Abstract
INTRODUCTION
Our previous studies have shown that amyloid β peptide (Aβ) is subject to complement-mediated clearance from the peripheral circulation, and that this mechanism is deficient in Alzheimer’s disease (AD). The mechanism should be enhanced by Aβ antibodies, which form immune complexes (ICs) with Aβ, and therefore may be relevant to current Aβ immunotherapy approaches.
METHODS
Multidisciplinary methods were employed to demonstrate enhanced complement-mediated capture of Aβ ICs compared to Aβ alone in both erythrocytes and THP1-derived macrophages.
RESULTS
Aβ antibodies dramatically increased complement activation and opsonization of Aβ, followed by commensurately-enhanced Aβ capture by human erythrocytes and macrophages. These in vitro findings were consistent with enhanced peripheral clearance of intravenously-administered Aβ ICs in non-human primates.
DISCUSSION
Together with our previous results showing significant AD deficits in peripheral Aβ clearance, the present findings strongly suggest that peripheral mechanisms should not be ignored as contributors to the effects of Aβ immunotherapy.
Keywords: Alzheimer’s disease, amyloid β peptide, Aβ immunotherapy, complement, complement receptor 1, macrophage, erythrocyte, blood, human
1. Background
Over the last decade, the most visible strategy for the treatment of Alzheimer’s disease (AD) has been amyloid β peptide (Aβ) immunotherapy [reviewed in 1]. Although the first efforts with Aβ immunotherapy failed to complete clinical trials [1], AD transgenic mice [2,3] and human AD patients [4,5] did show significantly reduced Aβ burden after treatment. Perhaps as a result, Aβ immunization approaches continue to be pursued [1].
A critical, unresolved issue with Aβ immunization is whether or not its presumed mechanism of action, enhanced glial clearance of brain Aβ [e.g., 6–8], provides a sufficient explanation for its reported effects. For example, an Aβ antibody, m266, that did not react with brain Aβ deposits and appeared to have most if not all of its effect in the periphery, nonetheless reduced brain Aβ levels in a transgenic AD mouse model [9]. This antibody formed immune complexes (ICs) with Aβ in the peripheral circulation [10] and appeared to induce efflux of brain Aβ to plasma [9,11], leading to the “peripheral sink” hypothesis [9–11]. Moreover, the penetration of Aβ antibodies into the CNS remains open to debate. Levites and colleagues [12], for example, reported that only 1 fmol/mg of Aβ antibody could be detected in AD transgenic mouse brain after a 500 μg intraperitoneal injection. This brain concentration of antibody is nearly 3 orders of magnitude less than estimates of total brain Aβ in the mice [12]. Cerebrospinal fluid (CSF) concentrations of bapineuzumab, a humanized monoclonal Aβ antibody, are also found to be, on a molar basis, approximately three orders of magnitude less than typical CSF Aβ concentrations [reviewed in 13].
The above considerations, of course, do not necessarily disallow direct CNS actions of Aβ immunotherapeutics. Golde [14], for example, has cogently argued that if endogenous antibodies can have material effects on the CNS, which is clearly the case [15,16], then exogenous antibodies should be able to do so as well. On the other hand, considering that only minute quantities of peripherally-administered Aβ antibodies reach the CNS, whereas they are wholly and directly exposed to circulating Aβ, it is difficult to understand how interactions of Aβ antibodies with circulating Aβ can be ignored as at least a potential, additional mechanism of action for Aβ immunotherapy.
We have explored specific mechanisms by which Aβ/Aβ antibody immune complexes (Aβ ICs) formed in blood in the course of Aβ immunization might enhance clearance of Aβ through enhanced interactions with the complement system. These studies were informed by the fact that major pathways for peripheral pathogen clearance in primates hinge on complement receptor 1 (CR1) [17], single nucleotide polymorphisms (SNPs) in which have been consistently identified as a significant risk factor for AD [18–22]. Compared to Aβ alone, we found that the presence of Aβ antibodies in the fluid phase dramatically increased virtually all steps in the major pathways for peripheral pathogen clearance in primates, including complement activation, formation of complement-opsonized complexes that are ligands for CR1, and peripheral capture and disposal of Aβ through CR1-mediated erythrocyte and macrophage mechanisms. Consistent with these in vitro results, clearance of Aβ from plasma and erythrocyte compartments in vivo was also robustly enhanced in non-human primates intravenously (IV) inoculated with Aβ ICs. Although, as noted, these findings do not disallow CNS actions of Aβ immunotherapy, they do strongly suggest that peripheral effects should be considered as well—particularly since peripheral strategies might avoid the CNS adverse effects that have been encountered in previous AD immunotherapy trials [1,4,5].
2. Methods
2.01 Subjects
Human erythrocytes for the various experiments were obtained from study investigators under an Institutional Review Board-approved protocol. Two male Cynomolgus macaque monkeys (25 years old, 7.0 kg weight, and 28 years old, 6.5 kg weight) received intravenous injections of Aβ and blood samples were taken from them at various intervals (see below). These studies were performed under an Institutional Animal Care and Use Committee-approved protocol.
2.02 Preparation of Aβ
Lyophilized human synthetic Aβ(1–42) (Genscript, Piscataway, NJ) or FITC-conjugated Aβ(1–42) (Bachem, Torrance, CA) was resuspended in sterile 100% DMSO (Sigma, St. Louis, MO) at 10 mg/ml, diluted to 2 mg/ml in sterile ddH2O, and then brought to 1 mg/ml in sterile 100 mM Tris, pH 7.4. The suspension was incubated overnight at room temperature (RT), in the dark, with shaking at 450 rpm. The resulting 1 mg/ml stock solution was then diluted with 100 mM Tris to achieve the concentrations employed in the experiments. Western blots of Aβ solutions prepared in this manner showed the presence of Aβ aggregates at multiple molecular weights, an important point since the monomeric form of Aβ poorly activates complement, if at all, whereas Aβ aggregates are relatively potent activators [23].
2.03 Serum complement activation
Various concentrations of Aβ prepared in 100 mM Tris, as above, were incubated with either Aβ antibody (4G8, Biolegend, San Diego, CA) or PBS, pH 7.2, after which the solutions were mixed with normal human serum (NHS) (CompTech, Tyler, Texas) for 30 minutes at 37°C. NHS plus 10 mM EDTA (Amresco, Solon, OH) (final concentration in the serum) was employed as a control. C3a production, one of several standard measures of complement activation, was assayed by ELISA (Affymetrix, Santa Clara, CA, #BHS2089) following the manufacturer’s protocol.
2.04 iC3b Western blots and densitometry
NHS was incubated with 300 μg/ml Aβ alone or Aβ ICs for 30 minutes at 37°C to permit complement activation, generation of complement opsonins, and their covalent binding to Aβ. To form Aβ ICs, a 9:1 molar ratio of Aβ:4G8 antibody was employed, as this ratio gave optimal complement activation (see Section 3.01, below). As a control to block complement activation and opsonization, 10 mM EDTA was added to NHS prior to incubation with Aβ or Aβ ICs. The solutions were run under reducing/denaturing conditions on SDS-PAGE 4–15% mini-PROTEAN TGX gels (BioRad, Hercules, CA, #146-1086), transferred to PVDF membranes (BioRad, #170-4156), blotted with a biotinylated (Thermo Fisher Scientific, Waltham, MA, #21326) iC3b antibody (Quidel, San Diego, CA), and imaged on an Odyssey Imaging System (LI-COR, Lincoln, NE). To control for any effects of endogenous Igs or iC3b, a parallel gel was also run and analyzed under the same conditions, except that NHS was pre-depleted of endogenous Ig and iC3b using Aβ antibody 4G8 (Biolegend), iC3b antibody (Quidel), protein A/G PLUS agarose beads (Santa Cruz Biotechnology, Dallas), and standard immunoprecipitation methods. Uncompressed images of both Western blots were analyzed using ImageJ software (https://imagej.nih.gov). Blot background was quantified by integrating the signal in 10 different regions where there were no visible bands on the membrane, and linear regression was used to determine the relationship between integrated area and integrated background intensity (R2 > 0.99 for both blots). For each band, the signal was integrated and the background (based on the area of the band) was subtracted to give final integrated optical densities.
2.05 Erythrocyte adhesion assay
To measure erythrocyte binding to Aβ alone and to Aβ ICs, a previously-published method for assaying erythrocyte CR1 binding to opsonized CR1 ligands was employed [24]. Aβ42 was prepared as described above and 50 μl was coated on a high-binding Costar 96-well plate (#07-200-39, Fisher Scientific, Waltham, MA) for 1 hour with shaking (450 rpm) at RT. The plate was washed 3X with 2/3 TBST (10 mM Tris, pH 7.2, 100 mM NaCl, 0.05% Tween-20), blocked with 100 μL 0.5% PEG-3350 (Sigma, # P4338-1KG) in 2/3 TBST for 1 hour, RT, with shaking, and washed 3X again before 50 μl of various concentrations of amyloid antibody 4G8 were added to each well so as to form Aβ/Aβ ICs, as in the previous experiment on complement activation. The plate was then incubated for 1 hour at RT with shaking, washed 3X, and incubated for 30 minutes at RT with 50 μl of NHS, diluted 1:32 in VBS++ (CompTech, #B112), as a complement source for opsonization of the Aβ and Aβ ICs. To demonstrate that Aβ binding is mediated by complement opsonization, parallel wells were incubated with heat-inactivated (56°C, 30 minutes) NHS or C1q-depleted NHS (Comp Tech), both of which block various stages of complement activation but at different points. Plates were washed 2X with 2/3 PBST and 1X with adhesion buffer (140 mM Dextrose, 100 mM NaCl, 450 uM CaCl2, 170 uM MgCl2, 8 mM Tris, pH 7.4). Packed human erythrocytes were then diluted 1:2667 in adhesion buffer, and 200 μl each of the suspensions were incubated with all plates for 45 minutes at RT. After washing, erythrocytes that remained bound to the plate were imaged (brightfield, 100X) using an inverted Olympus IX71 microscope (Olympus, Center Valley, PA) and quantified using investigator-independent ImageJ software.
2.06 Cell culture and differentiation
As in many other published studies to investigate various effects on macrophages in vitro [reviewed in 25], THP-1 cells (TIB-202, ATCC, Manassas, VA), a monocytic leukemia cell line, were cultured for fewer than 30 passages following ATCC recommendations, then differentiated to cells bearing immunologic, morphologic, and functional characteristics of macrophages [reviewed in 25]. All media used fetal bovine serum (FBS, ATCC) that was heat-inactivated for 30 minutes at 56°C in order to prevent complement activation. To differentiate THP-1 monocytes, cells were collected by centrifugation at 500 x g for 5 minutes at 23°C and resuspended in fresh media with 0.01 μM phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich #P1585, St. Louis, MO) and 1 μM retinoic acid (RA, Sigma #R4643). After a brief vortex, 1 mL of the suspended cells was dispensed to each well of a tissue-culture-treated 24-well plate (Costar #3526, Corning, Corning, NY), followed by incubation for 24 hours at 5% CO2 and 37°C, at which point the cells became adherent and morphologically distinct. The media was aspirated off and replaced with 1 mL media containing 1 μM RA. The plate was then further incubated at 5% CO2 and 37°C for 78 hours, a time when our pilot studies showed cellular expression of typical macrophage markers (e.g., CD11b, CD14, HLA-DR, CD35, CD36) and maximal expression of CR1.
2.07 Aβ capture and uptake by THP-1-derived macrophages
THP-1-derived macrophages were incubated for 1.5 hours at 5% CO2 and 37°C with 10% NHS in RPMI-1640 (#30-2001, ATCC) containing 1 μM FITC-conjugated Aβ alone or 1 μM FITC-conjugated Aβ plus 0.1 μM Aβ antibody 4G8 to permit formation of Aβ ICs. In order to control for specificity to complement reactions, parallel wells employed heat-inactivated or C1q-depleted NHS, and Fc receptor-mediated and non-specific antibody binding were blocked in all wells by replacing the culture media with 1 μM human IgG (Sigma, #I4506) in RPMI-1640 for 30 minutes prior to assay. After incubation of THP-1-derived macrophages with Aβ alone or Aβ ICs under the various experimental and control conditions, PBS was added to each well and adherent cells were disassociated from the plate by pipetting. Cells were transferred to 1.5 mL microcentrifuge tubes and spun for 5 minutes at 500 x g at 23°C, resuspended in 2% paraformaldehyde in PBS, incubated 20 min, spun again, resuspended in PBS, and assayed for Aβ using flow cytometry. Here, the cells were incubated with fluorophore-conjugated primary antibodies directed against five common macrophage antigens: PE-conjugated mouse monoclonal anti-CR1 (Biolegend, San Diego, CA, Clone E11 PE: #333406), PE/Cy5-conjugated rat monoclonal anti-CD11b (Biolegend, Clone M1/70 APC-Cy7: #101226), Pacific Blue-conjugated mouse monoclonal anti-CD14 (Biolegend, Clone M5E2 PacBlue: #301815), APC/Cy7-conjugated mouse monoclonal anti-HLA-DR (Biolegend, Clone L243 APC-Cy7: #307606), and APC-conjugated mouse monoclonal anti-CD36 (Biolegend, Clone 5-271 APC, #336208). Incubation was for 30 minutes with rapid shaking at 4°C, after which the cells were spun 3 minutes at 1000 x g, washed once with PBS, and resuspended in PBS. Compensation controls were run with IgG antibody beads (Becton-Dickinson #552843 or 552845, San Jose, CA) appropriate for antibody host species, and received the same treatment as the cells. To determine compensation for fluorescent Aβ, fluorescent Aβ and mouse anti-human Aβ antibody (6E10, Biolegend) were added to regular mouse compensation beads. Cells were run on a Becton-Dickinson LSR II flow cytometer and analyzed with FlowJo v10.1 (Ashland, OR). Only cells that gated positive for all five of the macrophage markers were included in the final analysis. Using these stringent criteria, median fluorescence of FITC-conjugated Aβ/cell was used as a measure of Aβ capture by THP-1-derived macrophages in each condition.
2.08 Histology
To complement the quantitative assessment by flow cytometry, uptake of Aβ and Aβ ICs in THP-1-derived macrophages was qualitatively assessed using fluorescence and confocal microscopy. Macrophages were differentiated as described above, but were grown on glass coverslips instead of culture plates. After exposure to 1 μM fluorescent Aβ or Aβ/4G8 ICs (1 μM Aβ plus 0.1 μM 4G8), as in the flow cytometry studies, the cells were stained with a cell membrane marker (Sigma, # PKH26) and fixed with 2% paraformaldehyde (Electron Microscopy Services, Hatfield PA, #15714-5) in PBS. The glass coverslips were inverted and mounted on glass slides with DAPI-containing mounting medium (ProLong Gold with DAPI (Thermo Fisher). Images were collected on a Nikon A1 confocal microscope.
2.09 Clearance of IV-administered Aβ and Aβ ICs in non-human primates
To demonstrate functional relevance of the above mechanisms, two Cynomolgus macaque monkeys, ages 28 and 25 years old, received saphenous vein injections of either 3.0 mg Aβ42 alone or 3.0 mg Aβ42 that had previously been incubated with 11.1 mg Aβ antibody 4G8 to form Aβ ICs. Although the latter amounts to only a 9:1 molar ratio of Aβ to antibody, our earlier complement activation assays (see Section 3.01, below) indicated near-asymptotic responses to this ratio, and higher titers of antibody would have been prohibitively expensive (a request to obtain relevant doses of bapineuzumab from the license holder was denied). Femoral artery blood samples were taken in EDTA tubes 5 minutes before and 1, 5, 10, 15, 20, and 40 minutes after IV infusion of Aβ or Aβ ICs. Plasma and erythrocyte samples were processed as previously described [26], and were assayed using Wako Aβ42 ELISA kits (#298-62401, Richmond, VA) following the manufacturer’s recommendations throughout. To control for baseline differences in plasma and erythrocyte Aβ levels in the animals, the data were normalized as percent of Aβ recovered in the erythrocyte compartment relative to the amount available in the plasma compartment.
2.10 Statistics
Parametric statistical tests (ANOVA, repeated measures ANOVA, Pearson’s R) were employed throughout. Significance levels were two-tailed.
3. Results
3.01 Aβ activation of complement is enhanced by formation of Aβ ICs
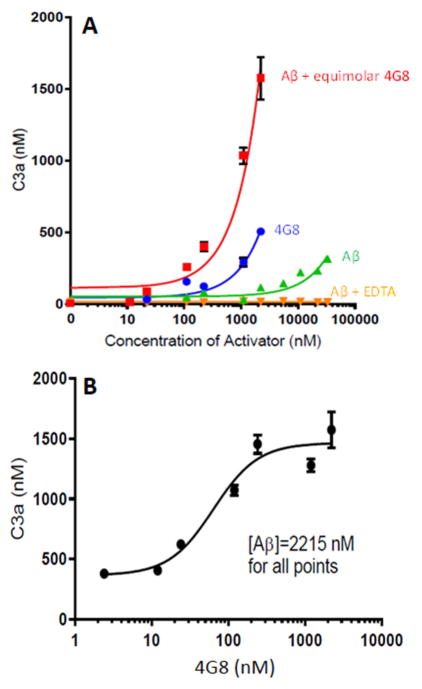
Replicating our previous work [23,26,27] and that of others [28,29], Aβ alone was found to activate complement in a dose-dependent fashion (F = 203.9, P < 0.001). Prior incubation of Aβ with Aβ antibody 4G8 to form Aβ/4G8 ICs significantly enhanced activation by as much as 34-fold compared to Aβ alone (Foverall = 110.6, P < 0.001) (Fig. 1A). Complement activation by Aβ ICs was significantly dose-dependent both when 4G8 concentration was equimolar to increasing concentrations of Aβ (F = 95.6, P < 0.001) (Fig. 1A) or when Aβ concentration was held constant and 4G8 concentrations were varied (F = 76.4, P < 0.001) (Fig. 1B). Notably, maximum enhancement of complement activation by Aβ ICs occurred at an Aβ:4G8 molar ratio of approximately 9:1 (Fig. 1B). This is consistent with the fact that Aβ aggregates are required to activate complement [23]; steric hindrance and epitope masking in Aβ aggregates would therefore make it unlikely that all Aβ moieties in an Aβ aggregate could simultaneously be bound by an Aβ Ig.
Fig. 1. Complement activation by Aβ and Aβ/4G8 ICs.
A) Aβ42 was incubated with equimolar Aβ antibody 4G8 to form Aβ/4G8 ICs, then reacted with NHS as a complement source. Significant dose-dependent complement activation (P < 0.001), as measured by C3a generated, was observed for Aβ ICs even at low nM concentrations. Aβ alone and 4G8 alone also dose-dependently activated complement (P < 0.001), but required μM concentrations to stimulate C3a production above background levels. EDTA abolished these effects, showing that they are specific to complement mechanisms. B) Complement activation by Aβ ICs could also be shown to be dose-dependent with respect to the amount of Aβ antibody available (P < 0.001). Here, a constant amount of Aβ (2215 nM) was incubated with varying concentrations of Aβ antibody 4G8, from low to equimolar concentrations relative to Aβ. Note that the X-axis is log-scaled in these graphs to include the wide range of concentrations. For both figures, each data point represents the mean of triplicate samples. Error bars denote standard error of the mean. Standard error for replicates is not shown when they are smaller than the symbols for a particular concentration.
Spontaneous aggregation of antibodies in vitro is known to provide the multiple binding sites necessary to activate C1 [c.f., 23]. Thus, Aβ antibody 4G8 alone also dose-dependently activated complement (F = 108.2, P < 0.001), though, by comparison, the activation was again far less than that for Aβ ICs (F = 181.7, P < 0.001) (Fig. 1A). EDTA, a standard control for specificity to complement reactions, completely abolished the effects in all conditions.
3.02 Opsonization of Aβ is enhanced by formation of Aβ ICs
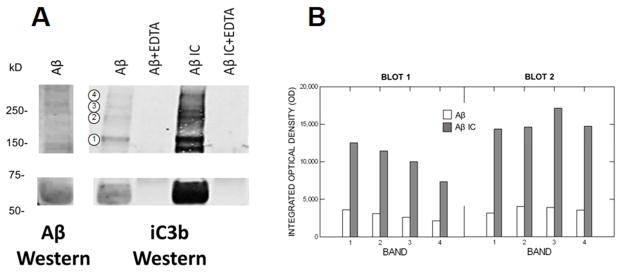
Following complement activation, complement opsonins such as iC3b bind back to the activating substrates. Because this binding is covalent, the opsonins remain attached to the activator even under the reducing/denaturing conditions of conventional SDS/PAGE Western blots. When Aβ alone or Aβ/4G8 ICs were incubated with NHS to permit complement activation, Aβ IC solutions exhibited markedly enriched iC3b immunoreactivity on iC3b Western blots compared to Aβ alone (Fig. 2A), consistent with the enhanced complement activation provided by Aβ ICs. As expected, immunoreactivity for iC3b (observed as iC3b fragments under reducing/denaturing conditions) was evident across multiple molecular weights, since multiple aggregate species of Aβ can activate and bind complement opsonins [23]. EDTA abolished iC3b immunoreactivity in both Aβ IC and Aβ samples (Fig. 2A), demonstrating that the iC3b was generated by complement reactions specific to Aβ ICs or Aβ. Pre-depletion of endogenous Igs and iC3b by immunoprecipitation in a second blot had no detectable effect on these results, and the depleted blot could not be distinguished visually or by densitometry (F = 1.12, P = 0.307) from the undepleted blot, showing that the opsonization observed is the result of de novo complement activation and Aβ IC formation in the experiments. Densitometry of the Ig/iC3b-depleted and undepleted blots gave a nearly identical pattern of results, with immunoreactivity for parallel bands in the Aβ IC condition exhibiting from 3-fold to 5-fold increases in iC3b compared to Aβ alone (F = 20.51, P = 0.001) (Fig. 2B). The addition of EDTA to Aβ and Aβ IC solutions reduced densitometry values to background or less.
Fig. 2. Complement opsonization of Aβ and Aβ/4G8 ICs.
A) After exposure to NHS to permit complement activation, Aβ alone and Aβ IC solutions were run on SDS reducing/denaturing Western blots to detect the complement opsonin iC3b. EDTA treatment (lanes 2 and 4 of the iC3b blot) abolished complement activation. The absence of iC3b immunoreactivity after EDTA treatment therefore shows that the iC3b detected in Aβ alone and Aβ IC conditions (lanes 1 and 3 of the iC3b blot) was specifically generated by Aβ and Aβ IC complement activation, and not by endogenous levels of iC3b. Compared to Aβ alone, iC3b immunoreactivity was markedly enhanced by Aβ ICs, consistent with enhanced complement activation by Aβ ICs in our earlier experiments. A parallel Western blot for Aβ (6E10 detection antibody) using the Aβ solution employed in the experiment is shown in the left-most lane. Note that bands for iC3b and Aβ aggregate forms typically coincide, as would be predicted given that iC3b is covalently bound to Aβ aggregate forms that can activate complement. B) Densitometry of the blot in panel A, as well as a second blot, prepared in the same way but pre-depleted of endogenous Igs and iC3b, gave a similar pattern of results that did not differ significantly from blot to blot (P = 0.307), with both showing highly-enriched iC3b immunoreactivity in Aβ IC compared to Aβ alone samples (F = 71.65, P = 0.001). For example, integrated optical density of the molecular weight bands designated by the four circled numbers on panel A were increased by 3-fold to 5-fold in the Aβ IC condition.
3.03 Erythrocyte capture of Aβ is enhanced by formation of Aβ ICs
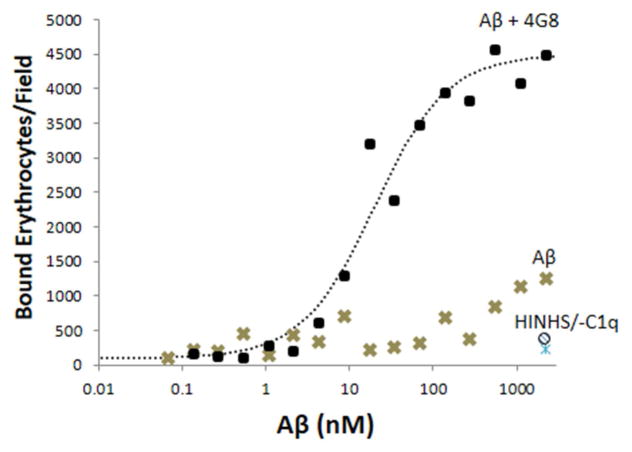
Once opsonized by complement, the opsonized complexes can bind to CR1 expressed on the surface of primate erythrocytes. They are then ferried to the liver and stripped off by Kupffer cells for degradation [30,31]. To assay erythrocyte binding, Aβ or Aβ ICs in various concentrations were coated on 96-well plates, incubated with serum, then incubated with erythrocytes. Consistent with the ability of Aβ ICs to enhance complement activation and opsonization, as shown above (Figs. 1, 2), binding of erythrocytes to Aβ ICs was dramatically enhanced compared to Aβ alone (F = 93.7, P < 0.001), with increases of more than 10-fold at many Aβ IC concentrations (Fig. 3). Specificity to complement opsonization, as opposed to non-specific interactions with Igs or capture by non-complement-mediated mechanisms, was demonstrated by use of heat-inactivated NHS and C1q-depleted NHS, both of which abolish complement opsonization and/or classical pathway complement activation and both of which reduced adhesion to background levels (Fig. 3).
Fig. 3. Erythrocyte capture of Aβ and Aβ ICs.
In erythrocyte adhesion assays, erythrocytes bound to Aβ-coated and Aβ/4G8 IC-coated plates in a dose-dependent manner (P < 0.001) and binding was significantly greater for Aβ ICs (P < 0.001). Abolition of effects by heat-inactivated NHS at the highest dose of Aβ ICs and Aβ alone (HINHS and ○ symbol at bottom right of graph) demonstrated specificity to complement reactions as opposed to erythrocyte binding by mechanisms other than complement. Abolition of effects by C1q-depleted serum (-C1q and △ symbol at bottom right of graph) confirmed this finding and, in addition, strongly suggested that erythrocyte capture of Aβ is primarily mediated by the classical, rather than the alternative complement pathway. Each data point represents the mean of duplicates. The vast majority of data points exhibited standard errors that were too small to show clearly. For the Aβ alone condition, the average standard error of the mean was 111.3 bound erythrocytes/field (range of 7.8 to 422.5). For the Aβ IC condition, the average standard error of the mean was 321.0 bound erythrocytes/field (range of 9.9 to 996.7).
3.04 Aβ capture and uptake by THP-1-derived macrophages is enhanced by formation of Aβ ICs
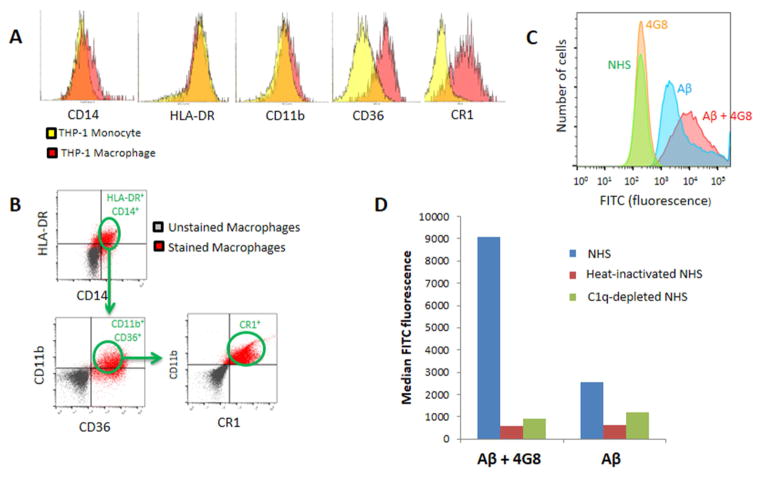
THP-1-derived macrophages were characterized by flow cytometry for expression of typical macrophage markers such as CD14, HLA-DR, CD11b, CD36, and CR1 (Fig. 4A). The cells were then exposed to fluorescence-labeled Aβ alone or fluorescence-labeled Aβ ICs in the presence of NHS, heat-inactivated NHS, or C1q-depleted NHS, followed by flow cytometry assay for capture of Aβ. By gating for expression of CD14, HLA-DR, CD11b, CD36, and CR1 (Fig. 4B), only cells that expressed appropriate macrophage markers were evaluated for Aβ. When incubated with NHS to permit complement opsonization, detection of cell-associated Aβ fluorescence was increased by nearly 4-fold in the Aβ IC condition compared to Aβ alone (Figs. 4C,4D). These results appeared to be mediated almost wholly by macrophage binding of complement-opsonized Aβ and Aβ ICs, as opposed to non-complement-dependent mechanisms, since THP-1-derived macrophages were pretreated with IgG to block Fc receptor-mediated and non-specific antibody binding. Moreover, Aβ uptake was reduced to background levels when heat-inactivated NHS and C1q-depleted NHS were employed as complement sources (Fig. 4D). Heat-inactivated NHS blocks all complement pathways, whereas C1q-depleted NHS blocks the classical pathway but should permit alternative pathway activation. These data for macrophages and the above data for erythrocytes therefore suggest that classical pathway activation is a key mechanism in THP-1-derived macrophage and erythrocyte binding of Aβ and Aβ ICs.
Fig. 4. Macrophage capture of Aβ and Aβ ICs.
A) Flow cytometry characterization of THP-1 monocytes and their differentiation to THP-1-derived macrophages revealed a transition to increased expression of inflammatory markers such as CD36, a mediator of macrophage phagocytosis [25], and, especially, CR1, the macrophage receptor for the major complement opsonins in primates [34,35]. B) To insure assessment of the macrophage-specific phenotype in subsequent experiments, all cells were gated for criterion expression of five different macrophage markers, CD14, HLA-DR, CD11B, CD36, and CR1. C) Fluorescence intensity distributions for samples pre-incubated with NHS. Distributions designated NHS and 4G8 represent fluorescence of the macrophage markers on which the cells were gated. Fluorescence distributions for gated, FITC-labeled Aβ and Aβ IC samples appear in the right-hand portion of the graph. A total of 100,000 cells were evaluated for each sample. D) Median fluorescence intensity of Aβ captured by THP-1-derived macrophages increased nearly 4-fold for Aβ ICs compared to Aβ alone. Only background readings were obtained for the cells when heat-inactivated NHS or C1q-depleted serum were substituted for NHS as a complement source.
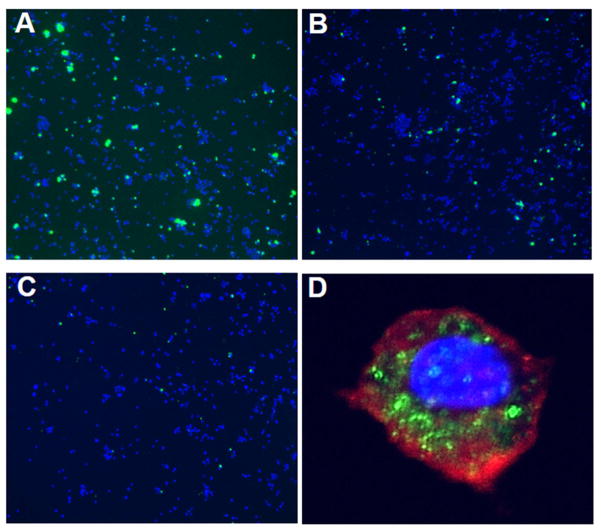
Enhanced uptake of Aβ ICs by THP-1-derived macrophages could also be demonstrated qualitatively using fluorescence microscopy (Figs. 5A–5C). Here, THP-1-derived macrophages were incubated with FITC-conjugated Aβ/4G8 ICs (1 μM Aβ plus 0.1 μM 4G8) (Fig. 5A) or FITC-conjugated Aβ alone (1 μM) (Fig. 5B) in the presence of NHS to permit complement activation. Aβ fluorescence was clearly more intense in Aβ/4G8 IC-treated macrophages, and was evident in many more cells compared to Aβ alone. Heat inactivation of serum, which abolishes complement activation, reduced Aβ detection to background (Fig. 5C). Evaluation by confocal microscopy (Fig. 5D) strongly suggested that Aβ and Aβ ICs were not only bound by THP-1-derived macrophages, but also were phagocytosed by the cells.
Fig. 5. Macrophage uptake of Aβ and Aβ ICs.
A–C) Representative fluorescence micrographs (10X) of THP-1-derived macrophages (DAPI nuclear counterstain) (blue) after exposure to 1 μM FITC-conjugated Aβ plus 0.1 μM Aβ antibody 4G8 to form Aβ ICs (A) or 1 μM FITC-conjugated Aβ alone (B). Aβ IC and Aβ alone solutions were previously incubated with NHS to permit complement activation. The number of cells co-localized with Aβ (green), as well as their fluorescence intensity, was clearly enhanced by Aβ ICs. Heat inactivation of the serum reduced staining to background (C). D) Representative high power (40X objective) confocal micrograph of a macrophage after exposure to FITC-conjugated Aβ plus 4G8 and NHS. PKH26 (red) was used to stain the cell membrane and DAPI (blue) was used to stain the nucleus. Localization of the FITC label for Aβ (green) was primarily intracellular, suggesting that the macrophages had phagocytosed the Aβ ICs.
3.05 Functional effects of Aβ ICs on peripheral clearance of Aβ in non-human primates
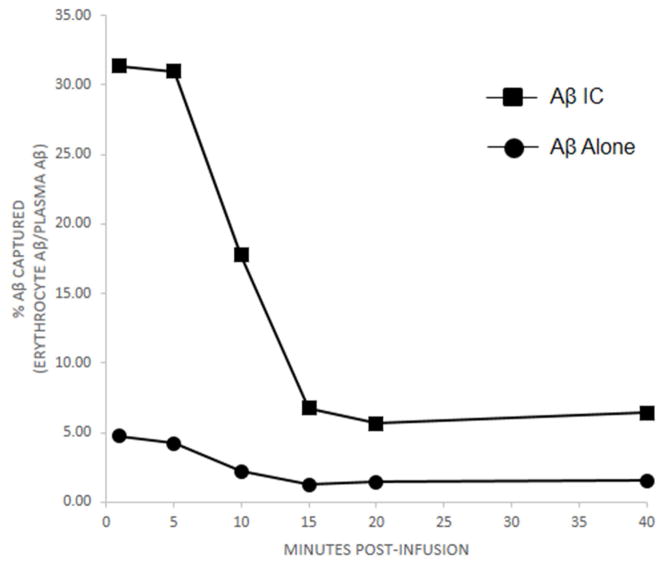
In order to study the effects of Aβ IC clearance in vivo, two Cynomolgus macaque monkeys received saphenous vein injections of either Aβ alone or Aβ that had previously been incubated with Aβ antibody 4G8 to form Aβ ICs. In both the Aβ IC and Aβ alone conditions, levels of IV-injected Aβ42 in the plasma and erythrocyte compartments rose steeply within 1 minute of infusion, the earliest time point that was possible to sample, and declined to near baseline over the next 15 minutes, similar to the kinetics we [26] and others [32] observed in a previous study with Aβ40. Compared to Aβ alone, erythrocyte capture of Aβ ICs from the plasma compartment was significantly increased overall (by repeated measures ANOVA, F = 1088.5, P < 0.001) and by more than 6-fold at the earliest time points (Fig. 6).
Fig. 6. Erythrocyte uptake and clearance of Aβ and Aβ ICs in non-human primates.
Relative to the amount of Aβ available in the plasma compartment, erythrocyte uptake and clearance of Aβ inoculated into two non-human primates was significantly greater for Aβ ICs compared to Aβ alone (P < 0.001). Because erythrocyte and plasma levels at baseline differed in the animals, the data in the figure have been normalized as percent of Aβ recovered in the erythrocyte compartment relative to the amount available in the plasma compartment. Clearance into the erythrocyte compartment was rapid over the first 15 minutes after Aβ or Aβ IC injection, a characteristic of immune adherence [41]. By 20 minutes, Aβ IC values had returned to baseline, but remained elevated by some 20% relative to baseline for Aβ alone. Samples at each time point in each animal were assayed in duplicate. Standard error bars for each time point for each animal were smaller than the symbols at each time point and are not shown. For Aβ alone, mean standard error was 0.13% (range of 0.01 to 0.39). For Aβ IC, mean standard error was 0.82% (range of 0.10 to 2.81).
4. Discussion
The present experiments follow up and are completely consistent with our prior findings [26] that blood Aβ is subject to peripheral clearance by a major pathway for circulating pathogens in primates, immune adherence [30,31]. CR1-mediated erythrocyte immune adherence, as well as CR1-mediated macrophage capture of pathogens, requires complement activation and opsonization. Although some pathogens, including Aβ [26–29], can, by themselves, stimulate complement activation and opsonization through binding of the pathogen to C1q, these processes are markedly enhanced when the pathogens form immune complexes, which bind with higher affinity to C1q. Thus, in the present experiments, the formation of Aβ ICs, as would occur with active and passive Aβ immunotherapy, increased Aβ activation of fluid phase complement by as much as 34-fold. This enhancement was predictably followed by multi-fold increases in complement opsonization, erythrocyte adherence, and macrophage phagocytosis. In vivo, capture of Aβ through the erythrocyte clearance pathway was also robustly increased for Aβ ICs compared to Aβ.
Whether generated in the blood by active immunization or infused into the blood by passive immunization, Aβ antibodies are, first and foremost, directly exposed to circulating Aβ, permitting the formation of Aβ ICs. Theoretically, there is, in fact, enough Aβ in the plasma compartment to form ICs with all the bapineuzumab infused into the average human being after a typical 1 mg/kg dose (assuming an average body weight and blood volume of 80.7 kg and 4.7 liters, respectively, and an average plasma Aβ level of 500 pg/ml [33–36]). Although, in practice, antibodies do not bind antigens with 100% efficiency (the KD for bapineuzumab is reported to be 89 nM for soluble Aβ40 [37]), the amount of Aβ antibody available to enter the brain should still be substantially limited by formation of Aβ ICs in the blood and their subsequent clearance by the mechanisms studied in this report. Antibodies surviving these processes would then also have to penetrate the blood-brain barrier. It may not be surprising, therefore, that the concentrations of Aβ antibodies that reach the brain are estimated to be <0.1% of the amount administered peripherally, and many orders of magnitude less than the molar amount of Aβ present in the AD brain [12–14].
Data on plasma Aβ levels after Aβ immunotherapy [reviewed in 38] and after experimental infusion of Aβ antibodies in AD transgenic mice [9,11] have been reported in some cases. Although these studies typically find increased plasma Aβ after treatment, they do not necessarily contradict the present results, and, in fact, may support them. Efflux of CNS Aβ into the plasma compartment after treatment appears to be rapid and extensive, and, after treatment, virtually all of the Aβ in plasma appears to be in the form of Aβ ICs [9]. However, if these high levels of Aβ ICs were simply “sequestered” in the circulation, as presently assumed, they would almost certainly lead to immune complex disorders, where high levels of uncleared immune complexes lodge in various organs and cause significant pathology [reviewed in 39]. Subsequent infusions of Aβ antibody, as in AD immunotherapy, would further compound this problem. Thus, the data on increased plasma Aβ after treatment with Aβ antibody antibodies actually demands an active peripheral clearance mechanism such as immune adherence in order to avoid immune complex disease in the recipients. The Aβ ICs cannot simply persist and build up. A more accurate representation would therefore be that the enhanced efflux of CNS Aβ into the plasma seen after Aβ infusion is interactive with the enhanced clearance of peripheral Aβ shown in the present experiments. AD immunotherapy studies on plasma alone do not (and cannot) measure this latter contribution, whereas our experiments, using infusions of Aβ ICs (as opposed to only the antibodies themselves) do so.
Although it is unquestionable that AD is a CNS disease, there is ample precedent for peripheral influences on brain disorders (e.g., effects of diabetes on the CNS) [reviewed in 40]. Whether considered in light of the “peripheral sink hypothesis” [9–11] or some other mechanism [32], impaired peripheral clearance of Aβ, as shown for AD subjects in our previous research [26], could not be favorable for brain concentrations of Aβ, whereas enhanced peripheral clearance by Aβ antibodies, as shown by the present data, should be beneficial. Accordingly, whether or not the Aβ antibodies provided by Aβ immunotherapy actually penetrate to the brain in sufficient amounts to assist removal of brain Aβ, the peripheral effects of such antibodies should not be ignored. Likewise, whether or not normal erythrocyte and macrophage peripheral clearance pathways are sufficient to deal with the increased plasma Aβ brought about by AD immunotherapy [38], approaches to enhance peripheral, complement-mediated removal of circulating pathogens have been reported and might be considered for Aβ. For example, a bispecific antibody that bound both the pathogen and the erythrocyte receptor for complement-opsonized complexes, CR1, has been shown to substantially enhance clearance of the pathogen [41].
RESEARCH IN CONTEXT.
Systematic Review
Although amyloid β peptide (Aβ) immunotherapy for Alzheimer’s disease (AD) continues to be pursued, critical questions still remain with respect to its mechanism and sites of action. This is particularly true given the incomplete penetration of peripherally-administered Aβ antibodies into the CNS compared to the full penetration of the antibodies in the peripheral circulation. Interpretation:Our findings demonstrate a heretofore unexplored mechanism wherein Aβ immune complexes (ICs) dramatically enhance virtually all steps in complement-mediated peripheral clearance of Aβ compared to Aβ alone. These enhanced effects of Aβ ICs apply to erythrocyte clearance of Aβ (immune adherence), as well as to macrophage clearance of Aβ. They therefore warrant consideration as additional mechanisms by which Aβ immunotherapy may act. Future Directions:Complement receptor 1 (CR1) plays a pivotal role in erythrocyte and macrophage clearance mechanisms, and polymorphisms in CR1 are a known risk factor for AD. Demonstrating that these polymorphisms predictably alter peripheral Aβ clearance, a task we are pursuing, would further indicate the potential importance of peripheral mechanisms in Aβ pathogenesis and therapeusis.
Acknowledgments
Experiments on human erythrocyte Aβ uptake were supported by the National Institute on Aging of the National Institutes of Health under award number RO1AG07367. Experiments on human CR1 were supported by the National Institute on Aging of the National Institutes of Health under award number RO1AG039750. Studies with non-human primates were supported by SRI International. We thank Priya Asok and Crystal Caldwell for phlebotomy services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lannfelt L, Relkin NR, Siemers ER. Amyloid-β-directed immunotherapy for Alzheimer’s disease. J Intern Med. 2014 Mar;275(3):284–95. doi: 10.1111/joim.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bussière T, Bard F, Barbour R, Grajeda H, Guido T, et al. Morphological characterization of Thioflavin-S-positive amyloid plaques in transgenic Alzheimer mice and effect of passive Abeta immunotherapy on their clearance. Am J Pathol. 2004 Sep;165(3):987–95. doi: 10.1016/s0002-9440(10)63360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bard F, Cannon C, Barbour R, Burke RL, Games D, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000 Aug;6(8):916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 4.Gilman S, Koller M, Black RS, et al. Clinical effects of A-beta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 5.Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol. 2010 Sep;120(3):369–84. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 6.Wes PD, Sayed FA, Bard F, Gan L. Targeting microglia for the treatment of Alzheimer’s Disease. Glia. 2016 Oct;64(10):1710–32. doi: 10.1002/glia.22988. [DOI] [PubMed] [Google Scholar]
- 7.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006 Nov;65(11):1040–8. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 8.Sha S, Xing XN, Cao YP. Active immunotherapy facilitates Aβ plaque removal following through microglial activation without obvious T cells infiltrating the CNS. J Neuroimmunol. 2014 Sep 15;274(1–2):62–70. doi: 10.1016/j.jneuroim.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 9.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001 Jul 17;98(15):8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci. 2002 May;5(5):452–7. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 11.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002 Mar 22;295(5563):2264–7. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 12.Levites Y, Smithson LA, Price RW, Dakin RS, Yuan B, Sierks MR, et al. Insights into the mechanisms of action of anti-Abeta antibodies in Alzheimer’s disease mouse models. FASEB J. 2006 Dec;20(14):2576–8. doi: 10.1096/fj.06-6463fje. [DOI] [PubMed] [Google Scholar]
- 13.Goure WF, Krafft GA, Jerecic J, Hefti F. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer’s disease immunotherapeutics. Alzheimers Res Ther. 2014 Jul 9;6(4):42. doi: 10.1186/alzrt272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golde TE. Open questions for Alzheimer’s disease immunotherapy. Alzheimers Res Ther. 2014 Jan 7;6(1):3. doi: 10.1186/alzrt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irani SR, Vincent A. NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11:298–304. doi: 10.1007/s11910-011-0186-y. [DOI] [PubMed] [Google Scholar]
- 16.Panzer J, Dalmau J. Movement disorders in paraneoplastic and autoimmune disease. Curr Opin Neurol. 2011;24:346–353. doi: 10.1097/WCO.0b013e328347b307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Niu ZX. The structure, genetic polymorphisms, expression and biological functions of complement receptor type 1 (CR1/CD35) Immunopharmacol Immunotoxicol. 2009;31(4):524–35. doi: 10.3109/08923970902845768. [DOI] [PubMed] [Google Scholar]
- 18.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 19.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedraza O, Allen M, Jennette K, Carrasquillo M, Crook J, Serie D, et al. Evaluation of memory endophenotypes for association with CLU, CR1, and PICALM variants in black and white subjects. Alzheimers Dement. 2014;10:205–13. doi: 10.1016/j.jalz.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keenan BT, Shulman JM, Chibnik LB, Raj T, Tran D, Sabuncu MR, et al. A coding variant in CR1 interacts with APOE-epsilon4 to influence cognitive decline. Hum Mol Genet. 2012;21:2377–88. doi: 10.1093/hmg/dds054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin C, Li W, Yuan J, Xu W, Cheng Z. Association of the CR1 polymorphism with late-onset Alzheimer’s disease in Chinese Han populations: a meta-analysis. Neurosci Lett. 2012;527:46–9. doi: 10.1016/j.neulet.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Webster S, Bradt B, Rogers J, Cooper N. Aggregation state-dependent activation of the classical complement pathway by the amyloid beta peptide. J Neurochem. 1997;69:388–98. doi: 10.1046/j.1471-4159.1997.69010388.x. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson-Weller A. C1q and C4b bind simultaneously to CR1 and additively support erythrocyte adhesion. J Immunol. 1999;163:5056–63. [PubMed] [Google Scholar]
- 25.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014 Nov;23(1):37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Brubaker WD, Crane A, Johansson JU, Yen K, Garfinkel K, et al. Peripheral complement interactions with amyloid β peptide (Aβ) in Alzheimer’s disease: 1. Erythrocyte clearance of Aβ. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2017.03.010. this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, et al. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992 Nov 1;89(21):10016–20. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J Exp Med. 1998;188:431–8. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H, Burdick D, Glabe CG, Cotman CW, Tenner AJ. beta-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J Immunol. 1994;152:5050–9. [PubMed] [Google Scholar]
- 30.Hess C, Schifferli JA. Immune adherence revisited: novel players in an old game. News Physiol Sci. 2003;18:104–8. doi: 10.1152/nips.01425.2002. [DOI] [PubMed] [Google Scholar]
- 31.Birmingham DJ, Hebert LA. CR1 and CR1-like: the primate immune adherence receptors. Immunol Rev. 2001;180:100–11. doi: 10.1034/j.1600-065x.2001.1800109.x. [DOI] [PubMed] [Google Scholar]
- 32.Mackic JB, Weiss MH, Miao W, Kirkman E, Ghiso J, Calero M, et al. Cerebrovascular accumulation and increased blood-brain barrier permeability to circulating Alzheimer’s amyloid beta peptide in aged squirrel monkey with cerebral amyloid angiopathy. J Neurochem. 1998;70:210–5. doi: 10.1046/j.1471-4159.1998.70010210.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuo YM, Kokjohn TA, Kalback W, Luehrs D, Galasko DR, Chevallier N, et al. Amyloid-beta peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun. 2000;268(3):750–6. doi: 10.1006/bbrc.2000.2222. [DOI] [PubMed] [Google Scholar]
- 34.Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, et al. Plasma amyloid beta-peptide 1–42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46(3):412–6. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 35.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57(1):100–5. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 36.Tamaoka A, Fukushima T, Sawamura N, Ishikawa K, Oguni E, Komatsuzaki Y, et al. Amyloid beta protein in plasma from patients with sporadic Alzheimer’s disease. J Neurol Sci. 1996;141(1–2):65–8. doi: 10.1016/0022-510x(96)00143-8. [DOI] [PubMed] [Google Scholar]
- 37.Miles LA, Crespi GA, Doughty L, Parker MW. Bapineuzumab capures the N-terminus of the Alzheimer’s disease amyloid-beta-peptide in a helical conformation. Sci Rep. 2013;3:1302. doi: 10.1038/srep01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreth J, Mavoungou C, Schindowski K. Is abeta a sufficient biomarker for monitoring anti-abeta clinical studies? A critical review. Front Aging Neurosci. 2013 Jul 2;5:25. doi: 10.3389/fnagi.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvidio G, Andres G. Immune deposits and immune complex disease. Clin Exp Rheumatol. 1986;4:281–8. [PubMed] [Google Scholar]
- 40.Mooradian AD. Central nervous system complications of diabetes mellitus--a perspective from the blood-brain barrier. Brain Res Brain Res Rev. 1997;23(3):210–8. doi: 10.1016/s0165-0173(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 41.Taylor RP, Martin EN, Reinagel ML, Nardin A, Craig M, Choice Q, et al. Bispecific monoclonal antibody complexes facilitate erythrocyte binding and liver clearance of a prototype particulate pathogen in a monkey model. J Immunol. 1997;159:4035–44. [PubMed] [Google Scholar]