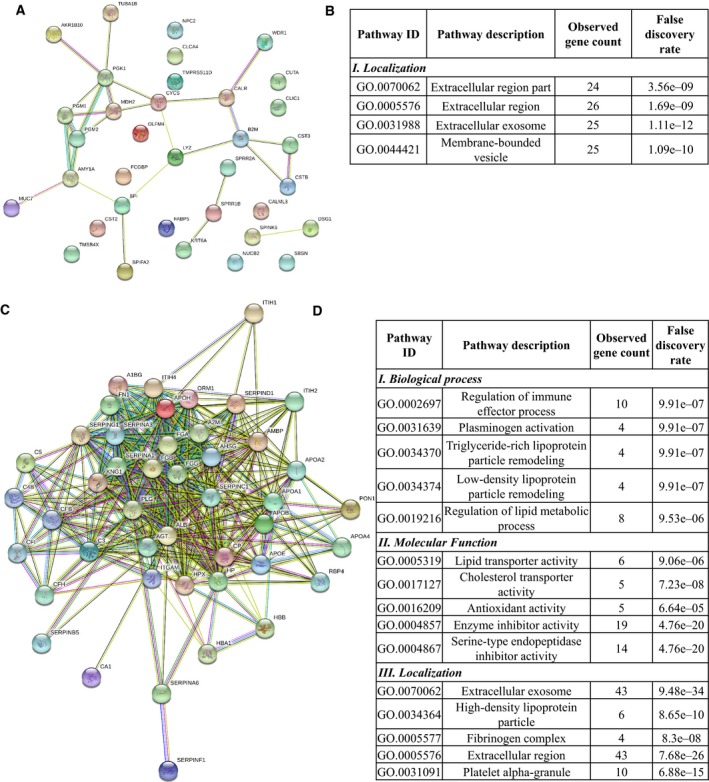
The protein–protein interaction network and functional classification of up‐ and downregulated proteins in OSCC. The network of downregulated (A) and upregulated (C) proteins in OSCC displayed by String 10.4 using default settings and medium stringency. Each node represents a protein and the edges represent protein–protein interactions based on different levels of evidence collected by String. The enrichment table of GO terms calculated by String in the case of downregulated (B) and upregulated (D) proteins is shown indicating the number of the proteins belonging to each term and the false discovery rate calculated by String.
To obtain more insights into the changes associated with OSCC, a literature search was performed to see which proteins have been associated with oncogenesis. Most of the proteins were already associated with OSCC, and 32 proteins were identified to be present in saliva in this pathological condition.
Complement C4B (C4B), complement factor B (CFB), complement C3, and alpha‐1‐antitrypsin were shown to be associated with the risk of developing OSCC according to a targeted proteomics study 36. The levels of apolipoproteins A and E; PON1; inter‐alpha‐trypsin inhibitor heavy chain H1, H2, and H4; kininogen 1; protein AMBP; nucleobindin‐2; SERPIND1; and SERPINA6 were found to be upregulated in OSCC in shotgun proteomics experiments carried out on saliva samples 23. The presence of APOE was related to the increased invasion potential of OSCC 37.
The alpha‐1‐acid glycoprotein, alpha‐1B glycoprotein, alpha‐amylase, beta‐2‐glycoprotein, carbonic anhydrase 1, cystatin‐SA, hemopexin, phosphoglycerate kinase, and serum albumin were identified as potential salivary markers of OSCC 14, 16, 22.
Some of the proteins found to be differentially expressed in our study, such as fibrinogen alpha, beta, and gamma chains, haptoglobin, SERPINB5, retinol‐binding protein 4, and ceruloplasmin, were shown to be plasma markers of OSCC, while the presence of integrin alpha‐M and fibronectin FN1 was demonstrated in the OSCC tissue 12, 38, 39, 40, 41, 42.
In the case of 36 proteins, no association with OSCC was found so far (Table 2). Angiotensinogen and plasminogen themselves were not found to be associated with OSCC, but the plasminogen activator system was shown to be a predictive marker for early OSCC, and by bioinformatics analysis, the angiotensin‐converting enzymes were associated with malignant epithelial neoplasia characteristic of OSCC 43, 44. In the case of six proteins, not the protein from our list, but another protein from the same family was already demonstrated to be differentially expressed in OSCC (Table 2). In the case of SERPINB5, there are contradictory data; in our study, the level of SERPINB5 was found to be elevated in OSCC; however, the SERPINB5 and different forms of SERPINS from clade B were found by other groups to be downregulated in OSCC on mRNA level and higher SERPINB5 levels were found to correlate with better prognosis of patients with oral cancer 45, 46.
Plasma protease C1 inhibitor (SERPING1), antithrombin III, and fibronectin were found to play a role in carcinogenesis, but their implication in oral cancer, especially in OSCC, has not been demonstrated yet 47, 48. The CFH was previously identified in lung adenocarcinoma and cutaneous squamous cell carcinoma, but not in OSCC 49, 50, and apoB100 was found in serum of patients with head and neck squamous cell carcinoma 51. No data were found on the presence of complement C5 and mucin‐7 in cancer; however, other components of the complement system and other forms of mucins were all identified in different forms of cancer and in OSCC as well 36, 52.
As for the involvement of cytochrome c, it was shown that the HIF‐1α‐dependent suppression of hypoxia‐induced apoptosis in OSCC happens through the inhibition of cytochrome c release 53.
Examination of the level of selected proteins by ELISA
Many of the studies published in the scientific literature are based on shotgun proteomics experiments. Only few of the proteins listed in Table 2 were verified or validated either using SRM‐based targeted or antibody‐based methods. Considering the proteins present only in OSCC based on our shotgun experiments, the data presented in the literature, and the availability of antibodies, SERPIND1 and C5 were selected for further studies. To test the utility of potential biomarkers identified in Asia for a European population, resistin reported to be a potential biomarker for OSCC in Taiwan 23 was also selected.
The concentrations of C5, SERPIND1, and resistin were examined in the saliva of patients with OSCC, MCTL, and YCTL using quantitative sandwich ELISA kits (Fig. 3). In the case of C5, the difference was significant but only when YCTL and patients with OSCC or YCTL and MCTL were compared, indicating that the level of C5 was age‐dependent or it was influenced by other factors. One such factor can be the inflammatory status related to poor oral hygiene often observed in the middle‐aged and elderly population in Hungary 54. This means that despite the differential expression of C5 in the OSCC group, the level of C5 does not discriminate between the target MCTL and the diseased group, and hence, it cannot be used as a biomarker for OSCC.
Figure 3.
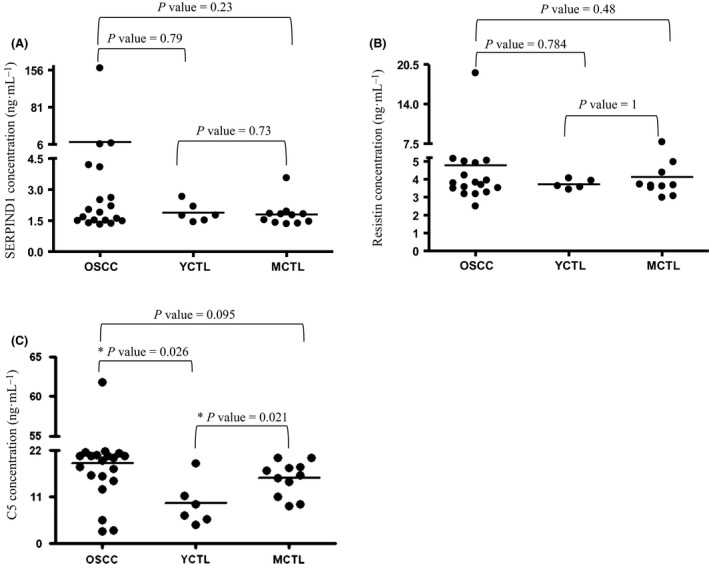
Examination of potential salivary biomarkers using ELISA. The concentration of SERPIND1 (A), resistin (B), and complement C5 (C) proteins in the saliva samples collected from patients with OSCC, YCTL, and MCTL. The P value is indicated; * refers to P < 0.05.
In the case of resistin and SERPIND1, no significant differences were found between the groups. Resistin was not up‐ or downregulated according to our shotgun experiments and did not show significant differences in the ELISA experiments either. In the case of SERPIND1, one possible explanation of the disagreement between the shotgun proteomics and ELISA data can be that the low number of samples (three for each group) tested by shotgun proteomics and the high individual variation of the saliva samples collected from the patients may lead to false‐positive results. This outcome highlights the importance of validation of the shotgun proteomics data on larger patient cohorts to decrease the false positivity of biomarker identifications. In a two‐stage experimental approach, starting with a shotgun proteomics experiment, the level of resistin was found to be significantly higher in the saliva samples of patients with OSCC compared to controls. However, following ELISAs showed that the median values in the OSCC group were only slightly elevated compared to the control group 23. In the same study, SERPIND1 was not validated but was shown to be upregulated in the saliva samples of patients with OSCC. In our study, a similar experimental setup was applied; in the shotgun experiment, the level of SERPIND1 was higher but the level of resistin did not change markedly in the OSCC group, and the validation of SERPIND1 and resistin shows that none of them turned to be useful potential biomarkers. The fact that resistin was identified as a biomarker for OSCC in Taiwan but not in Hungary gives further evidence for the importance of regional studies highlighted in our previous work 55.
Conclusions
Global analysis of salivary samples from patients with OSCC and controls contributes to the better understanding of the disease, including the interaction of tumor cells with their environment and the influence of cancer lesion on salivary protein ecology. Salivary proteins, characterizing patients with OSCC in this study, highlighted the importance of networks involving the immune system and proteolysis in this disease. Six proteins were only detected in OSCC samples by proteomics analyses and two of them were further examined using ELISA, but none of the proteins turned to be a potential biomarker in OSCC in our study group. The fact that resistin was shown to be a possible biomarker in Taiwan but not in our study highlights the importance of regional or population‐tailored studies.
Author contributions
IM, EC, and CK designed the experiments; IM and JN performed stomatologic examination of patients; BM and ZD carried out the experiments; BM, ZD, and EC evaluated the data and wrote the manuscript; BM, ES, and EC prepared the figures and tables; and JT, KM, CK, and IM reviewed the manuscript.
Supporting information
Table S1. List of identified proteins.
Acknowledgements
This work was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, Hungarian Scientific Research Fund OTKA K105034; TÁMOP‐4.2.2.D‐15/1/KONV‐2015‐0016 project implemented through the New Széchenyi Plan cofinanced by the European Social Fund; and the National Research, Development and Innovation Office [GINOP‐2.3.2‐15‐2016‐00020 and GINOP‐2.3.2‐15‐2016‐00001]. The work of Kamilla Sólyom and Jánosné Tóth is greatly acknowledged.
References
- 1. Massano J, Regateiro FS, Januário G and Ferreira A (2006) Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102, 67–76. [DOI] [PubMed] [Google Scholar]
- 2. Argiris A, Karamouzis MV, Raben D and Ferris RL (2008) Head and neck cancer. Lancet 371, 1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P and Boyle P (2008) Tobacco smoking and cancer: a meta‐analysis. Int J Cancer 122, 155–164. [DOI] [PubMed] [Google Scholar]
- 4. Bagnardi V, Blangiardo M, La Vecchia C and Corrao G (2001) A meta‐analysis of alcohol drinking and cancer risk. Br J Cancer 85, 1700–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenquist K (2005) Risk factors in oral and oropharyngeal squamous cell carcinoma: a population‐based case‐control study in southern Sweden. Swed Dent J Suppl, 179, 1–66. [PubMed] [Google Scholar]
- 6. Suba Z, Mihályi S, Takács D and Gyulai‐Gaál S (2009) Oral cancer: morbus Hungaricus in the 21st century. Fogorv Sz 102, 63–68. [PubMed] [Google Scholar]
- 7. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J and Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108. [DOI] [PubMed] [Google Scholar]
- 8. Myers JN, Elkins T, Roberts D and Byers RM (2000) Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg 122, 44–51. [DOI] [PubMed] [Google Scholar]
- 9. Garavello W, Bertuccio P, Levi F, Lucchini F, Bosetti C, Malvezzi M, Negri E and La Vecchia C (2010) The oral cancer epidemic in central and eastern Europe. Int J Cancer 127, 160–171. [DOI] [PubMed] [Google Scholar]
- 10. Santosh ABR, Jones T and Harvey J (2016) A review on oral cancer biomarkers: understanding the past and learning from the present. J Cancer Res Ther 12, 486–492. [DOI] [PubMed] [Google Scholar]
- 11. Sparano A, Weinstein G, Chalian A, Yodul M and Weber R (2004) Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg 131, 472–476. [DOI] [PubMed] [Google Scholar]
- 12. Shpitzer T, Hamzany Y, Bahar G, Feinmesser R, Savulescu D, Borovoi I, Gavish M and Nagler RM (2009) Salivary analysis of oral cancer biomarkers. Br J Cancer 101, 1194–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shpitzer T, Bahar G, Feinmesser R and Nagler RM (2007) A comprehensive salivary analysis for oral cancer diagnosis. J Cancer Res Clin Oncol 133, 613–617. [DOI] [PubMed] [Google Scholar]
- 14. Sannam Khan R, Khurshid Z, Akhbar S and Faraz Moin S (2016) Advances of salivary proteomics in oral squamous cell carcinoma (OSCC) detection: an update. Proteomes 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah FD, Begum R, Vajaria BN, Patel KR, Patel JB, Shukla SN and Patel PS (2011) A review on salivary genomics and proteomics biomarkers in oral cancer. Indian J Clin Biochem 26, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winck FV, Prado Ribeiro AC, Ramos Domingues R, Ling LY, Riaño‐Pachón DM, Rivera C, Brandão TB, Gouvea AF, Santos‐Silva AR, Coletta RD et al. (2015) Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci Rep 5, 16305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Csősz É, Kalló G, Márkus B, Deák E, Csutak A and Tőzsér J (2017) Quantitative body fluid proteomics in medicine — A focus on minimal invasiveness. J Proteomics 153, 30–43. [DOI] [PubMed] [Google Scholar]
- 18. Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ and Wong DTW (2013) Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev 26, 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagler R, Bahar G, Shpitzer T and Feinmesser R (2006) Concomitant analysis of salivary tumor markers–a new diagnostic tool for oral cancer. Clin Cancer Res 12, 3979–3984. [DOI] [PubMed] [Google Scholar]
- 20. Schulz BL, Cooper‐White J and Punyadeera CK (2013) Saliva proteome research: current status and future outlook. Crit Rev Biotechnol 33, 246–259. [DOI] [PubMed] [Google Scholar]
- 21. Grassl N, Kulak NA, Pichler G, Geyer PE, Jung J, Schubert S, Sinitcyn P, Cox J and Mann M (2016) Ultra‐deep and quantitative saliva proteome reveals dynamics of the oral microbiome. Genome Med 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu S, Arellano M, Boontheung P, Wang J, Zhou H, Jiang J, Elashoff D, Wei R, Loo JA and Wong DT (2008) Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res 14, 6246–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu C‐C, Chu H‐W, Hsu C‐W, Chang K‐P and Liu H‐P (2015) Saliva proteome profiling reveals potential salivary biomarkers for detection of oral cavity squamous cell carcinoma. Proteomics 15, 3394–3404. [DOI] [PubMed] [Google Scholar]
- 24. Yu J‐S, Chen Y‐T, Chiang W‐F, Hsiao Y‐C, Chu LJ, See L‐C, Wu C‐S, Tu H‐T, Chen H‐W, Chen C‐C et al. (2016) Saliva protein biomarkers to detect oral squamous cell carcinoma in a high‐risk population in Taiwan. Proc Natl Acad Sci 113, 11549–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 26. Guan S, Price JC, Prusiner SB, Ghaemmaghami S and Burlingame AL (2011) A data processing pipeline for mammalian proteome dynamics studies using stable isotope metabolic labeling. Mol Cell Proteomics 10, M111.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saldanha Alok J (2004) Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248. [DOI] [PubMed] [Google Scholar]
- 28. Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P et al. (2017) The STRING database in 2017: quality‐controlled protein‐protein association networks, made broadly accessible. Nucleic Acids Res 45, D362–D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta‐Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP et al. (2015) STRING v10: protein‐protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43, D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li S‐J, Peng M, Li H, Liu B‐S, Wang C, Wu J‐R, Li Y‐X and Zeng R (2009) Sys‐BodyFluid: a systematical database for human body fluid proteome research. Nucleic Acids Res 37, D907–D912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swaak AJ, Visch LL and Zonneveld A (1988) Diagnostic significance of salivary levels of beta 2‐microglobulin in Sjögren's syndrome. Clin Rheumatol 7, 28–34. [DOI] [PubMed] [Google Scholar]
- 32. Guo T, Rudnick PA, Wang W, Lee CS, DeVoe DL and Balgley BM (2006) Characterization of the human salivary proteome by capillary isoelectric focusing/nanoreversed‐phase liquid chromatography coupled with ESI‐tandem MS. J Proteome Res 5, 1469–1478. [DOI] [PubMed] [Google Scholar]
- 33. Rossetti DV, Martelli C, Longhi R, Iavarone F, Castagnola M and Desiderio C (2013) Quantitative analysis of thymosin β 4 in whole saliva by capillary electrophoresis‐mass spectrometry using multiple ions monitoring (CE‐MIM‐MS). Electrophoresis 34, 2674–2682. [DOI] [PubMed] [Google Scholar]
- 34. Morzel M, Chabanet C, Schwartz C, Lucchi G, Ducoroy P and Nicklaus S (2014) Salivary protein profiles are linked to bitter taste acceptance in infants. Eur J Pediatr 173, 575–582. [DOI] [PubMed] [Google Scholar]
- 35. Alves DBM, Bingle L, Bingle CD, Lourenco SV, Silva AA, Pereira DL and Vargas PA (2017) BPI‐fold (BPIF) containing/plunc protein expression in human fetal major and minor salivary glands. Braz Oral Res 31, e6. [DOI] [PubMed] [Google Scholar]
- 36. Kawahara R, Bollinger JG, Rivera C, Ribeiro ACP, Brandão TB, Leme AFP and MacCoss MJ (2016) A targeted proteomic strategy for the measurement of oral cancer candidate biomarkers in human saliva. Proteomics 16, 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jayakar SK, Loudig O, Brandwein‐Gensler M, Kim RS, Ow TJ, Ustun B, Harris TM, Prystowsky MB, Childs G, Segall JE et al. (2017) Apolipoprotein E promotes invasion in oral squamous cell carcinoma. Am J Pathol 187, 2259–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, Azman SN, Kerishnan JP, Zain RB, Chen YN and Wong YL (2014) Identification of host‐immune response protein candidates in the sera of human oral squamous cell carcinoma patients. PLoS ONE 9, e109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jayadeep A, Raveendran Pillai K, Kannan S, Nalinakumari KR, Mathew B, Krishnan Nair M and Menon VP (1997) Serum levels of copper, zinc, iron and ceruplasmin in oral leukoplakia and squamous cell carcinoma. J Exp Clin Cancer Res 16, 295–300. [PubMed] [Google Scholar]
- 40. Pro Tung CL, Lin ST, Chou HC, Chen YW, Lin HC, Tung CL, Huang KJ, Chen YJ, Lee YR and Chan HL (2013) Proteomics‐based identification of plasma biomarkers in oral squamous cell carcinoma. J Pharm Biomed Anal 75, 7–17. [DOI] [PubMed] [Google Scholar]
- 41. Ohara T, Kawashiri S, Tanaka A, Noguchi N, Kitahara H, Okamune A, Kato K, Hase T, Nakaya H and Yoshizawa K (2009) Integrin expression levels correlate with invasion, metastasis and prognosis of oral squamous cell carcinoma. Pathol Oncol Res 36, 429–436. [DOI] [PubMed] [Google Scholar]
- 42. Yen CY, Huang CY, Hou MF, Yang YH, Chang CH, Huang HW, Chen CH and Chang HW (2013) Evaluating the performance of fibronectin 1 (FN1), integrin α4β1 (ITGA4), syndecan‐2 (SDC2), and glycoprotein CD44 as the potential biomarkers of oral squamous cell carcinoma (OSCC). Biomarkers 18, 63–72. [DOI] [PubMed] [Google Scholar]
- 43. Magnussen S, Rikardsen OG, Hadler‐Olsen E, Uhlin‐Hansen L, Steigen SE and Svineng G (2014) Urokinase plasminogen activator receptor (uPAR) and plasminogen activator inhibitor‐1 (PAI‐1) are potential predictive biomarkers in early stage oral squamous cell carcinomas (OSCC). PLoS ONE 9, e101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Carvalho Fraga CA, Farias LC, Jones KM, Batista de Paula AM and Guimaraes ALS (2017) Angiotensin‐converting enzymes (ACE and ACE2) as potential targets for malignant epithelial neoplasia: review and bioinformatics analyses focused in oral squamous cell carcinoma. Protein Pept Lett 24, 784–792. [DOI] [PubMed] [Google Scholar]
- 45. Shiiba M, Nomura H, Shinozuka K, Saito K, Kouzu Y, Kasamatsu A, Sakamoto Y, Murano A, Ono K, Ogawara K et al. (2010) Down‐regulated expression of SERPIN genes located on chromosome 18q21 in oral squamous cell carcinomas. Oncol Rep 24, 241–249. [DOI] [PubMed] [Google Scholar]
- 46. Xia W, Lau YK, Hu MC, Li L, Johnston DA, Sheng S, El‐Naggar A and Hung MC (2000) High tumoral maspin expression is associated with improved survival of patients with oral squamous cell carcinoma. Oncogene 19, 2398–2403. [DOI] [PubMed] [Google Scholar]
- 47. Kocsis J, Mészáros T and Madaras B (2011) High levels of acute phase proteins and soluble 70 kDa heat shock proteins are independent and additive risk factors for mortality in colorectal cancer. Cell Stress Chaperones 16, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daly M, O'Meara A and Hallinan FM (1987) Identification and characterization of a new antithrombin III familial variant (AT Dublin) with possible increased frequency in children with cancer. Br J Haematol 65, 457–462. [DOI] [PubMed] [Google Scholar]
- 49. Cui T, Chen Y, Knosel T, Yang L, Zoller K, Galler K, Berndt A, Mihlan M, Zipfel PF and Petersen I (2011) Human complement factor H is a novel diagnostic marker for lung adenocarcinoma. Int J Oncol 39, 161–168. [DOI] [PubMed] [Google Scholar]
- 50. Riihilä PM, Nissinen LM, Ala‐aho R, Kallajoki M, Grénman R, Meri S, Peltonen S, Peltonen J and Kähäri V (2014) Complement factor H: a biomarker for progression of cutaneous squamous cell carcinoma. J Invest Dermatol 134, 498–506. [DOI] [PubMed] [Google Scholar]
- 51. Li G, Da M, Zhang W, Wu H, Ye J, Chen J, Ma L, Gu N, Wu Y and Song X (2016) Alteration of serum lipid profile and its prognostic value in head and neck squamous cell carcinoma. J Oral Pathol Med 45, 167–172. [DOI] [PubMed] [Google Scholar]
- 52. Li P, Xiao LY and Tan H (2015) Muc‐1 promotes migration and invasion of oral squamous cell carcinoma cells via PI3K‐Akt signaling. Int J Clin Exp Pathol 8, 10365–10374. [PMC free article] [PubMed] [Google Scholar]
- 53. Sasabe E, Tatemoto Y, Li D, Yamamoto T and Osaki T (2005) Mechanism of HIF‐1α‐dependent suppression of hypoxia‐induced apoptosis in squamous cell carcinoma cells. Cancer Sci 96, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bánóczy J and Rigó O (1991) Prevalence study of oral precancerous lesions within a complex screening system in Hungary. Community Dent Oral Epidemiol 19, 265–267. [DOI] [PubMed] [Google Scholar]
- 55. Csősz É, Lábiscsák P, Kalló G, Márkus B, Emri M, Szabó A, Tar I, Tőzsér J, Kiss C and Márton I (2017) Proteomics investigation of OSCC‐specific salivary biomarkers in a Hungarian population highlights the importance of identification of population‐tailored biomarkers. PLoS ONE 12, e0177282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of identified proteins.


