Abstract
Objectives
Our goal was to evaluate stereotactic ablative radiotherapy (SABR) as a salvage option for isolated recurrence of non-small-cell lung cancer (NSCLC) in the lung parenchyma following definitive treatment of stage I-III disease.
Methods
Patients who had histologically confirmed, positron emission tomography (PET) staged, isolated NSCLC recurring locally or metastasis in the lung parenchyma (≤3 cm, suitable for SABR) after previous definitive treatment, were prospectively enrolled in this trial and treated with volumetric, image-guided SABR to 50 Gy in four fractions. Patients were then followed with computed tomography (CT) or PET/CT. Primary endpoints included the pattern of failure following salvage SABR, overall survival (OS), and progression-free survival (PFS).
Results
Fifty-nine recurrent patients were treated with salvage-SABR. The median age was 70 years (range: 45-86 years), and the median follow-up time after salvage-SABR was 58.3 months. Nineteen patients (32%) developed re-recurrence after salvage-SABR. Measuring from the date of salvage-SABR, the estimated 5-year rates of local, regional and distant failure were 5.2%, 10.3% and 22.4%, respectively; the estimated PFS was 46.2% at 3 years and 41.1% at 5 years; and OS rates were 63.5% at 3 years and 56.5% at 5 years. A high post-SABR neutrophil-to-lymphocyte ratio was found to predict poor survival. Three patients (5%) developed grade 3 treatment-related adverse events. No patient had grade 4 or 5 events.
Conclusion
Our study showed salvage-SABR provides excellent 5-year OS, local control, and PFS rates with minimal toxicity for patients with isolated NSCLC recurrence in the lung parenchyma. These results are striking and comparable to historically reported outcomes of patients with primary early-stage NSCLC treated with definitive SABR. SABR appears to be a very effective and safe salvage option for patients with isolated lung parenchyma recurrent disease following definitive treatment and should be considered along with surgery as a potential first-line option for patients with local lung parenchymal recurrent disease.
Keywords: Stereotactic ablative radiotherapy, Non-small-cell lung cancer, Pattern of failure, Toxicity, Recurrent lung cancer
Introduction
Uncontrolled tumors are a major source of seeding to distant organs that can cause treatment failure in patients with recurrent or second primary lung cancer. Undoubtedly, definitive local treatment is required to achieve optimal outcomes when isolated recurrence and possibly new primaries emerge following definitive treatment for any stage of non-small-cell lung cancer (NSCLC). About 5%-40% of patients with NSCLC develop a local tumor recurrence even after receiving definitive standard treatment, and the likelihood of recurrence increases in patients with more advanced disease.1–3 In addition, a substantial proportion of patients experience isolated lung parenchymal recurrence/metastasis, which occurs in a different lobe/lung than the primary tumor,4 or a second primary lung cancer (estimated risk: 1%-6% per person-year).5, 6 Thus, there is a great need for effective and aggressive local treatments that can produce durable local control and a potential cure if recurrence occurs. Such treatments should have minimal toxicity because many NSCLC patients have reduced tolerance of toxic effects after having undergone aggressive treatment for their primary lung cancer.
Currently, the National Comprehensive Cancer Network guidelines for NSCLC recommend re-resection as the preferred modality for patients with resectable loco-regional recurrence that is based upon data from small retrospective studies, and the indications, effectiveness, feasibility, safety and long-term outcomes of re-resection remain unclear.7–9 Particularly, there is very limited report about salvage surgery for isolated recurrence or isolated distant metastasis in lung parenchyma, as many patients after definitive treatment might have poor lung capacity and/or co-morbidities. 10 Stereotactic ablation radiotherapy (SABR), with its high local control rate and minimal toxicity, has emerged as an alternative local modality for recurrent or second primary NSCLC.11–13 However, large prospective studies with robust long-term data are needed to illustrate patient survival, toxic effects, and disease control outcomes after SABR in this unique patient cohort.
This is the first report of such a study. Our prospective phase II clinical trial investigated patterns of treatment failure, patient survival, and safety in patients with isolated lung parenchymal recurrence of NSCLC treated with salvage SABR who were followed for 5 years thereafter.
Materials and Methods
Patients and Study Design
From November 2005 to March 2013, patients with an isolated lung parenchymal recurrence of NSCLC who were treated at The University of Texas MD Anderson Cancer Center and met all inclusion criteria were eligible to enroll in this SABR trial. This study was approved by the Institutional Review Board of MD Anderson, and all patients provided written informed consent to participate.
The inclusion criteria for the study were as follows. Patients had to have a histologically documented history of prior NSCLC and isolated lung parenchymal recurrence of NSCLC, ≤3 cm and suitable for SABR including meeting with dose volume constraints, able to follow instructions during the procedure and etc., without evidence of lymph node involvement or extra-thoracic disease after definitive treatment for prior NSCLC. Operable patients who elected to have SABR were also eligible. Histological confirmation of recurrent NSCLC is highly recommended. If the recurrent lesion was confirmed historically, it had to have the same histological characteristics as the initial NSCLC and could be located in the same lobe (termed as local recurrence) of the lung or in a different lobe (including the opposite lung) following an otherwise tumor-free interval. Since we can’t rule out the possibility of recurrence for patients with > 4 years after completion of treatment, and second lesion with the same histology that arise <6 months after the diagnosis of first lesion, these patients were eligible for and included in the study, although the secondary primary or synchronous tumors are among the differential diagnosis11, 14. Patients who received systemic therapy in addition to SABR were also eligible. Patients who had a new tumor with different pathological characteristics (which is for sure a second primary lung tumor) were excluded.
The restaging workup included computed tomography (CT), 18F-fluorodeoxyglucose positron emission tomography (PET)/CT, and brain magnetic resonance imaging within 3 months prior to SABR. Clinical staging of the previous NSCLC was done according to the sixth edition of the American Joint Committee on Cancer’s TNM staging system. Patients with hilar or mediastinal lymph nodes >1 cm on CT or abnormal PET studies underwent endobronchial ultrasonography (EBUS) to rule out regional disease.
SABR
For SABR, all patients underwent four-dimensional CT-based simulation with free-breathing, gated techniques to manage tumor motion. Repeated breath-hold scans were acquired when indicated (motion > 1 cm). The internal gross target volume (IGTV) was contoured according to the maximal intensity projection. 15 Image-guided stereotactic body radiotherapy with a dose of 50 Gy in four fractions was prescribed to the planning target volume (PTV). For patients with local recurrence (in the field of previous radiotherapy), compromised PTV coverage or an alternative prescription dose is allowed to meet with normal tissue dose constraints. We optimized our unmodulated, three-dimensional, conformal radiotherapy or intensity-modulated radiotherapy SABR plans using 6 to 12 coplanar or non-coplanar 6-MV photon beams. A prescribed isodose line was required to cover more than 95% of the PTV and 100% of the IGTV without margin. We defined centrally located lesions, conducted detailed stereotactic body radiotherapy simulations, and constrained the planning and dose volumes as described previously.16, 17
Follow-up Evaluations
Patient follow-up included chest CT 6 weeks after salvage SABR, then every 3 months for the first 2 years, then every 6 months for the next 3 years, and then annually. PET/CT was required 3 to 12 months after SABR. Local recurrence (LR) was defined as a recurrence or new lesion that occurred in the same lobe over time that corresponded to avid areas on PET/CT and/or confirmed histologically after SABR. LR was classified as in-field recurrence (occurring in the area inside the PTV), involved lobe failure (occurring in the same lobe outside the PTV), or marginal failure (recurrent lesion located within 1 cm of the PTV in any direction).
Regional recurrences (RRs) and distant metastases (DMs) after salvage SABR were documented. A RR was defined as any intrathoracic lymph node relapse outside the PTV. A recurrence appearing in a different lobe or in an extra-thoracic site was considered DM. Any adverse events definitely or possibly associated with SABR were graded according to the National Cancer Institute Common Toxicity Criteria (CTC) Version 3.0.
Statistical Analysis
Primary endpoint was progression-free survival (PFS). Secondary endpoints were overall survival (OS), pattern of failure, toxicity and predictive factors associated to prognosis as an exploratory analysis. PFS was calculated from the beginning date of salvage SABR to the date of any new recurrence (the date of the first image that showed abnormalities) or death (without confirmed recurrence). OS was calculated from the beginning date of salvage SABR to the date of death from any cause or last follow-up. The final data analyses were based on information received as of November 1, 2016.
We compared categorical data with χ2 test statistics; continuous data were compared with t tests. The median follow-up duration was estimated by the reverse Kaplan-Meier method. 18 According to the Kaplan-Meier method, survival curves were compared using the log-rank test. Univariate and multivariate Cox proportional hazards models were performed to obtain prognostic factors and corresponding hazard ratios (HRs) for different factors with 95% confidence intervals (CIs). Clinical variables in univariate analysis included age, gender, tumor size, EBUS, ECOG status, histology, maximum standardized uptake value (SUVmax) of tumor, IGTV, PTV, diagnosis interval between primary and recurrent tumor, pre- and post-SABR pulmonary function, and recurrence event. Pre- and post-SABR immunologic and inflammatory markers in peripheral blood including neutrophil and lymphocyte counts, as potential indicators reflecting immune status, were included in this exploratory analysis. Factors with P values <0.05 in univariate analysis were entered into multivariate analysis.
When the probability of any recurrence event was estimated, we calculated the probability of death as a competing risk event to overcome the overestimated probabilities of both events by using competing risk analysis. 19 All analyses was performed using IBM SPSS 21.0 software (SPSS, Inc., Chicago, IL, USA) with a macro to calculate the cumulative incidence competing risk 20. All significance tests were two-tailed, with significance set at P <0.05.
Results
Patient Characteristics
Among 65 patients screened, 59 patients were eligible and evaluable for the study (see Figure, Supplemental Data 1, which is flowchart of patients enrolled in the trial). Table 1 summarizes patient and disease characteristics. All patients had PET-CT staging, and 57 recurrent tumors (97%) were biopsied to confirm the same histology as prior NSCLC. Two lesions without biopsy due to severe co-morbidities were considered to have recurrent disease based on serial CT and PET/CT images. Six patients (10%) underwent hilar and mediastinal pathologic staging (EBUS procedure) prior to salvage SABR. The median interval between the diagnosis of prior NSCLC and of recurrence was 30 months (range, 2-132 months), and most patients (69%) developed recurrence within 4 years.
Table 1.
Clinical Characteristics of 59 Patients (59 tumors)
| Characteristic | Number of patients/tumors (%)* |
|---|---|
| Mean age | 69.4 years |
| Median age (range) | 70 years (45-86 years) |
| Sex | |
| Male | 32 (54) |
| Female | 27 (46) |
| Past or current smoker | 55 (93) |
| ECOG performance status | |
| 0 or 1 | 48 (81) |
| 2 | 11 (19) |
| Histology | |
| Adenocarcinoma | 36 (61) |
| Squamous cell carcinoma | 16 (27) |
| NSC NOS | 5 (8) |
| No pathologic findings† | 2 (3) |
| Prior stage | |
| I | 31 (53) |
| II | 15 (25) |
| III | 13 (22) |
| Interval between prior NSCLC diagnosis and recurrence | |
| Median (range) | 30 months (2-132 months) |
| < 4 years | 41 (69) |
| ≥ 4 years | 18 (31) |
| Treatment for prior NSCLC‡ | |
| Surgery, chemotherapy, radiation | 14 (24) |
| Surgery only | 13 (22) |
| Chemoradiation | 12 (20) |
| Surgery, chemotherapy | 11 (19) |
| Radiation only | 6 (10) |
| Other combinations | 3 (5) |
| Recurrent lesion location relative to prior tumor | |
| Contralateral lung | 33 (56) |
| Different lobe in ipsilateral lung | 17 (29) |
| Same lobe | 9 (15) |
| Maximum tumor diameter of recurrent lesion§ | |
| Mean | 1.5 cm |
| Median (range) | 1.4 cm (0.7-3.0 cm) |
| Tumor location | |
| Peripheral | 47 (80) |
| Central | 12 (20) |
| Median IGTV (range) | 3.84 cm3 (0.49-36.24 cm3) |
| Median PTV (range) | 38.47 cm3 (4.71-147.08 cm3) |
| SUVmax of recurrent tumor | |
| Mean | 4.5 |
| Median (range) | 4.1 (0-21.8) |
| SABR dose (BED) | |
| 50 Gy/4 fractions (112.5 Gy) | 57 (97) |
| 40 Gy/4 fractions (80 Gy)⊥ | 2 (3) |
Unless otherwise specified.
Two lesions with typical imaging feature were highly suspicious of malignancy. One lesion was involved lobe failure with SUVmax value of 9.2 and another lesions was in-field recurrence with SUVmax value of 5.0. They were not confirmed histologically due to the high risk of biopsy.
Forty patients were treated with lobectomy/limited resection and one patient was treated with pneumonectomy. Four patients had twice undergone thoracic surgery, and one patient had undergone thoracic surgery three times.
Tumor was measured on CT imaging within 2 months prior to SABR.
Two patients who had undergone thoracic irradiation previously received 40 Gy in 4 fractions in the same lung to meet normal-tissue dose constraints. Abbreviations: BED, biological equivalent dose; ECOG, Eastern Cooperative Oncology Group; IGTV, internal gross target volume; NOS, not otherwise specified; NSC, non-small-cell carcinomas; NSCLC, non-small-cell lung cancer; PTV, planning target volume; SABR, stereotactic ablative radiotherapy; SUVmax, maximum standardized uptake value.
As listed in Table 1, forty-one patients (69%) had received thoracic surgery, and thirty-three patients (56%) had been previously treated with thoracic radiation: five had received prior SABR (50 Gy in 4 fractions for four patients, 50 Gy in 5 fractions for one patient) and 28 had received conventional external beam radiotherapy with a median delivered dose of 66 Gy (range, 48.8-87.5 Gy). The median time from the end date of previous radiotherapy to the start of salvage SABR was 27.5 months (range, 2-61 months). One third (34%) of patients were potentially operable pre-SABR and the rest of patients were considered inoperable based on lung capacity and/or co-morbidities.
Two patients received chemotherapy after salvage SABR. Nine tumors (15%) treated with salvage SABR were locally recurrent disease in the same lobe, and most tumors (50; 85%) developed in a different lobe. Only two patients had overlapping PTV volumes between SABR and prior radiotherapy.
Pattern of Treatment Failure and Patient Survival After SABR
At a median follow-up of 58.3 months (interquartile range, 43.9-85.3 months), a total of 19 patients (32%) experienced recurrence at a median of 13.3 months after salvage SABR (range, 4.2-28.3 months; Figure 1A). Only three patients (5%) had LR as a first event (two had in-field recurrence and one had involved lobe failure); this occurred 8.5, 10.6 and 25.9 months after SABR, respectively. The SUVmax of the three recurrent lesions was 1.3, 19.4 and 17.3, respectively, and the first two lesions were confirmed pathologically. The estimated cumulative incidence rates for LR, which were calculated using the competing risk method, were 3.5% at 1 year, 5.2% at 3 years, and 5.2% at 5 years (Figure 1A).
Figure 1.
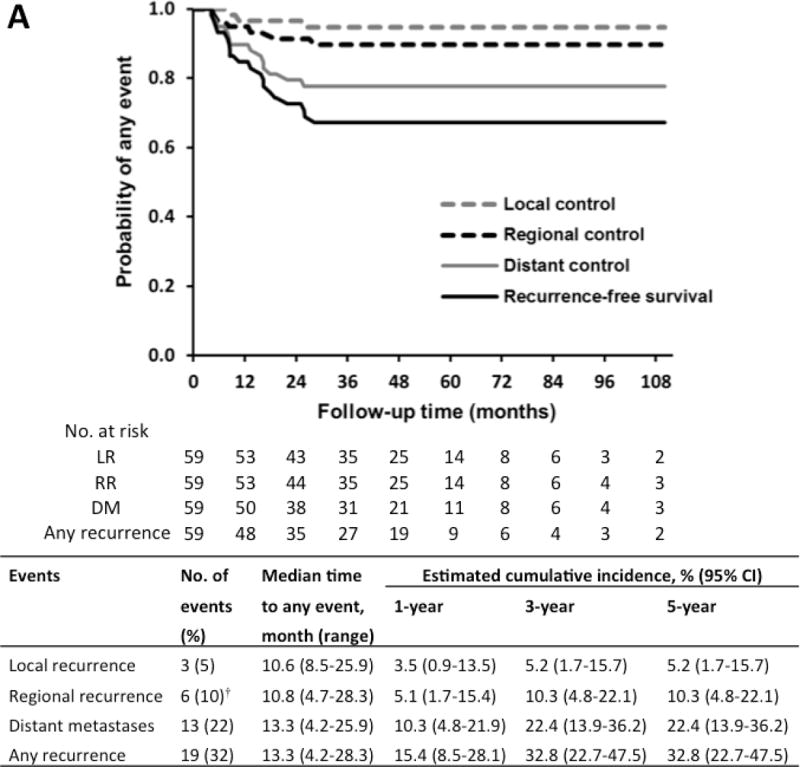

(A) Freedom from any recurrence and estimated cumulative incidence of any recurrence after salvage stereotactic ablation radiotherapy as determined by competing risk analysis. (B) Kaplan-Meier curves and estimated cumulative incidences of overall survival (OS), progression-free survival (PFS), and lung cancer-specific survival after SABR.
CI, confidence interval. † Simultaneous RR and DM occurred in 3 patients. * Estimation of 95% CI is limited.
Six patients (10%) had RR as the first event at a median interval of 10.8 months (range, 4.7-28.3 months), including simultaneous RR and DM occurred in three patients. DM as a first event occurred in 13 patients (22%) at a median interval of 13.3 months (range, 4.2-25.9 months), and the most common metastatic sites were the lung (nine patients; 69%). The estimated cumulative incidence rates for RR were 5.1% at 1 year and 10.3% at 3 years; for DM, they were 10.3% at 1 year and 22.4% at 3 years. The corresponding 5-year rates were the same as the 3-year rates because no events occurred after 3 years.
Twenty-nine patients (49%) treated with salvage SABR had died; 16 (27%) of them died of lung cancer. The estimated OS rate at 5 years was 56.5%, and the median OS was 63.8 months (Figure 1B). The OS rate reached as high as 74.1% at 5 years and 60.7% at 7 years (median OS, 119.9 months) if it was calculated from the diagnosis of the prior tumor instead of from the date of salvage SABR. When comparing survival rates by prior NSCLC stage, patients with initial stage I disease had significantly longer PFS and trend of longer OS than did patients with stage II or Ш disease (median PFS, [not reached] vs. 28.3 months, P=0.013; median OS, 81.7 vs. 41.9 months, P=0.072; Figure 2A, B).
Figure 2.

Kaplan-Meier curves of progression-free survival (A) and overall survival (B) after salvage stereotactic ablation radiotherapy by prior disease stage, and (C) overall survival by recurrence event after salvage SABR.
LRR, local or regional recurrence only; DM, distant metastasis.
Among the 6 patients who developed only LR or RR after salvage SABR, 4 received second local salvage treatment, including surgery, radiation, or chemoradiation, 1 received chemotherapy and 1 didn’t received salvage treatment; four patients developed a subsequent recurrence (1 had RR and 3 had DM). Among 13 patients who had distant failure after SABR, 8 patients received treatment: 4 had target therapy, 2 had SABR and 2 had chemotherapy. Twelve patients (92%) developed a subsequent recurrence (4 had a subsequent LR and 8 had a subsequent DM). Median OS time in the 13 patients with DM after salvage SABR was significantly poorer than that in patients without recurrence and patients with loco-regional recurrence alone (LR/RR) (30.9 months vs. [not reached], P=0.006; P=0.023 among the three groups; Figure 2C). However, there was no difference of OS between patients with LR/RR and patients without recurrence (P=0.811).
Prognostic and/or Predictive Factors of Survival
The potential predictors of PFS and OS following salvage SABR were analyzed using a Cox proportional hazards model (Table 2). Univariate analysis showed that several clinical factors, including Eastern Cooperative Oncology Group (ECOG) performance status, histologic types, and pre- and post-SABR carbon monoxide diffusing capacity (DLCO) are all significantly associated with PFS and OS (P<0.05). Notably, the post-SABR neutrophil-to-lymphocyte ratio (NLR), an immunologic and inflammatory marker, was significantly associated with both PFS and OS, and a high post-SABR NLR value predicted poor prognosis (P<0.05). The median post-SABR NLR value was 3.44 (range, 1.39-14.19) and the mean value was 4.00. The median pre-and post-SABR absolute lymphocyte count (ALC) values were 1.49 (range, 0.48-4.29) and 1.16 (range, 0.36-3.51), and the corresponding mean values are 1.57 and 1.34, respectively. We also analyzed the survival impact of the time from the prior lung cancer until the diagnosis for the recurrence, the result showed no significant difference with p value of 0.7.
Table 2.
Exploratory Cox Regression Analysis of Survival Predictors after Salvage SABR
| Variable* | Progression-free survival | Overall Survival | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P | HR (95% CI) | P | |
| Univariate analysis | ||||
| Tumor size | 1.90 (1.12-3.21) | 0.017 | 1.71 (0.96-3.05) | 0.067 |
| ECOG status (0-1 vs 2-3) | 0.31 (0.14-0.66) | 0.003 | 0.20 (0.09-0.46) | <0.001 |
| Histology (squamous vs adenocarcinoma) | 2.23 (1.09-4.59) | 0.029 | 2.59 (1.20-5.62) | 0.016 |
| Post-SABR NLR | 1.25 (1.04-1.50) | 0.019 | 1.32 (1.11-1.57) | 0.002 |
| Pre-SABR ALC | 0.69 (0.42-1.13) | 0.139 | 0.62 (0.35-1.10) | 0.099 |
| Post-SABR ALC | 0.71 (0.40-1.25) | 0.234 | 0.49 (0.24-1.01) | 0.053 |
| Pre-SABR FEV1 | 1.00 (0.98-1.02) | 0.764 | 0.99 (0.97-1.00) | 0.117 |
| Post-SABR FEV1 | 0.99 (0.98-1.01) | 0.537 | 0.98 (0.96-1.00) | 0.021 |
| Pre-SABR DLCO | 0.98 (0.96-1.00) | 0.038 | 0.97 (0.95-0.99) | 0.004 |
| Post-SABR DLCO | 0.98 (0.96-1.00) | 0.034 | 0.97 (0.94-0.99) | 0.008 |
| Multivariate analysis | ||||
| ECOG status (0-1 vs 2-3) | 0.56 (0.21-1.51) | 0.253 | 0.45 (0.16-1.24) | 0.122 |
| Post-SABR NLR | 1.25 (0.99-1.58) | 0.065 | 1.33 (1.06-1.65) | 0.012 |
| Pre-SABR DLCO | 0.99 (0.97-1.01) | 0.284 | 0.98 (0.96-1.00) | 0.092 |
| Tumor size | 1.77 (1.00-3.13) | 0.052 | N/A | |
To avoid possible collinearity between pre- and post-SABR DLCO in the multivariate model, we chose a variable that had smaller P value and most available cases in univariate analysis. Age and other clinical factors with P values >0.2 in univariate analysis are not shown.
ECOG status and histologic findings were treated as categorical variables; all other variables were treated as continuous variables. Abbreviations: ALC, absolute lymphocyte count; CI, confidence interval; DLCO, carbon monoxide diffusing capacity; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in the first second of expiration; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; SABR, stereotactic ablative radiotherapy.
In the multivariate analysis, tumor size and post-SABR NLR were not significant predictors for PFS (P = 0.052 and 0.065). The only significant prognostic factor for OS was the post-SABR NLR (HR = 1.33; 95% CI, 1.06-1.65; P = 0.012); each one-unit increase in NLR increased the risk of death by about 33%. ECOG status and pre-SABR DLCO, which indicate a patient’s health status, were not significantly associated to OS (P=0.122 and 0.092). Due to small sample size, all of above analysis should be considered as exploratory and validation is needed.
Adverse Events
A comparison between pre- and post-SABR pulmonary function tests showed a significant difference in DLCO, which may be associated with SABR (P < 0.001). However, there was no difference between pre- and post-SABR FEV1s (Table 3). At baseline assessment, 24 patients (41%) had dyspnea or shortness of breath because of comorbidities and/or prior treatment; five patients (8%) had dyspnea classified as grade 3 owing to the need for oxygen (see Table, Supplemental Data 2, which shows this symptom before and after SABR). Other less-frequent symptoms at baseline included cough, fatigue, and weakness. If dyspnea is present before SABR, accurate toxicity grading after SABR is difficult to determine because the CTC definition of pulmonary toxicity does not distinguish between baseline dyspnea and changes in the condition and dyspnea after SABR could reflect normal progression of pulmonary disease.
Table 3.
Comparison of Pulmonary Function Test Before and After SABR
| Baseline
|
Median test interval after SABR (range) | After SABR
|
P value | |||||
|---|---|---|---|---|---|---|---|---|
| No. of patient | Median (range) | Mean | No. of patient | Median (range) | Mean | |||
| FEV1 | 59 | 71% (14-122%) | 67.8% | 5.0 months (1.6-16.0 months) | 55 | 66% (12-112%) | 66.0% | 0.094 |
| DLCO | 55 | 67% (31-135%) | 66.3% | 50 | 58.5% (14-116%) | 60.0% | <0.001 | |
Pre-SABR pulmonary function tests were performed within 3 months before SABR. DLCO was unable to perform on four patients. Post-SABR pulmonary function test results were unknown in three patients, and the test was unable to perform in one patient. Post-SABR DLCO testing was unable to perform in another five patients. Abbreviations: DLCO, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in the first second of expiration; SABR, stereotactic ablation radiotherapy.
The most common acute adverse events after SABR were radiation pneumonitis and chest wall pain (Table 4). When five patients with grade 2 dyspnea at baseline were excluded, a total of 18 patients (31%) had grade 2 adverse events after SABR; 14 of them had undergone prior thoracic radiation, and nine had had prior surgery. In all, only three patients (5%) experienced grade 3 SABR-related adverse events: one (2%), who had received prior thoracic radiation with a dose of 66 Gy in 33 fractions had dermatitis, and two (3%) had radiation pneumonitis. One of the two patients with radiation pneumonitis had had surgery twice and had SABR delivered to the same lobe that had been treated with a prior wedge resection, and the other patient had previously received radiotherapy with 63 Gy in 35 fractions, both patients had grade 1 dyspnea at baseline. No patient experienced grade 4 or 5 adverse events. During long-term follow-up, only four patients experienced grade 2 late adverse events (two [3%] had pulmonary fibrosis and two [3.3%] had rib fractures).
Table 4.
Grade 2 and 3 Adverse Events after SABR
| Adverse event* | No. (%)
|
|
|---|---|---|
| Grade 2 | Grade 3 | |
| Acute adverse event | ||
| Dermatitis | 3 (5) | 1 (2) |
| Radiation pneumonitis | 14 (24) | 2 (3) |
| Dyspnea/shortness of breath† | 2 (3) | 0 |
| Chest wall pain | 8 (14) | 0 |
| Brachial plexopathy | 3 (5) | 0 |
| Esophagitis | 1 (2) | 0 |
| Late adverse event | ||
| Rib fracture | 2 (3) | 0 |
| Pulmonary fibrosis | 2 (3) | 0 |
Each symptom was scored separately (i.e., each patient could have several adverse events).
Patients who had dyspnea at baseline were not included.
Discussion
This prospective clinical study showed excellent local control of 94.8% at 5 years using SABR as salvage treatment for isolated recurrence of NSCLC in the lung parenchyma. Further, the post-salvage cumulative incidence of RR was only 10.3% at 5 years, mirroring the reported data for RR rates following primary early-stage NSCLC treated with definitive SABR or surgery.2, 21, 22 However, despite the excellent loco-regional control with salvage SABR, the cumulative DM rate (22.4% at 3 years) appears higher than what is expected for patients treated definitively for primary early-stage disease.2, 21–23 In addition, the median intervals to recurrence were relatively short after salvage SABR (about 1 year) as compared with primary early stage NSCLC treated with SABR (typically about 1.5 years). The fact that all recurrence events occurred within 2.5 years after salvage SABR also differs from the outcomes for patients with primary early-stage disease treated with definitive surgery or SABR, in whom late recurrence events after 4 years have been reported.2, 24 Therefore, while salvage is extremely effective for local-regional control, these recurrent patients are still at relatively high risk for distant failure.
Ultimately, it is difficult to judge whether the DM events we observed were attributable to failed salvage-SABR or to pre-existing distant seeding from the prior tumor. Indeed, patients who developed subsequent DM after salvage SABR had a worse OS than patients who did not. Consequently, the high DM rate, the short interval to recurrence, and the associated morbidity of such events after salvage SABR, reflect the intrinsic nature of recurrent disease. Together, it suggests that recurrence or presence of a new lung lesion heralds a more aggressive disease course and biology. Identifying that most recurrences occurred within 2.5 years following salvage SABR however helps clinical physicians to recognize that vigilant screening and follow-up during this time is prudent and patients to become better informed about the risks and expectations for disease recurrence. Further, given the relatively high DM rate (1 in 4 patients will experience DM within 2.5 years), these results suggest that many patients may benefit from systemic treatment in addition to local therapy at the time of local recurrence. However, identifying which subsets of patients would benefit from the addition of systemic therapy, and which systemic therapy, remains to be revealed.
It is highly noteworthy that, in spite of the high DM rate, in the present study, the 5-year OS rate after salvage SABR was as high as 56.5%, which is similar to the OS rate for patients with primary stage І NSCLC treated with definitive SABR. 25–27 When calculating from the date of initial primary NSCLC diagnosis, the OS rate was even higher, at an impressive 74.1% at 5 years. This suggests that, overall, the majority of patients with isolated local lung parenchymal disease are likely to do as well as patients with primary early stage lung cancer on the whole, and their disease is still highly curable. These findings echo recent studies that report better cancer-specific outcomes are achieved when bulky disease burden is aggressively locally controlled, such as in the oligo-metastatic setting, and closely represent the 5-year OS rates following diagnosis of a second lung primary after definitive treatment.4, 5, 11 Given the high OS rates shown in our study, eradicating local tumors is an essential step in obtaining a cure or better prognosis.
In attempting to understand which patients will fare better with salvage-SABR, we found that several important clinical factors were associated with survival outcomes. Notably, an exploratory analysis of the post-SABR NLR, which partially reflects the balance between the host’s pro-tumor inflammatory status and anti-tumor immune status, was found to correlate with OS. It has been documented that the anti-tumor effects of immunotherapy and radiation are carried out by lymphocytes (specifically, CD8+ effector cells), 28 and some researchers have argued that the lasting effects of SABR may, in fact, be partly mediated by immune mechanisms.29, 30 Therefore, it is not surprising that a high NLR, which indicates that a patient has a lower number of lymphocytes, the cells responsible for anti-tumor effects relative to neutrophils, may be a marker of poor response and survival following SABR. This exploratory result needs further validation and may potentially help to guide our future research directions.
Finally, with respect to SABR toxicity, our study showed that salvage-SABR was well tolerated and that it had a very low toxicity profile; only 5% of patients experienced grade 3 adverse events. Even counting the three patients whose need for oxygen increased after SABR compared with their need at baseline, only 5 patients (8%) had grade 3 radiation pneumonitis. It will be important for future studies to recognize symptom changes in patients who had severe symptoms at baseline and to apply specific criteria for toxicity grading. A reasonable explanation of the low toxicity we observed is that most tumors in our patients were peripheral small tumors, and almost half of our patients had not previously received radiotherapy; in contrast, most other published studies included patients who had previously received radiotherapy. 31 In the 54% of patients in our study who had previously received radiotherapy, SABR was delivered to lesions with minimal overlap of the prior target volume, so that normal tissue would receive a relatively acceptable cumulative dose of radiation.
There are several limitations in the present study. First, due to challenges in confirming the origin of the cancer, we can’t rule out the possibility that some of our recurrent cases may represent secondary lung cancer even though the histology is the same. 14 Second, this is a highly selective patient population, with isolated lung parenchymal lesion ≤3 cm; it represents very early stage of recurrence or isolated metastasis. Third, determining who may fail shortly after salvage SABR and need systemic therapy after SABR remains investigational.
In conclusion, this study represents one of the largest studies, and the first prospective study, of salvage SABR for isolated lung parenchymal recurrence of NSCLC. Our results demonstrated excellent local control, low toxicity, and very promising survival rates with a long-term follow-up of nearly 5 years. Importantly, most patients did not develop recurrence after SABR. These results indicate that the survival of patients with either isolated NSCLC recurrence or possibly a second primary NSCLC could be enhanced through both close surveillance after initial treatment to detect lesions at an early stage, and treatment of new lesions with SABR to obtain optimal local control. On the basis of our findings, we consider SABR to be a very effective and safe salvage strategy that should be strongly recommended for patients with isolated small (≤3 cm) lung parenchymal recurrent NSCLC and may be considered as a first line treatment along with surgery for isolated lung parenchymal recurrence.
Supplementary Material
Acknowledgments
We thank The University of Texas MD Anderson Cancer Center’s Thoracic Radiation Oncology section and Thoracic Care Center for their help and support, and we also thank Ms. Laura L. Russell of the Department of Scientific Publications for editorial assistance.
Funding sources: This research was supported in part by the National Cancer Institute at the National Institutes of Health through a Cancer Center Support Grant (P30CA016672) and through a Clinical and Translational Science Award (UL1 RR024148) to The University of Texas MD Anderson Cancer Center.
Footnotes
ClinicalTrials.gov: NCT00489008; https://clinicaltrials.gov/ct2/show/NCT00489008.
Conflicts of interest: None declared.
References
- 1.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. The Lancet Oncology. 2012;13:802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hishida T, Yoshida J, Aokage K, et al. Postoperative oligo-recurrence of non-small-cell lung cancer: clinical features and survival. Eur J Cardiothorac Surg. 2016;49:847–853. doi: 10.1093/ejcts/ezv249. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. Journal of the National Cancer Institute. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 6.Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. The Journal of thoracic and cardiovascular surgery. 2013;145:75–82. doi: 10.1016/j.jtcvs.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Schreiner W, Dudek W, Lettmaier S, et al. Should salvage surgery be considered for local recurrence after definitive chemoradiation in locally advanced non-small cell lung cancer? J Cardiothorac Surg. 2016;11:9. doi: 10.1186/s13019-016-0396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CF, Meyerhoff RR, Stephens SJ, et al. Long-term outcomes of lobectomy for non-small cell lung cancer after definitive radiation treatment. Ann Thorac Surg. 2015;99:1914–1920. doi: 10.1016/j.athoracsur.2015.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimada Y, Suzuki K, Okada M, et al. Feasibility and efficacy of salvage lung resection after definitive chemoradiation therapy for Stage III non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2016;23:895–901. doi: 10.1093/icvts/ivw245. [DOI] [PubMed] [Google Scholar]
- 10.Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol. 2013;31:1384–1390. doi: 10.1200/JCO.2012.45.9651. [DOI] [PubMed] [Google Scholar]
- 11.Chang JY, Liu YH, Zhu Z, et al. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer. 2013;119:3402–3410. doi: 10.1002/cncr.28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen D, Olivier KR, Mayo CS, et al. Outcomes of stereotactic body radiotherapy (SBRT) treatment of multiple synchronous and recurrent lung nodules. Radiat Oncol. 2015;10:43. doi: 10.1186/s13014-015-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parks J, Kloecker G, Woo S, et al. Stereotactic body radiation therapy as salvage for intrathoracic recurrence in patients with previously irradiated locally advanced non-small cell lung cancer. Am J Clin Oncol. 2016;39:147–153. doi: 10.1097/COC.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 14.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 15.Liu H, Zhang X, Vinogradskiy YY, et al. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. International journal of radiation oncology, biology, physics. 2012;84:1017–1023. doi: 10.1016/j.ijrobp.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JY, Liu H, Balter P, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiation oncology (London, England) 2012;7:152. doi: 10.1186/1748-717X-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JY, Bezjak A, Mornex F. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10:577–585. doi: 10.1097/JTO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 18.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controlled clinical trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Verduijn M, Grootendorst DC, Dekker FW, et al. The analysis of competing events like cause-specific mortality–beware of the Kaplan-Meier method. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2011;26:56–61. doi: 10.1093/ndt/gfq661. [DOI] [PubMed] [Google Scholar]
- 21.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2012;83:348–353. doi: 10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 22.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. The Lancet Oncology. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo Y, Shibuya K, Nagata Y, et al. Preliminary report of late recurrences, at 5 years or more, after stereotactic body radiation therapy for non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2012;7:453–456. doi: 10.1097/JTO.0b013e31823c5b29. [DOI] [PubMed] [Google Scholar]
- 25.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:2449–2455. doi: 10.1200/JCO.2013.50.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 27.Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. International journal of radiation oncology, biology, physics. 2015;93:989–996. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T-cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nature reviews Clinical oncology. 2016;13:516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Ruysscher D, Faivre-Finn C, Le Pechoux C, et al. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014;15:e620–624. doi: 10.1016/S1470-2045(14)70345-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


