Although juvenile dermatomyositis (JDM) is the most common pediatric inflammatory myopathy, it is a rare disease, which has impeded our recognition of the extent of the variation in both JDM symptoms and pathophysiology. Convincing new evidence has recently emerged documenting that myositis specific antibodies (MSA) are uniquely effective in identifying specific subsets of inflammatory myopathies in children. This review will focus on the impact that these MSAs have made on our understanding of both the clinical and laboratory features of JDM and will summarize some of our options for therapy.
The common laboratory and diagnostic features of JDM have been previously described (1, 2). In brief, JDM is a systemic autoimmune vasculopathy, with a mean age of onset of 6.7 years (boys), and 7.3 years (girls); the female: male ratio is 2.3:1 (3). At diagnosis, both boys and girls with JDM are shorter and lighter than their age and sex matched controls (4). One defining clinical manifestation of JDM is symmetrical proximal muscle weakness. The second major symptom, the characteristic rash, occurs over the joints and extremities, and the shawl region of the chest (Figure 1). This rash also localizes to the area around the eyes, as well as the lids themselves, and the malar area, including the bridge of the nose. Younger children may be edematous and have scalp involvement, resulting in alopecia. In untreated JDM, elevated serum levels of muscle derived enzymes (aldolase, CPK, AST, ALT, LDH) are time dependent; they tend to normalize by 4.5 months after diagnosis (4). MRI identifies the patchy muscle inflammation and can help direct the physician to biopsy an inflammatory site. The typical JDM muscle biopsy displays perifasicular atrophy, muscle fiber size variation, increased expression of major histocompatibility complex (MHC) Class 1, infiltration of primarily mononuclear cells (5), extensive muscle capillary drop out and damaged mitochondria (6). This evidence aids diagnosis and the choice of immunosuppressive therapy (5).
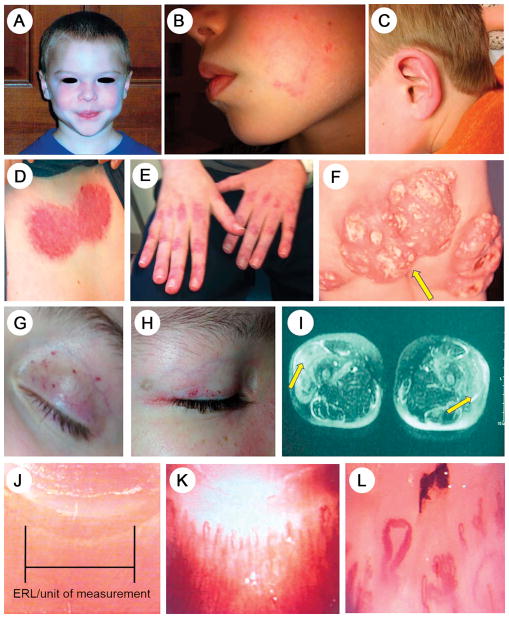
Figure 1. Clinical features, common and uncommon, of children with juvenile dermatomyositis.
A. Heliotrope rash on the face, characteristic of active JDM
B. A very small patch of persistent erythema on cheek in a child as the only rash of JDM positive for MDA-5
C. Erythema of the pinna of the ear in a child as the only sign indicating a flare of JDM positive for p155/140
D. Inflammation in the shawl area on the upper anterior chest indicating both acute and chronic changes
E. Linear erythema over the metacarpal-phalangeal (MCP) as well as the proximal (PIP) and distal intercarpal phalangeal (DIP) joints of the hands. Dilated nailfold end row capillary loops are visible.
F. Microvasculopathy dilated capillaries on the upper eyelid and eyelid margin in active JDM
G. Healing telangiectasia of the eyelid of a child with JDM
H. Dilated capillaries close to the edge of the eyelid, resolving; healed medial canthus infarct
I. Closely spaced normal nailfold capillary end row loops, showing unit of measure
J. Moderate nailfold capillary dropout with vessel tortuosity
K. Severe nailfold capillary dropout, with dilated loops and fewer end row loops/mm
Juvenile dermatomyositis etiology: genetics and environment
A working hypothesis is that JDM is a type-1 interferon-driven inflammatory process, triggered by one or more environmental stimuli, such as infection, exposure to smoking or ultraviolet rays (UVB), targeting a genetically susceptible child. Families of the JDM child may have a history of autoimmune disease, most often systemic lupus erythematosus (SLE) (7), but it is rare to have more than one case of inflammatory myopathy in a family. Disease susceptibility is attributed to the human leukocyte antigen (HLA) locus on chromosome 6 (8), similar to other autoimmune diseases, which was determined by testing both pediatric and adult dermatomyositis (DM) patients of European ancestry using genome wide association (GWAS) methodology (9). Further testing of Caucasians with a range of inflammatory myopathies defined additional loci; PTPN22 was associated with genome-wide significance for polymyositis (PM), but not DM or JDM (10). In Japanese myositis patients, HLA – DRB1*08:03 confers risk (11), which differs from the Han Chinese myositis patients, who are more likely to carry the HLA-DQA1*01:04, and HLADRB1*07 alleles (12). Each of these risk genes displays unique differences in the peptide-binding pocket, modifying their ability to attract and bind antigenic peptides which subsequently stimulate an immune response (13). As in SLE, some JDM children have decreased gene copy number for C4 (A>B), resulting in low production of C4 (14), which may also be diminished by complement consumption. The increase incidence of JDM in girls appears to be associated with a synergy between osteopontin and the TNF-α locus (15).
A range of potential facilitating factors are under active consideration: seasonality of birth; sun/UVB exposure; prenatal smoke/pollution exposure; urban vs rural dwelling; life stressor; immunizations and medications. Of these conditions, seasonality of birth has been reported (16), which may be confounded by ante-partum exposure of the fetus to cigarette smoke and traffic pollution (17, 18). Antecedent infection may precipitate the autoimmune process (19–24), as well as a flare of disease symptoms (20), and is implicated in both JDM (19–21) and DM (22). Detection of the specific antigen (infectious agent?) in untreated biopsies has not yet been achieved. Global warming (23) may have obscured the reported seasonality of onset (spring and fall in the Midwestern USA) (21). In the 3 months prior to the first symptom of JDM, respiratory and/or gastrointestinal infection predominate (24), whereas in the 6 months preceding a JDM flare, gastroenteritis (P = .04) and urinary tract infections (p=0.005) are more frequent (20). Sun exposure (OR=3.5; p=0.049) preceded a flare, despite photoprotective agents (20). Vaccinations may also trigger the myopathic process (25).
Pathophysiology of juvenile dermatomyositis
The duration, or length of time of the disease process, influences both the child’s symptoms and laboratory data at diagnosis (4). The duration of disease also impacts immunologic data, ranging from gene expression in the muscle biopsy of untreated children (26) to the display of specific apoptotic pathways in their muscle (27). The child’s age at disease onset is also associated with specific gene activation (28). The inflammatory infiltrate in the affected JDM muscle is predominantly composed of mononuclear cells—both lymphoid and phagocytic; lymphoid aggregates are associated with severe disease (29). Along with CD3+CD4+ T cells, there is an increase in FOXP3+regulatory T cells (30) as well as mature plasmacytoid dendritic cells, which secrete type 1 interferons. Recently recognized, myogenic precursor cells (MPCs) in JDM muscle also synthesize type 1 interferons (32). The MPCs also modulate the loss of microvasculature in muscle characteristic of JDM (6). Mast cells predominate in JDM skin biopsies, even in uninvolved areas, compared with inflamed muscle from the same child (32). In JDM, there may also be a role for natural killer (NK) cells in inflicting damage (33). The absolute count of CD3−CD16−56− NK cells appears to be a useful biomarker for some forms of inflammatory myopathy, such as orbital myositis (34). The levels of proinflammatory cytokines reflect immune activation. Biomarkers, such as serum tumor necrosis factor receptor type 2 or Galactin 9 (35), and/or the17-related cytokines (36) may be useful to guide the child’s therapy. Deposition of the terminal membrane attack complex, C5b-9, on the muscle microvasculature is associated with perifasicular atrophy in both adults and children with DM (37). The precise sequence of these immune events is not yet known, and appears to vary with the myositis specific antibodies (MSAs). RNA sequencing of peripheral blood mononuclear cells from untreated JDM showed a specific pattern of gene activation with p155/140+ MSA, differing from peripheral blood mononuclear cells from MJ+ children (38).
Autoantibodies identify JDM subsets as well as myositis overlap syndromes
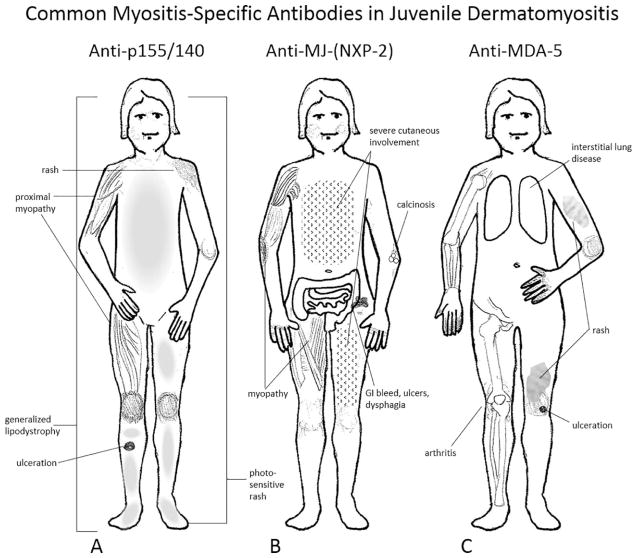
There are 2 major groups of autoantibodies: 1) myositis specific antibodies (MSAs), which define subsets of JDM children, and 2) myositis associated antibodies (MAAs), which identify children with additional symptoms of other connective tissue diseases. The major advance is the identification of specific MSAs, which characterize individual sub-groups of JDM children who each have specific phenotypes (Figure 2, A–C) and prognoses (39) (Table). It is generally agreed that protein immunoprecipitation is the most sensitive and consistent method to identify these antibodies (39, 40) but line–blot assays (41) or highly standardized ELISA assays may be used as well (39). These MSAs are present in 54% of children with juvenile myositis (JM) in the United States (US) (42) and about 51% in the United Kingdom (UK) (39). The most common MSA is directed against transcriptional intermediary factor 1 gamma (TIF-1-γ)/p155/140, and is associated with severe cutaneous disease, a chronic disease course and lipodystrophy, which can range from focal to generalized (43) (Figure 2, A). The second most frequent MSA is called anti-MJ in the US, but is labelled the anti-nuclear matrix protein-2 (NXP-2) in the UK and Europe, and is highly associated with the development of calcinosis at any age (Figure 2, B). The severity of the calcinosis is worse in the younger child, age 4 years or less (44). Of note, 25% of children with JDM in the US, both boys and girls, are below age 4 years at disease onset (24). Children with anti-MJ are more likely to have a chronic disease course with more severe muscle disease, GI bleeding, ulcers and dysphagia, with worse disease outcome and impaired functional status (42, 44). The next, less common MSA, anti-MDA-5, is directed against the melanoma differentiation associated gene 5 (also known as CADM-140), (Figure 2, C, Table). Anti-MDA-5 is associated with rapidly progressive interstitial lung disease (ILD) and a high mortality rate, complicated by arthritis and ulceration (45) in the Japanese population. This antibody is present in 54% of Japanese children with JDM; higher titers are associated with elevated levels of interleukin (IL)-18, IL-6, and ferritin and very severe ILD (46), unlike children with JDM with MDA-5 (7–8%) in the US, who rarely develop ILD. The most benign MSA is anti-Mi-2, present in 3–5% of patients with JDM in the US (42), 4% in the UK (39), and its target is a nucleosome remodeling deacetylase complex (NuRD). Children with Mi-2+ demonstrate “classic” JDM symptoms, and respond well to standard therapies with an excellent prognosis. In contrast to adults with DM or PM, <5% of children have antibody to the t-RNA-synthetase antigens (39) (Table 1). The antigenic targets are listed in Table 1, and, as in adults, anti-Jo-1 is the most reported. The anti t-RNA-synthetase syndrome is characterized by myositis, interstitial lung disease, fever, mechanic’s hands, Raynaud’s phenomena and arthritis; it occurs in older children who have an increased risk of mortality. A relatively new group is composed of two severe necrotizing autoimmune myopathies: anti-signal recognition particle (SRP) (47) and anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) (48, 49). Both of these necrotizing myopathies respond poorly to our current modes of therapy. SRP antibodies are more frequent in African American children who have frequent cardiac involvement and are often wheel chair bound (47, 48). Both types of myopathies have very high levels of muscle derived enzymes and display muscle cell necrosis on biopsy with scant inflammatory infiltrate. Children with HMGCR antibody may not have the typical dermatomyositis skin involvement (49), while adults often have a history of exposure to statins (50–51). Finally, the teenager that develops myositis may have an “overlap” syndrome with MAA antibodies to PmScl (3–5%) or U-RNP (5–15%) (Table 1). Other antigens in the MAA group include polymyositis/scleroderma (PM/Scl), or antibodies to U-RNP antigens which define the “overlap syndromes”. To complicate matters, a child with any of the MSAs may also have a MAA, for example anti-Ro-52 (6%), associated with ILD (52). Reversal of ILD with aggressive medical therapy was reported in a child positive for anti-PL-12 (53).
Figure 2. Phenotypes associated with the 3 most common myositis specific antibodies (MSAs) in children with myositis: anti-p155/140 (A), anti-MJ (B) and anti-MDA-5 (C).
A) Anti-p155/140, present in 18–30% of idiopathic juvenile inflammatory myopathies display an extensive photosensitive rash which ulcerates, a chronic disease course and generalized lipodystrophy. B) 15–23% of children positive for anti-MJ (NXP2 in the UK) may have disease onset at a younger age, have dysphonia, muscle cramps, atrophy and contractures, with increased weakness, and they are more likely to develop calcifications and gastrointestinal symptoms; their rash often spares the truncal area. C) Anti-MDA-5 is increased in the Japanese population (33%) vs the UK (6%) and is associated with inflammatory lung disease, oral and cutaneous ulcers, arthritis and a milder form of muscle involvement. Adapted with permission from Rider et al (42).
Table.
Clinical Associations: Myositis-Specific Autoantibodies (MSA) and Myositis-Associated Antibodies (MAA) in juvenile-onset myositis. Adapted with permission from Tansley (145).
| Autoantibody | Target autoantigen | Prevalence in patients with juvenile-onset myositis | Clinical associations |
|---|---|---|---|
| Common myositis-specific autoantibodies are found in 45–55% of patients with juvenile-onset myositis | |||
| Anti-Mi2 | Nucleosome remodeling deacetyalse complex (NuRD) | 3–4% [42, 39] | ‘Classic’ dermatomyositis. Responds well to standard therapies. Favorable prognosis [5, 138] |
| Anti-TIF1g (p155/140, TRIM33) | Transcriptional intermediary factor 1 gamma (TIF1-γ) | 18–35% [39, 42] | Severe cutaneous disease. Rashes in photo-exposed pattern. Chronic disease course. Lipodystrophy [48, 140, 141, 142, 43] |
| Anti-NXP2 (p140, MJ) | Nuclear matrix protein 2 (NXP2) | 15–22% [39, 42] | Calcinosis. More severe muscle disease. Gastrointestinal bleeding, ulcers and dysphagia. Worse disease outcome and functional status [138, 48, 44] |
| Anti-MDA5 (CADM-140) | Melanoma differentiation-associated gene 5 (MDA5) | 6% [39] | More common in east Asia where associated with clinically amyopathic myositis, rapidly progressive interstitial lung disease and a high mortality. In Caucasian populations associated with mild muscle disease, interstitial lung disease, arthritis and ulceration [149, 45, 46] |
| Rare but clinically important myositis-specific autoantibodies are found in 5–8% of patients with juvenile-onset myositis Antisynthetases (Jo-1, PL12, PL7, OJ, EJ, KS, Zo and Ha) | |||
| Antisynthetases | Antisynthetase syndrome: myositis, interstitial lung disease, fever, mechanics hands, Raynaud’s phenomenon and arthritis; occurs in older children. Increased mortality [48] | ||
| - Jo-1 | - Histidyl | 2–3% [39, 42] | |
| - PL12 | - Alanyl | 2–3% [39, 42] | |
| - PL7 | - Theronyl | 2–3% [39, 42] | |
| - OJ | - Isoleucyl | 2–3% [39, 42] | |
| - EJ | - Glycyl | 2–3% [39, 42] | |
| - KS | - Asparaginyl | 2–3% [39, 42] | |
| - Zo | - Phenylalanyl | 2–3% [39, 42] | |
| - Ha | - Tyrosyl | 2–3% [39, 42] | |
| Anti-SRP | Signal recognition particle (SRP) | 2% [39, 42] | Necrotizing autoimmune myositis. Severe weakness. Cardiac involvement. Occurs in older children. May be refractory to standard treatment [48] |
| Anti-HMGCR | HMGCR | 1% [39] | Necrotizing autoimmune myositis [143, 144] |
| Anti-SAE | Small ubiquitin-like modifier activating enzyme (SAE) | 1% [39] | Initially amyopathic disease with muscle involvement occurring later |
| Myositis-associated autoantibodies are found in 16–20% of patients with juvenile-onset myositis. Some may occur in conjunction with a myositis-specific autoantibody | |||
| Anti-PmScl | Exsome associated PM- Scl-75; PM-Scl-100; C1D [146] | 5% [39] | Overlap syndromes [48] |
| Anti-U1RNP | U1RNP [147] | 2% [42] | Overlap syndromes [48] |
| Anti-Ro52 | Ro52 [148] | 5% [42] | Overlap syndromes. May be found in conjunction with other MSA, particularly antisynthetases [48] |
Common MSAs are present in 45–55% of the United States pediatric population with juvenile onset myositis. Rare MSAs are present in 5–8%. Myositis-Associated Autoantibodies (MAA) are found in 16–20% of juvenile-onset myositis, with or without an accompanying MSA. Adapted with permission from Tansley (145).
The source of the antigens that elicit these antibodies is under investigation. Anti-Mi-2 is directed against antigens emerging on the regenerating myofibers in the areas of muscle perifasicular atrophy (54); other myopathic antigens are expressed by regenerating muscle fibers (55). Of note, inclusion body myositis occurs in people ≥50 years of age, not children (56).
Diagnostic and clinical characteristics of children with JDM
The common clinical findings and differential diagnosis are well established (1, 2). Figure 2 highlights the typical symptoms expressed by JDM children with anti-p155/140 (1A), anti-MJ (1B), or anti-MDA-1 (1C) antibodies. The classic features of JDM, symmetrical, proximal muscle weakness, and the typical heliotrope rash (Figure 1, A) vary with and within each MSA group. Lesser skin involvement may occur: a child positive for MDA-5 displayed only a small area of persistent erythema on the cheek (Figure 1, B), and another child positive for p155/140+ had only erythema of the pinna the ear (Figure 1, C). The shawl sign involves the skin of the upper chest, and can display both acute and chronic inflammation (Figure 1, D). Erythema tends to occur where the skin bends, as over the joints of the hands (Figure 1, E), knees, and elbows. Classic Gottron papules can appear on the hands, elbows and elsewhere, become atrophic and scarred, and not infrequently, calcify. Calcifications can become exuberant, as seen in the buttocks of a 2 year old child (Figure 1, F). Microvasculopathy can be identified as either frank telangiectasia (Figure 1, G), and/or dilated capillaries at the eyelid margin (Figure 1, H). Symmetrical proximal muscle involvement, MRI image, Figure 2, I, is typical of JDM, differing from overlap syndromes, which are often unilateral. Quantitation of the number of the child’s nailfold end row capillary loops reflects disease improvement or flare—Figure 2, J shows normal end row capillary loops; Figures 1, K and 1, L depict moderate and severe capillary dropout and deformation, respectively.
Soft tissue calcifications, a major contributor to morbidity and mortality, vary in frequency from 17% (57) to 44% (58), and are more severe in children of African descent (59). These dystrophic calcifications are associated with chronic cutaneous inflammation (60) as well as lipoatrophy, either focal or generalized (43). The calcifications vary in shape and location, are composed of osteopontin, osteonectin and bone sialoprotein (60) and contain small integrin-binding ligand N-linked glycoprotein (SIBLING) proteins (61); hydroxyapatite was the only mineral detected (60). The calcifications often occur at pressure points, but may be deep in the connective tissue. Response to therapy can be measured by a decrease in the calcification volume over time by use of low dose, single slice CT; the radiation dose is equivalent to a chest x-ray (62).
Childhood and adult onset dermatomyositis have differing features
Differences in epidemiology
The inflammatory myopathies are 10 times more common in adults: incidence = 20 cases/million people/year vs 2.3 cases/million people/year for children in the US. JDM is the most common of the inflammatory myopathies in children, 75%, compared with 14–28% of adults with DM. In the UK, the prevalence of dermatomyositis is 30/100,000 for adults compared with 6/100,000 children (63). In the US, the prevalence of adult inflammatory myopathy ranges from 17–32/100,000; it has not been published for children (64). Adult DM-PM with anti-p155/140 (anti-TIF-1-γ) antibody frequently (17.5%) develop cancer. In contrast, children with JDM, even those with anti-p155/140, do not develop malignancy, although sporadic lymphomas have been reported (65). Patients with JDM have more calcinosis; adult patients with DM develop more lung disease (66).
Differences in pathophysiology
There are clues to the differing pathophysiology in children and adults. Assays of miRNA expression in muscle of untreated children with JDM show a marked downregulation of miRNA-10a (67), not reported for adult DM muscle (some of whom were treated) (68). RNA-10a, a master regulator of proinflammatory cytokines via the NFkB pathway, also controls vascular system components (69). Studies of endothelial cells from adults with DM or PM showed a decrease in PM only, not in DM (70). Similarly, the number of endothelial precursor cells were normal in children with JDM (71), providing evidence that production of the endothelial cells is not the problem in JDM. It is accepted that the endothelial cells are damaged in both the inflamed muscle (6, 72) as well as the capillary end row loops in the fingernails (73, 74). What is open to speculation is the identity of the agent, hypoxia, targeted viral infection, or the circulating and tissue based pro-inflammatory cytokines, such as TNF-α or type-1 interferons (75). Although reports of cytokine data often combined results from adults and children with DM (76, 77), when untreated patients with DM and JDM were compared, a marked increase in IRF-4, retinoic related orphan receptor γ, IL-6, IL-17F, IL-23A, IL-21, GATA3 and IL1β were increased in JDM blood at baseline and Stat3 and BCL6 were increased in adult DM blood. JDM muscle had higher levels of GATA3, IL-13 and STAT5B than adult DM muscle (36), suggesting significant differences in disease pathways between DM children and adults.
Compelling new data shows that 49% of children with juvenile myositis in the UK are positive for one or more MSA, specifically determined by radio-labelled immunoprecipitation and previously validated ELISA’s. Furthermore, these MSA were exclusively limited to children with inflammatory myositis, not other forms of pediatric rheumatic disease (juvenile idiopathic arthritis, juvenile onset SLE or those with muscular dystrophies) and healthy controls (39). Importantly, these assays can confirm the diagnosis of a specific type of inflammatory myopathy. Treatment of juvenile dermatomyositis
Corticosteroids
The advent of corticosteroids has improved the outcome for JDM children, although the dose/route of corticosteroid administration appears to be highly variable (78). Prior to their usage, 1/3 of the JDM had calcinosis, 1/3 died, and 1/3 survived (79). As more information became available about the range of JDM symptoms, consensus derived, comparative research proposals for treatment were developed by pediatric rheumatologists who were members of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) (80–83). Standardized criteria for evaluating improvement in response to therapy was also created (84). Our center usually starts with high dose intravenous methyl prednisolone (30 mg/kg, one gram maximum dose), daily for three or more consecutive daily doses once the diagnosis of JDM is confirmed. This is followed by oral corticosteroids, as presented in a recent Paediatric Rheumatology International Trials Organization (PRINTO) study (85). However, the absorption of oral prednisone is greatly impaired in JM patients with nailfold capillary loss (86), and it takes 2–3 months after recognized JDM onset to develop this nailfold drop out (87), but the intravenous route of high dose corticosteroid therapy reduces the incidence of calcinosis (57, 88). Unfortunately, adverse reactions to corticosteroids are a major cause of morbidity in JDM patients (57) and a slow corticosteroid taper should be initiated after disease stabilization. Reliable and documentable biomarkers are sorely needed to guide this process.
Methotrexate (MTX)
A folic acid analogue that inhibits nucleic acid synthesis, MTX is a first line agent for treatment of moderate to severe JDM patients (80). A recent randomized trial of 139 children with JDM compared 3 treatment plans in 22 countries: after an induction period consisting of three daily doses of IV methylprednisolone at 30 mg/kg, the children were given for 2 years either oral prednisone alone, prednisone + MTX, or prednisone + cyclosporine A (CyA). The study concluded that combined prednisone + either MTX or CyA performed better than prednisone alone; more adverse reactions occurred with a 4–5 mg/kg dose of CyA, which was then discontinued (85). Because 30% of patients with JDM do not respond to MTX, research now focuses on identifying biomarkers that both reflect a significant clinical response (89) and critical polymorphisms in the multiple genes controlling MTX transport and glutamination pathways (90). The dosage of MTX is 15mg/M2 every week (max=25 mg/week) with 1mg/day of folic acid, except on the day when MTX is given. The most common adverse reaction is nausea and vomiting; less usual complaints include mouth sores, bone marrow suppression, elevated liver enzyme and mild lung dysfunction. Pregnancy is a contraindication—drug contact induces fetal malformations. If serology tests for hepatitis B and C is positive, another drug should be used.
Cyclosporine A (CyA)
Another corticosteroid sparing agent, CyA has a higher frequency of clinically significant side effect than MTX. It is often given to those JDM who are corticosteroid resistant or have a persistent rash (83). An inhibitor of T cell activation, the pro-drug of CyA becomes activated after complexing with cyclophillin. This intracytoplasmic protein complex then inhibits calcineurin, a phosphatase that mediates the pharmacologic effects. Lipophilic in nature, a raised serum lipid level increases clearance of the drug, which should be given before mealtime every 12 hours; CyA trough levels should also be obtained before eating (91). In most children with JDM, CyA at the dose of 3 mg/kg, can maintain an 11th hour target trough level of 80–110 ng/ml.
Intravenous immunoglobulin (IVIG)
A range of mechanisms are proposed for the action of IVIG, including cytokine and autoantibody neutralization and saturation of the Fc receptor, blocking receptor activation (92). Monthly administration of IVIG (1–2 grams/kg) is recommended for children with JDM who continue to have rash as a persistent complaint (83). A retrospective study of 78 JDM concluded that IVIG controlled JDM disease activity, particularly in corticosteroid dependent cases (93). Testing for immunoglobulin A (IgA) deficiency should be obtained prior to the administration of intravenous immunoglobulin. Reactions to IVIG, flushing, flu like symptoms, such as headache and fatigue, commonly occur about 24 hours after the infusion and may last as long as 3 days. Using IVIG products that are low in IgA content may decrease these side effects (94). For steroid resistant patients with DM, the use of IVIG + corticosteroids was cost saving (95). Replacement IgG may be required, after rituximab treatment (see below) if the child develops a significantly low level of IgG (96). Hyaluronidase-facilitated immunoglobulin allows higher doses of immunoglobulin to be given subcutaneously (97).
Hydroxychloroquine (HCQ)
This drug targets NADPH oxidase and strongly reduces or completely prevents the induction of endosomal NOX by TNFα, IL-1β and aPL in human monocytes and MonoMac1 as well as blocking TLR7/9 on plasmacytoid dendritic cells, reducing the type 1 interferon signature (98). It also has an anti-thrombotic effect and is associated with a significant reduction in total cholesterol, triglycerides and low-density lipoprotein (LDL) levels (98). Patients with JDM given HCQ had an improvement in their rash within 3 months (99). HCQ was included in all three consensus clinical treatment plans by CARRA for the treatment of skin prominent disease (82). Recommendations from The American Academy of Ophthalmology regarding screening for HCQ retinopathy were changed in 2016 from “annual” to 5 years after starting the drug, when a standard complete examination should be performed (100). The dosage is 5 mg/kg/day; adverse reactions include abdominal pain ± nausea. Very rare adverse effects include cardiomyopathy, cardiac arrhythmia, elevated liver function tests, bone marrow suppression and myopathy.
Mycophenolate mofetil (MMF)
MMF is a reversible, selective, and non-competitive inhibitor of inosine monophosphate, a critical enzyme in the de novo purine synthesis pathway required for lymphocyte proliferation (101). There are no guidelines to identify who will benefit from MMF (102). MMF given to 50 children with JDM was both corticosteroid sparing and decreased muscle and skin inflammation without leukopenia or an increased number of infections (103). Adult DM with ILD responded well to MMF (104). Associated with congenital malformations, MMF should be discontinued 7 weeks before a planned pregnancy. Diarrhea, bone marrow suppression and reactivation of hepatitis B and C can occur; screening for hepatitis is recommended. MMF is given every 12 hours at 20mg/kg (maximum of 1,000 mg/24 hours).
Biologic Agents: rituximab, TNF inhibitors, abatacept
The biologic agents are a promising mode of intervention; the targets are specific components of the inflammatory cascade (105, 106), but the specific pathways/mechanisms have yet to be defined for each MSA/MAA combination.
Rituximab
Rituximab, a monoclonal antibody directed against CD20, depletes B cells via several mechanisms, including complement fixation, antibody-dependent cellular cytotoxicity and signaling for apoptosis (105). The initial reports (107, 108) were followed by a large multi-center randomized clinical trial to evaluate the effectiveness of rituximab in both adult and pediatric patients with refractory myositis (109–111). Reanalysis of the data suggested that specific MSAs, anti-Jo-1 and anti-Mi-2, or youth (JDM vs DM) predicted a better response to rituximab. The visual analog scale for muscle and inflammatory cytokine levels improved after 16 weeks of therapy (111). Rituximab is the most commonly used biologic in the treatment of JDM (106). Serum immunoglobulin G (IgG) levels should be monitored; 30–50% of children may develop hypogammaglobinemia in the first year of therapy (112, 113). The child’s B cell response to rituximab can be evaluated by sequential CD19+ absolute counts (flow cytometry).
TNF-inhibitors
Etanercept appeared to be ineffective in JDM (114), but other TNF-antibody inhibitors fared better (115). Of note, TNF inhibitors used to treat other autoimmune diseases may rarely precipitate the development of an inflammatory myopathy (116).
Abatacept
This fusion protein between immunoglobulin and CTLA-4, when used in conjunction with thiosulfate was successful in diminishing the calcific lesions (117).
Autologous stem cell transplant
Symptoms of DM and PM are well known “distinctive features” of chronic graft vs host disease as well as autologous bone marrow transplantation (BMT) (118–122). CD3/CD19 depleted autologous BMT successfully treated 2 patients with JDM after immunoablative conditioning with fludarabine, cyclophosphamide and anti-thymocyte globulin (118). A child with refractory PM responded to immune ablation with Campath -1H (anti-CD52) antibody alone, thus avoiding the expense and trauma of BMT (122).
Vitamin D
Low vitamin D serum levels occur in both the general population (123) and in patients with JDM (124). Essential therapy for immune system maintenance, vitamin D modulates monocyte maturation to immature dendritic cells and other antigen presenting cells as well as B and T cells (125), via 1α-hydroxylase (CYP27B1) (123). Because the sun is the major source of UVB, which promotes the formation of vitamin D, and JDM children are asked to avoid UVB exposure, supplementation with exogenous vitamin D is usually required. The recommended blood level for vitamin D is 30 IU or above, but others suggest 60 IU (123). Vitamin D supplementation is usually about 1–2,000 IU/day; more may be needed to raise the blood level to the desired range.
Physical therapy (PT)
Implementation of PT has reversed from advising bed rest for months at a time, to initiating graded resistance early in the course of illness (126). Truncal muscles are weakened in JM, impairing ventilatory capacity (52). Muscle weakness contributes to low bone mass, aerobic deconditioning and exercise intolerance, but can respond to a home-based regimen (127), decreasing travel demands on the family.
Outcomes
Although the outcome of JDM has improved—the mortality rate is now 1–2% in the US—it is elevated elsewhere, approximately 8% (58). Weakness and reduced endurance was identified in 32–45% of subjects 16.8 years after the diagnosis in childhood of JDM; MRI confirmed muscle damage in 52% (128), predicted by skin inflammation 6 months after diagnosis (129). Cardiovascular disease is present in adults who had JDM in childhood (130), and may be extensive (131). Unlike SLE, levels of SLE anticoagulant are normal in girls with DM (132). Chinese women with myositis have increased rates of miscarriage (133) and studies of fertility in women who had JDM in childhood showed evidence of compromise (134).
Hope for the Future
In summary, the documentation that MSAs identify specific disease patterns of JDM will lead to discovery of MSA specific disease mechanisms and individualized therapy (135). With increased awareness, aided by The Myositis Association and the Cure JM Foundation® (136), children with JDM will obtain medical therapy shortly after symptom onset, promoting a more successful outcome. We hope that the emotional burden now sustained by both the JDM children and their families (137) will respond to more effective interventions.
Acknowledgments
Supported by the Cure JM Foundation and NIAMS (R21AR066846).
The authors appreciate the assistance of Brittany Hudanick, for manuscript preparation, and Katherine Carmichael, for Figure 2, and the pediatric rheumatology community collaboration.
ABBREVIATIONS
- JDM
Juvenile Dermatomyositis
- JM
Juvenile Myositis
- MSA
Myositis Specific Antibodies
- MHC
Major Histocompatibility Complex
- SLE
Systemic Lupus Erythematosus
- NuRD
Nucleosome Remodeling Deacetylase Complex
- DM
Dermatomyositis
- PM
Polymyositis
- SRP
Signal Recognition Particle
- HMGCR
3-hydroxy-3-methylglutaryl-coenzyme A reductase
- MAA
Myositis Associated Antibody
- NK
Natural Killer
- CARRA
Childhood Arthritis & Rheumatology Research Alliance
- MMF
Mycophenolate mofetil
- IVIG
Intravenous Immunoglobulin Therapy
- IgA
Immunoglobulin A
- CyA
Cyclosporine A
- IIM
Idiopathic Inflammatory Myopathies
- IgG
Immunoglobulin G
- ILD
Interstitial Lung Disease
- US
United States
- UK
United Kingdom
- MTX
Methotrexate
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauren M. Pachman, Professor of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, Stanley Manne Children’s Research Institute; Director, Cure JM Center of Excellence in Juvenile Myositis (JM) Research; Department of Pediatrics, Ann & Robert H. Lurie Children’s Hospital of Chicago, Illinois, USA.
Amer M. Khojah, Allergy-Rheumatology-Immunology Fellow, Ann & Robert H. Lurie, Children’s Hospital of Chicago, Illinois, USA.
References
- 1.Feldman BM, Reed AM, Rider LG, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–12. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 2.Rider LG, Katz JD, Jones OY. Developments in the classification and treatment of the juvenile idiopathic inflammatory myopathies. Rheum Dis Clin N Am. 2013;39:877–904. doi: 10.1016/j.rdc.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendez EP, Lipton R, Ramsey-Goldman, Roettcher P, Bowyer S, Dyer A, et al. Us Incidence of Juvenile Dermatomyositis, 1995–1998: Results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300–305. doi: 10.1002/art.11122. [DOI] [PubMed] [Google Scholar]
- 4.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148:247–53. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Deakin CT, Yasin SA, Simou S, Arnold KA, Tansley SL, Betteridge ZE, et al. Muscle biopsy in combination with myositis-specific autoantibodies aids prediction of outcomes in juvenile dermatomyositis. Arthritis Rheumatol. 2016;68:2806–16. doi: 10.1002/art.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter S, Karpati G, Rothman S, Watters G. The childhood type of dermatomyositis. Neurology. 1976;26:952–62. doi: 10.1212/wnl.26.10.952. [DOI] [PubMed] [Google Scholar]
- 7.Niewold T, Wu S, Smith M, Morgan GA, Pachman LM. Familial aggregation of autoimmune disease in juvenile dermatomyositis. Pediatrics. 2011;127:e1239–46. doi: 10.1542/peds.2010-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pachman LM, Jonasson O, Cannon RA, Friedman JM. Increased frequency of HLA-B8 in juvenile dermatomyositis. Lancet. 1977;8037:567–568. doi: 10.1016/s0140-6736(77)90716-4. [DOI] [PubMed] [Google Scholar]
- 9.Miller FW, Cooper RG, Ivencovsky J, Rider LG, Danko K, Wedderburn LR, et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum. 2013;65:3239–47. doi: 10.1002/art.38137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothwell S, Cooper RG, Lundberg IE, Miller FW, Gregersen PK, Bowes J, et al. Dense genotyping of immune-related loci in the idiopathic inflammatory myopathies confirms HLA alleles as strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann Rheum Dis. 2016 doi: 10.113/62015-208119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuya T, Hakoda M, Tsuchiya N, Kotake S, Ichikawa N, Nanke Y, et al. Immunogenetic features in 120 Japanese with idiopathic inflammatory myopathy. J Rheumatol. 2004;31:1766–74. [PubMed] [Google Scholar]
- 12.Gao X, Han L, Yuan L, Yang Y, Gou G, Sun H, Lu L, Bao L. HLA class II alleles may influence susceptibility to adult dermatomyositis and polymyositis in a Han Chinese population. BMC Dermatol. 2014;14:9. doi: 10.1186/1471-5945-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zen M, Gatto M, Domeneghetti M, Palma L, Borella E, Iaccarino L, et al. Clinical guidelines and definitions of autoinflammatory diseases: Contrasts and comparisons with autoimmunity—a comprehensive review. Clin Rev Allerg Immunol. 2013;45:227–235. doi: 10.1007/s12016-013-8355-1. [DOI] [PubMed] [Google Scholar]
- 14.Lintner KE, Patwardhan A, Rider LG, Abdul-Aziz R, Wu YL, Lundstrom E, et al. Gene copy-number variations (CNVs) of complement C4 and C4A deficiency in genetic risk and pathogenesis of juvenile dermatomyositis. Ann Rheum Dis. 2016;75:1599–606. doi: 10.1136/annrheumdis-2015-207762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Gene-gene-sex interaction in cytokine gene polymorphisms revealed by serum interferon alpha phenotype in juvenile dermatomyositis. J Pediatr. 2010;157:653–7. doi: 10.1016/j.jpeds.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vegosen LJ, Weinberg CR, O’Hanlon TP, Targoff IN, Miller FW, Rider LG. Seasonal birth patterns in myositis subgroups suggest an etiologic role of early environmental exposures. Arthritis Rheum. 2007;56:2719–28. doi: 10.1002/art.22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernatsky S, Smargiassi A, Barnabe C, Svenson LW, Brand A, Martin RV, et al. Fine particulate air pollution and systemic autoimmune rheumatic disease in two Canadian provinces. Environ Res. 2016;146:85–91. doi: 10.1016/j.envres.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Orione MA, Silva CA, Sallum AM, Campos LM, Omori CH, Braga AL, et al. Risk factors for juvenile dermatomyositis: exposure to tobacco and air pollutants during pregnancy. Arthritis Care Res (Hoboken) 2014;66:1571–5. doi: 10.1002/acr.22358. [DOI] [PubMed] [Google Scholar]
- 19.Manlhiot C, Liang L, Tran D, Bitnun A, Tyrrell PN, Feldman BM. Assessment of an infectious disease history preceding juvenile dermatomyositis symptom onset. Rheumatology (Oxford) 2008;47:526–9. doi: 10.1093/rheumatology/ken038. [DOI] [PubMed] [Google Scholar]
- 20.Mamyrova GRL, Ehrich A, Jones O, Pachman LM, Nickeson R, Criscone-Schreiber Jung L, Miller FW, et al. Environmental factors associated with disease flares in juvenile and adult dermatomyositis. Rheumatology (Oxford) 2017;56:1342–7. doi: 10.1093/rheumatology/kex162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen ML, Pachman LM, Schneiderman R, Patel DC, Friedman JM. Prevalence of Coxsackie B virus antibodies in patients with juvenile dermatomyositis. Arthritis Rheum. 1986;29:1365–70. doi: 10.1002/art.1780291109. [DOI] [PubMed] [Google Scholar]
- 22.Svensson J, Arkema EV, Lundberg IE, Holmqvist M. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology (Oxford, England) 2017;56:802–10. doi: 10.1093/rheumatology/kew503. [DOI] [PubMed] [Google Scholar]
- 23.Liang L, Gong P. Climate change and human infectious diseases: A synthesis of research findings from global and spatio-temporal perspectives. Environ Int. 2017;103:99–108. doi: 10.1016/j.envint.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Care Res. 2005;53:166–172. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 25.Limaye V, Smith C, Koszyca B, Blumbergs P, Otto S. Infections and vaccinations as possible triggers of inflammatory myopathies. Muscle Nerve. 2017 doi: 10.1002/mus.25628. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y-W, Shi R, Geraci N, Shrestha S, Gordish-Dressman H, Pachman LM. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile dermatomyositis. BMC Immunol. 2008 doi: 10.1186/1471-2172-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Fedczyna TO, McVicker V, Caliendo J, Li H, Pachman LM. Apoptosis in the skeletal muscle of untreated children with juvenile dermatomyositis: impact of duration of untreated disease. Clin Immunol. 2007;125:165–72. doi: 10.1016/j.clim.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deakin C, Bowes J, Marshall L, Johnson C, Mamyrova G, Curiel R, et al. Interaction between genetic risk factors and age of disease onset in juvenile dermatomyositis. Arthritis Rheumatol. 2017;69(suppl 10) Abst # 2341. [Google Scholar]
- 29.López De Padilla CM, Vallejo AN, Lacomis D, McNallan K, Reed AM. Extranodal lymphoid microstructures in inflamed muscle and disease severity of new-onset juvenile dermatomyositis. Arthritis Rheum. 2009;60:1160–72. doi: 10.1002/art.24411. [DOI] [PubMed] [Google Scholar]
- 30.Vercoulen Y, Bellutti Enders F, Meerding J, Plantinga M, Elst EF, Varsani H, et al. Increased presence of FOXP3+ regulatory T cells in inflamed muscle of patients with active juvenile dermatomyositis compared to peripheral blood. PLoS One. 2014;9:e105353. doi: 10.1371/journal.pone.0105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gitiaux C, Latroche C, Weiss-Gayet M, Rodero MP, Duffy D, Bader Meunier B, et al. Myogenic progenitor cells exhibit IFN type I-driven pro-angiogenic properties and molecular signature during juvenile dermatomyositis. Arthritis Rheumatol. 2017 doi: 10.1002/art.40328. [DOI] [PubMed] [Google Scholar]
- 32.Shrestha S, Wershil B, Sarwark JF, Niewold TB, Philipp T, Pachman LM. Lesional and nonlesional skin from patients with untreated juvenile dermatomyositis displays increased numbers of mast cells and mature plasmacytoid dendritic cells. Arthritis Rheum. 2010;62:2813–22. doi: 10.1002/art.27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller ML, Lantner R, Pachman LM. Natural and antibody-dependent cellular cytotoxicity in chldren with systemic lupus erythematosus and juvenile dermatomyositis. J Rheumatol. 1983;10:640–2. [PubMed] [Google Scholar]
- 34.Briones MR, Morgan GA, Amoruso MC, Rahmani B, Ryan ME, Pachman LM. Decreased CD3-CD16+CD56+ Natural Killer absolute cell counts in children with Orbital Myositis: a clue to disease activity. RMD Open. 2017;3:e000385. doi: 10.1136/rmdopen-2016-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellutti Enders F, van Wijk F, Scholman R, Hofer M, Prakken BJ, van Royen-Kerkhof A, et al. Correlation of CXCL10, tumor necrosis factor receptor type II, and galectin 9 with disease activity in juvenile dermatomyositis. Arthritis Rheum. 2014;66:2281–9. doi: 10.1002/art.38676. [DOI] [PubMed] [Google Scholar]
- 36.Lopez De Padilla CM, Crowson CS, Hein MS, Pendegraft RS, Strausbauch MA, Niewold TB, et al. Gene expression profiling in blood and affected muscle tissues reveals differential activation pathways in patients with new-onset juvenile and adult dermatomyositis. J Rheumatol. 2017;44:117–24. doi: 10.3899/jrheum.160293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahoria R, Selcen D, Engle AG. Microvascular alterations and the role of complement in dermatomyositis. Brain. 2016;139:1891–1903. doi: 10.1093/brain/aww122. [DOI] [PubMed] [Google Scholar]
- 38.Huang C-C, Hans V, Xu D, Curran ML, Morgan GA, Roberson DO, Galat V, Galat K, Pachman LM. RNASeq detection of gene dysregulaton in PBMCs for Juvenile Dermatomyositis, postitive for p155/140 myositis specific antibody. Arthritis Rheumatol. 2017;69 [abst # 852.] [Google Scholar]
- 39.Tansley SL, Simou S, Shaddick G, Betteridge ZE, Almeida B, Gunawardena H, et al. Autoantibodies in juvenile-onset myositis: Their diagnostic value and associated clinical phenotype in a large UK cohort. J Autoimmun. 2017 doi: 10.1016/j.jaut.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trieu EP, Targoff IN. Immunoprecipitation: Western Blot for Proteins of Low Abundance. In: Kurien B, Scofield R, editors. Western Blotting. Methods in Molecular Biology. Vol. 1312. Humana Press; New York, NY: 2015. [DOI] [PubMed] [Google Scholar]
- 41.Ghirardello AI, Rampudda M, Ekholm L, Bassi N, Tarricone E, Zampieri S, et al. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology (Oxford) 2010;49:2370–4. doi: 10.1093/rheumatology/keq281. [DOI] [PubMed] [Google Scholar]
- 42.Rider LG, Nistala K. The juvenile idiopathic inflammatory myopathies: pathogenesis, clinical and autoantibody phenotypes, and outcomes. J Intern Med. 2016;280:24–38. doi: 10.1111/joim.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bingham A, Mamyrova G, Rother KI, Oral E, Cochran E, Premkumar A, et al. Predictors of acquired lipodystrophy in juvenile-onset dermatomyositis and a gradient of severity Medicine. 2008;87:70–86. doi: 10.1097/MD.0b013e31816bc604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tansley SL, Betteridge ZE, Shaddick G, Gunawardena H, Arnold K, Wedderburn LR, et al. Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology (Oxford) 2014;53:2204–8. doi: 10.1093/rheumatology/keu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi I, Okura Y, Yamada M, Kawamura N, Kuwana M, Ariga T. Anti-melanoma differentiation-associated Gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J Pediatr. 2011;158:675–7. doi: 10.1016/j.jpeds.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi N, Takezaki S, Kobayashi I, Iwata N, Mori M, Nagai K, Nakano N, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology (Oxford, England) 2015;54:784–91. doi: 10.1093/rheumatology/keu385. [DOI] [PubMed] [Google Scholar]
- 47.Rouster-Stevens KA, Pachman LM. Autoantibody to signal recognition particle in African American girls with juvenile polymyositis. J Rheumatol. 2008;35:927–9. [PubMed] [Google Scholar]
- 48.Rider LG, Shah M, Mamyrova G, Huber AM, Rice MM, Targoff IN, Miller FW Childhood Myositis Heterogeneity Collaborative Study Group. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013 Jul;92(4):223–43. doi: 10.1097/MD.0b013e31829d08f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kishi T, Rider LG, Pak K, Barillas-Arias L, Henrickson M, McCarthy PL, et al. Association of Anti-3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Autoantibodies With DRB1*07:01 and Severe Myositis in Juvenile Myositis Patients. Arthritis Care Res (Hoboken) 2017;69:1088–1094. doi: 10.1002/acr.23113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–21. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiniakou E, Christopher-Stine L. Immune-mediated necrotizing myopathy associated with statins: history and recent developments. Current opinion in rheumatology. 2017;29:604–611. doi: 10.1097/BOR.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 52.Prestridge A, Morgan G, Ferguson L, Huang CC, Pachman LM. Pulmonary function tests in idiopathic inflammatory myopathy: Association with clinical parameters in children. LID - Arthritis Care Res. 2013;65:1424–143. doi: 10.1002/acr.22014. [DOI] [PubMed] [Google Scholar]
- 53.Vega P, Ibarra M, Prestridge A, Pachman LM. Autoantibody to PL-12 (Anti-Alanyl-tRNA synthetase) in an African American girl with juvenile dermatomyositis and resolution of interstitial lung disease. J Rheumatol. 2011;38:394–5. doi: 10.3899/jrheum.100608. [DOI] [PubMed] [Google Scholar]
- 54.Mammen AL, Casciola-Rosen LA, Hall JC, Christopher-Stine L, Corse AM, Rosen A. Expression of the dermatomyositis autoantigen Mi-2 in regenerating muscle. Arthritis Rheum. 2009;60:3784–93. doi: 10.1002/art.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson C, Piguet V, Choy E. The pathogenesis of dermatomyositis. Br J Dermatol. 2017 doi: 10.1111/bjd.15807. [DOI] [PubMed] [Google Scholar]
- 56.Keller CW, Schmidt J, Lunemann JD. Immune and myodegenerative pathomechanisms in inclusion body myositis. Ann Clin Transl Neurol. 2017;4:422–45. doi: 10.1002/acn3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pachman LM, Curran ML, Morgan GA, Targoff I, Huang H, Xu D, Huang CC. 101 Juvenile Myositis Patients Characterized By Myositis Specific Antibodies: Disease Activity and Damage over 60 Months. Arthritis Rheumatol. 2017;69 [abst #1272] [Google Scholar]
- 58.Okong’o LO, Esser M, Wilmshurst J, Scott C. Characteristics and outcome of children with juvenile dermatomyositis in Cape Town: a cross-sectional study. Pediatr Rheumatol Online J. 2016;14:60. doi: 10.1186/s12969-016-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillippi K, Hoeltzel M, Robinson AB, Kim S. Race, income, and disease outcomes in juvenile dermatomyositis. J Pediatr. 2017;184:38–44. doi: 10.1016/j.jpeds.2017.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pachman LM, Veis A, Stock S, Abbott K, Vicari F, Patel P, et al. Composition of calcifications in children with juvenile dermatomyositis: association with chronic cutaneous inflammation. Arthritis Rheum. 2006;54:3345–50. doi: 10.1002/art.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urganus AL, Zhao YD, Pachman LM. Juvenile dermatomyositis calcifications selectively displayed markers of bone formation. Arthritis Rheum. 2009;61:501–8. doi: 10.1002/art.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ibarra M, Rigsby C, Morgan GA, Sammet CL, Huang CC, Xu D, et al. Monitoring change in volume of calcifications in juvenile idiopathic inflammatory myopathy: a pilot study using low dose computed tomography. Pediatr Rheumatol Online J. 2016;14:64. doi: 10.1186/s12969-016-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tansley S, Wedderburn LR. Comparing and contrasting clinical and serological features of juvenile and adult-onset myositis: implications for pathogenesis and outcomes. Curr Opin Rheumatol. 2015;27:601–7. doi: 10.1097/BOR.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 64.Meyer A, Meyer N, Schaeffer M, Gottenberg J-E, Geny B, Sibilia J. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology. 2015;54:50–63. doi: 10.1093/rheumatology/keu289. [DOI] [PubMed] [Google Scholar]
- 65.Stübgen JP. Juvenile dermatomyositis/polymyositis and lymphoma [review] J Neurol Sci. 2017;377:19–24. doi: 10.1016/j.jns.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 66.Tansley S, McHugh NJ, Weddeburn LR. Adult and juvenile dermatomyositis: are the distinct clinical features explained by our current understanding of serological subgroups and pathogenic mechanisms? Arthritis Res Ther. 2013;15:211. doi: 10.1186/ar4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu D, Huang CC, Kachaochana A, Morgan GA, Bonaldo MF, Soares MB, et al. MicroRNA-10a regulation of proinflammatory mediators: an important component of untreated juvenile dermatomyositis. J Rheumatol. 2016;43:161–8. doi: 10.3899/jrheum.141474. [DOI] [PubMed] [Google Scholar]
- 68.Eisenberg I, Alexander MS, Kunkel LM. miRNAS in normal and diseased skeletal muscle. J Cell Mol Med. 2009;13:2–11. doi: 10.1111/j.1582-4934.2008.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–5. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ekholm L, Kahlenberg JM, Barbasso Helmers S, Tjarnlund A, Yalavarthi S, Zhao W, et al. Dysfunction of endothelial progenitor cells is associated with the type I IFN pathway in patients with polymyositis and dermatomyositis. Rheumatology (Oxford) 2016;55:1987–92. doi: 10.1093/rheumatology/kew288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu D, Kacha-Ochana A, Morgan GA, Huang CC, Pachman LM. Endothelial progenitor cell number is not decreased in 34 children with juvenile dermatomyositis: a pilot study. Pediatr Rheumatol Online J. 2017;15:42. doi: 10.1186/s12969-017-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banker BQ. Dermatomyositis of childhood, ultrastructural alterations of muscle and intramuscular blood vessels. J Neuropathol Exp Neurol. 1975;34:46–75. doi: 10.1097/00005072-197501000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Spencer-Green G, Crowe WE, Levinson JE. Nailfold capillary abnormalities and clinical outcome in childhood dermatomyositis. Arthritis Rheum. 1982;25:954–8. doi: 10.1002/art.1780250807. [DOI] [PubMed] [Google Scholar]
- 74.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with Juvenile Dermatomyositis. Arthritis Rheum. 2008;58:571–576. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huard C, Gullà SV, Bennett DV, Coyle AJ, Vleugels RA, Greenberg SA. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon β in dermatomyositis. Br J Dermatol. 2017;176:1224–1230. doi: 10.1111/bjd.15006. [DOI] [PubMed] [Google Scholar]
- 76.Reed AM, Peterson E, Bilgic H, Ytterberg SR, Amin S, Hein MS, et al. Changes in novel biomarkers of disease activity in juvenile and adult dermatomyositis are sensitive biomarkers of disease course. Arthritis Rheum. 2012;64:4078–86. doi: 10.1002/art.34659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baechler EC, Bilgic H, Reed AM. Type I interferon pathway in adult and juvenile dermatomyositis. Arthritis Res Ther. 2011;13:249. doi: 10.1186/ar3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stringer E, Bohnsack J, Bowyer SL, Griffin TA, Huber AM, Lang B, et al. Treatment approaches to juvenile dermatomyositis (JDM) across North America: The Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM Treatment Survey. J Rheumatol. 2010;37:1953–61. doi: 10.3899/jrheum.090953. [DOI] [PubMed] [Google Scholar]
- 79.Bitnum S, Daeschner C, Travis L, Dodge WF, Hopps H. Dermatomyositis. J Pediatr. 1964;64:101–31. doi: 10.1016/s0022-3476(64)80325-5. [DOI] [PubMed] [Google Scholar]
- 80.Huber AM, Giannini EH, Bowyer SL, Kim S, Lang B, Lindsley CB, et al. Protocols for the initial treatment of moderately severe juvenile dermatomyositis: results of a Children’s Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res. 2010;62:219–25. doi: 10.1002/acr.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huber AM, Robinson AB, Reed AM, Abramson L, Bout-Tabaku S, Carrasco R, et al. Consensus treatments for moderate juvenile dermatomyositis: beyond the first two months. Results of the second Childhood Arthritis and Rheumatology Research Alliance consensus conference. Arthritis Care Res. 2012;64:546–53. doi: 10.1002/acr.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim S, Kahn P, Robinson AB, Lang B, Shulman A, Oberle EJ, et al. Childhood Arthritis and Rheumatology Research Alliance consensus clinical treatment plans for juvenile dermatomyositis with skin predominant disease. Pediatr Rheumatol Online J. 2017;15:1. doi: 10.1186/s12969-016-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huber AM, Kim S, Reed AM, Carrasco R, Feldman BM, Hong SD, et al. Childhood Arthritis and Rheumatology Research Alliance consensus clinical treatment plans for juvenile dermatomyositis with persistent skin rash. J Rheumatol. 2017;44:110–6. doi: 10.3899/jrheum.160688. [DOI] [PubMed] [Google Scholar]
- 84.Rider LG, Aggarwal R, Pistorio A, Bayat N, Erman B, Feldman BM, et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in juvenile dermatomyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation collaborative initiative. Arthritis Rheumatol. 2017;69:911–23. doi: 10.1002/art.40060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruperto N, Pistorio A, Oliveira S, Zulian F, Cuttica R, Ravelli A, et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet. 2016;387:671–8. doi: 10.1016/S0140-6736(15)01021-1. [DOI] [PubMed] [Google Scholar]
- 86.Rouster-Stevens KA, Gursahaney A, Ngai KL, Daru JA, Pachman LM. Pharmacokinetic study of oral prednisolone compared with intravenous methylprednisolone in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;59:222–6. doi: 10.1002/art.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ostrowski RA, Sullivan CL, Seshadri R, Morgan GA, Pachman LM. Association of normal nailfold end row loop numbers with a shorter duration of untreated disease in children with juvenile dermatomyositis. Arthritis Rheum. 2010;62:1533–8. doi: 10.1002/art.27379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fisler RE, Liang MG, Fuhlbrigge RC, Yalcindag A, Sundel RP. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol. 2002;47:505–11. doi: 10.1067/mjd.2002.122196. [DOI] [PubMed] [Google Scholar]
- 89.Roszkiewicz J, Smolewska E. In the pursuit of methotrexate treatment response biomarker in juvenile idiopathic arthritis-are we getting closer to personalised medicine? Curr Rheumatol Rep. 2017;19:19. doi: 10.1007/s11926-017-0646-8. [DOI] [PubMed] [Google Scholar]
- 90.Liu SG, Gao C, Zhang RD, Zhao XX, Cui L, Li WJ, et al. Polymorphisms in methotrexate transporters and their relationship to plasma methotrexate levels, toxicity of high-dose methotrexate, and outcome of pediatric acute lymphoblastic leukemia. Oncotarget. 2017;8:37761–72. doi: 10.18632/oncotarget.17781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chighizola CB, Ong VH, Meroni PL. The use of Cyclosporine A in rheumatology: a 2016 comprehensive review. Clin Rev Allergy Immunol. 2017;52:401–23. doi: 10.1007/s12016-016-8582-3. [DOI] [PubMed] [Google Scholar]
- 92.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–89. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 93.Lam CG, Manlhiot C, Pullenayegum EM, Feldman BM. Efficacy of intravenous Ig therapy in juvenile dermatomyositis. Ann Rheum Dis. 2011;70:2089–94. doi: 10.1136/ard.2011.153718. [DOI] [PubMed] [Google Scholar]
- 94.Manlhiot C, Tyrrell PN, Liang L, Atkinson AR, Lau W, Feldman BM. Safety of intravenous immunoglobulin in the treatment of juvenile dermatomyositis: adverse reactions are associated with immunoglobulin A content. Pediatrics. 2008;121:e626–e630. doi: 10.1542/peds.2007-1218. [DOI] [PubMed] [Google Scholar]
- 95.Bamrungsawad N, Chaiyakunapruk N, Upakdee N, Pratoomsoot C, Sruamsiri R, Dilokthornsakul P. Cost-utility analysis of intravenous immunoglobulin for the treatment of steroid-refractory dermatomyositis in Thailand. Pharmacoeconomics. 2015;33:521–31. doi: 10.1007/s40273-015-0269-8. [DOI] [PubMed] [Google Scholar]
- 96.Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, et al. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun. 2015;57:60–5. doi: 10.1016/j.jaut.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Speth F, Haas JP, Hinze CH. Treatment with high-dose recombinant human hyaluronidase-facilitated subcutaneous immune globulins in patients with juvenile dermatomyositis who are intolerant to intravenous immune globulins: a report of 5 cases. Pediatr Rheumatol Online J. 2016;14:52. doi: 10.1186/s12969-016-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Müller-Calleja N, Manukyan D, Canisius A, Strand D, Lackner KJ. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann Rheum Dis. 2017;76:891–897. doi: 10.1136/annrheumdis-2016-210012. doi:10.1136. [DOI] [PubMed] [Google Scholar]
- 99.Olson NY, Lindsley CB. Adjunctive use of hydroxychloroquine in childhood dermatomyositis. J Rheumatol. 1989;16:1545–7. [PubMed] [Google Scholar]
- 100.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision) Ophthalmology. 2016;123:1386–94. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 101.Strathie Page SJ, Tait CP. Mycophenolic acid in dermatology a century after its discovery. Australas J Dermatol. 2015;56:77–83. doi: 10.1111/ajd.12259. [DOI] [PubMed] [Google Scholar]
- 102.Mendoza-Pinto C, Pirone C, van der Windt DA, Parker B, Bruce IN. Can we identify who gets benefit or harm from mycophenolate mofetil in systemic lupus erythematosus? A systematic review. Semin Arthritis Rheum. 2017;47:65–78. doi: 10.1016/j.semarthrit.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Rouster-Stevens KA, Morgan GA, Wang D, Pachman LM. Mycophenolate mofetil: a possible therapeutic agent for children with juvenile dermatomyositis. Arthritis Care Res (Hoboken) 2010;62:1446–51. doi: 10.1002/acr.20269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koyama RVL, Braga TKK, da Silva Dias GA, Fujihara S, Fuzii HT, Yoshikawa GT. Hypomyopathic dermatomyositis associated with interstitial lung disease and good response to mycophenolate mofetil: case-based review. Clin Rheumatol. 2017;36:1919–26. doi: 10.1007/s10067-017-3671-0. [DOI] [PubMed] [Google Scholar]
- 105.Sheng J, Srivastava S, Sanghavi K, Lu Z, Schmidt BJ, Bello A, et al. Clinical Pharmacology Considerations for the Development of Immune Checkpoint Inhibitors. J Clin Pharmacol. 2017 Oct;57(Suppl 10):S26–S42. doi: 10.1002/jcph.990. [DOI] [PubMed] [Google Scholar]
- 106.Spencer CH, Rouster-Stevens KA, Gewanter H, Syverson G, Modica R, Schmidt K, et al. Biologic therapies for refractory juvenile dermatomyositis: five years of experience of the Childhood Arthritis and Rheumatology Research Alliance in North America. Pediatric Rheumatology. 2017;15:50. doi: 10.1186/s12969-017-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levine TD. Rituximab in the Treatment of Dermatomyositis:An Open-Label Pilot Study. Arthritis Rheum. 2005;52:601–607. doi: 10.1002/art.20849. [DOI] [PubMed] [Google Scholar]
- 108.Bader-Meunier B, Decaluwe H, Barnerias C, Gherardi R, Quartier P, Faye A, et al. Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol. 2011;38:1436–40. doi: 10.3899/jrheum.101321. [DOI] [PubMed] [Google Scholar]
- 109.Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DR, Levesque MC, Fertig N. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: A randomized, placebo-phase trial. Arthritis Rheumatol. 2013;65:314–24. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aggarwal R, Bandos A, Reed AM, Ascherman DP, Barohn RJ, Feldman BM, et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66:740–9. doi: 10.1002/art.38270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reed AM, Crowson CS, Hein M, de Padilla, Olazagasti JM, Aggarwal R. Biologic predictors of clinical improvement in rituximab-treated refractory myositis. BMC Musculoskeletal Disorders. 2015;16:257. doi: 10.1186/s12891-015-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13:106–11. doi: 10.1016/j.clml.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khojah A, Fuleihan R, Miller M, Curran M, Klein-Giitelman M. Rituximab-induced hypogammaglobulinemia in pediatric patients with autoimmune diseases [abstract] Arthritis Rheumatol. 2017;69(suppl 10) doi: 10.1186/s12969-019-0365-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rouster-Stevens KA, Ferguson L, Morgan G, Huang CC, Pachman LM. Pilot study of etanercept in patients with refractory juvenile dermatomyositis. Arthritis Care Res. 2014;66:783–7. doi: 10.1002/acr.22198. [DOI] [PubMed] [Google Scholar]
- 115.Riley P, McCann LJ, Maillard SM, Woo P, Murray KJ, Pilkington CA. Effectiveness of infliximab in the treatment of refractory juvenile dermatomyositis with calcinosis. Rheumatology. 2008;47:877–80. doi: 10.1093/rheumatology/ken074. [DOI] [PubMed] [Google Scholar]
- 116.Kim NN, Lio PA, Morgan GA, Jarvis J, Pachman LM. Double trouble: therapeutic challenges in patients with both juvenile dermatomyositis and psoriasis. Arch Dermatol. 2011 Mar 21; doi: 10.1001/archdermatol.2011.49. Online. [DOI] [PubMed] [Google Scholar]
- 117.Arabshahi B, Silverman RA, Jones OY, Rider LG. Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J Pediatr. 2012;160:520–2. doi: 10.1016/j.jpeds.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holzer U, van Royen-Kerkhof A, Van Der Torre P, Kemmerle-Deschner J, Well C, Handgretinger R, et al. Successful autologous stem cell transplantation in two patients with juvenile dermatomyositis. Scand J Rheumatol. 2010;39:88–92. doi: 10.3109/03009740903096622. [DOI] [PubMed] [Google Scholar]
- 119.Ollivier I, Wolkenstein P, Gherardi R, Wechsler J, Kuentz M, Cosnes A, Revuz J, Bagot M. Dermatomyositis-like graft-versus-host disease. Br J Dermatol. 1998;138:558–9. doi: 10.1046/j.1365-2133.1998.02155.x. [DOI] [PubMed] [Google Scholar]
- 120.Ruzhansky KM, Brannagan TH., 3rd Neuromuscular complications of hematopoietic stem cell transplantation. Muscle Nerve. 2015;52:480–7. doi: 10.1002/mus.24724. [DOI] [PubMed] [Google Scholar]
- 121.Enders FB, Delemarre EM, Kuemmerle-Deschner J, van der Torre P, Wulffraat NM, Prakken BP, van Royen-Kerkhof A, van Wijk F. Autologous stem cell transplantation leads to a change in proinflammatory plasma cytokine profile of patients with juvenile dermatomyositis correlating with disease activity. Ann Rheum Dis. 2015;74:315–7. doi: 10.1136/annrheumdis-2014-206287. [DOI] [PubMed] [Google Scholar]
- 122.Reiff A, Shaham B, Weinberg KI, Crooks GM, Parkman R. Anti-CD52 antibody-mediated immune ablation with autologous immune recovery for the treatment of refractory juvenile polymyositis. J Clin Immunol. 2011;31:615–22. doi: 10.1007/s10875-011-9533-7. [DOI] [PubMed] [Google Scholar]
- 123.Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 124.Altieri B, Muscogiuri G, Barrea L, Mathieu C, Vallone CV, Mascitelli L, et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord. 2017;18:335–346. doi: 10.1007/s11154-016-9405-9. [DOI] [PubMed] [Google Scholar]
- 125.Robinson AB, Thierry-Palmer M, Gibson KL, Rabinovich CE. Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J Pediatr. 2012;160:297–302. doi: 10.1016/j.jpeds.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Omori CH, Silva CAA, Sallum AM, Rodrigues Pereira RM, Lúciade Sá Pinto A, Roschel H, et al. Exercise training in juvenile dermatomyositis. Arthritis Care Res. 2012;64:1186–94. doi: 10.1002/acr.21684. [DOI] [PubMed] [Google Scholar]
- 127.Habers GE, Bos GJ, van Royen-Kerkhof A, Lelieveld OT, Armbrust W, Takken T, et al. Muscles in motion: a randomized controlled trial on the feasibility, safety and efficacy of an exercise training programme in children and adolescents with juvenile dermatomyositis. Rheumatology (Oxford) 2016;55:1251. doi: 10.1093/rheumatology/kew026. –62.129. [DOI] [PubMed] [Google Scholar]
- 128.Sanner H, Kirkhus E, Merckoll E, Tollisen A, Roisland M, Lie BA, et al. Long-term muscular outcome and predisposing and prognostic factors in juvenile dermatomyositis: A case-control study. Arthritis Care Res. 2010;62:1103–11. doi: 10.1002/acr.20203. [DOI] [PubMed] [Google Scholar]
- 129.Schwartz T, Sanner H, Gjesdal O, Flato B, Sjaastad I. In juvenile dermatomyositis, cardiac systolic dysfunction is present after long-term follow-up and is predicted by sustained early skin activity. Ann Rheum Dis. 2014;73:1805–10. doi: 10.1136/annrheumdis-2013-203279. [DOI] [PubMed] [Google Scholar]
- 130.Eimer M, Brickman W, Seshardri R, Ramsey-Goldman R, McPherson D, Smuleitz B, et al. Clinical status and cardiovascular risk profile of adults with a history of juvenile dermatomyositis. J Pediatr. 2011;159:795–801. doi: 10.1016/j.jpeds.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barth Z, Nomeland Witczak B, Schwartz T, Gjesdal K, Flatø B, Koller A, et al. In juvenile dermatomyositis, heart rate variability is reduced, and associated with both cardiac dysfunction and markers of inflammation; a cross-sectional study median 13. 5 years after symptom onset. Rheumatology (Oxford) 2016;55:535–43. doi: 10.1093/rheumatology/kev376. [DOI] [PubMed] [Google Scholar]
- 132.Gedalia A, Molina JF, Garcia CO, Doggett S, Espinoza LR, Gharavi AE. Anticardiolipin antibodies in childhood rheumatic disorders. Lupus. 1998;7:551–3. doi: 10.1191/096120398678920659. [DOI] [PubMed] [Google Scholar]
- 133.Zhong Z, Lin F, Yang J, Zhang F, Zeng X, You X. Pregnancy in polymyositis or dermatomyositis: retrospective results from a tertiary centre in China. Rheumatology (Oxford) 2017;56:1272–1275. doi: 10.1093/rheumatology/kex070. [DOI] [PubMed] [Google Scholar]
- 134.de Souza FH, Shinjo SK, Yamakami LY, Viana VS, Baracat EC, Bonfa E, et al. Reduction of ovarian reserve in adult patients with dermatomyositis. Clin Exp Rheumatol. 2015;33:44. –9.136. [PubMed] [Google Scholar]
- 135.Haq SA, Tournadre A. Idiopathic inflammatory myopathies from immunopathogenesis to new therapies. Int J Rheum Dis. 2015;18:818–25. doi: 10.1111/1756-185X.12736. [DOI] [PubMed] [Google Scholar]
- 136.Rider LG, Pachman LM, Miller FW, Bollar H. Myositis and you: A guide to juvenile dermatomyositis for patients, families, and healthcare providers. Washington, DC: TMA Press; 2007. [Google Scholar]
- 137.Kountz-Edwards S, Aoki C, Gannon C, Gomez R, Cordova M, Packman W. The family impact of caring for a child with juvenile dermatomyositis. Chronic Illn. 2017 doi: 10.1177/174239517690034. [DOI] [PubMed] [Google Scholar]
- 138.Espada G, Maldonado Cocco JA, Fertig N, Oddis CV. Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J Rheumatol. 2009;36:2547–2551. doi: 10.3899/jrheum.090461. [DOI] [PubMed] [Google Scholar]
- 139.Wedderburn LR, McHugh NJ, Chinoy H, et al. HLA class II haplotype and autoantibody associations in children with juvenile dermatomyositis and juvenile dermatomyositis-scleroderma overlap. Rheumatology (Oxford, England) 2007;46:1786–1791. doi: 10.1093/rheumatology/kem265. [DOI] [PubMed] [Google Scholar]
- 140.Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology (Oxford, England) 2008;47:324–328. doi: 10.1093/rheumatology/kem359. [DOI] [PubMed] [Google Scholar]
- 141.Shah M, Targoff IN, Rice MM, et al. Brief report: ultraviolet radiation exposure is associated with clinical and autoantibody phenotypes in juvenile myositis. Arthritis Rheum. 2013;65:1934–1941. doi: 10.1002/art.37985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Habers GE, Huber AM, Mamyrova G, et al. Brief Report: association of myositis autoantibodies, clinical features, and environmental exposures at illness onset with disease course in juvenile myositis. Arthritis Rheumatol (Hoboken, NJ) 2016;68:761–768. doi: 10.1002/art.39466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Allenbach Y, Drouot L, Rigolet A, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine (Baltimore) 2014;93:150–157. doi: 10.1097/MD.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tansley SL, Betteridge ZE, Simou S, et al. 174 A diagnostic and treatment challenge: the prevalence and clinical associations of anti-HMG-CoA reductase autoantibodies in a large UK juvenile-onset myositis cohort. Rheumatology. 2016;55(Suppl):i132–i133. [Google Scholar]
- 145.Tansley SL. Antibodies in juvenile-onset myositis. Curr Opin Rheumatol. 2016;28:645–50. doi: 10.1097/BOR.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 146.Schilders G, Egberts WV, Raijmakers R, Pruijn GJ. C1D is a major autoantibody target in patients with the polymyositis-scleroderma overlap syndrome. Arthritis Rheum. 2007;56(7):2449–54. doi: 10.1002/art.22710. [DOI] [PubMed] [Google Scholar]
- 147.Bernstein RM. Autoantibodies in myositis. Baillieres Clin Neurol. 1993;2(3):599–615. [PubMed] [Google Scholar]
- 148.Pourmand N, Pettersson I. The Zn2+ binding domain of the human Ro 52 kDa protein is a target for conformation-dependent autoantibodies. J Autoimmun. 1998;11(1):11–7. doi: 10.1006/jaut.1997.0171. [DOI] [PubMed] [Google Scholar]
- 149.Tansley SL, Betteridge ZE, Gunawardena H, et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther. 2014;16:R138. doi: 10.1186/ar4600. [DOI] [PMC free article] [PubMed] [Google Scholar]