Abstract
Objective
To explore gender differences in real-world outcomes after catheter ablation of atrial fibrillation (AF).
Background
Compared to men, women with AF have greater thromboembolic risk and tend to be more symptomatic. Catheter ablation is generally more effective than antiarrhythmic drug therapy alone. However, there is limited data on the influence of gender on AF ablation outcomes.
Methods
We analyzed medical claims of 45 million United States patients enrolled in a variety of employee-sponsored and fee-for-service plans. We identified patients who underwent an AF ablation from 2007 to 2011 and evaluated 30-day safety and one-year effectiveness outcomes.
Results
Of the 21,091 patients who underwent an AF ablation, 7,460 (29%) were female. Women, compared to men, were older (62±11 vs. 58±11 years), had higher CHADS2 (1.2±1.1 vs. 1.0±1.0), higher CHA2DS2-VASc (2.9±1.5 vs. 1.6±1.4), and higher Charlson comorbidity index scores (1.2±1.3 vs. 1.0±1.2)(p<0.001 for all). Following ablation, women had higher risk of 30-day complications of hemorrhage (2.7 vs. 2.0%,p<0.001) and tamponade (3.8 vs. 2.9%,p<0.001). In multivariable analyses, women were more likely to have a re-hospitalization for AF (adjusted HR 1.12,p=0.009), but less likely to have repeat AF ablation (adjusted HR 0.92,p=0.04) or cardioversion (adjusted HR 0.75,p<0.001).
Conclusion
Women have increased hospitalization rates after AF ablation and are more likely to have a procedural complication. Despite the higher rate of hospital admissions for AF after ablation, women were less likely to undergo repeat ablation or cardioversion. These data call for greater examination of barriers and facilitators to sustain rhythm control strategies in women.
Keywords: atrial fibrillation, catheter ablation, disparities, rhythm control, women
1. Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in the adult population with estimates that one in four women will develop the arrhythmia at some point in their lifetime.(1) In 2014, female gender was incorporated into risk stratification guidelines for stroke prevention in AF based on the CHA2DS2-VASc score, highlighting the importance of gender in AF management.(2) Not only are women at an increased risk of thromboembolic complications, they tend to be more symptomatic than men, and less likely to respond to pharmacologic therapy.(3–6) Catheter ablation may therefore seem particularly attractive in women. However, there are limited data on the influences of gender on AF ablation procedures. We therefore sought to explore gender influences on long-term outcomes of catheter ablation of AF using data from multiple insurance health plans throughout the United States.
2. Methods
We analyzed data from the Truven Health MarketScanR Commercials Claims and Encounters and Medicare Supplemental Databases (Truven Health Analytics Inc., Ann Arbor, MI). This data source contains de-identified medical and pharmacy claims data on inpatient, outpatient, and prescription drug experience of over 45 million enrolled employees, dependents and retirees covered under a variety of fee-for-service (FFS) and managed care health plans, including health maintenance organizations, preferred and exclusive provider organizations, and point-of-service and consumer-directed health plans.(7) Linked datasets include the Inpatient Admissions file, which contains all diagnoses and procedures in the service records related to a hospital admission; the Outpatient Services file, which contains information on encounters and claims for services delivered at a doctor’s office, hospital outpatient facility, emergency room, or other outpatient facility; and the Outpatient Pharmaceutical Claims file, which includes complete records of mail-order or card program prescription drug claims. Medical claims are linked to outpatient prescription drug claims and person-level enrollment information. These data sources have been used extensively in health services research of heart rhythm disorders, including for catheter ablation.(8–10)
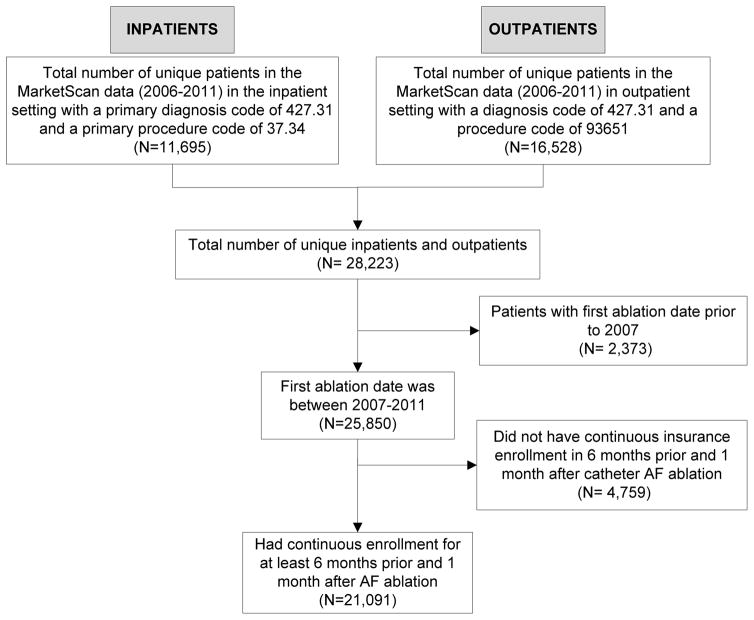
We identified all adult patients with non-valvular AF who received a catheter ablation between January 1, 2007 and December 31, 2011 (Figure 1). Patients were required to have a primary diagnosis of AF (International Classification of Diseases, 9th Revision [ICD-9] code 427.31) associated with any inpatient or outpatient encounter as well as a documented catheter ablation procedure (ICD-9 code 37.34 or Current Procedural Terminology, 4th Edition [CPT-4] code 93651). To avoid bias in ascertainment of baseline comorbidities, drug prescriptions and in post-ablation outcomes, we excluded patients without continuous health plan enrollment for at least six months prior and one month after the index ablation procedure date.
Figure 1.
Cohort Selection Diagram
Clinical covariates
Baseline comorbidities were determined using comorbidity-specific ICD-9 codes up to one year prior to the catheter ablation date, based on the Agency for Healthcare Research and Quality (AHRQ) Clinical Classification System. We additionally assessed comorbidity using the Charlson and Selim Comorbidity Indices using validated methods.(11) CHADS2 and CHA2DS2- VASc scores were calculated by summing the component comorbidities. Pharmacological therapies received in the 120 days prior to AF ablation were ascertained using the MarketScanR Outpatient Pharmaceutical Claims file.
Procedural Complications
We ascertained 30-day post-procedural complications based on hospital discharge diagnoses or procedure codes that would be used to manage a complication (e.g. pericardiocentesis). This approach has been previously applied to AF treatment studies using the same data source.(8,12) One-year clinical outcomes were similarly ascertained and included hospitalization for AF, cardioversion (inpatient or outpatient), or repeat catheter ablation of AF. For the endpoint of AF hospitalization, we considered any inpatient encounter associated with a primary diagnosis for AF, which occurred after the initial inpatient or outpatient AF ablation procedure. We evaluated cause-specific hospitalization for acute coronary syndrome or myocardial infarction, heart failure, supraventricular tachycardia, ventricular arrhythmia, syncope, stroke or transient ischemic attack, and intracranial hemorrhage.
Statistical analysis
We compared differences in baseline characteristics and 30-day complications between females and males using t-tests for continuous variables and chi-squared tests for categorical variables. Cox proportional hazards regression was used to examine the association between gender and each outcome at one year (hospitalization for AF, cardioversion, repeat catheter ablation of AF), while adjusting for potential confounders. We included the following baseline covariates in multivariate models: age, sex, Charlson Comorbidity Index score, CHF, hypertension, diabetes, stroke/TIA, prior MI, anemia, peripheral artery disease (PAD) or vascular disease, year of ablation, insurance plan, and receipt of concomitant drug therapies (warfarin, dabigatran, clopidogrel, Class I and Class III anti-arrhythmic drugs, amiodarone, beta blockers, and calcium channel blockers). The Cox model assumption of proportional hazards was found to be valid by using the Schoenfeld test. We also used the Kaplan-Meier method and Log-rank tests to calculate differential probability of event-free survival using the composite endpoint of AF hospitalization, cardioversion, or repeat AF ablation within one year.
The study was approved by the local Institutional Review Board. All analyses were performed using SAS, version 9.1 (Cary, NC) and STATA, version 11.0 (College Station, TX). The funding agencies were not involved in any way with study design, data analysis, interpretation of results, or manuscript preparation.
3. Results
We identified 21,091 patients with an index AF ablation meeting inclusion criteria. Although procedure volume increased from over the study period (2007–2011), 30% of the ablations were performed in women, and this proportion did not substantively change over the observation period. At the time of catheter ablation, women, compared to men, were older (62±11 vs. 58±11 years, p<0.001), and had higher CHADS2 (1.2±1.1 vs. 1.0±1.0, p<0.001), CHA2DS2-VASc scores (2.9±1.5 vs. 1.6±1.4, p<0.001) and Charlson comorbidity index scores (1.2±1.3 vs. 1.0±1.2, p<0.001) (Table 1). Women, compared to men, also had a higher prevalence of hypertension (66.2% vs 61.8%, p<0.001), diabetes (22.8% vs 20.7%, p<0.001), prior stroke or transient ischemic attack (TIA) (5.7% vs 3.5%, p<0.001), and anemia (13.6% vs 8.2%, p<0.001) but had a lower prevalence of prior myocardial infarction (4.5% vs 5.6%, p<0.001). There was no significant difference in prevalence of heart failure (33.4% vs 33.6%, p=NS) or chronic kidney disease (8.0% vs 7.4%, p=NS).
Table 1.
Baseline Characteristics
| Female(N=6,137) | Male(N=14,954) | p | |
|---|---|---|---|
| Inpatient/Outpatient Status | 0.001 | ||
| Inpatient | 2,524 (41.1%) | 5,787 (38.7%) | |
| Outpatient | 3,613 (58.9%) | 9,167 (61.3%) | |
|
| |||
| Age, years | 61.9 ± 11.4 | 58.1 ± 10.5 | <0.001 |
|
| |||
| Comorbidity index | |||
| Charlson comorbidity index | 1.2 ± 1.3 | 1.00 ± 1.2 | <0.001 |
| Selim comorbidity index | 2.3 ± 2.3 | 2.5 ± 2.1 | <0.001 |
|
| |||
| CHADS2 score | 1.2 ± 1.1 | 1.0 ± 1.0 | <0.001 |
|
| |||
| CHADS2 score group | <0.001 | ||
| CHADS2 0 | 1,753 (28.6%) | 5,174 (34.6%) | |
| CHADS2 1 | 2,376 (38.7%) | 5,943 (39.7%) | |
| CHADS2 2 | 1,199 (19.5%) | 2,594 (17.4%) | |
| CHADS2 3 | 564(9.2%) | 904 (6.1%) | |
| CHADS2 4 | 174 (2.8%) | 237 (1.6%) | |
| CHADS2 5 | 59 (0.96%) | 87 (0.58%) | |
| CHADS2 6 | 12 (0.20%) | 15 (0.10%) | |
|
| |||
| CHA2DS2-VASc score | 2.9 ± 1.5 | 1.6 ± 1.4 | <0.001 |
|
| |||
| Congestive heart failure | 2,038 (33.2%) | 5,031 (33.6%) | 0.54 |
|
| |||
| Hypertension | 4,061 (66.2%) | 9,246 (61.8%) | <0.001 |
|
| |||
| Diabetes | 1,400 (22.8%) | 3,097 (20.7%) | 0.0007 |
|
| |||
| Prior stroke or transient ischemic attack | 347 (5.7%) | 529 (3.5%) | <0.001 |
|
| |||
| Prior myocardial infarction | 276 (4.5%) | 831 (5.6%) | 0.001 |
|
| |||
| Anemia | 832 (13.6%) | 1,232 (8.2%) | <0.001 |
|
| |||
| Prior bleeding | 733 (11.9%) | 1,676 (11.2%) | 0.13 |
|
| |||
| Peripheral artery disease | 304 (5.0%) | 603 (4.0%) | 0.003 |
|
| |||
| CKD | 489 (8.0%) | 1,104 (7.4%) | 0.14 |
|
| |||
| Region | 0.002 | ||
| Northeast | 771 (13.0%) | 2,187(14.6%) | |
| North Central | 1,763 (28.7%) | 4,075 (27.3%) | |
| South | 2,453 (40.0%) | 5,873 (39.3%) | |
| West | 1,067 (17.4%) | 2,606 (17.4%) | |
| Unknown | 83 (1.3%) | 213 (1.4%) | |
|
| |||
| Ablation Year | 0.03 | ||
| 2007 | 656 (10.7%) | 1,800 (12.0%) | |
| 2008 | 882 (14.4%) | 2,239 (15.0%) | |
| 2009 | 1,103 (18.0%) | 2,569 (17.2%) | |
| 2010 | 1,382 (22.5%) | 3,358 (22.5%) | |
| 2011 | 2,114 (34.5%) | 4,988 (33.4%) | |
|
| |||
| Insurance Plan Type | <0.001 | ||
| Comprehensive | 1087 (17.7%) | 1,599 (10.7%) | |
| EPO | 79 (1.3%) | 204 (1.4%) | |
| HMO | 661 (10.8%) | 1,609 (10.8%) | |
| POS | 346 (5.6%) | 996 (6.7%) | |
| PPO | 3466 (56.5%) | 9,148 (61.2%) | |
| POS with capitation | 23 (0.37%) | 91 (0.61%) | |
| CDHP | 132 (2.2%) | 377 (2.5%) | |
| HDHP | 53 (0.86%) | 193 (1.3%) | |
| Missing | 290 (4.7%) | 737 (4.9%) | |
|
| |||
| Cardiovascular medications | |||
| Aspirin | 20 (0.33%) | 26 (0.17%) | 0.03 |
| Warfarin | 3,475 (56.6%) | 8,599 (57.5%) | 0.24 |
| Dabigatran | 578 (9.4%) | 1,531 (10.2%) | 0.07 |
| Rivaroxaban | 2 (0.03%) | 1 (0.01%) | 0.20 |
| Clopidogrel | 271 (4.4%) | 930 (6.2%) | <0.001 |
| ACE inhibitor or angiotensin receptor blocker | 2,017 (32.9%) | 4,873 (32.6%) | 0.69 |
| Diuretics | 2,082 (33.9%) | 3,585 (24.0%) | <0.001 |
| Niacin or fibrates | 98 (1.6%) | 576 (3.9%) | 0.007 |
| Statins | 1,498 (24.4%) | 4,275 (28.6%) | <0.001 |
|
| |||
| Antiarrhythmic drugs | |||
| All Class I | 1,716 (28.0%) | 3,561 (23.8%) | <0.001 |
| Class III (Sotalol/Dofetilide) | 1,252 (20.4%) | 2,952 (19.7%) | 0.28 |
| Amiodarone | 851 (13.9%) | 2,396 (16.0%) | <0.001 |
|
| |||
| Rate-controlling drugs | |||
| Metoprolol | 1,997 (32.5%) | 4,657 (31.1%) | 0.80 |
| Carvedilol | 356 (5.8%) | 1,175 (7.9%) | <0.001 |
| Atenolol | 467 (7.6%) | 814 (5.4%) | <0.001 |
|
| |||
| Calcium-channel blockers | |||
| Diltiazem | 1,314 (21.4%) | 2,459 (16.4%) | <0.001 |
| Verapamil | 199 (3.2%) | 320 (2.1%) | <0.001 |
Prior to ablation, women were more likely to be treated with rate-control agents (70.6% vs. 63.0%, p<0.001) and class I antiarrhythmic agents (28.0% vs. 23.8%, p<0.001) but less likely to be treated with amiodarone (13.9% vs. 16.0%, p<0.001). Women had similar use of class III antiarrhythmic agents (20.4% vs. 19.7%, P=NS) and oral anticoagulation (66.0% vs. 67.8%, P=NS).
30-day Complications
In the 30 days following the ablation, women had an increased risk of complications, including vascular complications (2.7% vs 2.0%, p<0.001), hematoma or hemorrhage (2.3% vs 1.6%, p<0.001), and perforation or tamponade (3.8% vs 2.9%, p<0.001) (Table 2). Women demonstrated a trend for increased all-cause hospitalization (9.4% vs 8.6%, p=0.07) and stroke or TIA (0.85% vs 0.64%, p=0.09). There were 7 in-hospital deaths (0.07% vs 0.02%, p= NS).
Table 2.
30-Day complications after AF ablation
| 30-Day complications | All patients (N=21,091) | Gender | ||
|---|---|---|---|---|
| Female (N=6.137) | Male (N=14,954) | p | ||
| All-cause hospitalization | 1,868 (8.9%) | 578 (9.4%) | 1,290(8.6%) | 0.07 |
| Vascular complication | 464 (2.2%) | 168 (2.7%) | 296 (2.0%) | 0.0007 |
| Hematoma or hemorrhage | 376 (1.8%) | 139 (2.3%) | 237 (1.6%) | 0.0007 |
| Perforation or tamponade | 661 (3.1%) | 234 (3.8%) | 427 (2.9%) | 0.0003 |
| Pneumothorax or hemothorax | 29 (0.14%) | 8(0.13%) | 21 (0.14%) | 0.86 |
| Stroke or transient ischemic attack | 147 (0.70%) | 52 (0.85%) | 95 (0.64%) | 0.09 |
| Pacemaker implantation | 2 (0.01%) | 1 (0.02%) | 1 (0.01%) | 0.50 |
| Implantable cardioverter defibrillator implantation | 0 (0.0%) | |||
| Congestive heart failure | 116 (0.55%) | 34 (0.55%) | 82 (0.55%) | 0.96 |
| In-hospital Death | 7 (0.03%) | 4 (0.07%) | 3 (0.02%) | 0.11 |
One-year outcomes
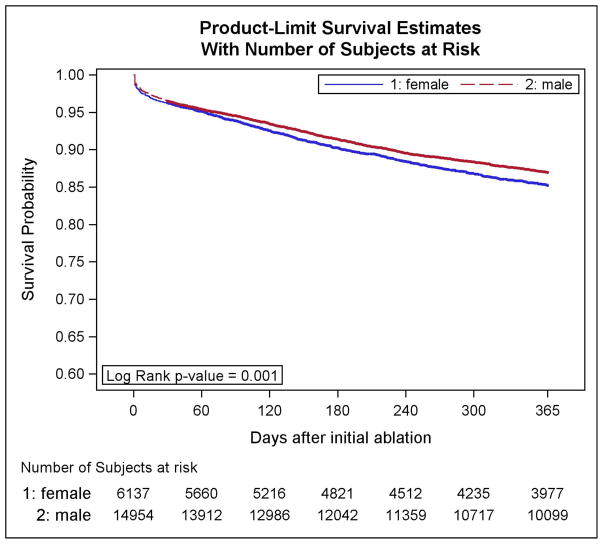
In the year following ablation, women were less likely to receive a cardioversion (17% vs 21%; adjusted HR 0.75, p<0.001) or repeat ablation procedure (13% vs 15%; adjusted HR 0.92, p<0.001) (Table 3). However, unadjusted and adjusted risk of one-year all-cause hospitalization was higher in women compared to men (32% vs 27%, p<0.001; adjusted HR 1.14, 95% CI 1.07–1.20, p<0.001) as was risk of hospitalization for AF (13% vs 12%; adjusted HR 1.12, 95% CI 1.03–1.22, p<0.001). In secondary analyses, we found that women were less likely to be re-hospitalized for myocardial infarction (0.7% vs 1.2%, adjusted HR 0.84 95% CI 0.42–0.84, p<0.05) and were more likely to be admitted with congestive heart failure over the following year (2.5% vs 1.8%, adjusted HR 3.45 95% CI 2.68–4.45, p<0.001) (Table 4). Cumulative event-free survival, defined as freedom from AF rehospitalization, was decreased among women compared to men (86.6% vs 89.0%, adjusted HR 0.88, 95% CI 0.78–0.97, p<0.01) as shown in Figure 2.
Table 3.
Primary outcomes in one year after initial ablation (N=21,091)
| One year outcome | Gender | # Patients | # Events | Unadjusted Incidence Rate (per 100 person-years) | Unadjusted Hazard Ratio* (95%CI) | p | Adjusted Hazard Ratio*+ (95%CI) | p |
|---|---|---|---|---|---|---|---|---|
| All-cause hospitalization | Female | 6,137 | 1,955 | 46.1 | 1.23 | <0.00 | 1.14 | <.0001 |
|
|
||||||||
| Male | 14,954 | 4,017 | 36.9 | (1.65- | 1 | (1.07–1.20) | ||
|
| ||||||||
| Atrial fibrillation rehospitalization | Female | 6,137 | 825 | 16.8 | 1.14 | 0.001 | 1.12 | 0.009 |
|
|
||||||||
| Male | 14,954 | 1,792 | 14.7 | (1.05- | (1.03–1.22) | |||
|
| ||||||||
| Cardioversion | Female | 6,137 | 1,050 | 22.4 | 0.78 | <.000 | 0.75 | <.0001 |
|
|
||||||||
| Male | 14,954 | 3,203 | 28.9 | (0.73- | 1 | (0.70–0.81) | ||
|
| ||||||||
| Repeat ablation for atrial fibrillation | Female | 6,137 | 805 | 16.2 | 0.90 | 0.008 | 0.92 | 0.04 |
|
|
||||||||
| Male | 14,954 | 2,195 | 18.1 | (0.82- | (0.841- | |||
Reference group is male
Adjusted for age, Charlson Comorbidity Index score, CHF, hypertension, diabetes, stroke/TIA, prior MI, anemia, peripheral artery disease (PAD) or vascular disease, chronic kidney disease, year of ablation, insurance plan, and receipt of concomitant drug therapies (warfarin, dabigatran, clopidogrel, Class I and Class III anti-arrhythmic drugs, amiodarone, beta blockers, and calcium channel blockers)
Table 4.
Secondary outcomes at one year after index ablation (N=21,091)
| Cause of inpatient admission at one year | Gender | Number of Patients | Number of Events | Unadjusted Incidence Rate (per 100 person- years) | Unadjusted Hazard Ratio* (95%CI) | p | Adjusted Hazard Ratio*+ (95%CI) | p |
|---|---|---|---|---|---|---|---|---|
| Myocardial infarction or unstable angina | Female | 6,137 | 44 (0.72%) | 0.82 | 0.63 (0.45–0.88) | 0.006 | 0.59 (0.42–0.84) | 0.003 |
|
| ||||||||
| Male | 14,954 | 172(1.2%) | 1.30 | |||||
|
| ||||||||
| Congestive heart failure | Female | 6,137 | 151 (2.5%) | 2.85 | 1.38 (1.13–1.69) | 0.001 | 3.45 (2.68–4.45) | <0.001 |
|
| ||||||||
| Male | 14,954 | 269(1.8%) | 2.05 | |||||
|
| ||||||||
| Supraventricular tachycardia | Female | 6,137 | 23 (0.37%) | 0.43 | 1.58 (0.93- 2.66) | 0.08 | 1.52 (0.88- 2.62) | 0.14 |
|
| ||||||||
| Male | 14,954 | 36 (0.24% ) | 0.27 | |||||
|
| ||||||||
| Ventricular arrhythmia | Female | 6,137 | 66 (1.08%) | 1.24 | 1.32 (0.98–1.77) | 0.07 | 1.30(0.95–1.77) | 0.44 |
|
| ||||||||
| Male | 14,954 | 124 (0.83%) | 0.94 | |||||
|
| ||||||||
| Stroke or transient ischemic attack | Female | 6,137 | 39 (0.64%) 70 | 0.73 | 1.38 (0.93– 2.03) | 0.11 | 1.18 (0.79–1.79) | 0.42 |
|
| ||||||||
| Male | 14,954 | (0.47%) | 0.53 | |||||
|
| ||||||||
| Intracranial hemorrhage | Female | 6,137 | 14 (0.23%) | 0.26 | 1.50(0.77–2.92) | 0.22 | 1.16 (0.56– 2.42) | 0.68 |
|
| ||||||||
| Male | 14,954 | 23 (0.15%) | 0.17 | |||||
|
| ||||||||
| Syncope | Female | 6,137 | 14 (0.23%) | 0.26 | 1.23 (0.65– 2.34) | 0.52 | 0.95 (0.48–1.92) | 0.89 |
|
| ||||||||
| Male | 14,954 | 28 (0.19%) | 0.21 | |||||
Reference group is male
Adjusted for age, Charlson Comorbidity Index score, CHF, hypertension, diabetes, stroke/TIA, prior MI, anemia, peripheral artery disease (PAD) or vascular disease, chronic kidney disease, year of ablation, insurance plan, and receipt of concomitant drug therapies (warfarin, dabigatran, clopidogrel, Class I and Class III anti-arrhythmic drugs, amiodarone, beta blockers, and calcium channel blockers)
Figure 2.
Survival free from AF rehospitalization.
4. Discussion
We evaluated the 30-day safety and one-year effectiveness outcomes in over 20,000 patients undergoing catheter ablation procedures for AF using medical claims data from a variety of representative healthcare plans throughout the Unites States. We found that gender was significantly associated with certain aspects of the procedure, including the baseline clinical characteristics of the patients, the safety profile, and long-term outcomes. Adjusting for risk factors, women revealed a paradoxical discordance between increased rehospitalizations for AF and decreased subsequent use of cardioversion or repeat ablation. Our data call for greater examination of barriers and facilitators to sustain rhythm control strategies in women with AF.
In agreement with prior studies, we found women undergoing catheter ablation of AF were older with increased comorbidities compared to men.(13–16) This pattern may be indicative of gender influences on the natural history of AF. Population studies, such as Framingham, have demonstrated that new-onset AF tends to occur at an age approximately five years older in women and are more likely to have comorbidities such as hypertension and diabetes at the time of diagnosis.(17,18) Although women display a similar overall prevalence of AF as men,(19) the older age at diagnosis may decrease the likelihood of undergoing catheter ablation. We also found that women were more likely to be treated with Class I antiarrhythmic medications, which may have been driven by a lower prevalence of prior myocardial infarction, a contraindication to this class of medication.
Compared to men, women were found to have a low, but modestly higher, risk of complications within 30 days of the catheter ablation procedures. These findings are consistent with prior studies and confirm the increased procedural risk for women in our large study population, which is representative of most patients receiving AF ablation today. (20) Prior studies have reported 30-day complication rates which range from 5–8% in women, roughly 1–3% higher compared to men.(12,21,22) The higher complication rate seen in women may be partially explained by the increased comorbidity burden. However, due to the low frequency of events, we did not perform multivariate adjustment. Other potential explanations may include differences in pharmacokinetics and body size. For example, women tend to require a lower heparin dose in order to obtain adequate anticoagulation, even after adjusting for weight.(23) Concordantly, women have demonstrated higher activated clotting times (ACT) during catheter ablation procedures. (24) Fortunately, the overall rates of both stroke (<1%) and death (<0.1%) were low and similar between genders.
With respect to gender differences with longer-term procedural success, prior studies have not revealed consistent findings.(13,14,25–27). Several studies have reported that women have a higher recurrence rate after the ablation procedure(13,14) (25) while other studies have found no significant difference between men and women.(26,27) Our study, which is the largest AF ablation study examining procedural outcomes by gender in the United States, found that women were significantly more likely to be re-hospitalized with AF within one-year after an ablation procedure but less likely to undergo cardioversion or repeat ablation. Although the nature of the claims data does not allow for evaluation of any AF recurrence, our findings, in context, may be indicative of potential barriers to optimal or sustained rhythm control strategies in women. For example, women are more likely than men to report having delayed care due to non-medical barriers, including time logistics and cost. (28) Furthermore, women are more likely to have their cardiac symptoms and risk of heart disease downplayed by their doctors; (29) and are less likely to receive implantable defibrillators. (30) In the case of atrial fibrillation, there is evidence that women exhibit a delayed referral pattern compared to men and fail more antiarrhythmic medications before proceeding to catheter ablation.(15,16) Delaying the time to ablation could promote a higher AF or comorbidity burden at the time of ablation which may have resulted in increased electrical and structural remodeling. However, it is unclear from our data if women were less likely to be offered rhythm-control strategies, or if women were more likely to decline the procedures. The underlying motivations driving clinical decision-making are complex and may vary by gender.(31) For example, a single-center study from Japan suggested women were more likely to refuse catheter ablation.(14) In context, these data highlight the importance of communication between patients and clinicians to ensure that fears, motivations, and perspectives of patients and caregivers are adequately addressed in order to deliver optimal and patient-centered care.
Our study has several significant limitations. First, we used de-identified administrative claims data. Although claims data has the advantages of performing large-scale analyses across multiple health care systems or payers, claims-based ascertainment of ablation procedures and outcomes may be imperfect. AF ablation ascertainment based on procedural and diagnosis could lead to misclassification and inappropriate inclusion of other ablations, such as atrial tachycardia (AT), which tend to be more prevalent in women, although cohort inclusion was conditioned upon a concurrent AF diagnosis.(32,33) However, because AF ablation is has higher risk than AT or SVT procedures, (34) this misclassification would bias complication risk in women toward the null. Second, unidentified confounders and covariates influencing patient outcomes such as repeat ablation or cardioversion, such as frailty or body mass index, may not be identified. Third, we are unable to measure differences in AF recurrence, AF burden, or quality of life, which are the key endpoints used in randomized trials of ablation. Fourth, we are unable to classify AF severity (e.g. paroxysmal vs. persistent), AF duration prior to ablation, heart failure severity, or ejection fraction from administrative data. The ICD-10 medical classification system has separate diagnoses codes for AF severity and may prove useful for future work. Finally, while the baseline differences and outcomes provide insight, the clinical circumstances or patient preferences associated with care cannot be identified. However, these findings provide the rationale for other study designs that may allow more granular and qualitative examination of these issues.
CONCLUSION
In 21,091 patients undergoing catheter ablation of AF across the U.S., women, compared to men, were older, with increased comorbidity burden, and had a low, but modestly increased risk of procedural complications. Adjusting for risk factors, women who underwent catheter ablation revealed a paradoxical discordance between increased rehospitalizations for AF and decreased subsequent use of cardioversion or repeat ablation. These data call for greater examination of barriers and facilitators to sustain rhythm control strategies in women.
Perspectives.
Competency in Medical Knowledge
Similar to stroke risk, women with atrial fibrillation have a low, but modestly higher rate of procedural complications in the 30 days following AF ablation.
Translational Outlook
The higher risk of 30-day complications in women may be mitigated by improved patient selection or tailoring aspects of the procedure to minimize complication rates. Additionally, a greater examination of barriers and facilitators of care and use of shared-decision making tools could help to minimize potential disparities in care and ensure that treatment decisions are in alignment with the clinical evidence and patient preferences.
Acknowledgments
Funding/Support:
Dr. Kaiser is supported by a grant from the National Heart, Lung and Blood Institute (1T32Hl098049). Dr. Turakhia is supported by a Veterans Health Services Research & Development Career Development Award (CDA09027-1), an American Heart Association National Scientist Development Grant (09SDG2250647), a VA Health Services and Development MERIT Award (IIR 09-092), the Gilead Sciences Cardiovascular Scholars Program, and the Stanford Center for Health Research on Women and Sex Differences in Medicine
ABBREVIATIONS
- AF
atrial fibrillation
- CPT
current procedural terminology
- FFS
fee-for-service
- HR
hazard ratio
- ICD-9
International Classification of Diseases, 9th Revision
- TIA
transient ischemic attack
Footnotes
Disclosures: There are no conflicts of interest to report.
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 3.Suttorp MJ, Kingma JH, Koomen EM, van 't Hof A, Tijssen JG, Lie KI. Recurrence of paroxysmal atrial fibrillation or flutter after successful cardioversion in patients with normal left ventricular function. The American journal of cardiology. 1993;71:710–3. doi: 10.1016/0002-9149(93)91015-a. [DOI] [PubMed] [Google Scholar]
- 4.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. The New England journal of medicine. 2002;347:1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 5.Rienstra M, Van Veldhuisen DJ, Hagens VE, et al. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: data of the Rate Control Versus Electrical Cardioversion (RACE) study. Journal of the American College of Cardiology. 2005;46:1298–306. doi: 10.1016/j.jacc.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 6.Kerr CR, Humphries K. Gender-related differences in atrial fibrillation. Journal of the American College of Cardiology. 2005;46:1307–8. doi: 10.1016/j.jacc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Danielson E. The MarketScan Data Databases. 2014. Health Research Data for the Real world. [Google Scholar]
- 8.Reynolds MR, Gunnarsson CL, Hunter TD, et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circulation Cardiovascular quality and outcomes. 2012;5:171–81. doi: 10.1161/CIRCOUTCOMES.111.963108. [DOI] [PubMed] [Google Scholar]
- 9.Hao SC, Hunter TD, Gunnarsson C, et al. Acute safety outcomes in younger and older patients with atrial fibrillation treated with catheter ablation. J Interv Card Electrophysiol. 2012;35:173–82. doi: 10.1007/s10840-012-9690-5. [DOI] [PubMed] [Google Scholar]
- 10.Ladapo JA, David G, Gunnarsson CL, et al. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. Journal of cardiovascular electrophysiology. 2012;23:1–8. doi: 10.1111/j.1540-8167.2011.02130.x. [DOI] [PubMed] [Google Scholar]
- 11.Selim AJ, Fincke G, Ren XS, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. The Journal of ambulatory care management. 2004;27:281–95. doi: 10.1097/00004479-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Deshmukh A, Patel NJ, Pant S, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128:2104–12. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XD, Tan HW, Gu J, et al. Efficacy and safety of catheter ablation for long-standing persistent atrial fibrillation in women. Pacing and clinical electrophysiology : PACE. 2013;36:1236–44. doi: 10.1111/pace.12212. [DOI] [PubMed] [Google Scholar]
- 14.Takigawa M, Kuwahara T, Takahashi A, et al. Differences in catheter ablation of paroxysmal atrial fibrillation between males and females. International journal of cardiology. 2013;168:1984–91. doi: 10.1016/j.ijcard.2012.12.101. [DOI] [PubMed] [Google Scholar]
- 15.Patel D, Mohanty P, Di Biase L, et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7:167–72. doi: 10.1016/j.hrthm.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Santangeli P, di Biase L, Pelargonio G, Natale A. Outcome of invasive electrophysiological procedures and gender: are males and females the same? Journal of cardiovascular electrophysiology. 2011;22:605–12. doi: 10.1111/j.1540-8167.2010.01920.x. [DOI] [PubMed] [Google Scholar]
- 17.Humphries KH, Kerr CR, Connolly SJ, et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–70. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study Jama. 1994;271:840–4. [PubMed] [Google Scholar]
- 19.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circulation Cardiovascular quality and outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg BA, Holmes DN, Ezekowitz MD, et al. Rate versus rhythm control for management of atrial fibrillation in clinical practice: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. American heart journal. 2013;165:622–9. doi: 10.1016/j.ahj.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spragg DD, Dalal D, Cheema A, et al. Complications of catheter ablation for atrial fibrillation: incidence and predictors. Journal of cardiovascular electrophysiology. 2008;19:627–31. doi: 10.1111/j.1540-8167.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 22.Piccini JP, Sinner MF, Greiner MA, et al. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–7. doi: 10.1161/CIRCULATIONAHA.112.109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell NR, Hull RD, Brant R, Hogan DB, Pineo GF, Raskob GE. Different effects of heparin in males and females. Clinical and investigative medicine Medecine clinique et experimentale. 1998;21:71–8. [PubMed] [Google Scholar]
- 24.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Atrial fibrillation ablation using open-irrigated tip radiofrequency: experience with intraprocedural activated clotting times </=210 seconds. Heart Rhythm. 2014;11:963–8. doi: 10.1016/j.hrthm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Kummer BR, Bhave PD, Merkler AE, Gialdini G, Okin PM, Kamel H. Demographic Differences in Catheter Ablation After Hospital Presentation With Symptomatic Atrial Fibrillation. J Am Heart Assoc. 2015;4:e002097. doi: 10.1161/JAHA.115.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagres N, Clague JR, Breithardt G, Borggrefe M. Significant gender-related differences in radiofrequency catheter ablation therapy. Journal of the American College of Cardiology. 2003;42:1103–7. doi: 10.1016/s0735-1097(03)00925-2. [DOI] [PubMed] [Google Scholar]
- 27.Forleo GB, Tondo C, De Luca L, et al. Gender-related differences in catheter ablation of atrial fibrillation. Europace. 2007;9:613–20. doi: 10.1093/europace/eum144. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau. Women's Health USA 2011. Rockville, Maryland: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 29.Frich JC, Malterud K, Fugelli P. Women at risk of coronary heart disease experience barriers to diagnosis and treatment: a qualitative interview study. Scand J Prim Health Care. 2006;24:38–43. doi: 10.1080/02813430500504305. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–32. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 31.Heidenreich PA, Shlipak MG, Geppert J, McClellan M. Racial and sex differences in refusal of coronary angiography. The American journal of medicine. 2002;113:200–7. doi: 10.1016/s0002-9343(02)01221-4. [DOI] [PubMed] [Google Scholar]
- 32.Porter MJ, Morton JB, Denman R, et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004;1:393–6. doi: 10.1016/j.hrthm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Wolbrette D, Naccarelli G, Curtis A, Lehmann M, Kadish A. Gender differences in arrhythmias. Clin Cardiol. 2002;25:49–56. doi: 10.1002/clc.4950250203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohnen M, Stevenson WG, Tedrow UB, et al. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011;8:1661–6. doi: 10.1016/j.hrthm.2011.05.017. [DOI] [PubMed] [Google Scholar]