SUMMARY
Objective
X-linked nephrogenic diabetes insipidus (XNDI), caused by mutations in the V2 vasopressin receptor (V2R), is clinically distinguished from central diabetes insipidus (CDI) by elevated serum vasopressin (AVP) levels and unresponsiveness to 1-desamino-8-D-arginine vasopressin (DDAVP). We report two infants with XNDI, and present the characterization and functional rescue of a novel V2R mutation.
Patients
Two male infants presented with poor growth and hypernatremia. Both patients had measurable pre-treatment serum AVP and polyuria that did not respond to DDAVP, suggesting NDI. However, both also had absent posterior pituitary bright spot on MRI, which is a finding more typical of CDI.
Methods
The AVPR2 gene encoding V2R was sequenced. The identified novel missense mutation was re-created by site-directed mutagenesis and expressed in HEK293 cells. V2R activity was assessed by the ability of transfected cells to produce cAMP in response to stimulation with DDAVP. Membrane localization of V2R was assessed by fluorescence microscopy.
Results
Patient 1 had a deletion of AVPR2; patient 2 had the novel mutation L57R. In transiently transfected HEK293 cells, DDAVP induced detectable but severely impaired L57R V2R activity compared to cells expressing wild-type V2R. Fluorescence microscopy showed that myc-tagged wild-type V2R localized to the cell membrane while L57R V2R remained intracellular. A non-peptide V2R chaperone, SR121463, partially rescued L57R V2R function by allowing it to reach the cell membrane.
Conclusions
L57R V2R has impaired in vitro activity that can be partially improved by treatment with a V2R chaperone. The posterior pituitary hyperintensity may be absent in infants with XNDI.
Keywords: diabetes insipidus, nephrogenic, posterior pituitary hyperintensity, bright spot, pharmacological chaperone
Introduction
Diabetes insipidus (DI), the inability to reabsorb free water, results in polyuria, hypo-osmolar urine and hypernatremia. DI can be central (CDI), due to deficiency of arginine vasopressin (AVP), or nephrogenic (NDI), due to a defect in AVP action in the kidneys. 1 CDI results from the destruction or abnormal development of the neurons that produce AVP, usually due to hypothalamic tumors, surgery, trauma, congenital malformations or autoimmune, infiltrative, metastatic, inflammatory or infectious processes. Very rarely, mutations in the vasopressin-neurophysin II gene can also cause CDI. 2
NDI is genetic or acquired. The genetic causes are X-linked (mutations in the AVPR2 gene) or autosomal (mutations in the AQP2 gene). 3 X-linked NDI (XNDI) accounts for about 90% of the genetic cases of NDI. AVPR2, located on chromosome Xp28, encodes the V2 vasopressin receptor (V2R), a G-protein-coupled receptor (GPCR) expressed in renal collecting duct cells. AVP acts via V2R to facilitate insertion of aquaporin-2 channels into the apical membrane of collecting duct cells and allow reabsorption of free water. 3 While most AVPR2 mutations compromise V2R function and cause XNDI, rare activating mutations of V2R, causing the syndrome of inappropriate antidiuresis, also occur. 4 Of the 211 reported AVPR2 mutations causing XNDI, approximately half are missense, and 31 of these have been characterized functionally. 5 Most V2R missense mutations result in a translated but misfolded protein that remains trapped in the endoplasmic reticulum. 6–8 Pharmacological chaperones can partially rescue the cell-surface expression and functional activity of misfolded mutant V2 receptors that would otherwise be targeted for degradation. 9–12
Among infants, CDI is far more common than NDI. The differential diagnosis of CDI includes trauma, midline defect, intracranial mass (germinoma, craniopharyngioma) and infiltrative process (Langerhans cell histiocytosis). 13 Therefore, magnetic resonance imaging (MRI) of the brain is indicated when CDI is suspected. MRI findings suggesting CDI include absent posterior pituitary hyperintensity (bright spot)14–17 and pituitary stalk thickening. 14, 18 Absent bright spot in the context of DI suggests a central cause. 19, 20 However, in a radiologic study the bright spot varied temporally and was absent in 8 to 25% of normal adults; 21 and conversely, isolated reports show that a bright spot can be present at the initial diagnosis of CDI. 22–24
Materials and Methods
Case Reports
Patient 1
A 2.8 kg newborn male gained weight normally for 4 months (50th percentile), but by 6 months was below the 3rd percentile and had delayed motor milestones. He was transferred to our institution with weight 5.4 kg (−2.5 SD) and serum Na 156 to 170 mEq/L despite IV hydration. At admission his serum Na was 163 mEq/L, K 4.9 mEq/L, Cl 128 mEq/L, serum osmolality was 339 mOsm/kg (normal 285–293) while urine osmolality was 275 mOsm/kg (normal 300–900), and urine output was high (12–35 ml/kg/h). Treatment with 0.025 µg/kg subcutaneous DDAVP caused a decreased urine output (8–10 ml/kg/h) in the first 24 hours, suggesting CDI. Brain MRI showed absent posterior pituitary hyperintensity on T1 weighted images (Figure 1). However, subsequently the urine output remained high and unresponsive to subcutaneous DDAVP, despite titration of the dose up to 0.1 µg/kg. Serum AVP was 5.1 pg/ml (normal range 1.0–13.3) when Na was 156 mEq/L. Within 48 hours of treatment with chlorothiazide (20 mg/kg/d) and indomethacin (2 mg/kg/d), urine output decreased to 2–5 ml/kg/h, serum Na normalized and oral intake improved. Repeat MRI six weeks later showed a normal posterior pituitary bright spot (Figure 1).
Figure 1.
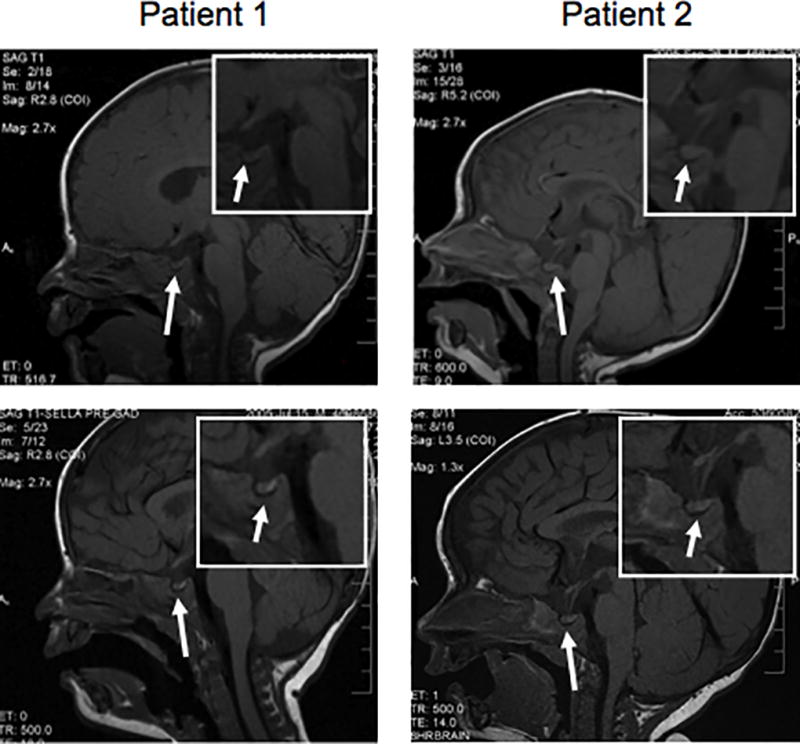
Pre-contrast sagittal T1-weighted images of the brain demonstrate a normal-appearing pituitary gland. No posterior pituitary bright spot is seen within the pituitary gland or in an ectopic location. The infundibulum demonstrates a normal thickness without evidence of abnormal enhancement after contrast administration. The suprasellar cistern is unremarkable. The hypothalamus demonstrates no evidence of mass. Insets are higher magnification of the sella. Lower panels show recurrence of the posterior pituitary bright spot in each patient, 6 weeks and 3 months after the initial diagnosis, respectively.
Patient 2
A 3.4 kg (25th percentile) newborn male presented at 7 months with weight 6 kg (−2.5 SD), hypotonia and gross motor delays. At admission his serum Na was 152 mEq/L, K 4.1 mEq/L, Cl 116 mEq/L. Serum osmolality was 308 mOsm/kg while urine osmolality was 158 mOsm/kg, and urine output was high (8–12 ml/kg/h). Polyuria and hypotonic urine with hypernatremia established the diagnosis of DI. The patient’s physical examination was noteworthy for frontal bossing, prominent occiput and a full anterior fontanelle. Therefore, a central etiology for DI was suspected, and brain MRI was performed to search for intracranial pathology. Hourly urine osmolalities remained low (109, 143 and 93 mOsm/kg) despite increasing doses of subcutaneous DDAVP, titrated from 0.025 to 0.1 µg/kg. The patient had two healthy older brothers. Serum AVP was 16.9 pg/ml (normal 1.0 to 13.3) when serum Na was 151 mEq/L and brain MRI showed absent posterior pituitary hyperintensity on T1 weighted images (Figure 1). Within 48 hours of treatment with chlorothiazide (20 mg/kg/d) and indomethacin (2 mg/kg/d), urine output decreased to 4–6 ml/kg/h. At discharge and subsequent follow-up, the patient had normal weight gain, serum Na, and developmental milestones. Repeat MRI three months after diagnosis showed a faintly visible posterior pituitary bright spot (Figure 1). The AVPR2 gene from both patients was sequenced (Quest Diagnostics, San Juan Capistrano, CA)
Generation of V2R constructs
An N-terminal-c-Myc-tagged wild-type (WT) V2R construct was made by PCR amplification of human V2R cDNA 25 with primers Forward: 5’ GCGAATTCGCCACC ATGGAACAAAAGCTAATAAGCGAAGAAGACCTAATGCTCATGGCGTCCACCACT 3’ and Reverse: 5’ATAGTTTAGCGGCCGCTCACGATGAAGTGT CCTTGGC 3’ and subcloned into pcDNA3.1 (Invitrogen). L57R was introduced into the WT V2R construct by oligonucleotide-mediated site-directed mutagenesis and verified by sequencing.
Cell culture and transient transfection
HEK293 cells (American Tissue Culture Collection) were grown in modified Eagle’s medium (MEM) with 10% calf serum, 2 mM L-glutamine, non-essential amino acids and antibiotics. Cells were transiently transfected in 6-well plates using Effectene (Qiagen), 45 ng/well for cAMP-inducible luciferase-expressing plasmid (pCREluc), 26 450 ng/well for WT V2R, L57R V2R or control, and 5 ng/well for Renilla luciferase expression plasmid (pRL-RSV) (Promega). Control DNA was pcDNA3.1 expressing β-galactosidase.
DDAVP dose response and treatment with a pharmacological chaperone
Cells were split 24h after transfection into 96-well plates for stimulation with DDAVP and into 48-well plates for the Renilla luciferase assays, then incubated for 24 h with or without 10−6 M SR121463 ((1-[4-(N-tert-butylcarbamoyl)-2-methoxybenzenesulfonyl]-5-ethoxy-3-spiro-[4-(2-morpholinoethoxy)cyclohexane]indol-2-one) cis-isomer, molecular weight 759.8) (Sanofi Recherche, France). 27, 28 After washing × 3 in MEM with 0.1 mg/ml bovine serum albumin, cells were incubated for 24 h in the same medium containing 0.25 mM isobutylmethylxanthine, and either 0, 10−13 to 10−7 M DDAVP or 1mM 8Br-cAMP. Luciferase activity was normalized to Renilla luciferase activity and expressed as percentage of maximum DDAVP-stimulated WT V2R activity. 26 Best-fit estimates of EC50 and 95% confidence intervals were obtained by nonlinear regression fitting of the sigmoidal dose response curves using GraphPad Prism 4.0 software (San Diego, CA). Statistical significance was determined by Unpaired t-test or ANOVA with Tukey’s Multiple Comparison Test as appropriate.
Membrane localization studies
HEK293 cells were transiently cotransfected with 250 ng myc-tagged WT or L57R V2R and 250 ng pIRES2 expressing a fluorescent protein mCherry farnesyl (generated by fusing the hRas farnesylation site to the C-terminus of mCherry 29 in pIRES2 (Clontech)). Cells were plated on chamber slides (Lab-Tek II, Nalge Nunc International) coated with poly-L-lysine and rat tail collagen, and incubated with or without 10−6 M SR121463 at 37 °C in 5% CO2. After 48h, the nearly confluent cells were fixed with 4% paraformaldehyde for 20 min at 4° C, washed twice with phosphate-buffered saline (PBS), incubated with 1:50 dilution of 200 µg/ml of the c-Myc mouse monoclonal antibody (9E10):sc-40 (Santa Cruz Biotechnology) at room temperature for 2 h, washed three times with PBS, biotinylated with 1:500 anti-mouse IgG Reagent (Vector MOM Kit) for 20 min, washed with PBS and incubated with 1:500 of 2 mg/ml Avidin Fluorescein conjugate (Invitrogen Molecular Probes) for 1 h. After washing with PBS, cells were mounted and imaged with Zeiss Confocal Fluorescence Microscope (LSM510).
Results
Clinical Studies
Both patients presented with hypernatremia and serum hyperosmolality with dilute urine, the classic signs of DI. Neither had a posterior pituitary bright spot, suggesting CDI; however, both had measurable serum AVP levels and did not respond to DDAVP, suggesting NDI. The diagnosis of XNDI was confirmed in patient 1 by the presence of an X-chromosome deletion encompassing AVPR2. The patient’s family refused further study to determine the endpoints of the patient’s deletion, and declined genetic screening to determine whether the patient’s mother is a carrier of the deletion. The novel missense V2R mutation L57R in patient 2 suggested the diagnosis of XNDI; hence we studied the functional significance of this mutation. This patient’s mother declined screening to determine whether she is a carrier of the L57R mutation.
Functional Study of L57R V2R
Since V2R is a stimulatory GPCR, intracellular cAMP increases when V2R is bound by ligand such as vasopressin or DDAVP. Therefore, we measured cAMP-inducible luciferase activity as an index of cAMP generated in response to DDAVP. Compared to WT control, L57R significantly impaired V2R activity (P < 0.001) (Figure 2). When maximally stimulated with 10−7 M DDAVP, L57R V2R had about 40% of the activity of WT V2R. The dose of DDAVP that induced a half-maximal response (EC50) by the L57R mutant was 2.61 × 10−9 M, more than two orders of magnitude greater than that of WT (1.77 × 10−11 M) (P = 0.002) (Figure 3).
Figure 2.
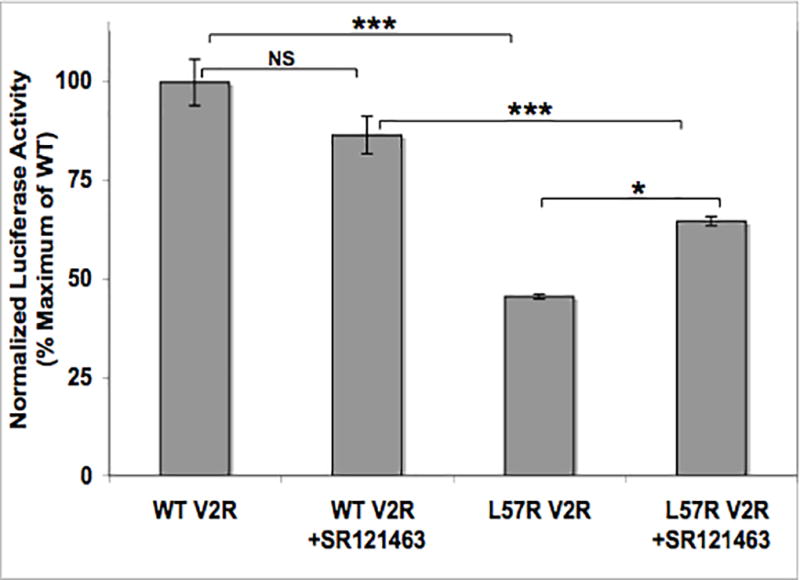
Maximum DDAVP-stimulated receptor activities. HEK293 cells expressing cAMP-inducible luciferase, Renilla luciferase and WT or L57R V2R were treated with 10−7 M DDAVP with and without 10−6 M SR121463. Luciferase activity, a measure of intracellular cAMP generation, is normalized to Renilla luciferase activity and expressed as percentage of maximum WT V2R activity. Data are mean +/− SEM of 2 experiments, each performed in triplicate. NS = not significant; * = P < 0.05; *** = P < 0.001.
Figure 3.
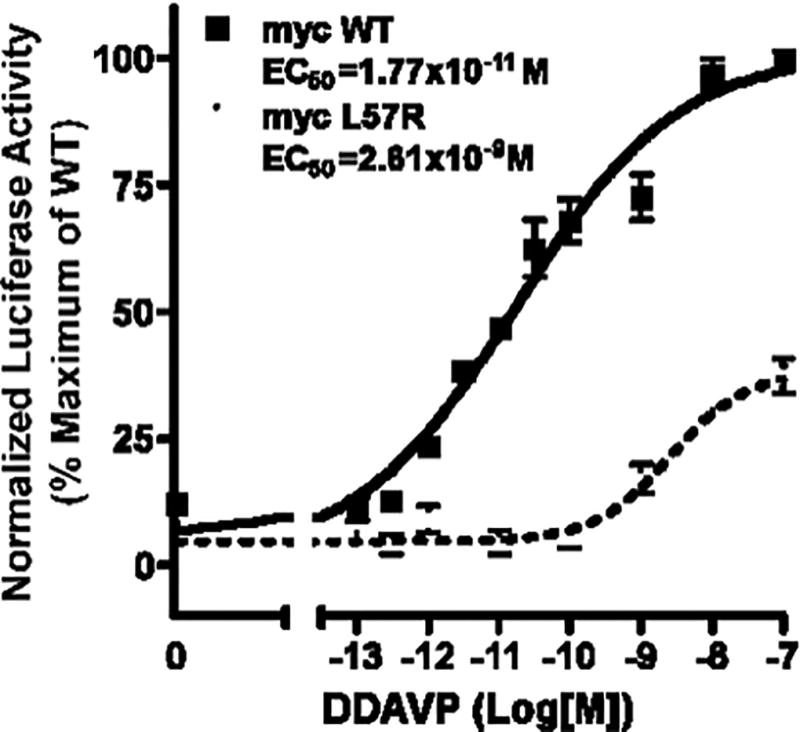
Dose-response curves. Basal (no DDAVP) and stimulated (10−13 to 10−7 M DDAVP) WT V2R and L57R V2R activities expressed as percentage of maximum WT V2R activity are plotted versus log of DDAVP concentration. The EC50 values for WT and mutant are significantly different (P = 0.002). Each point represents the mean +/− SEM of 3 experiments, each performed in triplicate.
Rescue with Pharmacological Chaperone SR121463
Pharmacological chaperones are non-peptide small molecules that bind and stabilize misfolded GPCRs to promote proper membrane localization and restore function 8, 9, 12. SR121463 is a potent V2R-specific antagonist that also acts as a V2R chaperone in that it increases cell-surface expression and AVP-stimulated function of some V2R mutants. 27 When cells expressing WT V2R or L57R V2R were incubated with SR121463, DDAVP-stimulated activity of L57R V2R increased significantly (P < 0.05), although this chaperone did not restore full activity (Figure 2).
Membrane Localization
To determine whether the partial rescue of L57R V2R activity by SR121463 is due to increased cell-surface localization of the mutant receptor, HEK293 cells were transfected with myc-tagged constructs of WT or L57R V2R. Cotransfection with farnesylated mCherry controlled for transfection efficiency and marked transfected cell membranes with red fluorescence. WT V2R was present at the membrane in all transfected cells both in the presence and absence of SR121463. L57R V2R cell-surface expression dramatically increased with SR121463 treatment (Figure 4).
Figure 4.
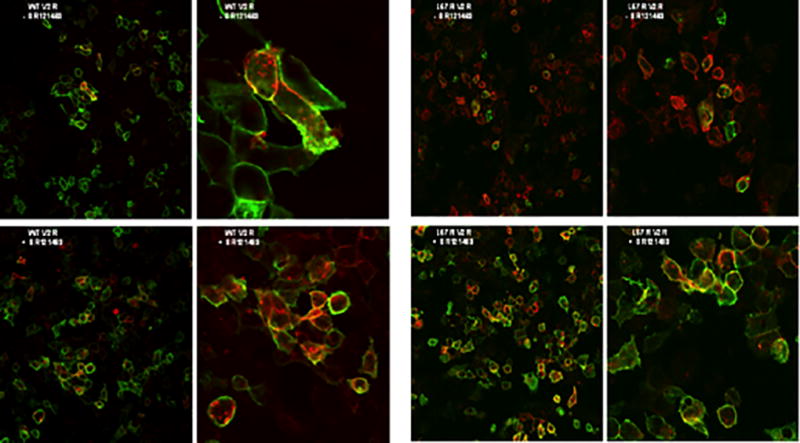
Membrane expression of V2R. Nonpermeabilized HEK 293 cells expressing myc-tagged WT or L57R V2R and farnesylated mCherry were incubated with and without 10−6 M SR121463 for 48h and imaged by fluorescence microscopy. Red fluorescence identifies the cell membrane of all transfected cells. Green fluorescence identifies the WT or mutant receptor when it is present at the cell membrane.
Discussion
We report two male infants with XNDI. The novel L57R V2R mutation identified in patient 2 had significantly decreased activity and cell-surface expression, which could be increased with SR121463. These data indicate that L57R, like other V2R missense mutations, compromises transport of the receptor from the endoplasmic reticulum (ER) to the cell membrane. 9, 12 SR121463 partially rescues L57R V2R function by diffusing into the cell and the ER lumen, binding to V2R and promoting its folding into a conformation that allows it to transit to the cell membrane. 12, 30 Our data suggest that SR121463 may be useful for treating XNDI. This compound is orally-active and well-tolerated in human subjects. 31 SR49059, a vasopressin-1a-specific receptor antagonist that has similar pharmacologic chaperone function as SR121463, was well-tolerated and effective in decreasing urine volume in five adults with severe XNDI.12 Thus, such pharmacologic chaperones offer specific and targeted therapy for XNDI caused by protein-misfolding due to missense mutations.
Brain MRIs, done early in the clinical evaluation to rule out an intracranial pathology, showed absent posterior pituitary bright spots in both our patients, suggesting CDI. However, neither patient responded to treatment with DDAVP, and both were subsequently found to have AVPR2 mutations and normal serum AVP levels, confirming the diagnosis of XNDI.
The posterior pituitary bright spot on MRI is usually present in NDI; its absence suggests neurohypophyseal dysfunction and a central etiology for diabetes insipidus. 15 However, the presence or absence of the bright spot is not a reliable way to distinguish between central and nephrogenic DI. Absence of the bright spot has been reported in normal individuals, especially older adults. 21, 32 By contrast, a small number of children with CDI have a bright spot identified by MRI at the time of diagnosis. 23, 24 Hence a normal bright spot cannot establish neurohypophyseal integrity, and its absence does not always imply CNS pathology.
Our study shows that infants with XNDI can have an absent bright spot at initial presentation. This observation has been made previously 33, 34 in a small number of adults, but is not widely appreciated in pediatrics. Experiments in water-deprived rabbits showed a direct correlation between the signal intensity of the bright spot and vasopressin concentration in the posterior pituitary, 35 and a negative correlation between the signal intensity and plasma vasopressin concentration, 36 suggesting that the hyperintensity on MRI is caused by vasopressin in neurosecretory granules. Depletion or absence of this stored vasopressin results in loss of the bright spot, which explains why the posterior pituitary bright spot disappears in states of AVP hypersecretion, such as untreated XNDI. Consistent with this explanation, the intensity of the bright spot image waxed and waned in parallel with the clinical courses of two patients who developed the triphasic response after pituitary surgery. 37
Thus, we report functional studies of a novel V2R mutation causing XNDI. We show that the mutant receptor had impaired in vitro activity that could be partially rescued by a pharmacological chaperone. We also show that infants with XNDI may present with absent posterior pituitary hyperintensity on MRI.
Acknowledgments
SAR and CCC were supported by Pediatric Endocrinology Training Grant T32-DK07161. CV is supported by NIH RO1 DK DK60540 and DK068152 as well as an established investigator award from the American Heart Association AHA#0740041N. We thank Professor A. J. Barkovich, Department of Radiology, University of California San Francisco, for interpretation of the brain MRIs in figure 1.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Verbalis JG. Diabetes insipidus. Reviews in Endocrine & Metabolic Disorders. 2003;4:177–185. doi: 10.1023/a:1022946220908. [DOI] [PubMed] [Google Scholar]
- 2.Miller WL. Molecular genetics of familial central diabetes insipidus. Journal of Clinical Endocrinology and Metabolism. 1993;77:592–595. doi: 10.1210/jcem.77.3.8370680. [DOI] [PubMed] [Google Scholar]
- 3.Knoers NV, Deen PM. Molecular and cellular defects in nephrogenic diabetes insipidus. Pediatric Nephrology. 2001;16:1146–1152. doi: 10.1007/s004670100051. [DOI] [PubMed] [Google Scholar]
- 4.Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, Lustig RH, Mathias RS, Portale AA, Miller WL, Gitelman SE. Nephrogenic syndrome of inappropriate antidiuresis. New England Journal of Medicine. 2005;352:1884–1890. doi: 10.1056/NEJMoa042743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spanakis E, Milord E, Gragnoli C. AVPR2 variants and mutations in nephrogenic diabetes insipidus: review and missense mutation significance. Journal of Cellular Physiology. 2008;217:605–617. doi: 10.1002/jcp.21552. [DOI] [PubMed] [Google Scholar]
- 6.Bichet DG, Birnbaumer M, Lonergan M, Arthus MF, Rosenthal W, Goodyer P, Nivet H, Benoit S, Giampietro P, Simonetti S, et al. Nature and recurrence of AVPR2 mutations in X-linked nephrogenic diabetes insipidus. American Journal of Human Genetics. 1994;55:278–286. [PMC free article] [PubMed] [Google Scholar]
- 7.Wenkert D, Schoneberg T, Merendino JJ, Jr, Rodriguez Pena MS, Vinitsky R, Goldsmith PK, Wess J, Spiegel AM. Functional characterization of five V2 vasopressin receptor gene mutations. Molecular and Cellular Endocrinology. 1996;124:43–50. doi: 10.1016/s0303-7207(96)03926-3. [DOI] [PubMed] [Google Scholar]
- 8.Pasel K, Schulz A, Timmermann K, Linnemann K, Hoeltzenbein M, Jaaskelainen J, Gruters A, Filler G, Schoneberg T. Functional characterization of the molecular defects causing nephrogenic diabetes insipidus in eight families. Journal of Clinical Endocrinology and Metabolism. 2000;85:1703–1710. doi: 10.1210/jcem.85.4.6507. [DOI] [PubMed] [Google Scholar]
- 9.Bernier V, Bichet DG, Bouvier M. Pharmacological chaperone action on G-protein-coupled receptors. Current Opinion in Pharmacology. 2004;4:528–533. doi: 10.1016/j.coph.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Bernier V, Lagace M, Lonergan M, Arthus MF, Bichet DG, Bouvier M. Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Molecular Endocrinology. 2004;18:2074–2084. doi: 10.1210/me.2004-0080. [DOI] [PubMed] [Google Scholar]
- 11.Morello JP, Salahpour A, Laperriere A, Bernier V, Arthus MF, Lonergan M, Petaja-Repo U, Angers S, Morin D, Bichet DG, Bouvier M. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. Journal of Clinical Investigation. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier V, Morello JP, Zarruk A, Debrand N, Salahpour A, Lonergan M, Arthus MF, Laperriere A, Brouard R, Bouvier M, Bichet DG. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. Journal of the American Society of Nephrology. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 13.Maghnie M. Diabetes insipidus. Hormone Research. 2003;59(Suppl 1):42–54. doi: 10.1159/000067844. [DOI] [PubMed] [Google Scholar]
- 14.Leger J, Velasquez A, Garel C, Hassan M, Czernichow P. Thickened pituitary stalk on magnetic resonance imaging in children with central diabetes insipidus. Journal of Clinical Endocrinology and Metabolism. 1999;84:1954–1960. doi: 10.1210/jcem.84.6.5745. [DOI] [PubMed] [Google Scholar]
- 15.Alter CA, Bilaniuk LT. Utility of magnetic resonance imaging in the evaluation of the child with central diabetes insipidus. Journal of Pediatric Endocrinology and Metabolism. 2002;15(Suppl 2):681–687. doi: 10.1515/jpem.2002.15.s2.681. [DOI] [PubMed] [Google Scholar]
- 16.Ghirardello S, Garre ML, Rossi A, Maghnie M. The diagnosis of children with central diabetes insipidus. Journal of Pediatric Endocrinology and Metabolism. 2007;20:359–375. doi: 10.1515/jpem.2007.20.3.359. [DOI] [PubMed] [Google Scholar]
- 17.Fujisawa I, Nishimura K, Asato R, Togashi K, Itoh K, Noma S, Kawamura Y, Sago T, Minami S, Nakano Y, et al. Posterior lobe of the pituitary in diabetes insipidus: MR findings. Journal of Computer Assisted Tomography. 1987;11:221–225. doi: 10.1097/00004728-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Mootha SL, Barkovich AJ, Grumbach MM, Edwards MS, Gitelman SE, Kaplan SL, Conte FA. Idiopathic hypothalamic diabetes insipidus, pituitary stalk thickening, and the occult intracranial germinoma in children and adolescents. Journal of Clinical Endocrinology and Metabolism. 1997;82:1362–1367. doi: 10.1210/jcem.82.5.3955. [DOI] [PubMed] [Google Scholar]
- 19.Gudinchet F, Brunelle F, Barth MO, Taviere V, Brauner R, Rappaport R, Lallemand D. MR imaging of the posterior hypophysis in children. American Journal of Roentgenology. 1989;153:351–354. doi: 10.2214/ajr.153.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Maghnie M, Cosi G, Genovese E, Manca-Bitti ML, Cohen A, Zecca S, Tinelli C, Gallucci M, Bernasconi S, Boscherini B, Severi F, Arico M. Central diabetes insipidus in children and young adults. New England Journal of Medicine. 2000;343:998–1007. doi: 10.1056/NEJM200010053431403. [DOI] [PubMed] [Google Scholar]
- 21.Brooks BS, el Gammal T, Allison JD, Hoffman WH. Frequency and variation of the posterior pituitary bright signal on MR images. American Journal of Neuroradiology. 1989;10:943–948. [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso G, Bergada I, Heinrich JJ. Resonancia magnética en niños y adolescentes con diabetes insípida central: hallazgos al diagnóstico y durante su seguimiento. Anales Españoles de Pediatría. 2000;53:100–105. [PubMed] [Google Scholar]
- 23.Kubota T, Yamamoto T, Ozono K, Shimotsuji T. Hyperintensity of posterior pituitary on MR T1WI in a boy with central diabetes insipidus caused by missense mutation of neurophysin II gene. Endocrine Journal. 2001;48:459–463. doi: 10.1507/endocrj.48.459. [DOI] [PubMed] [Google Scholar]
- 24.Maghnie M, Genovese E, Bernasconi S, Binda S, Arico M. Persistent high MR signal of the posterior pituitary gland in central diabetes insipidus. American Journal of Neuroradiology. 1997;18:1749–1752. [PMC free article] [PubMed] [Google Scholar]
- 25.Klein U, Muller C, Chu P, Birnbaumer M, von Zastrow M. Heterologous inhibition of G protein-coupled receptor endocytosis mediated by receptor-specific trafficking of beta-arrestins. Journal of Biological Chemistry. 2001;276:17442–17447. doi: 10.1074/jbc.M009214200. [DOI] [PubMed] [Google Scholar]
- 26.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. Journal of Clinical Investigation. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serradeil-Le Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, Chicot D, Jard S, Vilain P, Garcia C, Marty E, Raufaste D, Brossard G, Nisato D, Maffrand JP, Le Fur G. Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. Journal of Clinical Investigation. 1996;98:2729–2738. doi: 10.1172/JCI119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serradeil-Le Gal C, Raufaste D, Double-Cazanave E, Guillon G, Garcia C, Pascal M, Maffrand JP. Binding properties of a selective tritiated vasopressin V2 receptor antagonist, [H]-SR 121463. Kidney International. 2000;58:1613–1622. doi: 10.1046/j.1523-1755.2000.00322.x. [DOI] [PubMed] [Google Scholar]
- 29.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnology. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 30.Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends in Endocrinology and Metabolism. 2004;15:222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Serradeil-Le Gal C, Wagnon J, Valette G, Garcia G, Pascal M, Maffrand JP, Le Fur G. Nonpeptide vasopressin receptor antagonists: development of selective and orally active V1a, V2 and V1b receptor ligands. Progress in Brain Research. 2002;139:197–210. doi: 10.1016/s0079-6123(02)39017-4. [DOI] [PubMed] [Google Scholar]
- 32.Colombo N, Berry I, Kucharczyk J, Kucharczyk W, de Groot J, Larson T, Norman D, Newton TH. Posterior pituitary gland: appearance on MR images in normal and pathologic states. Radiology. 1987;165:481–485. doi: 10.1148/radiology.165.2.3659370. [DOI] [PubMed] [Google Scholar]
- 33.Moses AM, Clayton B, Hochhauser L. Use of T1-weighted MR imaging to differentiate between primary polydipsia and central diabetes insipidus. American Journal of Neuroradiology. 1992;13:1273–1277. [PMC free article] [PubMed] [Google Scholar]
- 34.Sato N, Ishizaka H, Yagi H, Matsumoto M, Endo K. Posterior lobe of the pituitary in diabetes insipidus: dynamic MR imaging. Radiology. 1993;186:357–360. doi: 10.1148/radiology.186.2.8421734. [DOI] [PubMed] [Google Scholar]
- 35.Kurokawa H, Fujisawa I, Nakano Y, Kimura H, Akagi K, Ikeda K, Uokawa K, Tanaka Y. Posterior lobe of the pituitary gland: correlation between signal intensity on T1-weighted MR images and vasopressin concentration. Radiology. 1998;207:79–83. doi: 10.1148/radiology.207.1.9530302. [DOI] [PubMed] [Google Scholar]
- 36.Lee MH, Choi HY, Sung YA, Lee JK. High signal intensity of the posterior pituitary gland on T1-weighted MR images. Correlation with plasma vasopressin concentration to water deprivation. Acta Radiologica. 2001;42:129–134. doi: 10.1034/j.1600-0455.2001.042002129.x. [DOI] [PubMed] [Google Scholar]
- 37.Fukino K, Yamada S, Ohta T, Takada K, Usui M. Serial MR intensity changes of the posterior pituitary in patients with diabetes insipidus after transsphenoidal surgery for pituitary adenomas: report of two cases. Pituitary. 2003;6:215–219. doi: 10.1023/b:pitu.0000023428.45413.62. [DOI] [PubMed] [Google Scholar]


