Abstract
NADPH oxidase is encoded by a small gene family (Respiratory burst oxidase homologs, Rbohs) and plays an important role in regulating various biological processes. However, little information about this gene family is currently available for strawberry. In this study, a total of seven Rboh genes were identified from strawberry through genomewide analysis. Gene structure analysis showed the number of exons ranged from 10 to 23, implying that this variation occurred in FvRboh genes by the insertion and distribution of introns; the order and approximate size of exons were relatively conserved. FvRbohC was predicted to localize to the thylakoid membrane of the chloroplast, while other members were computed to localize to the plasma membrane, indicating different functions. Amino acid sequence alignment, conserved domain, and motif analysis showed that all identified FvRbohs had typical features of plant Rbohs. Phylogenetic analysis of Rbohs from strawberry, grape, Arabidopsis, and rice suggested that the FvRbohs could be divided into five subgroups and showed a closer relationship with those from grape and Arabidopsis than those from rice. The expression patterns of FvRboh genes in root, stem, leaf, flower, and fruit revealed robust tissue specificity. The expression levels of FvRbohA and FvRbohD were quickly induced by cold stress, followed by an increase in NADPH oxidase activity, leading to accumulation and triggering the antioxidant reaction by the transient increases in SOD activity. This suggested these two genes may be involved in cold stress and defense responses in strawberry.
Keywords: cold stress, expression profiles, NADPH oxidase, phylogenetic analysis, Rbohs, strawberry
Abbreviations
- APX
ascorbate peroxidase
- CAT
catalase
- CTAB
cetyltrimethylammonium bromide
- EDTA
ethylenediaminetetraacetic acid
- H2O2
hydrogen peroxide
- MDA
malondialdehyde
superoxide anion
- OH.
hydroxyl radical
- POD
peroxidase
- Rbohs
respiratory burst oxidase homologs
- ROS
reactive oxygen species
- SOD
superoxide dismutase
NADPH oxidase, an enzyme complex that catalyzes the NADPH‐dependent one‐electron reduction of molecular oxygen to the superoxide anion , is also responsible for the accumulation of reactive oxygen species (ROS) associated with an abrupt rise in oxygen consumption as the respiratory burst oxidase 1, 2, 3. The oxidase complex comprises a membrane‐bound heterodimer, called flavocytochrome b558, consisting of gp91phox and p22phox, and four cytosolic components (p47phox, p67phox, p40phox, and the small GTPase Rac) in phagocytes. Once the cell is stimulated, cytosolic components will interact with flavocytochrome b558 to activate NADPH oxidase and then generate , subsequently ending with the secondary production of other ROS such as hydrogen peroxide (H2O2) and hydroxyl radical (OH.) 4, 5, 6. ROS plays an important role in immunity and cell growth, but excessive accumulation of ROS can cause cellular damage and might be toxic 7, 8. Still, some evidences have shown that ROS at low concentration could be the ‘alarm’ signal to activate defense responses in plants 9, 10. A balance between generation and elimination of the free radicals can keep cellular ROS homeostasis. Plants have evolved complex antioxidant defense systems including nonenzymatic (low molecular weight antioxidant compounds) and enzymatic (SOD, CAT, POD, and APX) components that scavenge excessively accumulated ROS under stress conditions 11, 12.
In plants, NADPH oxidase is known as respiratory burst oxidase homologs (Rbohs), which encodes a homolog of mammalian phagocyte gp91 phox. It was proved that plant Rbohs are plasma membrane enzymes. The main structure of Rbohs contains six conserved transmembrane domains with C‐terminal FAD and NADPH hydrophilic domains, two heme groups, and two N‐terminal Ca2+‐binding EF‐hand motifs which account for being regulated by Ca2+ 1. Rbohs, a small gene family, have been identified and isolated in a wide range of plants. Arabidopsis has ten members (AtRbohA–J), with a tissue‐specific expression pattern: AtRbohA–G and AtRbohI in the roots, AtRbohH and AtRbohJ in pollens, and AtRbohD and AtRbohF throughout the plant, suggesting that differential expression profile and functions are formed in plant growth 1, 13, 14. NADPH oxidase has not only been linked with plant development, but also responds to different abiotic or biotic stress conditions mainly by adjusting the ROS generation 15. ROS signals derived by orthologs of two AtRbohs (AtRbohH and AtRbohJ) are involved in pollen tube growth in tobacco 16. Interestingly, the same function was not testified in Arabidopsis thaliana. Moreover, Rboh‐dependent ROS generation has been associated with root and hypocotyl elongation, stomatal movement, seed germination, and fruit ripening 17, 18. Several studies have evinced that NADPH oxidases mediate an oxidative burst to respond to stress. The tomato SlRboh1 (homologous to AtRbohF) expression level increased, followed by much ROS accumulation, to adapt to the higher CO2 concentration environment 19, and it was also related to the regulation of stomatal movements in endurance to high temperature stress mediated by the phytohormones, abscisic acid (ABA), and brassinosteroid (BR) 20, 21. AtRbohD could affect H2O2 accumulation and involved in the release from suppression of root elongation by ethylene signaling during hypoxic stress 22. In most instances, an inhibition of NADPH oxidase contributes to the decrease in ROS production, then leading to the change of reaction to cell death and stress resistance. For example, DPI (diphenylene iodonium) inhibited NADPH oxidase activity, which decreased the production of H2O2 so as to lower ethylene‐induced cell death rates in rice 23 and resulted in diminishment of expression of several defense‐related genes in response to abiotic stress, such as wounding, oligosaccharides, systemin, and methyl jasmonate in Lycopersicon esculentum 24.
Strawberry is a perennial herb belonging to berry fruit crops. It is a model plant with high economic and nutritional value in Rosaceae genomics research. However, strawberry is often subjected to extreme sporadic chilling injury in the short term, which leads to huge economic loss because insulation measures are not strictly applied. Based on the significance of NADPH oxidase in the regulation of plant growth, development, and adaption to the environment, in this study, we identified NADPH oxidase family members from strawberry and analyzed their conserved motif, homology, and phylogenetic relationship with other plants. Enzyme activity and expression profiles in response to cold stress are also presented to provide scientific and theoretical basis for strawberry cultivation.
Materials and methods
Plant materials and treatments
Different tissues (root, stem, leaf, flower, and ripe fruit) of strawberry (Fragaria×ananassa cv. Toyonaka) were collected to verify NADPH oxidase family genes' tissue‐specific expression. Meanwhile, the strawberry seedlings from current‐year stem tip were grown in 12 cm × 10 cm pots filled with a 1 : 1 (v/v) mixture of soil and perlite, subjected to two‐month routine management from March 2014 in the greenhouse of Sichuan Agricultural University. Subsequently, the potted seedlings with vigorous and uniform growth status were used in the following experiment. They were moved to growth chambers (RXZ‐260B) with controlled environmental conditions (25 ± 1 °C, 108 μmol·m−2·s−1·16 h·d−1, and 70 ± 5% relative humidity) for 2 weeks. After that, the plants were subjected to cold stress at 4 °C. The samples (leaves) were collected at 0, 6, 12, 24, 48, 72, and 96 h after cold stress treatment and prepared in triplicate.
Identification and annotation of FvRboh homologs in the strawberry genome
Ten protein sequences of Arabidopsis AtRbohA–J obtained from The Arabidopsis Information Resource (https://www.arabidopsis.org/) and simultaneously the Hidden Markov Model (HMM) profile of NADPH_Ox (PF08414) downloaded from the Pfam protein family database (http://pfam.sanger.ac.uk) were used as queries to search against the strawberry genome v.1.0 hybrid gene proteins. Subsequently, protein, gene, and cDNA sequences were all retrieved and examined using the National Center for Biotechnology Information (NCBI) BLAST tool with the default cutoff parameters.
Bioinformatics analysis
The exon–intron structure of the FvRbohs was identified using the online Gene Structure Display Server v.2.0 (http://gsds.cbi.pku.edu.cn/) based on alignments of their coding sequences with corresponding genomic sequences. The protein molecular weight, theoretical pI, instability, aliphatic index, and grand average of hydropathicity (GRAVY) index were obtained using ExPASy ProtParam tool (http://web.expasy.org/protparam/). Putative signal peptides and subcellular location were predicted in the SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/) and ProtComp v.9.0 (http://linux1.softberry.com/berry.phtml?topic=protcomppl&group=programs&subgroup=proloc). DNAMAN v.8.0 software was used to perform multiple sequence alignment of FvRboh protein amino acid sequences. Additionally, SMART program (http://smart.embl-heidelberg.de), NCBI CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml), TMHMM Server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/), and TMpred (http://www.ch.embnet.org/software/TMPRED_form.html) were applied to speculate EF‐hands, transmembrane domains (TMs), and conserved binding sites for flavin adenine dinucleotide (FAD), NAD pyrophosphate, and NADP ribose. Phylogenetic relationship of Rbohs between strawberry and other species was constructed using Clustal X v.2.0 and MEGA v.6.0 software with the neighbor‐joining (NJ) method under the Poisson model, and 1000 bootstrap test replicates would evaluate the reliability of interior branches. The basic sequence information for bioinformatics analysis was described in Appendix S1.
NADPH oxidase extraction and assays
A two‐phase aqueous polymer partition system was used to isolate the leaf plasma membranes. The purity of the plasma membrane was assessed by assaying the activities of marker enzymes (vanadate‐sensitive ATPase). A total of one milliliter reaction mixture contained 50 mm Tris/HCl buffer (pH 7.5), 0.5 mm XTT, 100 μm EDTA, 15–20 μg membrane protein, and 100 μm NADPH. After the addition of 100 μm NADPH, the reaction was monitored at 470 nm with absorbance coefficient of 21.6 mm −1·cm−1. The NADPH oxidase activity was assayed based on the reduction of XTT by radicals 25, 26. Corrections were made for background production in the presence of 50 units of SOD.
Expression profiles of FvRbohs
Total RNA was extracted from samples using the improved CTAB method 27. After quality assessment, 1 μg of total RNA was reverse‐transcribed into the complementary DNA (cDNA) with PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Japan) according to the manufacturer's instructions. The expression level of NADPH oxidase genes in different tissues and cold stress was determined by semiquantitative RT‐PCR and quantitative RT‐PCR, respectively. All quantitative real‐time PCRs were performed using SYBR Green Premix Ex Taq™ (Takara, Japan) on the CFX96 real‐time PCR system (Bio‐Rad, USA) in triplicate of each sample. Total 20 μL reaction contained 0.6 μL of each primer (10 μm), 10 μL SYBR Premix (Takara, Japan), 2 μL cDNA (10 ng) template, and 6.8 μL of RNase‐free water. Reaction protocol was set with two‐step cycling conditions: 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s, and 60 °C for 30 s. Fluorescence was monitored at the end of the annealing step each cycle. Melting curve was inserted, ramping from 65 °C to 95 °C (increment 0.5 °C/5 s) after the final cycle. The relative expression level was analyzed with the 2−▵▵Ct method. FaActin was used as the reference gene to standardize the raw data. Primers for Rbohs and Actin genes are listed in Table 1.
Table 1.
Primers for semiquantitative RT‐PCR and quantitative RT‐PCR
| Gene name | Forward primers (5′ to 3′) | Reverse primers (5′ to 3′) | Product size (bp) |
|---|---|---|---|
| FvRbohA | CTCGTCCAATAGTAGAATCC | ATTATTCTGAGAAGCAATCG | 172 |
| FvRbohB | GGATATGAGACAGTGAAGAT | GAAGTAATTGAGAACGGATG | 164 |
| FvRbohC | CCAGAAGATCATATCGGAGAAG | CTCGTTGTCGGAGTGATACT | 75 |
| FvRbohD | TGTTGATGACCATAGCATTC | AGGAGAGCGTAGACTATAAC | 142 |
| FvRbohE | TATAATGCTGAGTGCTTCTG | TGGTCTGCTATAGTCTATGTAA | 172 |
| FvRbohF | TGGCGACGAGCATGGATAGTTT | AGGGTTTCAGCAGCACCTTTGG | 143 |
| FvRbohH | TGCACGGTCTGCGCTTATTA | TCCGGCTTTGTGAGACAACA | 80 |
| FvActin | TTCACGAGACCACCTATAACTC | GCTCATCCTATCAGCGATT | 122 |
Determination of production rate and SOD enzyme activity
A total of 0.1 g of the leaf powder was homogenized in 1 mL ice‐cold potassium phosphate buffer (50 mm, pH 7.8) containing 1% (w/v) of polyvinylpolypyrrolidone (PVP). The homogenate was centrifuged at 10 000 × g for 10 min at 4 °C. The supernatant fraction was prepared for determination of content and SOD activity. Total soluble protein contents of the extracts were determined according to Bradford 28, and bovine serum albumin was used as a standard.
Superoxide anion () was determined according to the method of Cai et al. 29. The supernatant (0.5 mL) was mixed with 0.5 mL of 50 mm potassium phosphate buffer (pH 7.8) and 1 mL of 1 mm hydroxylamine hydrochloride and then incubated at 25 °C for 1 h. After incubation, 1 mL of 17 mm p‐aminophenylsulfonic acid (in glacial acetic acid/H2O (3 : 1)) and 1 mL of 7 mm α‐naphthylamine (in glacial acetic acid/H2O (3 : 1)) were added into the mixture for a further 20 min at 25 °C, followed by immediate measurement of absorbance at 530 nm. A standard curve with was used to calculate the production rate of from the chemical reaction of and hydroxylamine hydrochloride.
Total SOD (http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/15/1/1.html) activity was measured by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium chloride (NBT) 30, 31. The 3 mL of reaction mixture was comprised of 50 mm potassium phosphate buffer (pH 7.8), 13 mm methionine, 75 μm NBT, 2 μm riboflavin, 100 μm EDTA, and 100 μL of enzyme extract. Subsequently, the reaction mixtures were illuminated under light intensity of 80 μmol·m−2·s−1 at 25 °C for 15 min. One unit of SOD activity was defined as the amount of enzyme that was required to cause 50% inhibition of the reduction of NBT as monitored at 560 nm.
Lipid peroxidation
Lipid peroxidation was estimated by determining the malondialdehyde (MDA) contents in the leaves using the thiobarbituric acid method 32, 33. A total of 0.2 g of leaf samples was homogenized in 4 mL of 10% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 10 000 × g for 10 min. An aliquot of 2 mL supernatant was mixed with 2 mL of 0.5% (w/v) 2‐thiobarbituric acid (TBA) made in 10% TCA. The mixture was boiled at 100 °C for 10 min and then quickly cooled on ice. Samples were centrifuged at 10 000 × g for 10 min. The supernatant absorbance was monitored at 450, 532, and 600 nm, respectively, and MDA concentration was expressed as μmol·g−1 fresh weight.
Results
Identification and gene structure of Rboh homologs in the strawberry genome
A total of seven Rboh genes were identified and then named FvRbohA, FvRbohB, FvRbohC, FvRbohD, FvRbohE, FvRbohF, and FvRbohH according to conserved domain and multiple sequence alignment with Arabidopsis (Table 2). As shown, five genes except for FvRbohC and FvRbohH were all mapped to a specific chromosome (1, 5, and 6, respectively). The length of 9381‐bp FvRbohA and 12025‐bp FvRbohC genes was much longer than that of other members. In addition, the open reading frame length ranged from 2376 to 5598 bp and deduced protein sequence lengths varied from 791 to 1865 amino acids.
Table 2.
List of FvRbohs identified in strawberry
| Gen name | Gene ID | Chromosome | Location | Gene length (bp) | ORF length (bp) | Amino acid length (aa) |
|---|---|---|---|---|---|---|
| FvRbohA | gene31855 | chr5 | 1932913–1942293 | 9381 | 3084 | 1027 |
| FvRbohB | gene22214 | chr6 | 5063705–5067964 | 4260 | 2661 | 886 |
| FvRbohC | gene01814 | –a | –a | 12025 | 5598 | 1865 |
| FvRbohD | gene00215 | chr5 | 6658520–6662290 | 3771 | 2808 | 935 |
| FvRbohE | gene12928 | chr1 | 7411220–7415355 | 4136 | 2649 | 882 |
| FvRbohF | gene26084 | chr5 | 7980576–7984305 | 3730 | 2376 | 791 |
| FvRbohH | gene14024 | –a | –a | 4296 | 2598 | 865 |
Unplaced scaffold.
The unrooted phylogenetic tree showed that FvRboh genes were placed in two well‐resolved clades. Five members including FvRbohA, FvRbohB, FvRbohC, FvRbohD, and FvRbohE shared one clade, which indicated they might share a common evolutionary history. The other clade only contained FvRbohH and FvRbohF (Fig. 1A). The number of exons ranged from 10 in FvRbohD to 23 in FvRbohC. FvRbohF and FvRbohH owned 14 exons, while other genes had different number of exons. Although the order and approximate size of exons among the FvRbohs were relatively conserved, the length of introns was variable, which lead to a diversity of gene structures. In particular, FvRbohC and FvRbohA, respectively, contained one and four long introns consistent with their big size (Fig. 1B).
Figure 1.
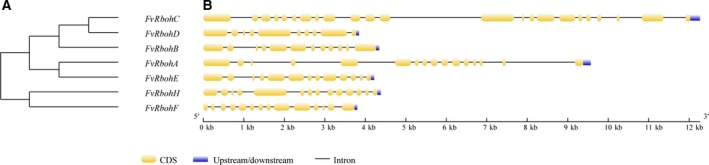
Phylogenetic tree (A) and exon–intron structures (B) of FvRboh genes. A phylogenetic tree of FvRboh genes was constructed with the neighbor‐joining method with 1000 bootstrap test replicates. Exons, introns, and untranslated regions (UTRs) are indicated by yellow boxes, gray horizontal lines, and blue boxes, respectively. The scale bar represents 12 kb.
Amino acid sequence and conserved motif analysis of FvRbohs
The relative molecular mass of the seven predicted FvRboh proteins ranged from 90.20 KDa (FvRbohF) to 206.97 KDa (FvRbohF), however, with similar corresponding isoelectric point around 9.0. Instability indexes higher than 40 showed that all family members of FvRbohs were unstable protein. Aliphatic indexes were predicted between 85.84 and 92.10. FvRbohs are inclined to be hydrophilic because grand average of hydropathy (GRAVY) negative values varied from −0.117 to −0.275. These proteins had no signal peptides, but contained six transmembrane helices (TMHs). Subcellular localization results indicated that most of the FvRbohs were localized to the plasma membrane, while only FvRbohC was computed to reside in the chloroplast thylakoid membrane (Table 3).
Table 3.
Protein properties of FvRbohs
| Protein | MW (kDa) | pI | Instability index | Aliphatic index | GRAVY | SignalP | TMHs | Location |
|---|---|---|---|---|---|---|---|---|
| FvRbohA | 116.25 | 8.94 | 50.16 | 86.83 | −0.255 | No | 6 | Plasma membrane |
| FvRbohB | 101.02 | 8.93 | 40.47 | 92.10 | −0.170 | No | 6 | Plasma membrane |
| FvRbohC | 206.97 | 8.88 | 46.45 | 88.53 | −0.240 | No | 6 | Chloroplast thylakoid membrane |
| FvRbohD | 105.46 | 9.14 | 40.47 | 85.84 | −0.275 | No | 6 | Plasma membrane |
| FvRbohE | 100.03 | 8.63 | 48.85 | 87.61 | −0.174 | No | 6 | Plasma membrane |
| FvRbohF | 90.20 | 8.74 | 50.13 | 85.94 | −0.117 | No | 6 | Plasma membrane |
| FvRbohH | 98.37 | 8.93 | 43.79 | 88.81 | −0.159 | No | 6 | Plasma membrane |
In addition, multiple sequence alignment of the seven FvRboh proteins was performed (Fig. 2), which demonstrated that these sequences were highly conservative and contained typical conserved domains of NADPH oxidase including two putative Ca2+‐binding EF‐hands and six transmembrane domains (TM1–6) in the N‐terminal region, and flavin adenine dinucleotide (FAD), NAD pyrophosphate, and NADP ribose conserved binding sites in the C‐terminal region. To further reveal the structural diversity and function prediction of FvRboh proteins, a total of eight conserved motifs were identified using online MEME tool and annotated based on Pfam, NCBI CDD, and PROSITE databases (Fig. 3). All seven FvRbohs shared eight motifs once, except for motif 3 in FvRbohH. Moreover, the order and distribution of the eight motifs in these proteins were almost the same as described above (Fig. 3A). Motif 1 was annotated as FAD‐binding domain; motifs 2, 6, and 8 were annotated as NAD‐binding region; and motif 5 was annotated as EF‐hands (Fig. 3B).
Figure 2.

Protein sequence multi‐alignment and domain structure of the Rbohs from strawberry. Conservative residues are highlighted by black shadings, and a lower level of conservations is indicated by lighter shadings. EF‐hands and conserved binding sites for FAD, NAD pyrophosphate, and NADP ribose are represented by straight lines. Transmembrane domains are indicated by boxes.
Figure 3.
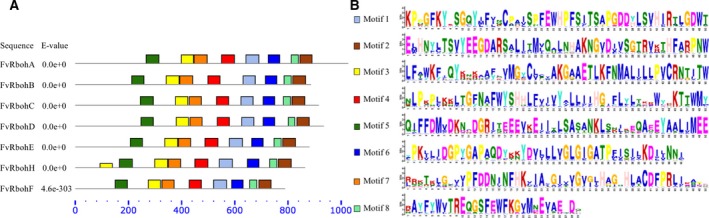
Conserved motifs distribution (A) and sequence (B) in FvRboh proteins. Motif of the FvRboh family members in strawberry was analyzed using the MEME web server. Different color boxes represent eight putative motifs, respectively. Names of all family members and E‐values are shown on the left side. The blue dotted line scale represents the length of amino acid. The height of a letter indicates its relative frequency at the given position in the motif.
Phylogenetic analysis of Rbohs in different plants
To analyze phylogenetic relationship of NADPH oxidase family members in strawberry, grape, Arabidopsis, and rice, the amino acid sequences of 33 Rboh proteins, including 7 from strawberry, 7 from grape, 10 from Arabidopsis, and 9 from rice, were aligned and used to construct an unrooted phylogenetic tree with the neighbor‐joining method (Fig. 4). The results indicated that 33 Rbohs could be assigned to five distinct subgroups (I, II, III, IV, and V). FvRbohC and FvRbohD were divided into the subgroup I. FvRbohF and FvRbohH were classified into the subgroup V. FvRbohA, FvRbohB, and FvRbohE belonged to subgroups IV, II, and III, respectively. Notably, FvRbohs were clustered together with grape or Arabidopsis Rbohs first, suggesting that strawberry Rboh proteins were more closely related to those from grape and Arabidopsis than to those of rice, which was consistent with the fact that strawberry, grape, and Arabidopsis are eudicots and diverged more recently from a common ancestor than from the lineage leading to monocots.
Figure 4.
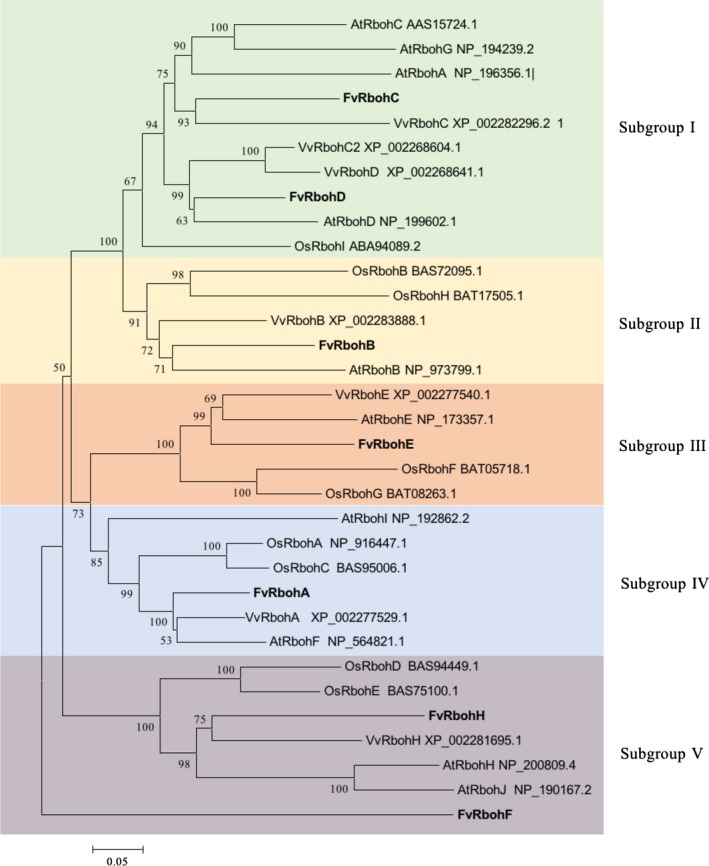
Phylogenetic analysis of Rbohs in strawberry, grape, Arabidopsis, and rice. The phylogenetic tree was constructed with the neighbor‐joining method based on the Poisson model. Tree reliability was assessed using 1000 bootstrap replicates. The numbers indicated for each clade represent bootstrap support values given as percentages. Five subgroups are shown as I, II, III, IV, and V.
NADPH oxidase activity and expression patterns of FvRbohs in response to cold stress
Expression of Rbohs in plant as previously reported was tissue‐specific. In our study, the results indicated FvRbohA, FvRbohC, FvRbohD, and FvRbohF could be detected in root, stem, leaf, flower, and fruit. FvRbohB and FvRbohE were expressed in root, stem, flower, and fruit, while FvRbohH was only observed in flower and fruit (Fig. 5A). Exposure to cold stress, NADPH oxidase activity in strawberry leaves showed a tendency: increased quickly and peaked at 48 h, and then had a decrease. Although the enzyme activity decreased in the late phase of treatment, it was still higher than that of the initial phase (25 °C). The relative expression levels of four members (FvRbohA, FvRbohC, FvRbohD, and FvRbohF) that could be detected in strawberry leaves were examined to explore their response to cold stress by real‐time qPCR analysis. As shown, FvRbohA and FvRbohD were keeping high expression levels, while FvRbohC and FvRbohF were difficult to be tested during cold treatment. Generally, the expression patterns of FvRbohA and FvRbohD were observed to be M‐shaped. The transcript abundances of RbohA and RbohD were highly induced and reached the maximum value after the first 6‐h low‐temperature exposure (Fig. 5B), so the peak value showed up earlier than that of NADPH oxidase activity.
Figure 5.
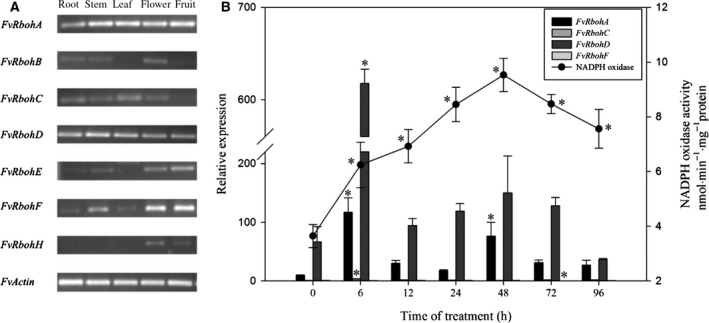
Expression patterns of FvRbohs in different tissues (A) and response to cold stress (B). The left and right Y‐axis is the scale of the relative transcript abundance level and NADPH oxidase activity, respectively. The X‐axis is the time course of 4 °C cold treatment. Values represent means ± standard error from three biological experiments (n = 3). Asterisks indicate significant differences based on one‐way ANOVA in SPSS 23.0 followed by the Dunnett t‐test (time 0 as the control, P < 0.05).
production rate, SOD enzyme activity, and lipid peroxidation
production rate increased slowly at the initial stage of cold stress and then followed a transient burst. After 72 h, a slight decrease in production occurred (Fig. 6A). The change trend of production rate was almost coinciding with NADPH oxidase activity. SOD activity had a quick increase and reached the peak value at 24 h, which inhibited the production. However, at the late stage of cold stress, SOD activity began to decrease (Fig. 6B). The MDA accumulation was often used as an indicator of lipid peroxidation. In general, the successive cold stress for 96 h caused a linear increase in MDA content (Fig. 6C).
Figure 6.
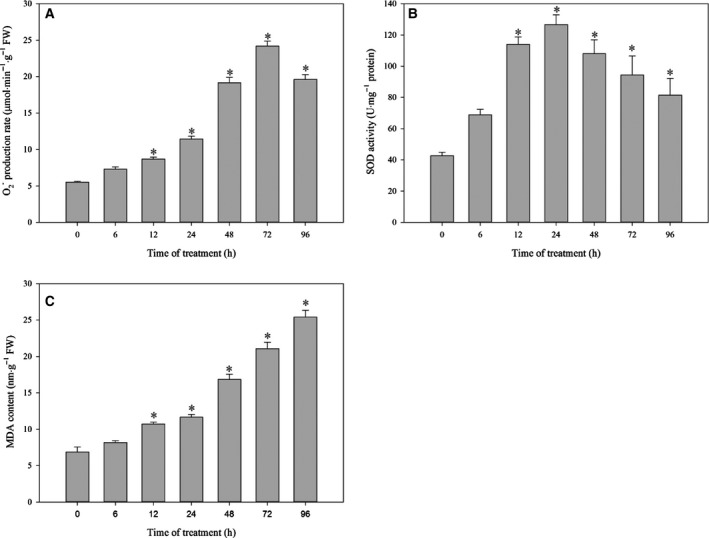
production rate (A), SOD enzyme activity (B), and lipid peroxidation (C). Each value represents mean ± standard error in histogram. Values represent means ± standard error from three biological experiments (n = 3). Asterisks indicate significant differences based on one‐way ANOVA in SPSS 23.0 followed by the Dunnett t‐test (time 0 as the control, P < 0.05).
Discussion
It was well known that ROS was toxic to biological organisms by oxidizing lipids, proteins, DNA, and carbohydrates, resulting in breakdown of normal cellular, membrane, and reproductive functions 34, but ROS when its concentration is in the appropriate range can act as a signal molecule to influence cell growth, organ development, and defense responses. In plant, NADPH oxidase‐catalyzed conversion of the superoxide anion () to other ROS, such as hydrogen peroxide, hydroxyl radicals, and perhydroxyl radicals, was the major source of ROS production 26. NADPH oxidase was coded by a small gene family of Rbohs. Benefiting from the availability of whole‐genome sequence in recent years, so far, a few model and crop plant NADPH oxidase families have been identified at the genomewide level, including 10 in Arabidopsis 1, 9 in rice 35, 6 in barley 36, 9 in apple 37, and 7 in grape 38. However, very little is known about this family in strawberry.
This study comprehensively identified and characterized seven FvRboh genes from strawberry at the genomewide level, which are mainly distributed in chromosomes 1, 5, and 6. Gene structure analysis showed FvRbohs included at least ten exons; FvRbohC had the most exons of 23, which was greatly surpassed that in other plants. Actually, the majority of Rbohs harbored 10–14 exons in Arabidopsis, rice, barley, and grape, except that AtRbohD contained eight exons and OsRbohD, VvRbohB, and VvRbohD had 15 exons in their coding DNA sequence (CDS) 39. The large structural variation of Rboh genes implied the significant genomic change during their evolutionary history, possibly as a result of highly diverse distribution and insertion of intronic regions amid the exonic sequences. In addition, FvRbohC protein was predicated to localize to the thylakoid membrane of the chloroplast, while other members were computed to localize to the plasma membrane, indicating the different functions. This case was also reported in the grape 38, in contrast to Arabidopsis and rice, where all the Rbohs were predicted to localize to the plasma membrane. The alignment of seven FvRbohs clearly showed the presence of two EF‐hands, six TMs, FAD, and NAD‐binding sites, which were known to be present in Rbohs from other plant species. EF‐hand regions that were absent from the mammalian phagocyte gp91phox protein can bind Ca2+, accounting for the direct regulation of plant Rbohs by Ca2+ 1, 14, 40. Thus, the regulation way of NADPH oxidases in plant might be different from that in mammalian phagocytes. Subsequently, the characterization and distribution of conserved motifs furtherly emphasized the importance of these structures. The phylogenetic tree of Rboh proteins from different plant species showed FvRbohs had a closer correlation with VvRbohs and AtRbohs than those from monocotyledonous rice, indicating the genes were established prior to the divergence of the corresponding taxonomic lineages. Moreover, those homologs clustered in the same group were possible to be involved in similar functions; however, further experimental analyses are necessary to confirm this.
The tissue‐specific expression patterns of Rboh genes have been reported in some species; however, there is no uniform expression pattern for the plant Rboh genes reported. In grape, seven VvRboh genes could be detected in all tissues involved in young leaves, roots, stems, inflorescences, berries, tendrils, and ovules 38, while four of the six and two of the ten Rboh genes had widespread constitutive spatial expression patterns in barley and Arabidopsis, respectively 1, 36. In our study, FvRbohA, FvRbohC, FvRbohD, and FvRbohF were tested in all tissues; FvRbohA and FvRbohD were the highly expressed genes. FvRbohB and FvRbohE had transcript abundances in most of the tissues except for leaves, while FvRbohH was expressed only in flower and fruit. These findings might suggest tissue‐specific function of these family members.
It has been documented that low temperature, as many other stressful environmental conditions, could trigger enhanced generation of ROS to disrupt cellular homeostasis. But ROS when its concentration is in the appropriate range can act as a signal molecule to initiate defense responses 41. Plasma membrane, peroxisomes, chloroplasts, and mitochondria are potential sources of ROS in plant cells 42. The plasma membrane‐located NADPH oxidases have been shown to mainly involve in ROS production and play critical roles in plant development and defense responses 15, 43. Our study showed that NADPH oxidase activity increased sharply at the early stage of strawberry seedlings exposed to cold stress and then had a slight decline, but still kept high level, compared to 0 h (25 °C), accompanied by the production of the superoxide anion. To alleviate the negative effects of stress and balance the ROS level, organism would initiate enzymatic and nonenzymatic mechanisms to maintain the cellular redox homeostasis. SOD was involved in dismutation of to H2O2, and its activity reflected the ability to adapt to stress in plant. In our study, SOD activity increased at the initial treatment and then decreased, which demonstrated that short‐term cold stress could induce antioxidative defense mechanism in the plant. However, the longer the stress time was, the more ROS produced, so that antioxidative enzyme activity was impaired and the ROS could not be removed effectively. Subsequently, peroxidation of lipids in the cell membrane resulted in a massive MDA accumulation, which was toxic to plant 44. Correspondingly, the Rboh genes (FvRbohA, FvRbohC, FvRbohD, and FvRbohF) which were specifically expressed in leaves were monitored during the cold treatment. FvRbohA and FvRbohD reacted quickly to cold stress by improving transcript levels, while FvRbohC and FvRbohF were keeping a low expression level during this process. CsRbohA was upregulated by low temperature in short time, which was consistent with our findings. AtRbohD in Arabidopsis thaliana was constitutively and ubiquitously expressed and showed a high degree of stress responsiveness 45, 46. Recently, it was reported that the expression level of AtRbohD had an extremely significant increase at an early stage in the hypoxia response. AtRbohD‐knockout mutant was used to further demonstrate that AtRbohD played a key role in regulating the transcript abundance of downstream hypoxia‐inducible genes at an early stage during hypoxic stress and could be a cross talk in ethylene modulating H2O2 signal transduction in the hypoxia response pathway 22. These facts hinted that FvRbohA and FvRbohD might be the dominate induction of antioxidant defense system in response to cold stress.
Conclusion
In this study, we totally identified seven Rboh genes from the strawberry genome and revealed classification, gene structure, evolution, conserved protein motif, and phylogenetic relationship by systematical bioinformatic analysis. NADPH oxidase was related to initiation of antioxidant system in leaves of strawberry against cold stress by regulating production. Expression profile analysis among different tissues (root, stem, leaf, flower, and fruit) showed that FvRboh genes had the tissue‐specific characteristic. Furthermore, FvRbohA and FvRbohD maintained the high expression in response to cold stress, implying that they played a crucial role in this process. This information provides some insights into potential functions of strawberry Rbohs and increases our understanding of the molecular basis of the acquired cold tolerance of strawberry and even the adaptability of strawberry to other stress conditions.
Author contributions
YTZ and HRT conceived and designed the experiment. YTZ, YLL, and YWH performed the experiment and analyzed the data. WJH collected the samples. YTZ wrote the manuscript. YZ, XRW, and HRT reviewed drafts of the manuscript. All authors read and approved the final version.
Supporting information
Appendix S1. Basic sequence information for bioinformatics analysis.
References
- 1. Sagi M and Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quinlan CL, Treberg JR, Perevoshchikova IV, Orr AL and Brand MD (2012) Native rates of superoxide production from multiple sites in isolated mitochondria measured using endogenous reporters. Free Radic Biol Med 53, 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandes RP, Weissmann N and Schroder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76, 208–226. [DOI] [PubMed] [Google Scholar]
- 4. Vignais PV (2002) The superoxide‐generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59, 1428–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quinn MT and Gauss KA (2004) Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 76, 760–781. [DOI] [PubMed] [Google Scholar]
- 6. Mizrahi A, Berdichevsky Y, Ugolev Y, Molshanski‐Mor S, Nakash Y, Dahan I, Alloul N, Gorzalczany Y, Sarfstein R, Hirshberg M et al (2006) Assembly of the phagocyte NADPH oxidase complex chimeric constructs derived from the cytosolic components as tools for exploring structure‐function relationships. J Leukoc Biol 79, 881–895. [DOI] [PubMed] [Google Scholar]
- 7. Panday A, Sahoo MK, Osorio D and Batra S (2015) NADPH oxidases: an overview from structure to innate immunity‐associated pathologies. Cell Mol Immunol 12, 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma P, Jha AB, Dubey RS and Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012, 1–26. [Google Scholar]
- 9. Reczek CR and Chandel NS (2015) ROS‐dependent signal transduction. Curr Opin Cell Biol 33, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foyer CH and Noctor G (2009) Redox regulation in photosynthetic organisms‐signaling, acclimation, and practical implications. Antioxid Redox Sign 11, 861–905. [DOI] [PubMed] [Google Scholar]
- 11. Del Rio LA (2015) ROS and RNS in plant physiology: an overview. J Exp Bot 66, 2827–2837. [DOI] [PubMed] [Google Scholar]
- 12. Liu N and Lin Z (2014) Reactive oxygen species and relative enzyme activities in the development of aerial roots of Chinese Banyan (Ficus microcarpa). J Plant Growth Regul 33, 160–168. [Google Scholar]
- 13. Torres MA and Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8, 397–403. [DOI] [PubMed] [Google Scholar]
- 14. Torres MA, Onouchi H, Hamada S, Machida C, Hammond‐Kosack KE and Jones JDG (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 14, 365–370. [DOI] [PubMed] [Google Scholar]
- 15. Marino D, Dunand C, Puppo A and Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17, 9–15. [DOI] [PubMed] [Google Scholar]
- 16. Potocký M, Jones MA, Bezvoda R, Smirnoff N and Žárský V (2007) Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 174, 742–751. [DOI] [PubMed] [Google Scholar]
- 17. Dunand C, Crevecoeur M and Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174, 332–341. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Chen C, Zhang D, Li H, Li P and Ma F (2014) Reactive oxygen species produced via plasma membrane NADPH oxidase regulate anthocyanin synthesis in apple peel. Planta 240, 1023–1035. [DOI] [PubMed] [Google Scholar]
- 19. Yi C, Yao K, Cai S, Li H, Zhou J, Xia X, Shi K, Yu J, Foyer CH and Zhou Y (2015) High atmospheric carbon dioxide‐dependent alleviation of salt stress is linked to RESPIRATORY BURST OXIDASE 1 (RBOH1)‐dependent H2O2 production in tomato (Solanum lycopersicum). J Exp Bot 66, 7391–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xia XJ, Gao CJ, Song LX, Zhou YH, Shi K and Yu JQ (2014) Role of H2O2 dynamics in brassinosteroid‐induced stomatal closure and opening in Solanum lycopersicum . Plant, Cell Environ 37, 2036–2050. [DOI] [PubMed] [Google Scholar]
- 21. Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen Z and Yu JQ (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65, 4371–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang CY and Hong CP (2015) The NADPH oxidase Rboh D is involved in primary hypoxia signalling and modulates expression of hypoxia‐inducible genes under hypoxic stress. Environ Exp Bot 115, 63–72. [Google Scholar]
- 23. Steffens B and Sauter M (2009) Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 21, 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orozco‐Cárdenas ML, Narváez‐Vásquez J and Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13, 179–191. [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou J, Wang J, Shi K, Xia XJ, Zhou YH and Yu JQ (2012) Hydrogen peroxide is involved in the cold acclimation‐induced chilling tolerance of tomato plants. Plant Physiol Biochem 60, 141–149. [DOI] [PubMed] [Google Scholar]
- 26. Sagi M and Fluhr R (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Q, Yu HW, Wang XR, Xie XL, Yue XY and Tang HR (2012) An alternative cetyltrimethylammonium bromide‐based protocol for RNA isolation from blackberry (Rubus L.). Genet Mol Res 11, 1773–1782. [DOI] [PubMed] [Google Scholar]
- 28. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 29. Cai C, Chen K, Xu W, Zhang W, Li X and Ferguson I (2006) Effect of 1‐MCP on postharvest quality of loquat fruit. Postharvest Biol Tec 40, 155–162. [Google Scholar]
- 30. Ali B, Tao Q, Zhou Y, Gill RA, Ali S, Rafiq MT, Xu L and Zhou W (2013) 5‐Aminolevolinic acid mitigates the cadmium‐induced changes in Brassica napus as revealed by the biochemical and ultra‐structural evaluation of roots. Ecotoxicol Environ Saf 92, 271–280. [DOI] [PubMed] [Google Scholar]
- 31. Beauchamp C and Irwin F (1971) Superoxide dismutase‐ improved assays and an assay applicable to acrylamide gels. Anal Biochem 44, 276–287. [DOI] [PubMed] [Google Scholar]
- 32. Zheng X and Tian S (2006) Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem 96, 519–523. [Google Scholar]
- 33. Shi H, Ye T and Chan Z (2013) Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 71, 226–234. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez R and Redman R (2005) Balancing the generation and elimination of reactive oxygen species. Proc Natl Acad Sci USA 102, 3175–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T et al (2007) Regulation of rice NADPH oxidase by binding of Rac GTPase to its N‐terminal extension. Plant Cell 19, 4022–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lightfoot DJ, Boettcher A, Little A, Shirley N and Able AJ (2008) Identification and characterisation of barley (Hordeum vulgare) respiratory burst oxidase homologue family members. Funct Plant Biol 35, 347–359. [DOI] [PubMed] [Google Scholar]
- 37. Cepauskas D, Miliute I, Staniene G, Gelvonauskiene D, Stanys V, Jesaitis AJ and Baniulis D (2015) Characterization of apple NADPH oxidase genes and their expression associated with oxidative stress in shoot culture in vitro . Plant Cell Tiss Org 124, 621–633. [Google Scholar]
- 38. Cheng C, Xu X, Gao M, Li J, Guo C, Song J and Wang X (2013) Genome‐wide analysis of respiratory burst oxidase homologs in grape (Vitis vinifera L.). Int J Mol Sci 14, 24169–24186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaur G and Pati PK (2016) Analysis of cis‐acting regulatory elements of Respiratory burst oxidase homolog (Rboh) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput Biol Chem 62, 104–118. [DOI] [PubMed] [Google Scholar]
- 40. Keller T, Damude HG, Werner D, Doerner P, Dixon RA and Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mittler R, Vanderauwera S, Gollery M and Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9, 490–498. [DOI] [PubMed] [Google Scholar]
- 42. Gill SS and Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48, 909–930. [DOI] [PubMed] [Google Scholar]
- 43. Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. [DOI] [PubMed] [Google Scholar]
- 44. Luo Y, Tang H and Zhang Y (2011) Production of reactive oxygen species and antioxidant metabolism about strawberry leaves to low temperatures. J Agr Sci 3, 89. [Google Scholar]
- 45. Suzuki N, Miller G, Morales J, Shulaev V, Torres MA and Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14, 691–699. [DOI] [PubMed] [Google Scholar]
- 46. Liu Y and He C (2016) Regulation of plant reactive oxygen species (ROS) in stress responses: learning from AtRBOHD. Plant Cell Rep 35, 995–1007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Basic sequence information for bioinformatics analysis.


