Abstract
Drosophila Dicer-2 processes RNA substrates into short interfering RNAs (siRNAs). Loquacious-PD (Loqs-PD), a dsRNA-binding protein that associates with Dicer-2, is required for processing of a subset of RNA substrates including hairpin RNAs into siRNAs. Inorganic phosphate—a small molecule present in all cell types—inhibits Dicer-2 from processing precursor of microRNAs (premiRNAs), which are processed by Dicer-1. Whether or how Loqs-PD modulates the inhibitory effect of inorganic phosphate on Dicer-2 processing of RNA substrates is unknown. To address this question, I performed in vitro hairpin RNA processing assay with Dicer-2 in the presence or absence of Loqs-PD and/or inorganic phosphate. I found that inorganic phosphate inhibits Dicer-2 alone, but not Dicer-2 + Loqs-PD, from processing blunt-end hairpin RNAs into siRNAs. Thus, Loqs-PD removes the inhibitory effect of inorganic phosphate on Dicer-2 processing of blunt-end hairpin RNAs, allowing siRNA production in the presence of inorganic phosphate.
Keywords: Dicer-2, Loquacious, siRNA, inorganic phosphate, dsRNA, RNA silencing
INTRODUCTION
Dicer enzymes produce microRNAs (miRNAs) and small interfering RNAs (siRNAs). In Drosophila, Dicer-1 processes precursor of miRNAs (pre-miRNAs) into miRNAs and Dicer-2 processes long dsRNAs into siRNAs (Lee et al. 2004).
Endogenous RNA substrates of Dicer-2 to make siRNAs (endo-siRNAs) include partially self-complementary hairpin RNA transcripts, transposon RNAs, and dsRNAs derived from convergent transcription of mRNAs (cis-natural antisense transcripts, cis-NAT) (Czech et al. 2008; Ghildiyal et al. 2008; Kawamura et al. 2008; Okamura et al. 2008a; Okamura et al. 2008b). The most abundant endo-siRNA in vivo is esi-2.1, a hairpin-derived endo-siRNA. This suggests that the esi-2.1 precursor hairpin RNA is a predominant substrate of Dicer-2. Dicer-2 also processes exogenous long dsRNAs derived from viral RNA genomes or intermediates of replication and those introduced artificially into exogenous siRNAs (exo-siRNAs) (Lee et al. 2004; Marques et al. 2013).
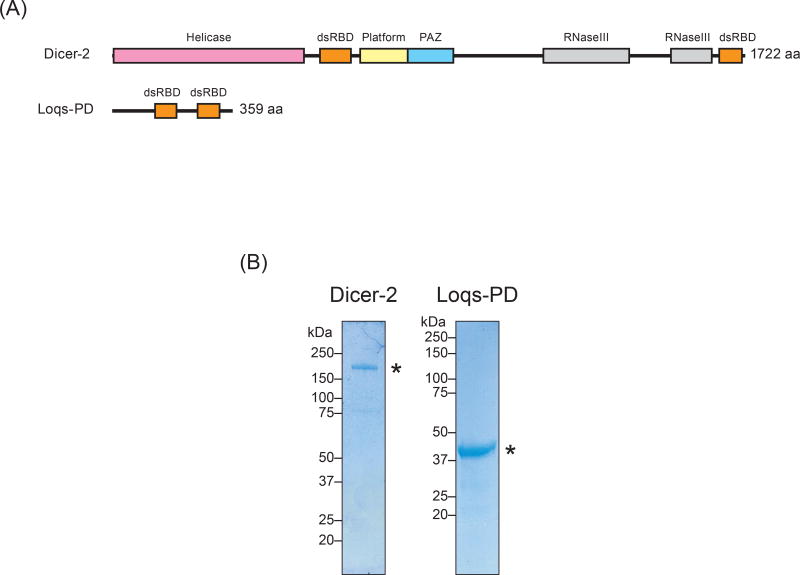
Dicer-2 has an N-terminal helicase domain, a central dsRNA-binding domain (dsRBD), a platform domain, a PAZ domain, two RNase III domains, and a C-terminal dsRBD (Fig. 1A). The Dicer-2 helicase domain binds and hydrolyzes ATP for processive siRNA production (Cenik et al. 2011; Welker et al. 2011). The Dicer-2 PAZ domain has a phosphate-binding pocket important for high-fidelity production of 21 nt siRNAs, which is important for efficient RNA silencing (Kandasamy and Fukunaga 2016). Each RNaseIII domain has an RNaseIII active site, and the two RNase active sites cleave dsRNA. The C-terminal dsRBD is crucial for efficient and high-fidelity production of siRNAs (Kandasamy et al. 2017). Recent cryo-electron microscopy structures of Dicer-2 showed that Dicer-2 adopts L-shape structure, in which the helicase domain forms the shorter arm and the PAZ, platform, and RNase III domains form the longer arm (Sinha et al. 2018).
Figure 1. Recombinant proteins of Drosophila Dicer-2 and Loqs-PD.
(A) Domain structures of Drosophila Dicer-2 and Loqs-PD.
(B) Coomassie-stained SDS-PAGE gels of purified recombinant Dicer-2 and Loqs-PD proteins.
Dicer-2 can be bound by a cofactor dsRNA-binding protein Loquacious-PD (Loqs-PD) (Hartig et al. 2009). Loqs-PD has two dsRBDs (Fig. 1A). The Cterminal region of Loqs-PD binds the Dicer-2 helicase domain (Hartig and Forstemann 2011; Trettin et al. 2017). Loqs-PD is required for efficient production of a subset of siRNAs in vivo (Fukunaga et al. 2012; Marques et al. 2013; Mirkovic-Hosle and Forstemann 2014). Loqs-PD is required for efficient production of hairpin-derived endo-siRNAs (esi-1.1, esi-1.2, and esi-2.1), cis-NAT-derived endo-siRNAs, and exo-siRNAs derived from an inverted repeat transgene (Fukunaga et al. 2012). In contrast, Loqs-PD is dispensable for production of transposon-derived endo-siRNAs (Fukunaga et al. 2012; Mirkovic-Hosle and Forstemann 2014) and virus-derived exo-siRNAs from certain RNA viruses (Marques et al. 2013). It is unknown why Loqs-PD is crucial for production of only a subset of siRNAs. It is also unknown what distinguishes the substrates that require Loqs-PD for efficient processing and those that do not.
We previously showed that inorganic phosphate—a small molecule found in all cell types—specifically inhibits Dicer-2 from processing precursor of microRNAs (pre-miRNAs) and short dsRNAs (Cenik et al. 2011; Fukunaga et al. 2014). Recombinant Dicer-2 alone can efficiently process pre-miRNAs into miRNA-like dsRNA products in vitro. Notably, these miRNA-like dsRNA products produced by Dicer-2 are shorter than the biologically relevant miRNAs produced by Dicer-1. Such miRNA-like dsRNA products should not be produced in vivo. In fact, a physiological concentration of inorganic phosphate inhibits Dicer-2 from processing pre-miRNAs and short dsRNAs (Cenik et al. 2011; Fukunaga et al. 2014). Phosphate inhibition of Dicer-2 is dose-dependent and specific; inorganic phosphate inhibits neither Dicer-2 from processing long dsRNAs into siRNAs nor Dicer-1 from processing pre-miRNAs into miRNAs, and other anions do not inhibit Dicer-2 from processing pre-miRNAs. These studies suggest that inorganic phosphate binds the phosphate-binding pocket in the Dicer-2 PAZ domain and inhibits access of pre-miRNAs and short dsRNAs to the Dicer-2 PAZ domain, inhibiting their cleavage (Fukunaga et al. 2014). However, how inorganic phosphate affects Dicer-2 processing of hairpin RNAs with an intermediate length remains unknown. Whether or how the inhibitory effect of inorganic phosphate on Dicer-2 is modulated by Loqs-PD is also unknown.
To address these questions, I performed in vitro esi-2.1 precursor hairpin RNA processing assay with Dicer-2 in the presence or absence of Loqs-PD and/or inorganic phosphate (Dicer-2 ± Loqs-PD ± inorganic phosphate). I tested hairpin RNA substrates with distinct end structures (5′ monophosphorylated vs 5′ hydroxyl and blunt end vs 3′ overhang end), considering that RNA substrate end structures play a crucial role in processing by Dicer-2 (Welker et al. 2011; Fukunaga et al. 2014; Sinha et al. 2015; Sinha et al. 2018). I found that inorganic phosphate inhibits Dicer-2 alone, but not Dicer-2 + Loqs-PD, from processing esi-2.1 precursor hairpin RNAs with a blunt end. Thus, Loqs-PD allows Dicer-2 to process blunt-end hairpin RNAs in the presence of inorganic phosphate, which may explain the in vivo requirement of Loqs-PD for production of hairpin-derived endo-siRNAs.
MATERIALS AND METHODS
Recombinant protein purification
Recombinant Dicer-2 and Loqs-PD proteins were purified from Sf9 cells and E. coli cells, respectively, as previously described (Cenik et al. 2011; Fukunaga et al. 2012; Fukunaga et al. 2014).
RNA substrates preparation
32P-body-labeled hairpin RNAs were prepared using in vitro T7 transcription system in the presence of α-[32P]ATP (800 Ci/mmol; PerkinElmer) and were gel purified, as previously described (Cenik et al. 2011; Fukunaga et al. 2012).
In vitro Dicing Assays
In vitro RNA processing reactions by Dicer-2 were performed using 100 nM 32P-body-labeled hairpin RNAs, 8 nM Dicer-2 ± Loqs-PD in the presence or absence of 1 mM ATP and/or 25 mM inorganic phosphate at 25°C and analyzed as described (Cenik et al. 2011; Fukunaga et al. 2012; Fukunaga et al. 2014; Liao and Fukunaga 2018). Aliquots of the reaction time course were run on denaturing urea-PAGE gels. Dried gels were exposed to image plates and analyzed with an FLA-9000 and ImageGauge 3.0 software (Fujifilm, Tokyo, Japan).
To determine rates of reaction, substrate processed versus time was fit to y = y0 + A(1 − e −kt), where dy/dt = Ake−kt using Igor Pro 6.31 (WaveMetrics, Lake Oswego, OR, USA). When t = 0, dy/dt = Ak; k gives the initial rate of reaction (Lu and Fei 2003).
Statistical test
Statistical tests were performed using unpaired two-tailed Student’s t-test using data obtained from three independently performed experiments. P-value <0.05 was used as a threshold for statistical significance.
RESULTS
Dicer-2 requires ATP to process hairpin RNAs
To perform in vitro hairpin RNA processing assay with Dicer-2 ± Loqs-PD ± inorganic phosphate, I purified recombinant Dicer-2 protein from Sf9 cells and recombinant Loqs-PD protein from E. coli cells (Fig. 1B). For substrates, I prepared four esi-2.1 endo-siRNA precursor hairpin RNAs with 64 or 62 bp long stems (Okamura et al. 2008b). The hairpin RNAs tested have one of four chemically distinct structures: (1) a 5′ monophosphorylated blunt end, (2) a 5′ hydroxyl blunt end, (3) a 5′ monophosphorylated, 3′ 2-nt overhang end, and (4) a 5′ hydroxyl, 3′ 2-nt overhang end (Fig. 2). I performed in vitro RNA processing assay using the purified recombinant proteins (Dicer-2 alone and Dicer-2 + Loqs8 Fukunaga PD) and these four RNAs in the presence or absence of ATP and/or inorganic phosphate.
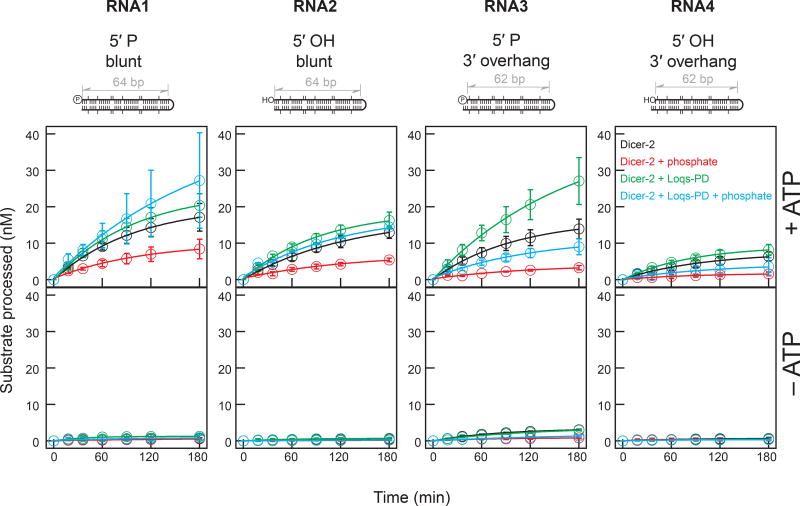
Figure 2. Time course of in vitro processing assay of hairpin RNAs by Dicer-2 ± Loqs-PD.
Hairpin RNAs (100 nM, uniformly 32P-radiolabeled) with (1) a 5′ monophosphorylated blunt end, (2) a 5′ hydroxyl blunt end, (3) a 5′ monophosphorylated, 3′ 2-nt overhang end, or (4) a 5′ hydroxyl, 3′ 2-nt overhang end were incubated with 8 nM Dicer-2 ± Loqs-PD in the presence or absence of 1 mM ATP and/or 25 mM inorganic phosphate. Data are mean ± s.d. for three independent trials.
In the absence of ATP, neither Dicer-2 alone nor Dicer-2 + Loqs-PD cleaved any of the RNA substrates appreciably with or without inorganic phosphate (Fig. 2). In contrast, Dicer-2 and Dicer-2 + Loqs-PD cleaved RNAs in the presence of ATP. I concluded that both Dicer-2 and Dicer-2 + Loqs-PD require ATP to process these hairpin RNA substrates.
Inorganic phosphate inhibits Dicer-2, but not Dicer-2 + Loqs-PD, from processing blunt-end hairpin RNAs
In the presence of ATP, the RNA processing rates differed among Dicer-2 ± Loqs-PD ± inorganic phosphate (Fig. 2). To better compare processing rates among the different conditions, using the time course data (Fig. 2), I determined initial velocities of the processing reactions (Fig. 3).
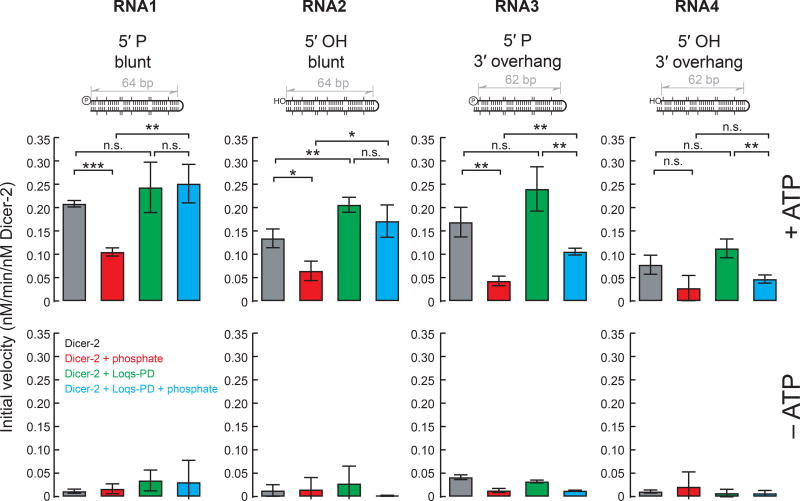
Figure 3. Initial velocities in the in vitro processing assay of hairpin RNAs by Dicer-2 ± Loqs-PD.
Initial velocities determined by the time course assay results shown in Figure 2. Data are mean ± s.d. for three independent trials. P-value <0.05, <0.01, and <0.001 are indicated by *, **, and ***, respectively (Student's t-test).
Inorganic phosphate inhibited Dicer-2 from cleaving RNA1 with a 5′ monophosphorylated blunt end (0.21 ± 0.01 min−1 vs 0.10 ± 0.01 min−1. P-value = 0.000091). Interestingly, however, inorganic phosphate did not inhibit Dicer-2 + Loqs-PD from cleaving RNA1 (0.24 ± 0.05 min−1 vs 0.25 ± 0.04 min−1. P-value = 0.85). Similarly, inorganic phosphate inhibited Dicer-2 (0.13 ± 0.02 min−1 vs 0.06 ± 0.02 min−1. P-value = 0.014), but not Dicer-2 +Loqs-PD (0.21 ± 0.02 min−1 vs 0.17 ± 0.03 min−1. P-value = 0.18) from cleaving RNA2 with a 5′ hydroxyl blunt end. Thus, Loqs-PD enables Dicer-2 to cleave blunt-end hairpin RNAs even in the presence of inorganic phosphate.
Inorganic phosphate inhibits both Dicer-2 and Dicer-2 + Loqs-PD from processing 3′ overhang-end hairpin RNAs
Unlike RNAs 1 and 2, inorganic phosphate inhibited both Dicer-2 (0.17 ± 0.03 min−1 vs 0.04 ± 0.01 min−1. P-value = 0.0028) and Dicer-2 + Loqs-PD (0.24 ± 0.05 min−1 vs 0.11 ± 0.01 min−1. P-value = 0.0083) from cleaving RNA3 with a 5′ monophosphorylated, 3′ overhang end. Inorganic phosphate also inhibited Dicer-2 + Loqs-PD from cleaving RNA4 with a 5′ hydroxyl, 3′ overhang end (0.11 ± 0.02 min−1 vs 0.05 ± 0.01 min−1. P-value = 0.0063). Inorganic phosphate also showed a trend to inhibit Dicer-2 from cleaving RNA4 while it did not reach a statistical significance (0.08 ± 0.02 min−1 vs 0.03 ± 0.03 min−1. P-value = 0.064). Thus, unlike blunt-end hairpin RNAs, Loqs-PD cannot enable Dicer-2 to cleave 3′ overhang-end hairpin RNAs in the presence of inorganic phosphate.
DISCUSSION
My studies here show that Loqs-PD enables Dicer-2 to cleave blunt-end hairpin RNAs in the presence of inorganic phosphate. Similarly, previous studies showed that Loqs-PD enables Dicer-2 to cleave RNA substrates normally refractory to cleavage, such as dsRNA with blocked, structured, or frayed ends (Sinha et al. 2015; Trettin et al. 2017).
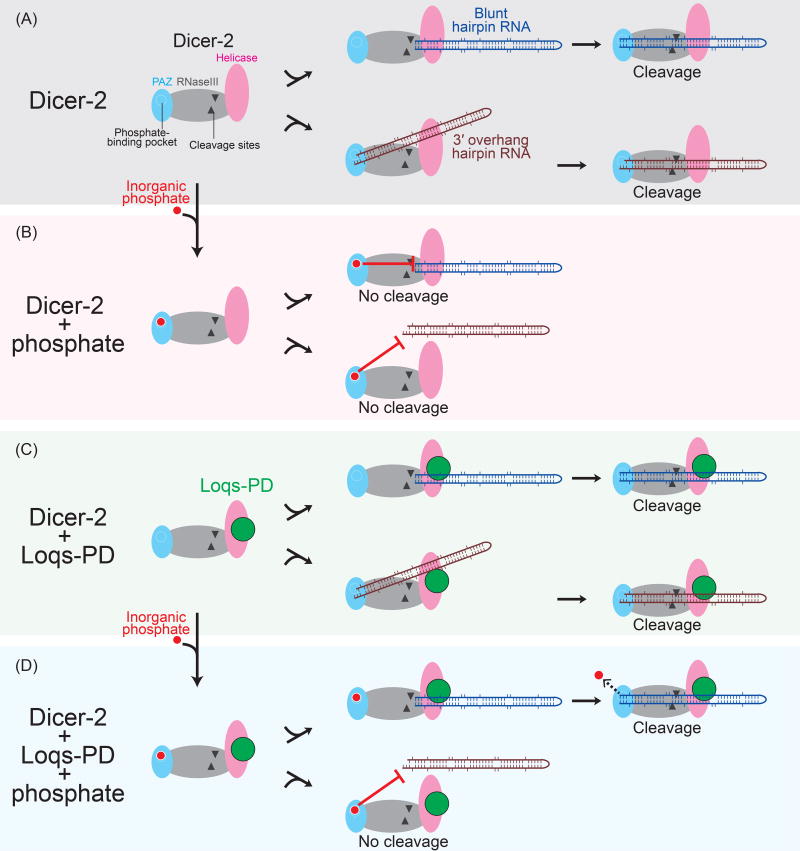
Based on the results in this study and past studies, I propose a model of how Loqs-PD enables Dicer-2 to process blunt-end hairpin RNAs in the presence of inorganic phosphate (Fig. 4). Previous biochemical and structural studies revealed that Dicer-2 processes blunt-end RNA substrates and 3′ overhang-end RNA substrates differently (Welker et al. 2011; Fukunaga et al. 2014; Sinha et al. 2015; Trettin et al. 2017; Sinha et al. 2018). Blunt-end hairpin RNA substrates are bound by the Dicer-2 helicase domain (Fig. 4A, top). The blunt-end hairpin RNA substrates are threaded through the Dicer-2 helicase domain until their ends reach the Dicer-2 PAZ domain. Then the blunt-end hairpin RNA substrates are cleaved by the Dicer-2 RNase III active sites. In contrast, 3′ overhang-end hairpin RNA substrates are bound by the Dicer-2 PAZ domain and then the 3′ overhang-end hairpin RNAs are aligned to the RNase III active sites for cleavage (Fig. 4A, bottom).
Figure 4. Model.
(A–D) Models how inorganic phosphate inhibits Dicer-2 from processing hairpin RNAs and how Loqs-PD removes this inhibitory effect. Models are shown for (A) Dicer-2 alone, (B) Dicer-2 + phosphate, (C) Dicer-2 + Loqs-PD, and (D) Dicer-2 + phosphate + Loqs-PD. The background colors of the panels (A-D) correspond to the colors used in Figures 2 and 3.
In vivo at a physiological concentration of inorganic phosphate, inorganic phosphate binds the phosphate-binding pocket in the Dicer-2 PAZ domain and inhibits access of RNA substrates to the PAZ domain, inhibiting their cleavage (Fig. 4B) (Fukunaga et al. 2014). The inhibition occurs to both blunt-end hairpin RNAs and 3′ overhang-end hairpin RNAs.
Loqs-PD binds the Dicer-2 helicase domain (Hartig and Forstemann 2011; Trettin et al. 2017). A blunt-end long dsRNA substrate and a 3′ overhang-end long dsRNA substrate are bound by the Dicer-2 helicase domain differently (Sinha et al. 2018). Therefore, Loqs-PD bound to the Dicer-2 helicase domain is also expected to interact blunt-end hairpin RNA substrates and 3′ overhang-end hairpin RNA substrates differently (Fig. 4C).
Loqs-PD increases the binding affinity of a blunt-end long dsRNA substrate to Dicer-2 (Trettin et al. 2017). The binding affinity increase of hairpin RNA substrates by Loqs-PD allows blunt-end hairpin RNAs to displace a bound inorganic phosphate from the binding pocket in the Dicer-2 PAZ domain, enabling efficient cleavage of the blunt-end hairpin RNAs (Fig. 4D, top). In contrast, due to a different binding mode of Loqs-PD between blunt-end hairpin RNAs and 3′ overhang-end hairpin RNAs, Loqs-PD cannot help 3′ overhang-end hairpin RNAs to displace a bound inorganic phosphate from the Dicer-2 PAZ domain (Fig. 4D, bottom). Thus, Loqs-PD enables Dicer-2 to process blunt-end hairpin RNAs, but not 3′ overhang-end hairpin RNAs, in the presence of inorganic phosphate.
Loqs-PD is required for efficient production of a subset of siRNAs in vivo (Fukunaga et al. 2012; Marques et al. 2013; Mirkovic-Hosle and Forstemann 2014). Different RNA substrate end structures may determine if Loqs-PD can enable Dicer-2 to process substrate RNAs in the presence of inorganic phosphate. For some RNA substrates, including blunt-end hairpin RNAs, processing by Dicer-2 is inhibited by phosphate while processing by Dicer-2 + Loqs-PD is not. Such RNA substrates require Loqs-PD for siRNA production. In contrast, for other RNA substrates, phosphate does not inhibit Dicer-2 from processing. Such RNA substrates do not require Loqs-PD for siRNA production. This may explain partially their different dependencies on Loqs-PD in siRNA production in vivo.
Regulation of Dicer activities by inorganic phosphate and its modulation by Loqs-PD orthologue may be widely conserved. Inorganic phosphate activates Arabidopsis Dicer-like 3 (DCL3) activity while it inhibits Arabidopsis Dicer-like 4 (DCL4) activity to process a 3′ overhang-end 50 nt dsRNA into siRNAs (Nagano et al. 2014). Phosphate deficiency in Arabidopsis seedlings inhibits DCL3 activity in a cell-free extract assay system (Seta et al. 2017). DCL3 activity in these phosphate-deficient seedling extract is activated directly by adding inorganic phosphate in the cell-free assay system (Seta et al. 2017). DCL3 binds a dsRNA-binding (DRB) protein cofactor DRB3 (Raja et al. 2014) while DCL4 binds DRB4, both of which are Drosophila Loqs-PD orthologues (Nakazawa et al. 2007). These Arabidopsis Dicer cofactor DRB proteins may modulate the effects of inorganic phosphate on the DCL3 and DCL4 activities. Similarly, inorganic phosphate may regulate human Dicer activity as well considering that crystal structures showed that inorganic phosphate binds the human Dicer PAZ domain (Tian et al. 2014). Human Dicer cofactor dsRNA-binding proteins TRBP and PACT (Loqs orthologues) may modulate effects of inorganic phosphate on human Dicer activity to generate small silencing RNAs.
Supplementary Material
Highlights.
Inorganic phosphate inhibits Dicer-2 processing of hairpin RNAs.
Phosphate does not inhibit Dicer-2 + Loqs-PD processing of blunt-end hairpin RNAs.
Loqs-PD removes phosphate inhibition of Dicer-2 processing of blunt hairpin RNAs.
Acknowledgments
I am grateful to Susan E. Liao and Li Zhu, members of Fukunaga lab, for their comments on the manuscript. This work was supported by the grants from American Heart Association [15SDG23220028] and the National Institutes of Health [R01GM116841] to RF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceptualization, Methodology, Investigation, Writing, Funding Acquisition, R.F.
Competing interests
The author has no conflicts of interest to declare.
References
- Cenik ES, Fukunaga R, Lu G, Dutcher R, Wang Y, Tanaka Hall TM, Zamore PD. Phosphate and R2D2 restrict the substrate specificity of Dicer-2, an ATP-driven ribonuclease. Molecular cell. 2011;42:172–184. doi: 10.1016/j.molcel.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, Perrimon N, Kellis M, Wohlschlegel JA, Sachidanandam R, et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R, Colpan C, Han BW, Zamore PD. Inorganic phosphate blocks binding of pre-miRNA to Dicer-2 via its PAZ domain. The EMBO journal. 2014;33:371–384. doi: 10.1002/embj.201387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R, Han BW, Hung JH, Xu J, Weng Z, Zamore PD. Dicer partner proteins tune the length of mature miRNAs in flies and mammals. Cell. 2012;151:533–546. doi: 10.1016/j.cell.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M, Seitz H, Horwich MD, Li C, Du T, Lee S, Xu J, Kittler EL, Zapp ML, Weng Z, et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science. 2008;320:1077–1081. doi: 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig JV, Esslinger S, Bottcher R, Saito K, Forstemann K. Endo-siRNAs depend on a new isoform of loquacious and target artificially introduced, high-copy sequences. The EMBO journal. 2009;28:2932–2944. doi: 10.1038/emboj.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig JV, Forstemann K. Loqs-PD and R2D2 define independent pathways for RISC generation in Drosophila. Nucleic acids research. 2011;39:3836–3851. doi: 10.1093/nar/gkq1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy SK, Fukunaga R. Phosphate-binding pocket in Dicer-2 PAZ domain for high-fidelity siRNA production. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:14031–14036. doi: 10.1073/pnas.1612393113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy SK, Zhu L, Fukunaga R. The C-terminal dsRNA-binding domain of Drosophila Dicer-2 is crucial for efficient and high-fidelity production of siRNA and loading of siRNA to Argonaute2. Rna. 2017;23:1139–1153. doi: 10.1261/rna.059915.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Saito K, Kin T, Ono Y, Asai K, Sunohara T, Okada TN, Siomi MC, Siomi H. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–797. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- Liao SE, Fukunaga R. Kinetic Analysis of Small Silencing RNA Production by Human and Drosophila Dicer Enzymes In Vitro. Methods in molecular biology. 2018;1680:101–121. doi: 10.1007/978-1-4939-7339-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WP, Fei L. A logarithmic approximation to initial rates of enzyme reactions. Analytical biochemistry. 2003;316:58–65. doi: 10.1016/s0003-2697(03)00034-4. [DOI] [PubMed] [Google Scholar]
- Marques JT, Wang JP, Wang X, de Oliveira KP, Gao C, Aguiar ER, Jafari N, Carthew RW. Functional specialization of the small interfering RNA pathway in response to virus infection. PLoS pathogens. 2013;9:e1003579. doi: 10.1371/journal.ppat.1003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic-Hosle M, Forstemann K. Transposon defense by endo-siRNAs, piRNAs and somatic pilRNAs in Drosophila: contributions of Loqs-PD and R2D2. PloS one. 2014;9:e84994. doi: 10.1371/journal.pone.0084994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano H, Fukudome A, Hiraguri A, Moriyama H, Fukuhara T. Distinct substrate specificities of Arabidopsis DCL3 and DCL4. Nucleic acids research. 2014;42:1845–1856. doi: 10.1093/nar/gkt1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Hiraguri A, Moriyama H, Fukuhara T. The dsRNA-binding protein DRB4 interacts with the Dicer-like protein DCL4 in vivo and functions in the trans-acting siRNA pathway. Plant molecular biology. 2007;63:777–785. doi: 10.1007/s11103-006-9125-8. [DOI] [PubMed] [Google Scholar]
- Okamura K, Balla S, Martin R, Liu N, Lai EC. Two distinct mechanisms generate endogenous siRNAs from bidirectional transcription in Drosophila melanogaster. Nature structural & molecular biology. 2008a;15:581–590. doi: 10.1038/nsmb.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Chung WJ, Ruby JG, Guo H, Bartel DP, Lai EC. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature. 2008b;453:803–806. doi: 10.1038/nature07015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja P, Jackel JN, Li S, Heard IM, Bisaro DM. Arabidopsis doublestranded RNA binding protein DRB3 participates in methylation-mediated defense against geminiviruses. Journal of virology. 2014;88:2611–2622. doi: 10.1128/JVI.02305-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta A, Tabara M, Nishibori Y, Hiraguri A, Ohkama-Ohtsu N, Yokoyama T, Hara S, Yoshida K, Hisabori T, Fukudome A, et al. Post-Translational Regulation of the Dicing Activities of Arabidopsis DICER-LIKE 3 and 4 by Inorganic Phosphate and the Redox State. Plant & cell physiology. 2017;58:485–495. doi: 10.1093/pcp/pcw226. [DOI] [PubMed] [Google Scholar]
- Sinha NK, Iwasa J, Shen PS, Bass BL. Dicer uses distinct modules for recognizing dsRNA termini. Science. 2018;359:329–334. doi: 10.1126/science.aaq0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha NK, Trettin KD, Aruscavage PJ, Bass BL. Drosophila dicer-2 cleavage is mediated by helicase- and dsRNA termini-dependent states that are modulated by Loquacious-PD. Molecular cell. 2015;58:406–417. doi: 10.1016/j.molcel.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Simanshu DK, Ma JB, Park JE, Heo I, Kim VN, Patel DJ. A Phosphate-Binding Pocket within the Platform-PAZ-Connector Helix Cassette of Human Dicer. Molecular cell. 2014 doi: 10.1016/j.molcel.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettin KD, Sinha NK, Eckert DM, Apple SE, Bass BL. Loquacious-PD facilitates Drosophila Dicer-2 cleavage through interactions with the helicase domain and dsRNA. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E7939–E7948. doi: 10.1073/pnas.1707063114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker NC, Maity TS, Ye X, Aruscavage PJ, Krauchuk AA, Liu Q, Bass BL. Dicer's helicase domain discriminates dsRNA termini to promote an altered reaction mode. Molecular cell. 2011;41:589–599. doi: 10.1016/j.molcel.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.