Abstract
Background
Many studies have evaluated predictors of postoperative complications, yet little is known about the development of multiple complications. The goal of this study was to assess complication timing in cascades of multiple complications and the risk of future complications given a patient’s first complication.
Methods
This study includes 30-day, postoperative complications from the American College of Surgeons National Surgical Quality Improvement Program for all patients who underwent major inpatient and outpatient operative procedures from 2005–2013. The timing and sequencing of complications were evaluated using χ2 analysis and pairwise comparisons.
Results
More severe postoperative complications (cardiac arrest or myocardial infarction, renal insufficiency or failure, stroke, intubation, septic shock, coma) had the greatest impact on the risk for developing further complications, increasing the relative risk of developing future, specific, severe complications by more than 40-fold. These more severe complications occur within a few days of other complications (whether as a preceding factor or an outcome), while less severe complications, such as surgical site infection and urinary tract infection, are linked less tightly to complication cascades.
Conclusion
This analysis highlights both the risk for secondary complications after an initial complication and when those future complications are likely to occur. Physicians can use this information to target interventions to prevent high-risk complications.
Surgical success requires managing preoperative risk factors and events during the operation, and reducing the occurrence of subsequent complications. Many studies have found that patients who suffer from postoperative complications have an increased risk of prolonged hospital stay, discharge to higher levels of care, greater rates of readmission and mortality, and greater cost of care.1–8
Recent work has investigated the postoperative timing of complications to understand critical points to monitor. These studies noted that many complications occur early, though different complications follow distinct temporal patterns.9–13 Wakeam et al14 found associations between the timing of complications and mortality, with different patterns depending on the type of complication.
Little is known, however, about how the timing of complications changes when multiple postoperative complications occur and how postoperative complications influence the risk of developing further complications. Tevis et al15 found that when multiple complications occur, there are associations between which complications occur postoperatively; however, the authors did not analyze the timing or sequence of complications. Assessing patterns in the development of multiple complications can identify opportunities to preemptively prevent complication cascades.
The purpose of this study was to characterize the timing of postoperative complications in the setting of multiple complications and assess how patient risk changes after complications occur. Our specific aims were to (1) evaluate how timing and relative ordering of complications changed when multiple complications occurred and (2) assess how each complication increased the risk of developing specific additional complications.
METHODS
Data
The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database includes preoperative risk factors, intraoperative variables, and 30-day postoperative outcomes for patients who underwent major inpatient and outpatient surgical procedures.16 ACS NSQIP data are collected by a trained Surgical Clinical Reviewer at each site and audited subsequently for reliability by the NSQIP program. This study includes cases from >435 institutions from 2005 to 2013. Patients included underwent both inpatient and outpatient procedures in surgical specialties tracked by the ACS NSQIP.
Complications included the 21 reported ACS NSQIP complications and postoperative mortality occurring within 30 days after the operation. Each recorded complication was annotated with the number of days after the operation that the complication was first diagnosed. If a complication was diagnosed multiple times postoperatively, only the first date of diagnosis was recorded.
Complications included infectious complications (superficial surgical site infection [SSI], deep SSI, organ space SSI, wound disruption, urinary tract infection (UTI), sepsis, and septic shock), physiologic complications (peripheral nerve injury, pneumonia, deep vein thrombosis or thrombophlebitis, pulmonary embolism, renal insufficiency, renal failure, stroke or cardiovascular incident, myocardial infarction [MI], cardiac arrest, or coma >24 hours), and interventional complications (unplanned intubation, cumulative ventilator-assisted respiration >48 hours, bleeding transfusion up to 72 hours postoperatively, and graft failure requiring intervention). Patients were excluded during quality control when the occurrence of complications did not fall within the specified time frame per NSQIP documentation.
Explanatory variables included patient characteristics, preoperative comorbidities, and operative factors. The following preoperative comorbidities as defined by ACS NSQIP were examined: weight loss, diabetes, smoking status, alcohol use, dyspnea, functional status, chronic obstructive pulmonary disease, hypertension, history of stroke, cancer, steroid use, and bleeding disorder. Examined operative factors included operation within the previous 30 days, wound classification, American Society of Anesthesiologists classification, intraoperative transfusion, whether it was an emergency operation, and operative time. Wound class included clean, clean contaminated, contaminated, and dirty.
Given the deidentified nature of the ACS NSQIP data, work with this data set has been deemed exempt by the University of Wisconsin Health Sciences Institutional Review Board.
Statistical analysis and characterization of complications
χ2 tests were used to compare candidate risk factors for patients with ≥2 complications to patients with zero or one complication. The frequency and timing of individual complications was determined for all patients diagnosed with ≥1 complication. The frequency of complications co-occurring with other complications was calculated by considering the percentage of patients diagnosed with each of the measured complications who also had other complications. The timing of each of these other complications was calculated relative to the date of the complication of interest. Descriptive statistics were analyzed in R (RStudio, Boston, MA) using “chisq” for χ2 calculations; all other data were analyzed with Matlab 2015a (MathWorks, Natick, MA).
To assess pairwise, sequential relationships between complications, we used the following approach. We evaluated the probability of each complication occurring initially given that specific other complications had occurred previously as follows: Let Ci(t) be a random variable representing whether or not the ith complication had occurred on or before day t for a given patient. We used ci(t) to denote the case where Ci(t) is true (ie, the complication has occurred) and ¬ci(t) to denote the case where it is false. The quantity represents the probability that the ith complication first occurred on day t given that the jth complication had occurred by day t−1. To consider relative risks, we also determined , which represents the probability of ith complication first occurring on day t, given that the jth complication has not yet occurred. To assess whether there was a sequential relationship between the pair of complications, we considered the ratio of these probabilities: . This analysis was done for all pairs of complications.
RESULTS
Study population profile and risk factors for multiple complications
The ACS NSQIP database contained 2,972,758 cases collected between 2005 and 2013. Of these, 390,646 cases (13.1%) experienced ≥1 complication, and 132,646 cases (4.5%) had multiple complications. Table I demonstrates patient characteristics, preoperative risk factors, and operative risk factors in association with multiple complications. The univariate analysis showed that patients with comorbidities, a dependent functional status, emergency operations, and greater operative times were found to have multiple complications more frequently.
Table I.
Risk factors for multiple complications (including mortality); demographics, preoperative risk factors, and operative risk factors
| All cases n (%) N = 2,972,758 |
0 complications n (%) N = 2,582,112 |
1 complication n (%) N = 257,970 |
2+ complications n (%) N = 132,646 |
P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Sex | |||||
| Female | 1,702,533 (57) | 1,493,294 (58) | 143,799 (56) | 65,440 (49) | |
| Male | 1,266,199 (43) | 1,085,331 (42) | 113,795 (44) | 67,073 (51) | |
| Unknown | 4,026 (<1) | 3,487 (<1) | 346 (<1) | 163 (<1) | <.001 |
| Race | |||||
| White | 1,268,771 (43) | 1,104,102 (43) | 104,332 (40) | 60,337 (45) | |
| Black | 172,163 (6) | 146,301 (6) | 15,272 (6) | 10,590 (8) | |
| Hispanic | 122,020 (4) | 110,595 (4) | 7,552 (3) | 3,873 (3) | |
| American Indian/Alaskan | 12,221 (<1) | 10,802 (<1) | 916 (<1) | 503 (<1) | |
| Asian/Pacific Islander | 43,078 (1) | 38,335 (1) | 3,075 (1) | 1,668 (1) | |
| Unknown | 1,354,505 (46) | 1,171,977 (45) | 126,823 (49) | 55,705 (42) | <.001 |
| Age (y) | |||||
| 18–64 | 1,961,461 (66) | 1,769,909 (69) | 131,859 (51) | 59,693 (45) | |
| 65+ | 1,011,288 (34) | 812,196 (31) | 126,109 (49) | 72,983 (55) | <.001 |
| Previous level of care | |||||
| Home | 2,850,319 (96) | 2,508,853 (97) | 232,961 (90) | 108,505 (82) | |
| Chronic care/nursing home | 33,330 (1) | 19,126 (<1) | 7,465 (3) | 6,739 (5) | |
| Outside ED | 27,739 (1) | 17,525 (<1) | 5,323 (2) | 4,891 (4) | |
| Acute care | 50,524 (2) | 28,540 (1) | 10,675 (4) | 11,309 (9) | |
| Other | 8,725 (<1) | 6,311 (<1) | 1,333 (<1) | 1,081 (<1) | |
| Unknown | 2,121 (<1) | 1,757 (<1) | 213 (<1) | 151 (<1) | <.001 |
| BMI | |||||
| <18.5 | 59,970 (2) | 45,168 (2) | 8,558 (3) | 6,244 (5) | |
| 18.5–24.9 | 767,500 (26) | 660,012 (25) | 69,851 (27) | 37,637 (28) | |
| 25–29.9 | 900,775 (30) | 790,183 (31) | 74,624 (29) | 35,968 (27) | |
| ≥30 | 1,173,237 (39) | 1,029,211 (40) | 96,969 (38) | 47,057 (35) | |
| Unknown | 71,276 (2) | 57,538 (2) | 7,968 (3) | 5,770 (4) | <.001 |
| Preoperative risk factors | |||||
| Diabetes | |||||
| Absent | 448,574 (15) | 359,113 (14) | 55,948 (22) | 33,513 (25) | |
| Present | 2,524,177 (85) | 2,222,992 (86) | 202,022 (78) | 99,163 (75) | <.001 |
| Smoker | |||||
| Absent | 2,398,372 (81) | 2,094,341 (81) | 203,784 (79) | 100,247 (76) | |
| Present | 574,361 (19) | 487,748 (19) | 54,184 (21) | 32,429 (24) | <.001 |
| Alcohol use | |||||
| Absent | 1,690,465 (57) | 1,482,116 (57) | 131,385 (51) | 76,964 (58) | |
| Present | 45,631 (2) | 37,500 (1) | 4,473 (2) | 3,658 (3) | |
| Unknown | 1,236,662 (42) | 1,062,496 (41) | 122,112 (47) | 52,054 (39) | <.001 |
| Dyspnea | |||||
| Absent | 2,715,169 (91) | 2,385,553 (92) | 224,201 (87) | 105,415 (79) | |
| With moderate exertion | 228,177 (8) | 18,137 (7) | 28,081 (11) | 18,717 (14) | |
| At rest | 29,385 (<1) | 15,160 (1) | 5,685 (2) | 8,540 (6) | <.001 |
| COPD | |||||
| Absent | 2,832,187 (95) | 2,481,606 (96) | 236,496 (92) | 114,085 (86) | |
| Present | 140,558 (5) | 100,494 (4) | 21,473 (8) | 18,591 (14) | <.001 |
| Hypertension | |||||
| Absent | 1,604,790 (54) | 1,452,626 (56) | 105,833 (41) | 46,331 (35) | |
| Present | 1,367,952 (46) | 1,129,473 (44) | 152,136 (59) | 86,343 (65) | <.001 |
| History of stroke | |||||
| Absent | 1,662,965 (56) | 1,464,927 (57) | 125,819 (49) | 72,219 (54) | |
| Present | 72,696 (2) | 54,313 (2) | 10,004 (4) | 8,379 (6) | <.001 |
| Cancer | |||||
| Absent | 2,911,212 (98) | 2,541,658 (98) | 245,739 (95) | 123,815 (93) | |
| Present | 61,529 (2) | 40,441 (2) | 12,228 (5) | 8,860 (7) | <.001 |
| Steroid use | |||||
| Absent | 2,874,212 (97) | 2,509,797 (97) | 243,260 (94) | 121,155 (91) | |
| Present | 98,529 (3) | 72,301 (3) | 14,708 (6) | 11,520 (9) | <.001 |
| Weight loss | |||||
| Absent | 2,920,420 (98) | 2,548,695 (99) | 247,872 (96) | 123,853 (93) | |
| Present | 52,320 (2) | 33,401 (1) | 10,096 (4) | 8,823 (7) | <.001 |
| Bleeding disorder | |||||
| Absent | 2,820,413 (95) | 2,476,912 (96) | 231,921 (90) | 111,580 (84) | |
| Present | 152,330 (5) | 105,187 (4) | 26,048 (10) | 21,095 (16) | <.001 |
| Functional status | |||||
| Independent | 2,823,382 (95) | 2,495,372 (97) | 227,492 (88) | 100,518 (76) | |
| Partially dependent | 104,250 (4) | 65,441 (3) | 20,929 (8) | 17,880 (13) | |
| Totally dependent | 34,097 (1) | 12,400 (<1) | 8,272 (3) | 13,425 (10) | |
| Unknown | 11,021 (<1) | 8,891 (<1) | 1,277 (<1) | 853 (<1) | <.001 |
| Operative risk factors | |||||
| Previous operation (30 days) | |||||
| Absent | 1,614,345 (54) | 1,423,994 (55) | 122,617 (48) | 67,734 (51) | |
| Present | 46,546 (2) | 30,086 (1) | 7,857 (3) | 8,603 (6) | <.001 |
| ASA classification | |||||
| 1 | 282,964 (10) | 274,364 (11) | 7,371 (3) | 1,229 (<1) | |
| 2 | 1,350,533 (45) | 1,256,136 (49) | 74,785 (29) | 19,612 (15) | |
| 3 | 1,133,113 (38) | 933,997 (36) | 133,146 (52) | 65,970 (50) | |
| 4 | 189,368 (6) | 107,840 (4) | 40,284 (16) | 41,244 (31) | |
| 5 | 7,164 (<1) | 892 (<1) | 1902 (<1) | 4,370 (3) | |
| Unknown | 9,616 (<1) | 8,883 (<1) | 482 (<1) | 251 (<1) | <.001 |
| Wound classification | |||||
| Clean | 1,577,064 (53) | 1,417,658 (55) | 117,525 (45) | 41,881 (32) | |
| Clean/contaminated | 1,015,721 (34) | 874,931 (34) | 92,019 (36) | 48,771 (37) | |
| Contaminated | 209,395 (7) | 173,544 (7) | 20,305 (8) | 15,546 (12) | |
| Dirty | 170,574 (6) | 115,975 (4) | 28,121 (11) | 26,478 (20) | |
| Unknown | 4 (<1) | 4 (<1) | 0 (0) | 0 (<1) | <.001 |
| Intraop transfusion | |||||
| Absent | 917,641 (31) | 828,367 (32) | 56,944 (22) | 32,330 (24) | |
| Present | 52,934 (2) | 29,581 (1) | 9,732 (4) | 13,621 (10) | <.001 |
| Emergency operation | |||||
| No | 2,638,865 (89) | 2,334,648 (90) | 214,274 (83) | 89,943 (68) | |
| Yes | 333,861 (11) | 247,433 (10) | 43,695 (17) | 42,733 (32) | <.001 |
| Operative time (mean, min) | 112 | 103 | 162 | 186 | <.001 |
ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ED, emergency department; Intraop, intraoperative.
Complication prevalence
Table II shows the frequency of complications in patients who experienced ≥1 complication. Bleeding requiring transfusion was the most common postoperative complication (37%), followed by superficial SSI (17%), sepsis (13%), and ventilator dependence >48 hours (13%), with infectious type complications prevalent overall.
Table II.
Frequency of individual complications out of all patients diagnosed with ≥1 postoperative complication
| Complication | Patients with complications N = 390,646 n (%) |
|---|---|
| Infection | |
| Superficial SSI | 65,711 (17) |
| Deep SSI | 19,952 (5) |
| Organ SSI | 34,890 (9) |
| Wound disruption | 14,914 (4) |
| UTI | 46,231 (12) |
| Sepsis | 50,047 (13) |
| Septic shock | 27,365 (7) |
| Physiologic | |
| Nerve injury | 1,077 (<1) |
| Pneumonia | 40,463 (10) |
| DVT | 19,299 (5) |
| Pulmonary embolism | 9,667 (3) |
| Renal insufficiency | 9,338 (2) |
| Acute renal failure | 11,723 (3) |
| Stroke/CVA | 6,806 (2) |
| MI | 9,942 (3) |
| Cardiac arrest | 10,690 (3) |
| Coma >24 h | 1,414 (<1) |
| Death | 39,575 (10) |
| Intervention | |
| Unplanned intubation | 33,496 (9) |
| On ventilator >48 h | 50,335 (13) |
| Bleeding transfusion | 140,413 (37) |
| Graft failure | 3,741 (1) |
CVA, Callout; DVT, deep vein thrombosis.
Relative timing and ordering of multiple complications
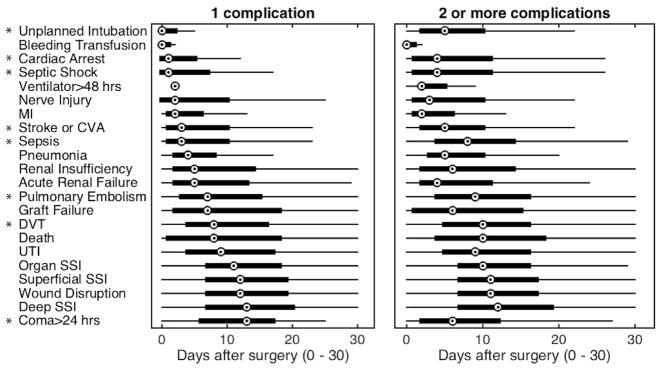
The distribution of the timing of complication is shown in Fig 1. Figure 1, A depicts the timing when only a single complication occurs, while Fig 1, B shows timing when multiple complications occur. Some complications occur with relatively consistent timing whether they occur in isolation or in patients suffering from multiple complications (eg, wound disruption and superficial SSI). The timing of other complications (such as septic shock and cardiac arrest) is very different in the setting of multiple complications. For example, superficial SSI occurs a median of 12 days after operation if only 1 complication occurs and at postoperative day 11 if there are multiple complications, whereas cardiac arrest changes from a median of 1 day postoperatively in isolation to 4 days postoperatively when there are multiple complications.
Fig 1.
Timing of the initial diagnosis of each complication after operation given that only one complication occurred or multiple complications (including mortality) occurred. The central mark of each boxplot is the median, box edges extend to the 25th and 75th percentiles, and whiskers extend to the most extreme data not considered outliers. Complications where the median timing changed by ≥2 days are marked (*).
To evaluate which complications occur as part of complication cascades, we evaluated the risk of experiencing multiple complications given a complication of interest (Table III). Complications, such as superficial SSI, nerve injury, and bleeding transfusion, occur with other complications approximately 30% of the time, while patients who experience coma and septic shock are much more likely to experience additional complications (98.7% and 93.4% of the time). In general, more severe complications occurred with other complications and less severe complications were more likely to occur in isolation.
Table III.
Risk for multiple complications (including mortality) given the occurrence of the listed complication
| Complication | Risk for multiple complications |
|---|---|
| Infection | |
| Superficial SSI | 29.6% |
| Deep SSI | 52.1% |
| Organ SSI | 65.6% |
| Wound Disruption | 63.4% |
| UTI | 46.7% |
| Sepsis | 77.6% |
| Septic shock | 93.4% |
| Physiologic | |
| Nerve injury | 30.3% |
| Pneumonia | 76.0% |
| DVT | 60.3% |
| Pulmonary embolism | 61.0% |
| Renal insufficiency | 73.2% |
| Acute renal failure | 87.5% |
| Stroke/CVA | 62.1% |
| MI | 68.6% |
| Cardiac arrest | 90.4% |
| Coma >24 h | 98.7% |
| Death | 74.2% |
| Intervention | |
| Unplanned intubation | 88.4% |
| On ventilator >48 h | 89.5% |
| Bleeding transfusion | 33.3% |
| Graft failure | 46.5% |
CVA, Cerebrovascular accident; DVT, deep vein thrombosis.
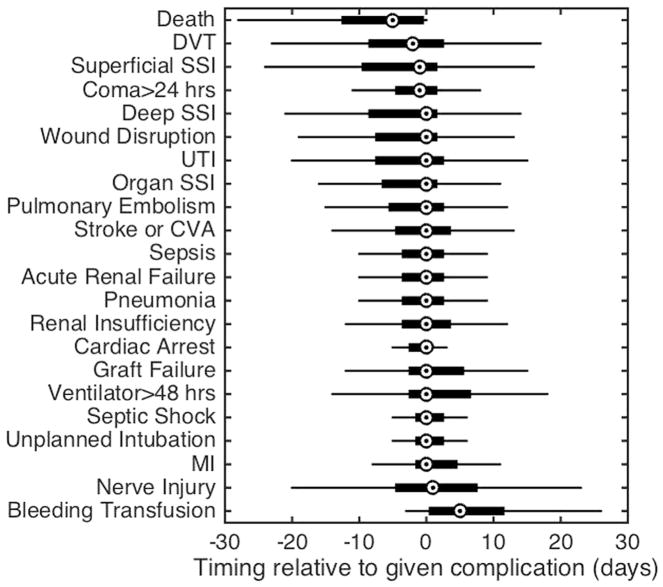
To further assess the ordering of multiple complications, we evaluated the timing of specific complications within a sequence (Fig 2). This analysis demonstrated that many complications occur or are listed as occurring on the same day as shown by timing differences with a median value of zero days. For example, deep SSI and wound disruption both have median values of zero days indicating that other complications are diagnosed on the same day; however, when examining the tails of the plot carefully, the analysis becomes much more interesting, because it reveals complications that occur early or late in the multiple complication cascade.
Fig 2.
Relative timing of other complications experienced given that patients had the listed complication. The central mark of each boxplot is the median, box edges extend to the 25th and 75th percentiles, and whiskers extend to the most extreme data not considered outliers.
For example, prolonged ventilation is remarkable, because while its median value is zero, the predominant tail is in the positive direction indicating that this complication occurs early in the complication cascade. In contrast, UTI also has a median value of zero, but its predominant tail is in the negative direction, indicating that UTIs tend to occur later in the cascade. Furthermore, even for complications that occur with equal prevalence, timing of other complications differs—for example, careful analysis of the complication “coma >24 hours” —demonstrates a median value with a tail that is predominantly negative, indicating that complications generally precede it. In contrast, septic shock has a median value of zero and a tail that is predominantly positive, indicating that it is diagnosed along with other complications or occurs before other complications.
Risk of a complication given a prior complication
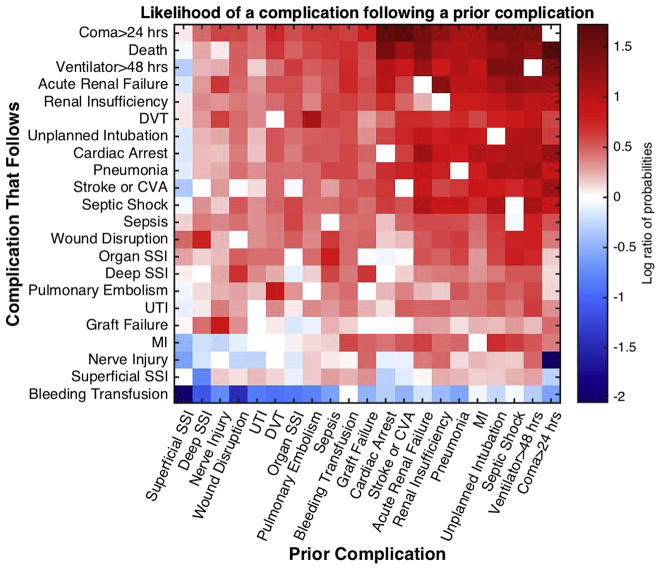
We then sought to assess which complications increased or decreased the risk of subsequent complications. Figure 3 demonstrates the extent to which the probability of a complication occurring changes if another specific complication occurred previously. In this heat map figure, red indicates the probability that a complication is increased by a specific prior complication, and the extent of the change is indicated by the depth of color. Careful analysis of these data indicates that some complications occur in isolation. For example, when superficial SSI is the first complication, it is often the only one with wound disruption having the greatest risk of occurrence after SSI.
Fig 3.
Relative risk of an additional complication given a prior complication. The probability of diagnosis of each complication after the diagnosis of a prior complication is divided by the overall probability of diagnosing that complication and plotted as a log ratio (base 10). Red indicates that the probability of a complication is increased by a given prior complication; blue indicates that the probability is decreased. (Color version of this figure is available online.)
In addition, the risk of suffering a superficial SSI after another complication is also not high. In contrast, if a patient suffers acute renal failure, the risk for developing another complication is quite high. Specifically, coma, death, or cardiac arrest are complications that are most likely to follow acute renal failure. Finally, when one examines those complications that are most likely to contribute to a complication cascade by looking at the rows of the heat map, not surprisingly, the serious complications, such as cardiac arrest or MI, renal insufficiency or failure, stroke, intubation, septic shock, and coma are in this list. This paired comparison shows which complications influence the risk of other complications occurring and the weight of each relationship.
DISCUSSION
This study demonstrated that temporal dependencies between multiple complications could be identified by analyzing the timing and ordering of complications. We found less severe complications were more likely to occur in isolation, while more severe complications tend to be associated with additional complications. We found further that complications, such as cardiac arrest or MI, renal insufficiency or failure, stroke, intubation, septic shock, and coma, increased the risk of complication cascades to a much greater extent than other complications—increasing the relative risk of subsequent complications by >40 times the risk if the prior complication had not occurred. Interestingly, the timing of specific complications changed if they occur as part of a cascade. Thus, using this analysis, we can see how the risks for future complications change after an initial complication and the riskiest time periods for those future complications to occur.
Previous studies have discussed how measures of poor overall health, such as older age, dependent functional status, American Society of Anesthesiologists classification, frailty, body mass index, and smoking, contribute to the development of complications.1,15,17–24 Our study found that similar factors measured in the NSQIP database predicted multiple complications. We also found that emergency operations had a greater risk of multiple complications (32% of patients with multiple complications had undergone an emergency operation vs only 10% of patients with ≤1 complications).
Our study expanded on work evaluating postoperative timing of complications.9–14 We found that 40% of complications occurred within the first 3 days after operation and noted similar trends as in previous work,11,13 such as earlier occurrences of severe complications (cardiac arrest and septic shock) and later occurrence of less severe complications (superficial SSI). In addition, we considered how the timing of some complications shifted when there were multiple complications. Coma occurred earlier with multiple complications; cardiac arrest, stroke, septic shock, and sepsis tended to shift to later times; and SSIs maintained similar timing regardless of other complications.
Some postoperative events, such as coma and septic shock, tend to occur within a few days of other complications, while there is a much greater range on the timing between SSIs and other complications, and those other complications tend to precede infections. Wakeam et al14 postulated that the correlation between late occurrence of severe complications, such as MI and stroke, and greater mortality may be present, because these complications are at the end of complication “cascades”; our work further expands on known temporal patterns among complications.
While Tevis et al15 found that there are predictable associations in which postoperative complications occur, we expanded this understanding by evaluating temporal dependencies in the sequences of multiple complications. Our analysis both highlighted similar relationships and brought forth new information about associations. Similar relationships included strong relationships between SSIs and the development of sepsis and between failure to wean from the ventilator and many other complications. New associations that we were able to measure by considering temporal dependencies included strong relationships between coma, MI or stroke, and other complications. Finally, this study moves from a retrospective analysis of a patient’s combination of multiple complications to allowing prospective assessment of likely postoperative complications.
More severe postoperative complications (cardiac arrest or MI, renal insufficiency or failure, stroke, intubation, septic shock, and coma) contribute to the development of further complications to the greatest extent and occur within a few days of other complications (whether as a preceding factor or an outcome). The complication cascades, in particular, the high-risk events highlighted in Fig 3, need to be expected, recognized, and acted on quickly.
Our study has limitations inherent to a retrospective analysis. We cannot detect if a patient was diagnosed with a complication multiple times, because the data set only includes the first date that the complication was diagnosed; thus, we considered the development of unique complications in this study instead of instances of repeat occurrences. Also, the data on complication diagnoses are only available at a temporal resolution of one day, which excludes evaluating dependencies between same day complications.
The standardization and size of the ACS NSQIP database also provides benefits that strengthen our study. The data are collected in a prospective manner by trained surgical clinical reviewers, complications are strictly defined in the database, and the national database has a large patient population, which allows us to assess the temporal dependencies in the <5% of patients who developed multiple complications.
Complication cascades contribute ultimately to poor outcomes and patient mortality. The timing and risk of progression after postoperative complications have been highlighted in this study. Future work will be required to evaluate the temporal and prognostic dependencies between complications through the development of a complication cascade. With knowledge of the greatest-risk complications and when associated complications are likely to occur, we may be better able to identify high-risk patients as postoperative risks increase and prevent the development of multiple complications.
Acknowledgments
Supported by the National Institutes of Health (NIH) T35 Training grant DK062709, NIH/NLM training grant T15 LM07359, NIH/NCATS grant UL1 TR000427, and the University of Wisconsin Department of Surgery.
We thank Leah Durbak and Ying Shan for assistance with acquiring the NSQIP data set.
Footnotes
The authors have no conflicts of interest to report.
Presented at the 11th Annual American Surgical Congress in Jacksonville, FL, February 2–4, 2016, as abstract ASC20160305 (“Big data in surgery: modeling how post-surgical complications increase risk for further complications”) and is a Society of University Surgeons paper.
References
- 1.Longo WE, Virgo KS, Johnson FE, Oprian CA, Vernava AM, Wade TP, et al. Risk factors for morbidity and mortality after co-lectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. doi: 10.1007/BF02237249. [DOI] [PubMed] [Google Scholar]
- 2.Kassin MT, Owen RM, Perez SD, Leeds I, Cox JC, Schnier K, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215:322–30. doi: 10.1016/j.jamcollsurg.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris AM, Baldivin LM, Matthews B, Dominitz JA, Barlow WE, Dobie SA, et al. Reoperation as a quality indicator in colorectal surgery - a population-based analysis. Ann Surg. 2007;245:73–9. doi: 10.1097/01.sla.0000231797.37743.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legner VJ, Massarweh NN, Symons RG, McCormick WC, Flum DR. The significance of discharge to skilled care after abdominopelvic surgery in older adults. Ann Surg. 2009;249:250–5. doi: 10.1097/SLA.0b013e318195e12f. [DOI] [PubMed] [Google Scholar]
- 5.Kohlnhofer BM, Tevis SE, Weber SM, Kennedy GD. Multiple complications and short length of stay are associated with postoperative readmissions. Am J Surg. 2014;207:449–56. doi: 10.1016/j.amjsurg.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson EH, Hall BL, Louie R, Ettner SL, Zingmond DS, Han L, et al. Association between occurrence of a postoperative complication and readmission: implications for quality improvement and cost savings. Ann Surg. 2013;258:10–8. doi: 10.1097/SLA.0b013e31828e3ac3. [DOI] [PubMed] [Google Scholar]
- 7.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA., Jr Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–7. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 8.McAleese P, Odling-Smee W. The effect of complications on length of stay. Ann Surg. 1994;220:740–4. doi: 10.1097/00000658-199412000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris MS, Deierhoi RJ, Richman JS, Altom LK, Hawn MT. The relationship between timing of surgical complications and hospital readmission. JAMA Surg. 2014;149:348–54. doi: 10.1001/jamasurg.2013.4064. [DOI] [PubMed] [Google Scholar]
- 10.Huebner M, Hübner M, Cima RR, Larson DW. Timing of complications and length of stay after rectal cancer surgery. J Am Coll Surg. 2014;218:914–9. doi: 10.1016/j.jamcollsurg.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Bohl DD, Webb ML, Lukasiewicz AM, Samuel AM, Basques BA, Ahn J, et al. Timing of complications after spinal fusion surgery. Spine (Phila Pa 1976) 2015;40:1527–35. doi: 10.1097/BRS.0000000000001073. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JS, Baxter BT, Allison JG, Johnson FE, Lee KK, Park WY. Temporal patterns of postoperative complications. Arch Surg. 2003;138:596–603. doi: 10.1001/archsurg.138.6.596. [DOI] [PubMed] [Google Scholar]
- 13.Sood A, Abdollah F, Sammon JD, Kapoor V, Rogers CG, Jeong W, et al. An evaluation of the timing of surgical complications following nephrectomy: data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) World J Urol. 2015;33:2031–8. doi: 10.1007/s00345-015-1564-x. [DOI] [PubMed] [Google Scholar]
- 14.Wakeam E, Hyder JA, Tsai TC, Lipsitz SR, Orgill DP, Finlayson SR. Complication timing and association with mortality in the American College of Surgeons’ National Surgical Quality Improvement Program database. J Surg Res. 2015;193:77–87. doi: 10.1016/j.jss.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Tevis SE, Cobian AG, Truong HP, Craven MW, Kennedy GD. Implicationsof multiple complications on thepostoperativere-covery of general surgery patients. Ann Surg. 2016;263:1213–8. doi: 10.1097/SLA.0000000000001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NSQIP. About ACS-NSQIP: participating sites; ACS NSQIP data; participant use data field. 2013 Available from: https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use.
- 17.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 18.de Silva S, Ma C, Proulx MC, Crespin M, Kaplan BS, Hubbard J, et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clin Gastroen-terol Hepatol. 2011;9:972–80. doi: 10.1016/j.cgh.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253:1223–9. doi: 10.1097/SLA.0b013e318214bce7. [DOI] [PubMed] [Google Scholar]
- 20.Hawn MT, Houston TK, Campagna EJ, Graham LA, Singh J, Bishop M, et al. The attributable risk of smoking on surgical complications. Ann Surg. 2011;254:914–20. doi: 10.1097/SLA.0b013e31822d7f81. [DOI] [PubMed] [Google Scholar]
- 21.Fischer JP, Wink JD, Nelson JA, Kovach SJ., 3rd Among 1,706 cases of abdominal wall reconstruction, what factors influence the occurrence of major operative complications? Surgery. 2014;155:311–9. doi: 10.1016/j.surg.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Turner PL, Saager L, Dalton J, Abd-Elsayed A, Roberman D, Melara P, et al. A nomogram for predicting surgical complications in bariatric surgery patients. Obes Surg. 2011;21:655–62. doi: 10.1007/s11695-010-0325-6. [DOI] [PubMed] [Google Scholar]
- 23.Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ., Jr Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93:1577–82. doi: 10.2106/JBJS.J.01048. [DOI] [PubMed] [Google Scholar]
- 24.Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg. 2009;208:1009–16. doi: 10.1016/j.jamcollsurg.2009.01.043. [DOI] [PubMed] [Google Scholar]